Abstract
Leishmania infection consists in two sequential events, the host cell colonization followed by the proliferation/dissemination of the parasite. In this review, we discuss the importance of two distinct sets of molecules, the secreted and/or surface and the nonsecreted antigens. The importance of the immune response against secreted and surface antigens is noted in the establishment of the infection and we dissect the contribution of the nonsecreted antigens in the immunopathology associated with leishmaniasis, showing the importance of these panantigens during the course of the infection. As a further example of proteins belonging to these two different groups, we include several laboratorial observations on Leishmania Sir2 and LicTXNPx as excreted/secreted proteins and LmS3arp and LimTXNPx as nonsecreted/panantigens. The role of these two groups of antigens in the immune response observed during the infection is discussed.
1. INTRODUCTION
Leishmaniasis are parasitic diseases, caused by protozoan parasites of the Leishmania genus, associated with significant morbidity and mortality in tropical and subtropical regions and in the Mediterranean basin. The disease has a wide range of clinical manifestations that depend not only on the infecting Leishmania species but also on the immune status of the host [1]. The most extensively studied leishmanial disease is the cutaneous form caused by L. major or L. tropica in the old world and L. braziliensis in the new world. It usually appears as a skin ulcer or dermal granuloma, which may take up to several months or years to heal [2]. With L. braziliensis, the infection may also spread to other cutaneous sites, like mucosal membranes giving origin to the mucocutaneous form of the disease. The most serious form of the disease is the visceral one that, if untreated, gives rise to a high mortality rate. It is characterized by fever, cachexia, hepatosplenomegaly, and hypergamaglobulinemia and is caused by members of the L. donovani complex (L. donovani in the old world, L. infantum in the Mediterranean basin and L. infantum chagasi in the New World) [3].
Leishmania is a digenetic protozoan that is transmitted to the mammalian host by sandflies of the genus Phlebotomus in the old world and Lutzomyia in the new world. In the alimentary tract of the insect vector, the parasite exists extracellularly as a flagellated motile form, the promastigote. During the insect blood meal, the infectious developmental form, metacyclic promastigotes, is injected into the dermis and phagocyted by resident macrophages within which the parasite differentiates into the nonmotile amastigote form and multiplies. Moreover, other cells such as fibroblasts and dendritic cells may also harbour parasites [4]. The cycle is completed when the sandfly takes another blood meal recovering free amastigotes or infected macrophages.
During an infection, the parasites have a remarkable adaptative capacity as they are able to survive inside phagocytic cells. These cells are responsible for the microbicidal and antigen-presenting functions however they serve as a safe habitat for the parasite. The existence of inbred mice, which are either susceptible (Balb/c) or resistant to infection (C57BL/6, CBA, C3H.HeJ) has helped to elucidate the protective or nonprotective role of cytokine and T-helper cell subsets and also the role of different leishmanial antigens in the immune evasion mechanism. Thus, it became generally accepted that resistance against leishmaniasis is associated with the production of IL-12 by antigen presenting cells (APC) macrophages and dendritic cells, leading to the differentiation and proliferation of the Th1-subset of CD4+ T-cells producers of IFN-γ. This will ultimately lead to the activation of parasite-infected macrophages that, through the induction of effector molecules as nitrogen and oxygen reactive species, will kill the intracellular parasites [5]. In contrast, failure to control the infection has been associated with the production of anti-inflammatory cytokines as IL-4, IL-10, IL-13 and TGF-β [6]. Given the ancient evolutionary divergence in Leishmania species, it is not surprising that the control of the different Leishmania driven diseases is related to different immunological properties. Hence, while in cutaneous leishmaniasis, IL-4 has been implicated in disease progression, in visceral leishmaniasis its importance has been ruled out [7]. In the latter, IL-10 has been shown to be the major immunosuppressive cytokine along with TGF-β. Overall, it suggests that it is the overshadowing of the Th2 response by a Th1 cell associated response that leads to the control of the infection [8]. Moreover, the real contribution of the humoral response is still under debate, however studies in different intracellular pathogens have shown that antibodies can also have a function in restricting the infection when the parasite is exposed to the extracellular milieu [9]. Consequently, in leishmaniasis, the induction of specific humoral responses to parasite antigens would, theoretically, be able to neutralize the parasite whether as free promastigotes, after the inoculum, or as amastigotes, when released from the infected macrophages, contributing to develop a protective response [10]. However, until now, no effective vaccine against human leishmaniasis is available for clinical use [3].
Leishmania parasites inside their hosts do not behave inertly. Rather, the virulence related to their pathology seems to be linked to an induced lack of immune response control. The parasite actively secretes proteases and other molecules that affect host immune system (cells and cytokines) facilitating the infection process. In addition, the parasite possesses intracellular nonsecreted antigens, members of conserved protein families, which are believed to contribute to the chronic immunopathology, observed in leishmaniasis. Here, we review these two groups of relevant parasite molecules, illustrated with laboratory observations of proteins belonging to the secreted and nonsecreted groups of antigens. Finally, we discuss their differential role in Leishmania infection and persistence as well in the development of a protective immune response.
1.1. The importance of the secreted versus nonsecreted antigens
Leishmania virulence has been explained using two different groups of parasite molecules, the secreted and surface and the intracellular molecules [11]. This model proposes that the secreted and surface molecules will be mostly important for the establishment of infection, protecting the parasite from the early action of the host immune system, acting as invasive/evasive determinants. According to this model, the intracellular molecules will be ultimately responsible for the disease phenotype [11].
1.2. Surface and secreted molecules
The secreted proteins have distinct functions during Leishmania infection. First, they play a role in the establishment of the infection [12] in conjunction with important elements existent in the saliva of the sandfly vector [13, 14]. In a second phase, they contribute to the maintenance of the infection by interfering with the macrophagic microbicidal functions, cytokine production, antigen presentation, and effector cells activation. This is achieved by repression of gene expression, post-translation protein modification or degradation, and by activation of suppressive pathways and molecules [15]. This macrophagic anergy enables the continuous multiplication of the amastigote form. The bulk of the knowledge on surface and secreted molecules of Leishmania is focused on lypophosphoglycan (LPG), on the promastigote surface protease named glycoprotein 63 (gp63), glycosylinositol phospholipids (GIPLs), cysteine peptidases and on a few others like β-mercaptoethanol activated proteases, acid phosphatases and chitinases. The importance of some of these molecules in the establishment of the infection is well documented [15, 16], but the real contribution of the secreted molecules remains elusive due to the difficulty of the intramacrophagic studies.
After entrance into a susceptible mammalian host, the Leishmania promastigotes are targeted by the host immune system. Serum components, like the complement system represent the first challenge following entrance into the bloodstream. Procyclic promastigotes are highly susceptible to complement action, unlike the metacyclic that can avoid complement mediated lysis [17]. This remarkable difference is mostly due to the surface molecule in Leishmania, the LPG. Composed mainly of repetitive units of a disaccharide and a phosphate, LPG is linked to the membrane by a glycosylphosphatidylinositol anchor [18]. The LPG is longer in metacyclic promastigotes preventing the attachment of C5b-C9 subunits of the complement complex avoiding its lytic action [17]. The relevance of LPG is not limited to complement resistance. Its importance is stated by several studies using either purified LPG or mutant strains. The LPG is implicated in several processes including the binding to the epithelial cells of the sandfly midgut [19], receptor mediated phagocytosis of macrophages through the CR3/CR1 ligand or the manose-fucose receptor (in conjunction with gp63) [20, 21], toll-like receptor 2 signalling [22], stimulation of NK cells [23], inhibition of phagosome-endosome fusion [24–26], and inhibition of phagosome-derived superoxide [27]. Several attempts to use LPG to confer protection were unproductive [28, 29]. Constitutively shed by several Leishmania species, the LPG is the paradigm molecule referred to as evasive and invasive. After the initial steps of infection, LPG is downregulated being almost absent from amastigotes [30].
Another molecule implicated in the invasive and evasive mechanisms is gp63. This protein is the most abundant in the parasite surface, although 10 fold less abundant than LPG [30]. In the promastigote form, gp63 is in the surface of the parasite under the LPG coat and is involved in L. donovani promastigote multiplication [31]. Like LPG, gp63 was shown to be implicated in complement resistance, in L. major and L. amazonensis, by mediating the interconvertion of C3b to C3bi [32]. This interconvertion favours the internalization via CR3 avoiding the oxidative burst. The binding of gp63 to fibronectin receptors favours the parasite uptake into the macrophage [33]. Furthermore, gp63 is an endopeptidase with the potential to degrade immunoglobulins, complement factors, and lysosomal proteins [34]. The optimal proteolitic activity of gp63 is at pH 4 that may indicate some active proteolitic function in the amastigote stage [34, 35]. Despite this, gp63 expression is downregulated in amastigotes [36]. In spite of being a virulence factor in most Leishmania species, immunization trials with gp63 were unable to protect mice from infectious challenge [37]. Moreover, gp63 mutation in L. major did not impair in vitro intramacrophagic survival [38]. So the importance of gp63 in the course of the infection remains elusive. The GIPLs are molecules 10 times more abundant than LPG on the parasite surface, although like gp63 they are physically under the LPG coat [39]. The GIPLs were described in L. major as having a protective role at the parasite surface by modulating the expression of nitric oxide synthetase in murine macrophages [40, 41]. Another interesting group of proteins are the cysteine proteases. In L. mexicana, this family of proteins seems to be associated with disease progression [42]. Cysteine protease activity can be found at the parasite surface or inside the macrophage endoplasmatic reticulum, probably associated with proteases released in the phagolysosome by Leishmania. The inhibition of major histocompatibility complex class II molecules in macrophages seems to involve, in L. amazonensis, the direct sequestering of these molecules following cysteine-peptidase-dependent degradation [43, 44]. Also, cysteine peptidase activity was demonstrated in L. mexicana to induce IL-12 repression and degradation of NF-kB [45]. It is still worthy to mention some other secreted proteins described as virulence factors, like the L. mexicana chitinase [46] and the L. donovani acid phosphatases [47–50]. An in depth study of the Leishmania secretome is missing. The most remarkable effort was done by Chenik and colleagues that were able to screen 33 different proteins using an L. major cDNA library and a rabbit immune sera raised against the secreted proteins [51]. Nine of them were already described as excreted/secreted proteins in Leishmania or other species, 11 corresponded to known proteins but not characterized as secreted and the other 13 were completely new and uncharacterized proteins [51]. This shows how little is known about the Leishmania secretome since only a few proteins are extensively characterized [52–56] . It is already known that total L. major secreted molecules, described as highly immunogenic [54, 57–59], can confer protection from infectious challenge [57, 59]. So it is obvious that somewhere among the Leishmania secreted proteins exist future candidates for vaccine design and drug targets. Nonetheless, one of the problems in vaccine design using surface or secreted/excreted proteins is the fact that these proteins are naturally exposed to the immune system. Chang et al. suggest that these secreted/excreted proteins were evolutionarily selected becoming immunologically “silent” [60]. This fact implies that secreted proteins that have a specific function in the establishment of the infection will be “silent,” allowing them to perform their vital functions unchecked by the host immune system [11, 12]. This will be more significant for the proteins involved in the first steps of infection, while the parasite is still exposed to the extracellular environment. As an example of this fact, we present three distinct proteins: a cytosolic tryparedoxin peroxidase of L. infantum (LicTXNPx) [61], the Leishmania silent information regulator 2 (Sir2) [52], and a tryparedoxin of L. infantum (LiTXN1) [62]. All are Leishmania secreted proteins (Figure 1) [52], that show distinct immunological properties. A high antibody titre against the LicTXNPx was detected in children [63]. This antibody titre is maintained during the Leishmania infection and decreases after its resolution [63]. Despite its high immunogenicity when tested in vitro or in vivo using the Balb/c model, this excreted/secreted protein did not show immunomodulatory properties (Figures 4, 5 and Table 1) and provided no protection against the infectious challenge (data not shown). On the other hand, the Leishmania Sir2 is a typical poorly immunogenic secreted antigen (Figure 2) characterized as a virulence factor [64]. Infectious challenge after Leishmania Sir2 immunization results in a decreased infectivity in the acute phase (Figure 3). This could be partially due to the production of lytic and neutralizing antibodies [65]. The immunization leads to a significant decrease of the spleen and liver parasite load at two weeks post infection (Figure 3) [65]. However, it is incapable by itself of resolving the infection, as seen six weeks after infection, where there is no significant difference between the immunized infected group and the infected control group (Figure 3). Certain secreted proteins seem to function as immunomodulatory components, acting as host immune evasive proteins. As an example, another excreted/secreted Leishmania protein, LiTXN1 (Figure 1), is capable to increase IL-10 splenocyte secretion (Table 1), a major immunosuppressive cytokine (manuscript in preparation). LiTXN1 can be among the proteins responsible for a transient immunosuppressive state that can favour the parasite internalization and colonization of the host cells. These examples show that among the secreted proteins we can find proteins naturally immunogenic, albeit nonprotective, like LicTXNPx while others less immunogenic show interesting properties in terms of protection probably due to the disruption of their in vivo functions, Leishmania Sir2, or by their immunomodulatory properties, LiTXN1. Unfortunately, the reduced immunogenicity of the most interesting secreted proteins probably will prevent their identification by serological based approaches [51].
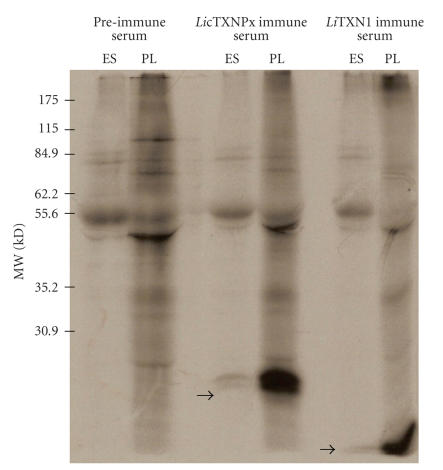
Figure 1.
The LicTXNPx and LiTXN1 are excreted/secreted proteins. Autoradiography of [35S] methionine labelled L. infantum promastigotes lysate (PL) and excreted/secreted antigens (ES), after 3 hours of incubation experiments, immunoprecipitated in the presence of immune anti-LicTXNPx or anti-LiTXN1 sera or with a preimmune serum.
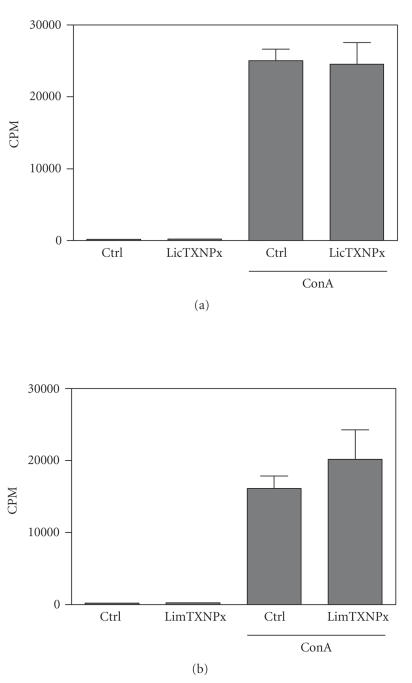
Figure 4.
No effect of rLicTXNPx and rLimTXNPx (a) on spleen cell proliferation. Spleen cells from normal Balb/c mice were cultured for 48 hours (2.5 × 105 cells/well) in the presence or absence of concanavalin A (ConA) (5 μg/ml) with or without rLicTXNPx and rLimTXNPx (b) (10 μg/ml). The cells were pulsed with [methyl-3H] thymidine in the last 8 hours of culture, and cpm (scintillations per minute) were determined. The data represent mean cpm and standard deviations from triplicate cultures of spleen cells from three mice analyzed individually. One of three independent experiments is depicted.
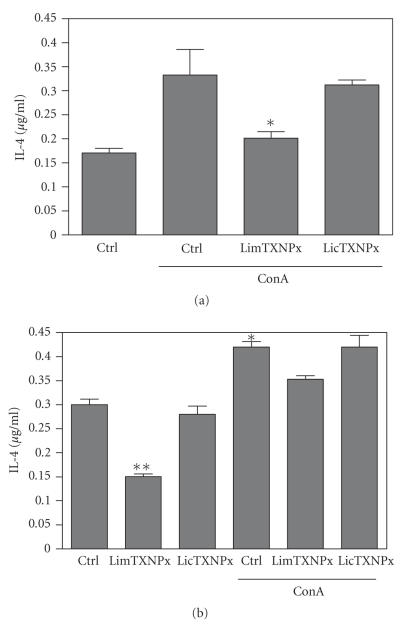
Figure 5.
Levels of IL-4 in the supernatants of spleen cells from rLicTXNPx or rLimTXNPx treated and untreated Balb/c mice. The spleen cells from untreated (a) and treated (b) Balb/c mice (50 μg of rLicTXNPx or rLimTXNPx i.p. injected once a week for 3 weeks followed by 2 weeks before the spleen cells were recovered) were incubated with rLicTXNPx or rLimTXNPx (10 μg/ml) in the presence or absence of ConA (5 μg/ml) for 48 hours. The levels of IL-4 were determined by ELISA in comparison with a standard curve using the recombinant IL-4. The data represent means and standard deviations for triplicate cultures of spleen cells from three mice. The results are from a representative experiment of three carried out independently. Statistical analysis was performed using Student t-test. Statistically, significant differences is indicated. *P < .05 and **P < .01.
Table 1.
Immunomodulatory properties of several Leishmania proteins.
| Protein | Properties | References |
| Leishmania Sir2 | Secreted, B-cell activator, induces lytic, and neutralizing antibodies | [64, 65] |
| LicTXNPx | Secreted, elicits strong humoral response and has no influence on | [63] |
| cytokine production | ||
| LimTXNPx | Nonsecreted, decreases IL-4 secretion both in vitro and in vivo | Figure 3 |
| LiTXN1 | Secreted, poorly immunogenic, induces IL-10 secretion both in vitro | (Manuscript in |
| and in vivo | preparation) | |
| LmS3arp | Nonsecreted, B-cell polyclonal activator, inhibits T-cell proliferation | [67] |
| and downregulate IL-2, 12 and IFN-γ in splenocytes | ||
Figure 2.
Antibodies against Leishmania SIR2 protein in the sera of chronically L. infantum infected Balb/c mice. Sera from 108 intraperitoneal (i.p.) L. infantum promastigotes infected Balb/c mice after 2, 8, 11, 15, 17, and 19 weeks, were used in a western blot against 1 μg of rLiSIR2 at two different dilutions, 1 : 200 and 1 : 50 (left and right lanes, respectively, for each different serum). A 0 weeks serum was obtained from noninfected mice.
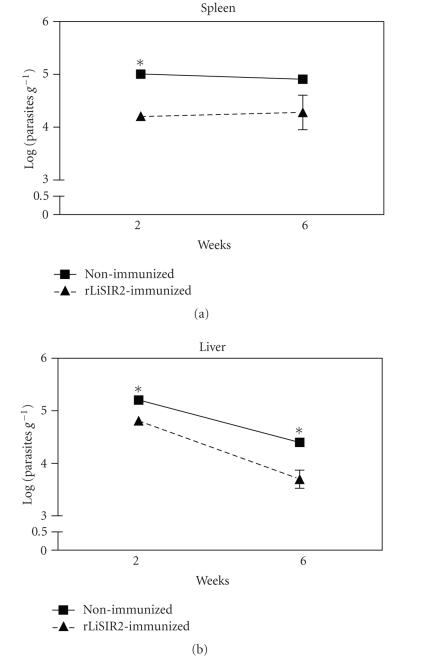
Figure 3.
The recombinant Leishmania SIR2 immunization reduces the parasite load in an acute phase of L. infantum Balb/c mice infection. The immunized mice (▴) received 3 i.p. injections of recombinant Leishmania SIR2 (50 μg) once a week and infected 2 weeks after the last immunization with 108 L. infantum stationary phase promastigotes. The nonimmunized mice (▪) were subjected to the same protocol but received PBS instead of recombinant Leishmania SIR2. The mice were sacrificed after 2 and 6 weeks of infection and the parasite load in the spleen and liver determined by the organ limiting dilution method [66]. The data represent means and standard deviations for three mice and are representative of two independent experiments. Statistical analysis was performed using Student t-test. Statistically, significant differences between immunized and nonimmunized mice are indicated. *P < .05.
The reduction of the secreted/excreted proteins to the given examples is an oversimplification. However, it is obvious that much more work is needed in this area, especially in the huge black hole of knowledge that concerns the interaction between host cell and Leishmania at a molecular level. Since most of the studies have been done using infection-phenotype approaches, little is known about the true agents involved in macrophagic disruption [16, 58, 68, 69]. We suggest that amastigote secreted proteins will be more immunogenic and can have interesting immunomodulatory properties since they have not been under the selective pressure as the promastigote secreted proteins. The selective pressure of the host immune system is a powerful driving force in evolution, as demonstrated in the case of Schistosoma mansoni that has the ability to completely evade the host immune system rendering itself “invisible” [70].
1.3. Panantigens—nonsecreted proteins
Human visceral leishmaniasis, unlike cutaneous leishmaniasis is characterized by high anti-Leishmania antibody titres [71, 72]. The role of these antibodies is still unclear as there seems to be no relation with the progression or resolution of the infection [58, 73, 74]. This exuberant humoral response against promastigote and amastigote antigens (fractions or total protein extract or specific Leishmania proteins) has been exploited for serodiagnosis with different degrees of success [58, 63, 74, 75]. Interestingly, one of the most sensitive techniques using recombinant Leishmania proteins does not involve surface molecules like LPG or gp63 but intracellular proteins like histones [75]. The screening of Leishmania expression libraries or total protein extract with serum from infected patients has unveiled several major immunogens [76–79]. Among these immunogens, nonsecreted proteins like heat shock proteins, ribosomal proteins and histones were described [76, 77, 80]. These highly-conserved proteins that elicit strong immune responses are generally designated as panantigens [81]. The elevated antibody titre against conserved proteins can be the direct result of B-lymphocytes polyclonal activation similar to what is found in Chagas disease [82, 83] or in autoimmune diseases [84]. Furthermore, in the Balb/c mouse model, an L. major protein homologue to the mammalian ribosomal protein S3a, LmS3arp, (Table 1) is able to elicit an unspecific activation of B-lymphocytes with the production of autoreactive antibodies [67]. Despite this, in natural infections, the humoral and cellular responses are highly specific with no significant autoantibody production [80, 81, 85]. Moreover, the epitope mapping of several Leishmania panantigens tends to reveal Leishmania unique epitopes that elicit strong immune responses [79–81, 86, 87]. There is practically no response to the homologous regions in these proteins, which argues against the nonspecific polyclonal activation as the source of reactivity against Leishmania panantigens [11, 81]. So, it is expected that these proteins are presented to the immune system during the natural course of the infection. Unlike secreted and surface proteins that are exposed and can be processed by the host immune system, the intracellular proteins are not. One must expect that the contact between the immune system and these proteins happens only upon the parasite destruction. Subsequently, one obvious source of intracellular proteins is the parasites from the initial inoculum some of which are destroyed. Furthermore, it was recently demonstrated that the presence of apoptotic parasites in the initial inoculum is a requisite for disease development [88]. Albeit the small number of parasites in the initial inoculum is not sufficient to explain the physical expansion of cell populations and immune mediators during the course of infection, it is a fact that panantigens are exposed long before the onset of any visible symptoms [88]. This initial release of panantigens may function in conjugation with the secreted and surface proteins acting as a transient “smoke screen” that enables the onset of the initial infection by viable parasites. The immune response developed against the panantigens may contribute to hide the parasite molecules involved in the invasion of the phagocytic cells. Moreover, the humoral profile suggests a steady release of panantigens during the infection [58, 73, 74]. It is also [81] suggested that panantigens originate from the residing parasite population either by the destruction of intracellular amastigotes by active macrophages or by the destruction of amastigotes that burst from macrophages or even by the spontaneous cytolysis of amastigotes inside the infected cells [11]. In active leishmaniasis, there seems to be a general anergy in infected macrophages that leads to impaired functioning [16, 89–92]. So, in this case, it is not expected that panantigens may result from the macrophage mediated elimination of Leishmania, as it will lead to the resolution of the infection. Although free amastigotes can infect macrophages directly, they are almost undetectable even in heavily infected hosts. Thus, their contribution to the pool of panantigens should be diminished [11]. The low speed of intracellular amastigotes multiplication and their capacity to delay apoptosis in heavily infected phagocytes [60] enables a lasting coexistence in infected macrophages. The most viable theory for the phased release of panantigens would be the spontaneous cytolysis (described as apoptosis by some authors) of intracellular amastigotes [93]. The effect of the panantigen release is gradual and more significant as the infection develops and the parasite burden augments explaining the increasing intense immunopathology associated with Leishmania infection [11]. This increase in panantigen release can be extrapolated in correlation with panantigen antibody titres and parasite burden as seen for the Leishmania kinesin like protein, k39 [81, 94]. Another protein that shows similar characteristics to k39 is the LicTXNPx which has also the ability to induce a high quantity of nonprotective antibodies both in natural or experimentally infected dogs (unpublished data) and in infected humans [63]. This induction can be done by direct activation on B-cell populations with clonal expansion as described for Leishmania Sir2 [65], which seems not to be the case since little or no antibodies for LicTXNPx are seen in HIV patients with leishmaniasis (unpublished data), as was observed for k39 [95]. This suggests the existence of specific T-cell epitopes in LicTXNPx. The nature of these epitopes will not be similar to those of k39, because the latter contain repetitive motifs that will contribute significantly to the clonal expansion of B-cells. For LicTXNPx, the strong immune response observed should be due to the formation of highly stable multimeric structures characteristic of this protein [96]. The nonprotective antibody titres induced by LicTXNPx seem to be transient and associated only with the immunopathology as they disappear after a period of time, unlike other Leishmania specific antibodies simultaneously in circulation [63]. These antibodies may contribute to the impairment of bone marrow and spleen [11].
The capacity of panantigens to modulate the immune system can be related to the fact that these intracellular proteins were not selected by the immune pressure, unlike the secreted and surface proteins. Hence, in the right conditions, they can provide the immunomodulatory properties needed for vaccine design. The most prominent intracellular proteins used in vaccine design are still LACK and LmSTI1 that are able to induce protective responses with a parasite-specific Th1 immune response (high IFN-γ but not IL-4 secretion) [87, 97]. Among the Leishmania proteins studied by our group, a mitocondrial tryparedoxin peroxidase (LimTXNPx; Table 1), homologous to LicTXNPx, is able to induce down regulation of IL-4, a Th2 cytokine, in splenocytes both in vitro and in vivo (Figure 5) though unable to induce significant protection (data not shown). It is noteworthy that similar proteins such as LicTXNPx and LimTXNPx are able to elicit distinct immune responses. LicTXNPx is secreted inducing only the production of nonprotective antibodies, while its related intracellular counterpart LimTXNPx has immunomodulatory properties interfering with cytokine production (Figure 5). This can be a good example of the type of evolutionary pressure induced by the immune system, in which two related proteins have distinct immunomodulatory properties (Figures 4, 5). It suggests that the host immune system selects characteristics in the exposed proteins that are either innocuous or nondeleterious to the parasite. Since this does not occur in the intracellular proteins they can retain distinct immunoregulatory properties that could be useful in vaccine design.
2. CONCLUDING REMARKS
Taken altogether, these observations support the idea that secreted and surface proteins tend to be poor or nonprotective immune modulators, like LicTXNPx. Nonetheless, their use in vaccine could induce short-lived protection probably due to the disruption of their biological activity or by production of lytic antibodies, as seen with Leishmania Sir2. Intracellular components like LmS3arp and LimTXNPx tend to have defined immunomodulatory properties. LmS3arp is able to induce polyclonal activation of B lymphocytes while LiLimTXNPxTXNPx confers a nonprotective dowregulation of IL-4 secretion by splenocytes.
Using the basic knowledge acquired in the study of the immune response against Leishmania in different murine models, one can look for proteins that induce the immunological phenotype needed for protection. Therefore, our data suggests that in vaccine development, the conjugation of secreted and surface proteins with intracellular components should provide a more efficient protection. Hence, the impairment of the parasite entrance in the host cells, either by lytic antibodies or by the disruption of protein function, will delay the onset of the immune suppression associated with Leishmania The parasite elimination could be achieved through a protective cellular response, induced by the intracellular parasite components present in the vaccine.
ACKNOWLEDGMENTS
This work was supported by FCT and Programa Opera cional Ciência e Inovação 2010 (POCI 2010) and FEDER in the projects: POCI/SAU-FCF/59837/2005 and POCI/CVT/59840/2004. R.S. and J.T. are supported by fellowships from FCT and FEDER number SFRH/BD/13120/2003 and SFRH/BD/18137/2004, respectively. The proteins, LicTXNPx, LimTXNPx, and LiTXN1, used in this works were purified from clones provided by A. Tomás.
References
- 1.Badaro R, Jones TC, Carvalho EM, et al. New perspectives on a subclinical form of visceral leishmaniasis. Journal of Infectious Diseases. 1986;154(6):1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 2.Salman SM, Rubeiz NG, Kibbi A-G. Cutaneous leishmaniasis: clinical features and diagnosis. Clinics in Dermatology. 1999;17(3):291–296. doi: 10.1016/s0738-081x(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO Report on Global Surveillance of Epidemic-Prone Infectious Diseases. Geneva, Switzerland: WHO; 2000. Leishmaniasis and Leishmania-HIV co-infection. [Google Scholar]
- 4.Solbach W, Laskay T. The host response to Leishmania infection. Advances in Immunology. 2000;74:275–317. doi: 10.1016/s0065-2776(08)60912-8. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan C, Gessner A, Werner S, Röllinghoff M. Invasion, control and persistence of Leishmania parasites. Current Opinion in Immunology. 1996;8(4):517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 6.Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunology Letters. 2005;99(1):17–23. doi: 10.1016/j.imlet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Satoskar A, Bluethmann H, Alexander J. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infection and Immunity. 1995;63(12):4894–4899. doi: 10.1128/iai.63.12.4894-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miralles GD, Stoeckle MY, McDermott DF, Finkelman FD, Murray HW. Th1 and Th2 cell-associated cytokines in experimental visceral Leishmaniasis. Infection and Immunity. 1994;62(3):1058–1063. doi: 10.1128/iai.62.3.1058-1063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends in Microbiology. 1998;6(3):102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 10.Ravindran R, Ali N. Progress in vaccine research and possible effector mechanisms in visceral leishmaniasis. Current Molecular Medicine. 2004;4(6):697–709. doi: 10.2174/1566524043360212. [DOI] [PubMed] [Google Scholar]
- 11.Chang KP, Reed SG, McGwire BS, Soong L. Leishmania model for microbial virulence: the relevance of parasite multiplication and pathoantigenicity. Acta Tropica. 2003;85(3):375–390. doi: 10.1016/s0001-706x(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 12.Rittig MG, Bogdan C. Leishmania-host-cell interaction: complexities and alternative views. Parasitology Today. 2000;16(7):292–297. doi: 10.1016/s0169-4758(00)01692-6. [DOI] [PubMed] [Google Scholar]
- 13.de Almeida MC, Vilhena V, Barral A, Barral-Netto M. Leishmanial infection: analysis of its first steps. A review. Memorias do Instituto Oswaldo Cruz. 2003;98(7):861–870. doi: 10.1590/s0074-02762003000700001. [DOI] [PubMed] [Google Scholar]
- 14.Kamhawi S. The biological and immunomodulatory properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes and Infection. 2000;2(14):1765–1773. doi: 10.1016/s1286-4579(00)01331-9. [DOI] [PubMed] [Google Scholar]
- 15.Gregory DJ, Olivier M. Subversion of host cell signalling by the protozoan parasite Leishmania . Parasitology. 2005;130(supplement 1):S27–S35. doi: 10.1017/S0031182005008139. [DOI] [PubMed] [Google Scholar]
- 16.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clinical Microbiology Reviews. 2005;18(2):293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puentes SM, Dwyer DM, Bates PA, Joiner KA. Binding and release of C3 from Leishmania donovani promastigotes during incubation in normal human serum. Journal of Immunology. 1989;143(11):3743–3749. [PubMed] [Google Scholar]
- 18.McConville MJ, Thomas-Oates JE, Ferguson MAJ, Homans SW. Structure of the lipophosphoglycan from Leishmania major . Journal of Biological Chemistry. 1990;265(32):19611–19623. [PubMed] [Google Scholar]
- 19.Davies CR, Cooper AM, Peacock C, Lane RP, Blackwell JM. Expression of LPG and gp63 by different developmental stages of Leishmania major in the sandfly Phlebotomus papatasi . Parasitology. 1990;101(3):337–343. doi: 10.1017/s0031182000060522. [DOI] [PubMed] [Google Scholar]
- 20.Sacks DL. Metacyclogenesis in Leishmania promastigotes. Experimental Parasitology. 1989;69(1):100–103. doi: 10.1016/0014-4894(89)90176-8. [DOI] [PubMed] [Google Scholar]
- 21.Mosser DM, Rosenthal LA. Leishmania-macrophage interactions: multiple receptors, multiple ligands and diverse cellular responses. Seminars in Cell Biology. 1993;4(5):315–322. doi: 10.1006/scel.1993.1038. [DOI] [PubMed] [Google Scholar]
- 22.de Veer MJ, Curtis JM, Baldwin TM, et al. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and toll-like receptor 2 signaling. European Journal of Immunology. 2003;33(10):2822–2831. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 23.Becker I, Salaiza N, Aguirre M, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Molecular and Biochemical Parasitology. 2003;130(2):65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 24.Miao L, Stafford A, Nir S, Turco SJ, Flanagan TD, Epand RM. Potent inhibition of viral fusion by the lipophosphoglycan of Leishmania donovani . Biochemistry. 1995;34(14):4676–4683. doi: 10.1021/bi00014a022. [DOI] [PubMed] [Google Scholar]
- 25.Desjardins M, Descoteaux A. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. Journal of Experimental Medicine. 1997;185(12):2061–2068. doi: 10.1084/jem.185.12.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dermine JF, Scianimanico S, Privé C, Descoteaux A, Desjardins M. Leishmania promastigotes require lipophosphoglycan to actively modulate the fusion properties of phagosomes at an early step of phagocytosis. Cellular Microbiology. 2000;2(2):115–126. doi: 10.1046/j.1462-5822.2000.00037.x. [DOI] [PubMed] [Google Scholar]
- 27.Lodge R, Diallo TO, Descoteaux A. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cellular Microbiology. 2006;8(12):1922–1931. doi: 10.1111/j.1462-5822.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 28.Tonui WK, Mpoke SS, Orago AS, Turco SJ, Mbati PA, Mkoji GM. Leishmania donovani-derived lipophosphoglycan plus BCG induces a Th1 type immune response but does not protect Syrian golden hamsters (Mesocricetus auratus) and BALB/c mice against Leishmania donovani . Onderstepoort Journal of Veterinary Research. 2003;70(4):255–263. doi: 10.4102/ojvr.v70i4.290. [DOI] [PubMed] [Google Scholar]
- 29.Tonui WK. Vaccination of BALB/c mice with Leishmania donovani derived lipophosphoglycan does not conver cross-protection to L. major infections. East African Medical Journal. 2003;80(5):260–263. doi: 10.4314/eamj.v80i5.8697. [DOI] [PubMed] [Google Scholar]
- 30.Pimenta PFP, Saraiva EMB, Sacks DL. The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major . Experimental Parasitology. 1991;72(2):191–204. doi: 10.1016/0014-4894(91)90137-l. [DOI] [PubMed] [Google Scholar]
- 31.Pandey S, Chakraborti P, Sharma R, Bandyopadhyay S, Sarkar D, Adhya S. Involvement of Leishmania donovani major surface glycoprotein gp63 in promastigote multiplication. Journal of Biosciences. 2004;29(1):15–22. doi: 10.1007/BF02702557. [DOI] [PubMed] [Google Scholar]
- 32.Brittingham A, Morrison CJ, McMaster WR, McGwire BS, Chang KP, Mosser DM. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. Journal of Immunology. 1995;155(6):3102–3111. [PubMed] [Google Scholar]
- 33.Brittingham A, Chen G, McGwire BS, Chang KP, Mosser DM. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infection and Immunity. 1999;67(9):4477–4484. doi: 10.1128/iai.67.9.4477-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhuri G, Chang KP. Acid protease activity of a major surface membrane glycoprotein (gp63) from Leishmania mexicana promastigotes. Molecular and Biochemical Parasitology. 1988;27(1):43–52. doi: 10.1016/0166-6851(88)90023-0. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhuri G, Chaudhuri M, Pan A, Chang KP. Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. Journal of Biological Chemistry. 1989;264(13):7483–7489. [PubMed] [Google Scholar]
- 36.Schneider P, Rosat JP, Bouvier J, Louis J, Bordier C. Leishmania major: differential regulation of the surface metalloprotease in amastigote and promastigote stages. Experimental Parasitology. 1992;75(2):196–206. doi: 10.1016/0014-4894(92)90179-e. [DOI] [PubMed] [Google Scholar]
- 37.Handman E, Button LL, McMaster RW. Leishmania major: production of recombinant gp63, its antigenicity and immunogenicity in mice. Experimental Parasitology. 1990;70(4):427–435. doi: 10.1016/0014-4894(90)90127-x. [DOI] [PubMed] [Google Scholar]
- 38.Joshi PB, Sacks DL, Modi G, McMaster WR. Targeted gene deletion of Leishmania major genes encoding developmental stage-specific leishmanolysin (gp63) Molecular Microbiology. 1998;27(3):519–530. doi: 10.1046/j.1365-2958.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson MAJ. The surface glycoconjugates of trypanosomatid parasites. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1997;352(1359):1295–1302. doi: 10.1098/rstb.1997.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proudfoot L, Schneider P, Ferguson MAJ, McConville MJ. Biosynthesis of the glycolipid anchor of lipophosphoglycan and the structurally related glycoinositolphospholipids from Leishmania major . Biochemical Journal. 1995;308, part 1:45–55. doi: 10.1042/bj3080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proudfoot L, Nikolaev AV, Feng GJ, et al. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):10984–10989. doi: 10.1073/pnas.93.20.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mottram JC, Coombs GH, Alexander J. Cysteine peptidases as virulence factors of Leishmania . Current Opinion in Microbiology. 2004;7(4):375–381. doi: 10.1016/j.mib.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 43.De Souza Leao S, Lang T, Prina E, Hellio R, Antoine JC. Intracellular Leishmania amazonensis amastigotes internalize and degrade MHC class II molecules of their host cells. Journal of Cell Science. 1995;108, part 10:3219–3231. doi: 10.1242/jcs.108.10.3219. [DOI] [PubMed] [Google Scholar]
- 44.Courret N, Frehel C, Prina E, Lang T, Antoine JC. Kinetics of the intracellular differentiation of Leishmania amazonensis and internalization of host MHC molecules by the intermediate parasite stages. Parasitology. 2001;122(3):263–279. doi: 10.1017/s0031182001007387. [DOI] [PubMed] [Google Scholar]
- 45.Cameron P, McGachy A, Anderson M, et al. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-κB signaling pathway. Journal of Immunology. 2004;173(5):3297–3304. doi: 10.4049/jimmunol.173.5.3297. [DOI] [PubMed] [Google Scholar]
- 46.Joshi MB, Rogers ME, Shakarian AM, et al. Molecular characterization, expression, and in vivo analysis of LmexCht1: the chitinase of the human pathogen, Leishmania mexicana . Journal of Biological Chemistry. 2005;280(5):3847–3861. doi: 10.1074/jbc.M412299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shakarian AM, Joshi MB, Yamage M, Ellis SL, Debrabant A, Dwyer DM. Members of a unique histidine acid phosphatase family are conserved amongst a group of primitive eukaryotic human pathogens. Molecular and Cellular Biochemistry. 2003;245(1-2):31–41. doi: 10.1023/a:1022851914014. [DOI] [PubMed] [Google Scholar]
- 48.Lovelace JK, Gottlieb M. Comparison of extracellular acid phosphatases from various isolates of Leishmania . American Journal of Tropical Medicine and Hygiene. 1986;35(6):1121–1128. doi: 10.4269/ajtmh.1986.35.1121. [DOI] [PubMed] [Google Scholar]
- 49.Ellis SL, Shakarian AM, Dwyer DM. Leishmania: amastigotes synthesize conserved secretory acid phosphatases during human infection. Experimental Parasitology. 1998;89(2):161–168. doi: 10.1006/expr.1998.4298. [DOI] [PubMed] [Google Scholar]
- 50.Doyle PS, Dwyer DM. Leishmania: immunochemical comparison of the secretory (extracellular) acid phosphatases from various species. Experimental Parasitology. 1993;77(4):435–444. doi: 10.1006/expr.1993.1103. [DOI] [PubMed] [Google Scholar]
- 51.Chenik M, Lakhal S, Ben Khalef N, Zribi L, Louzir H, Dellagi K. Approaches for the identification of potential excreted/secreted proteins of Leishmania major parasites. Parasitology. 2006;132(4):493–509. doi: 10.1017/S0031182005009546. [DOI] [PubMed] [Google Scholar]
- 52.Yahiaoui B, Taibi A, Ouaissi A. A Leishmania major protein with extensive homology to silent information regulator 2 of Saccharomyces cerevisiae . Gene. 1996;169(1):115–118. doi: 10.1016/0378-1119(95)00785-7. [DOI] [PubMed] [Google Scholar]
- 53.Wiese M, Ilg T, Lottspeich F, Overath P. Ser/Thr-rich repetitive motifs as targets for phosphoglycan modifications in Leishmania mexicana secreted acid phosphatase. The EMBO Journal. 1995;14(6):1067–1074. doi: 10.1002/j.1460-2075.1995.tb07089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webb JR, Campos-Neto A, Ovendale PJ, et al. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infection and Immunity. 1998;66(7):3279–3289. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Symons FM, Murray PJ, Ji H, et al. Characterization of a polymorphic family of integral membrane proteins in promastigotes of different Leishmania species. Molecular and Biochemical Parasitology. 1994;67(1):103–113. doi: 10.1016/0166-6851(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 56.Sereno D, Vanhille L, Vergnes B, Monte-Allegre A, Ouaissi A. Experimental study of the function of the excreted/secreted Leishmania LmSIR2 protein by heterologous expression in eukaryotic cell line. Kinetoplastid Biology and Disease. 2005;4:1. doi: 10.1186/1475-9292-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tonui WK, Mejia JS, Hochberg L, et al. Immunization with Leishmania major exogenous antigens protects susceptible BALB/c mice against challenge infection with L. major . Infection and Immunity. 2004;72(10):5654–5661. doi: 10.1128/IAI.72.10.5654-5661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryan JR, Smithyman AM, Rajasekariah G-H, Hochberg L, Stiteler JM, Martin SK. Enzyme-linked immunosorbent assay based on soluble promastigote antigen detects immunoglobulin M (IgM) and IgG antibodies in sera from cases of visceral and cutaneous leishmaniasis. Journal of Clinical Microbiology. 2002;40(3):1037–1043. doi: 10.1128/JCM.40.3.1037-1043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemesre JL, Holzmuller P, Cavaleyra M, Gonçalves RB, Hottin G, Papierok G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine. 2005;23(22):2825–2840. doi: 10.1016/j.vaccine.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 60.Chang KP, Reed SG, McGwire BS, Soong L. Leishmania model for microbial virulence: the relevance of parasite multiplication and pathoantigenicity. Acta Tropica. 2003;85(3):375–390. doi: 10.1016/s0001-706x(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 61.Castro H, Sousa C, Santos M, Cordeiro-da-Silva A, Flohé L, Tomás AM. Complementary antioxidant defense by cytoplasmic and mitochondrial peroxiredoxins in Leishmania infantum . Free Radical Biology and Medicine. 2002;33(11):1552–1562. doi: 10.1016/s0891-5849(02)01089-4. [DOI] [PubMed] [Google Scholar]
- 62.Castro H, Sousa C, Novais M, et al. Two linked genes of Leishmania infantum encode tryparedoxins localised to cytosol and mitochondrion. Molecular and Biochemical Parasitology. 2004;136(2):137–147. doi: 10.1016/j.molbiopara.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 63.Santarém N, Tomás A, Ouaissi A, et al. Antibodies against a Leishmania infantum peroxiredoxin as a possible marker for diagnosis of visceral leishmaniasis and for monitoring the efficacy of treatment. Immunology Letters. 2005;101(1):18–23. doi: 10.1016/j.imlet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Vergnes B, Sereno D, Madjidian-Sereno N, Lemesre JL, Ouaissi A. Cytoplasmic SIR2 homologue overexpression promotes survival of Leishmania parasites by preventing programmed cell death. Gene. 2002;296(1-2):139–150. doi: 10.1016/s0378-1119(02)00842-9. [DOI] [PubMed] [Google Scholar]
- 65.Silvestre R, Cordeiro-da-Silva A, Tavares J, Sereno D, Ouaissi A. Leishmania cytosolic silent information regulatory protein 2 deacetylase induces murine B-cell differentiation and in vivo production of specific antibodies. Immunology. 2006;119(4):529–540. doi: 10.1111/j.1365-2567.2006.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vergnes B, Sereno D, Tavares J, et al. Targeted disruption of cytosolic SIR2 deacetylase discloses its essential role in Leishmania survival and proliferation. Gene. 2005;363(1-2):85–96. doi: 10.1016/j.gene.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 67.Cordeiro-da-Silva A, Borges MC, Guilvard E, Ouaissi A. Dual role of the Leishmania major ribosomal protein S3a homologue in regulation of T- and B-cell activation. Infection and Immunity. 2001;69(11):6588–6596. doi: 10.1128/IAI.69.11.6588-6596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vannier-Santos MA, Martiny A, de Souza W. Cell biology of Leishmania spp.: invading and evading. Current Pharmaceutical Design. 2002;8(4):297–318. doi: 10.2174/1381612023396230. [DOI] [PubMed] [Google Scholar]
- 69.Saha S, Mondal S, Banerjee A, Ghose J, Bhowmick S, Ali N. Immune responses in kala-azar. Indian Journal of Medical Research. 2006;123(3):245–266. [PubMed] [Google Scholar]
- 70.Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of Schistosomiasis mansoni in mice and men. Immunology Today. 2000;21(9):465–466. doi: 10.1016/s0167-5699(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 71.Neogy AB, Nandy A, Ghosh Dastidar B, Chowdhury AB. Antibody kinetics in kala-azar in response to treatment. Annals of Tropical Medicine and Parasitology. 1987;81(6):727–729. doi: 10.1080/00034983.1987.11812177. [DOI] [PubMed] [Google Scholar]
- 72.Pearson RD, Sousa AQ. Clinical spectrum of leishmaniasis. Clinical Infectious Diseases. 1996;22(1):1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 73.Ghosh AK, Dasgupta S, Ghose AC. Immunoglobulin G subclass-specific antileishmanial antibody responses in Indian kala-azar and post-kala-azar dermal leishmaniasis. Clinical and Diagnostic Laboratory Immunology. 1995;2(3):291–296. doi: 10.1128/cdli.2.3.291-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anam K, Afrin F, Banerjee D, et al. Differential decline in Leishmania membrane antigen-specific immunoglobulin G (IgG), IgM, IgE, and IgG subclass antibodies in Indian kala-azar patients after chemotherapy. Infection and Immunity. 1999;67(12):6663–6669. doi: 10.1128/iai.67.12.6663-6669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maalej IA, Chenik M, Louzir H, et al. Comparative evaluation of ELISAs based on ten recombinant or purified Leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene. 2003;68(3):312–320. [PubMed] [Google Scholar]
- 76.Soto M, Requena JM, Garcia M, Gomez LC, Navarrete I, Alonso C. Genomic organization and expression of two independent gene arrays coding for two antigenic acidic ribosomal proteins of Leishmania . Journal of Biological Chemistry. 1993;268(29):21835–21843. [PubMed] [Google Scholar]
- 77.MacFarlane J, Blaxter ML, Bishop RP, Miles MA, Kelly JM. Identification and characterisation of a Leishmania donovani antigen belonging to the 70-kDa heat-shock protein family. European Journal of Biochemistry. 1990;190(2):377–384. doi: 10.1111/j.1432-1033.1990.tb15586.x. [DOI] [PubMed] [Google Scholar]
- 78.Dea-Ayuela MA, Rama-Iñiguez S, Bolás-Fernández F. Proteomic analysis of antigens from Leishmania infantum promastigotes. Proteomics. 2006;6(14):4187–4194. doi: 10.1002/pmic.200600101. [DOI] [PubMed] [Google Scholar]
- 79.Burns JM, Jr, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soto M, Requena JM, Quijada L, et al. Antigenicity of the Leishmania infantum histones H2B and H4 during canine viscerocutaneous leishmaniasis. Clinical and Experimental Immunology. 1999;115(2):342–349. doi: 10.1046/j.1365-2249.1999.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Requena JM, Alonso C, Soto M. Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitology Today. 2000;16(6):246–250. doi: 10.1016/s0169-4758(00)01651-3. [DOI] [PubMed] [Google Scholar]
- 82.Minoprio P, Burlen O, Pereira P, et al. Most B cells in acute Trypanosoma cruzi infection lack parasite specificity. Scandinavian Journal of Immunology. 1988;28(5):553–561. doi: 10.1111/j.1365-3083.1988.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 83.Cordeiro-da-Silva A, Espinoza AG, Taibi A, Ouaissi A, Minoprio P. A 24,000 MW Trypanosoma cruzi antigen is a B-cell activator. Immunology. 1998;94(2):189–196. doi: 10.1046/j.1365-2567.1998.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunology Today. 1991;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 85.Quijada L, Requena JM, Soto M, Alonso C. During canine viscero-cutaneous leishmaniasis the anti-Hsp70 antibodies are specifically elicited by the parasite protein. Parasitology. 1996;112(3):277–284. doi: 10.1017/s0031182000065793. [DOI] [PubMed] [Google Scholar]
- 86.Soto M, Requena JM, Quijada L, et al. Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunology Letters. 1995;48(3):209–214. doi: 10.1016/0165-2478(95)02473-5. [DOI] [PubMed] [Google Scholar]
- 87.Mougneau E, Altare F, Wakil AE, et al. Expression cloning of a protective Leishmania antigen . Science. 1995;268(5210):563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 88.van Zandbergen G, Bollinger A, Wenzel A, et al. Leishmania disease development depends on the presence of apoptotic promastigotes in the virulent inoculum. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13837–13842. doi: 10.1073/pnas.0600843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bacellar O, Brodskyn C, Guerreiro J, et al. Interleukin-12 restores interferon-γ production and cytotoxic responses in visceral leishmaniasis. Journal of Infectious Diseases. 1996;173(6):1515–1518. doi: 10.1093/infdis/173.6.1515. [DOI] [PubMed] [Google Scholar]
- 90.Buates S, Matlashewski G. General suppression of macrophage gene expression during Leishmania donovani infection. Journal of Immunology. 2001;166(5):3416–3422. doi: 10.4049/jimmunol.166.5.3416. [DOI] [PubMed] [Google Scholar]
- 91.Ghalib HW, Whittle JA, Kubin M, et al. IL-12 enhances Th1-type responses in human Leishmania donovani infections. Journal of Immunology. 1995;154(9):4623–4629. [PubMed] [Google Scholar]
- 92.Reiner NE, Ng W, McMaster WR. Parasite-accessory cell interactions in murine leishmaniasis. II. Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. Journal of Immunology. 1987;138(6):1926–1932. [PubMed] [Google Scholar]
- 93.Lee N, Bertholet S, Debrabant A, Muller J, Duncan R, Nakhasi HL. Programmed cell death in the unicellular protozoan parasite Leishmania . Cell Death and Differentiation. 2002;9(1):53–64. doi: 10.1038/sj.cdd.4400952. [DOI] [PubMed] [Google Scholar]
- 94.Singh S, Gilman-Sachs A, Chang KP, Reed SG. Diagnostic and prognostic value of K39 recombinant antigen in Indian leishmaniasis. Journal of Parasitology. 1995;81(6):1000–1003. [PubMed] [Google Scholar]
- 95.Alvar J, Cañavate C, Gutiérrez-Solar B, et al. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clinical Microbiology Reviews. 1997;10(2):298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flohé L, Hecht HJ, Steinert P. Glutathione and trypanothione in parasitic hydroperoxide metabolism. Free Radical Biology and Medicine. 1999;27(9-10):966–984. doi: 10.1016/s0891-5849(99)00172-0. [DOI] [PubMed] [Google Scholar]
- 97.Webb JR, Kaufmann D, Campos-Neto A, Reed SG. Molecular cloning of a novel protein antigen of Leishmania major that elicits a potent immune response in experimental murine leishmaniasis. Journal of Immunology. 1996;157(11):5034–5041. [PubMed] [Google Scholar]