Abstract
Background and objectives
Continuous perineural femoral analgesia has been reported to reduce numeric rating pain scores (NRS, scale zero to 10) after anterior cruciate ligament reconstruction (ACLR). In the current study, we determined rebound pain scores in autograft ACLR outpatients after nerve block analgesia resolved.
Methods
After standardized spinal anesthesia and perioperative multimodal analgesia, patients received a femoral perineural catheter and 50 hours of saline or levobupivacaine. All patients received levobupivacaine (30 mL of 0.25% as a bolus) before the infusion. Patients completed a pain diary for 6 days, indicating serial NRS scores and perceptions of when nerve block analgesia resolved. Block duration and rebound pain scores were computed.
Results
Data from 84 participants’ pain diaries were analyzed. Patients receiving saline infusion reported mean nerve block duration of 37 hours, versus 59 hours for patients receiving the levobupivacaine infusion (P<0.001). Mean Rebound Pain Scores increased by 2.0 (95% CI: 1.6, 2.4). Based on the computations used to derive block duration and rebound pain scores, each hour of additional block duration was predictive of a 0.03 unit reduction in rebound pain scores.
Conclusions
In an anesthesia care protocol consisting of spinal anesthesia and multimodal analgesia during and after autograft ACL reconstruction, approximately 33 hours of additional nerve block duration were required to reduce rebound pain scores by one unit. Further study is required to determine rebound pain score differences when other local anesthetics and anesthetic/analgesic plans are being used, and when other surgeries are being performed.
Keywords: acute pain, anterior cruciate ligament reconstruction, continuous nerve block, femoral nerve block, ketamine, spinal anesthesia, rebound pain
Introduction
For knee surgery and a myriad of other types of outpatient orthopedic surgery, it is well known that single-injection peripheral nerve blocks and perineural catheters can significantly reduce postoperative pain at home, and also reduce opioid consumption.1–4 What is less known is the extent to which patients encounter “Rebound Pain,” or a quantifiable difference in pain scores when the block is working, versus the increase in acute pain that is encountered during the first few hours after the effects of perineural single-injection or continuous infusion local anesthetics resolve. In an effort to minimize postoperative pain scores at home (i.e., keeping pain scores as “less than moderate”), it is important to quantify the extent to which extending nerve block duration would reduce Rebound Pain Scores (RPS, defined as the pain score difference between when the block was working and when the nerve block wore off). To our knowledge, little has been published about RPS, or attempts to quantify the extent to which nerve block duration suppresses Rebound Pain. By being able to quantify this value, anesthesiologists may be better able to determine which patients are more suitable for a continuous perineural infusion versus a single-injection nerve block, and/or which postoperative multimodal oral analgesics would be indicated to provide sustained postoperative analgesia at home after nerve block effects dissipate.
It should be noted that we have recently reported differences in numeric rating scores (NRS) for pain with movement, and in opioid consumption, for the study population of patients undergoing allograft and autograft anterior cruciate ligament (ACL) reconstruction.5 The purpose of this retrospective re-analysis of the original prospectively collected data in the current manuscript is to (i) focus specifically on pain responses of patients undergoing autograft procedures (hamstring or patellar tendon), and to direct attention to the concept of Rebound Pain. Our hypothesis is that increasing nerve block duration decreases RPS.
Methods
After achieving approval by the Institutional Review Board of the University of Pittsburgh Medical Center, and obtaining informed consent, ACL reconstruction patients underwent a standardized multimodal analgesia regimen (ketamine 0.2 mg/kg IV, plus intra-articular meperidine 100 mg with neostigmine 0.5 mg and ketorolac 15 mg) and anesthetic (ipsilateral hyperbaric spinal with bupivacaine) technique.5 Other inclusion and exclusion criteria from the prospective study were detailed in the original manuscript.5 Patients were randomized to one of the following femoral nerve block catheter treatment groups: (i) saline bolus (30 mL) plus saline infusion (270 mL at 5 mL/hr, placebo); (ii) levobupivacaine (0.25%) bolus with saline infusion (group LbSi), or (iii) levobupivacaine (0.25%) bolus plus levobupivacaine (0.25%) infusion (group LbLi), according to the method described previously.5 Only patients in groups LbSi and LbLi were included in the present analysis (placebo patients were excluded).
Postoperative analgesia consisted of rofecoxib (50 mg orally daily for 6 days, before the voluntary product recall by its manufacturer), controlled-release oxycodone (CR-OXY, 10–30 mg orally every 12 hours, titrated to patients’ NRS scores; Oxycontin©, Purdue Pharma, Stamford, CT), and generic, immediate-release oxycodone (5–10 mg orally every 3–6 hours as needed for breakthrough pain).
Data collection procedures
During the preoperative interview and after informed consent for the study was obtained, additional demographic information was elicited (age, gender, ethnicity, height, weight, ASA/PS classification, smoking status, baseline NRS with movement). Surgeon, tourniquet use, tourniquet time, and case duration were also recorded during the intraoperative course.
Postoperative course
After surgery, all patients were transferred to the PACU for placement of a perineural femoral catheter, the details of which having been previously described.5
Patients were given a pain diary before hospital discharge on the same day after surgery. There were 6 columns of data for patients to enter: time of day; NRS (0–10); “is the nerve block providing pain relief?” (no-yes); and columns for each of the 3 oral analgesics for recording number of tablets consumed. Patients were asked to complete the pain diary four times per day for 6 full days after surgery.
During the first 3 days after surgery, participants were recommended to take a minimum of 10 mg CR-OXY every 12 hours to prevent possible excruciating “breakthrough” pain or “rebound” pain. Recommended CR-OXY doses were based on NRS at the time of administration. Beginning on day 4, CR-OXY 10 mg every 12 hours was recommended only if NRS reached a minimum score of 2–3. These dosing maneuvers were implemented in response to our Institutional Review Board, who voiced a concern about the presence of a placebo bolus group, as well as a placebo infusion after an active bolus (i.e., group LbSi).
The lead author and/or study coordinator instructed participants how to answer the pain diary question that pertained to the perception that nerve block was providing pain relief (no-yes), as follows. Understanding that CR-OXY was being taken on a regular schedule, participants were asked to evaluate their nerve block as “providing pain relief” or “not providing pain relief” based on: (i) any gradual or abrupt increases in knee pain not necessarily related (temporally) to an increase in activity (such as physical therapy exercises); (ii) the need or absence of need for a dose of immediate-release oxycodone beyond “breakthrough” doses needed by that point (if at all); and (iii) presence (or absence) of a distinct numbness/heavy sensation of the thigh when compared with the non-operative contralateral lower extremity.
The time of nerve block placement was determined from anesthesia and recovery room medical record source documents. The pain diary time of the last “yes” (i.e., the nerve block is providing pain relief) was recorded, along with its corresponding NRS score. The corresponding time of the first “no” response (i.e., the nerve block is not providing pain relief) was similarly recorded, along with its corresponding NRS score. NRS scores during the first 12 hours after the first “no” response were also recorded. Pain diaries were returned in a packet of mailings that included other surveys and administrative materials.
Statistics
A sample size of 90 patients per nerve block treatment group (270 total, including all graft types) was justified previously.5 From these, only the participants receiving treatments LbSi and LbLi, and undergoing ACL reconstruction with either patellar tendon autograft or hamstring autograft, were included in the present analysis. Data were first explored to determine demographic and day-of-surgery equivalence between treatment groups. The demographic variables of gender, age, race/ethnicity, body mass index, ASA/PS, smoking history, baseline NRS score with movement, surgeon, length of surgery, use of thigh tourniquet intraoperatively were analyzed for differences between nerve block treatment groups (LbSi versus LbLi) using independent samples t-test (for continuous variables) or the chi-square test (for categorical variables).
Nerve block success
We were not able - and did not attempt - to determine block success on the day of surgery before discharge home. This is because (i) block boluses were dosed before knee pain was experienced (but after return of quadriceps function), and (ii) we wanted to maintain patient blinding regarding nerve block treatment group. In an effort to derive nerve block success “after the fact,” pain diaries were reviewed to determine whether patients reported any perceived benefit from both the single-injection bolus (for LbSi and LbLi patients) and continuous infusion (for LbLi patients only). Success rates are reported as “bolus successful” (pain scores lower initially versus later postoperatively, and/or with no repeated doses of oral immediate-release oxycodone) and “infusion successful” (for LbLi patients only, in which the nerve block infusion provided a consistent level of analgesia while the catheter was in place).
Nerve Block Duration
Nerve block duration (in hours) was calculated using the following method: the time at which the patient declared “no” (i.e., the nerve block is not providing pain relief) minus the recorded time of nerve block insertion on the day of surgery.
Rebound Pain Score
RPS after nerve block resolution was determined using the following formula: the highest NRS score recorded within the first 12 hours after the first report of “no” (i.e., the nerve block is not providing pain relief) minus the NRS score that was reported at the time the patient reported the last “yes” (i.e., the nerve block is providing pain relief). RPS was analyzed parametrically, based on the report of Dexter and Chestnut (1995) which allows for the parametric analysis of verbal pain scores (on a scale of 0 to 10). 6
After providing descriptive statistics based on nerve block treatment group (LbSi versus LbLi), RPS was analyzed first using univariate linear regression equations, to determine the extent to which demographic variables (see section “Data Collection Procedures” above) influenced RPS. Sequential regression models were run using RPS as the dependent variable. Univariate regressions were run first, using one variable at a time. Next, variables that were significant predictors at a P<0.2 level were considered together, along with nerve block duration in a backward multivariable linear regression model.
P-values less than or equal to 0.05 are considered statistically significant. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS for Windows, version 13.0, Chicago, IL).
Results
Data from 106 patients (assigned to either the LbSi or LbLi treatment group) were included for analysis. One surgeon performed 58 procedures, another surgeon performed 34 procedures, and 3 other surgeons performed the remaining 14 procedures. Pain diaries and other supporting study documents were used to help determine nerve block success rate in all 106 patients, but in only 84 patients were the pain diary data sufficiently complete to determine nerve block duration and RPS.
Based on pain diary reports, success rate of nerve block bolus was 52/54 (96%) in the LbSi group, and 51/52 (98%) in the LbLi group. The infusion success rate was 47/52 (90%) in the LbLi group: one catheter did not thread at all, and the other 4 infusion failures (based on clinical outcomes in pain diary reports) were likely due to the perineural catheter being threaded to a final location that was not perineural. Patient data in the setting of block failure defined above were included in all subsequent analyses according to the originally-assigned treatment group, following intent-to-treat principles.
There were no significant differences between treatment groups with respect to gender, age, race/ethnicity, body mass index, ASA/PS, smoking history, baseline preoperative pain score, use of thigh tourniquet, or length of surgery. There were no significant differences in proportions of participants who did not return their pain diaries, and there were no demographic differences between participants who returned their pain diaries (or return incomplete diaries for the purpose of this post hoc analysis), versus those who did not.
Patients in the LbLi treatment group reported significantly longer nerve block duration than did patients in the LbSi treatment group. Mean nerve block duration was 59 hours in the LbLi group, versus 37 hours in the LbSi group (P<0.001, Figure 1). There were no significant differences in Nerve Block Duration based on graft type. The mean RPS increase (and 95% confidence interval) was 2.0 (1.6, 2.4).
Figure 1.
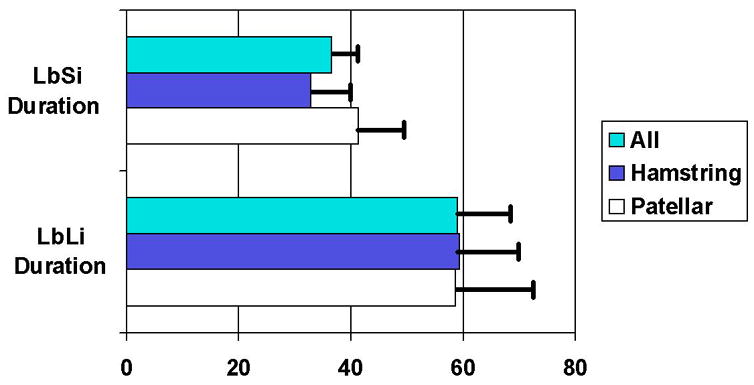
Duration (in hours) of femoral nerve blocks in the 2 treatment groups, for patients undergoing autograft ACL reconstruction. The differences in duration between LbSi and LbLi were statistically significant (P<0.001). The differences in LbSi duration between hamstring and patellar tendon autografts were not statistically significant (P=0.131). Significance values for graft type differences in nerve block duration for LbLi patients was P=0.80.
Factors (based on univariate linear regression analyses) that were included in the multivariable linear regression analyses for RPS were age, body mass index, surgeon, autograft type, and block duration (Table 1). Patients’ preoperative baseline NRS scores with movement were not predictive of RPS in the univariate linear regression model, so this factor and all other non-significant demographic variables were excluded from the multivariable analysis. Each additional hour of nerve block duration was predictive of a lower RPS by 0.03 units (P<0.001). There were no other factors that were predictive of RPS (Table 1).
Table 1.
Predictors of Rebound Pain Scores based on Linear Regression
| Univariate predictors (with P<0.2) evaluated |
|
| Multivariable Predictors of Rebound Pain Score | |
| Constant (95% CI) | 3.61 (2.86, 4.36) |
| Predictor 1 | Block Duration per hour |
| B coefficient (95% CI) | −0.03 (−0.02, −0.05) |
| Significance | <0.001 |
| Other Predictors | None |
Discussion
In this retrospective analysis of prospectively collected pain diary data, we have shown that patient reports of pain with movement appear to be reduced with each additional hour of nerve block duration. However, based on our data, 33 additional hours of nerve block duration are required to lower Rebound Pain NRS scores by a mere one point on a zero-to-10 scale. It is reassuring to report, however, that increasing nerve block duration does not increase Rebound Pain.
Clinical significance
In our opinion, it is a questionable practice to subject outpatients to the risk of moderate-to-severe pain after a nerve block resolves at home. Oral analgesics that are prescribed to facilitate “transitional analgesia” after blocks resolve should ensure that patients can sustain “less-than-moderate” pain scores (4 or less on a scale of 0–10). There may be several factors that attenuated RPS in our study. One may have been our multimodal analgesic protocol (pre- and postoperative rofecoxib 50 mg daily, postoperative oxycodone and cryotherapy, and intraoperative spinal anesthesia, low-dose ketamine, and intraarticular meperidine/neostigmine/ketorolac). For a frame of reference, we will refer to the excellent study by Mulroy et al. (2001), who had 3 treatment groups receiving only single-injection femoral nerve block (25 mL of bupivacaine 0.25%, versus 0.5%, versus sham block consisting of assembled equipment and sterile skin preparation, with palpation and shaking of the quadriceps femoris muscle). The pain scores in their sham treatment group gradually increased over the 8 postoperative days to a visual analog pain score of 5 out of 10 by day 8, versus scores of 3 in the bupivacaine groups.1 This is in contrast to our recent report 5 which showed all of our treatment groups (including our saline placebo SbSi treatment group) having their pain scores return to those of our active LbSi and LbLi treatment groups (median NRS score of 2) by the 7th postoperative day. Mulroy et al. (2001) did not specifically quantify “Rebound Pain” in their bupivacaine treatment groups, but their patients’ average pain scores went from 1 out of 10 on the day of surgery postoperatively, to 2 out of 10 on postoperative days 1–2, and 3 out of 10 on postoperative day 3. Meanwhile their sham group pain scores were successively 2.5, 3, 3.5, and 4 on the same days. These low pain scores on days 2 and 3 were notable in that bupivacaine-treated patients in their study reported a mean single-injection block duration of approximately 24 hours,1 therefore, favorable pain score effects appeared to outlast the actual block resolution time, which we have also previously reported in our patients.5
With respect to single-injection nerve block duration, we also found that our levobupivacaine duration (i.e., in the LbSi treatment group) averaged 37 hours, versus the 24 hour duration reported by Mulroy et al.(2001).1 It seems unlikely that an additional 5 mL of initial bolus in our study, or the use of levobupivacaine in our study instead of bupivacaine by Mulroy et al., would explain 13 additional hours of analgesia. Again, our multimodal analgesic strategy used for all of our study patients may have contributed to the perceived LbSi block duration of 37 hours. White et al. (2003), in a study of popliteal sciatic catheter infusions (with bupivacaine or placebo) initiated before surgery,7 speculated that placebo catheter patients (who received a preoperative bupivacaine bolus) may have had the efficacy of the bupivacaine bolus reduced by the dilutional effects of intraoperative perineural saline infusion.7 This speculation arose because placebo infusion patients in the study by White et al. had an immediate postoperative pain mean score of 7.5, versus 2.5 in the bupivacaine infusion group.7 Importantly, there is no evidence of dilutional effects reducing femoral perineural block efficacy or duration in our study patients.
Another important clinical consideration is determining types of procedures likely to have the highest RPS after blocks resolve. In outpatient orthopedic surgery, evaluating RPS after complex knee surgery (including ACL reconstruction) versus shoulder surgery may show significantly different RPS outcomes. For example, in our 1998–1999 clinical pathway database of patients undergoing complex knee surgery (n=167 respondents) or shoulder surgery (n=213 respondents), we found RPS to be 4.6 (95% CI: 4.2, 5.1) after shoulder surgery, and 3.6 (3.1, 4.0, P<0.001) after complex knee surgery. In this historical time frame, neither perineural catheters nor the multimodal analgesic scheme used in the present study were employed. It appears that site and type of surgery will greatly influence RPS, but further study is required.
Methodological considerations
To our knowledge, this is the first report of quantifying the RPS in outpatients. The need to develop and standardize such methodology is important due to the incidence and prevalence of outpatient surgery amenable to regional anesthesia techniques, including continuous peripheral nerve blocks. Evaluating outpatients’ anesthesia outcomes is quite difficult. However, using the parameters of “time block placed” and “time when block no longer provided pain relief” are both easy to determine and clinically relevant. We acknowledge that the patient’s perspective of the latter parameter may be confounded by coexisting systemic analgesics, but we suggest that the patient’s perspective of satisfactory analgesic outcome (including low RPS scores and minimal side effects) is more important to the individual patient than is a precise determination of nerve block duration.
Certainly, other parameters can be collected to compute nerve block duration and RPS in outpatients. When drafting this manuscript, we also analyzed nerve block duration data that considered the midpoint between the “last yes” and “first no” response to “is the nerve block providing pain relief?” The logic with this potential time point to determine resolution is that if the “last yes” and “first no” was separated by the patient sleeping, then defining the block resolution time would seem less accurate. In preparing this manuscript, we found, however, that the nerve block durations using these 2 different methods of calculation correlated extremely highly (Pearson’s r = 0.991, P<0.001). With respect to calculating RPS, the NRS score at the time of the “last yes” was what we ultimately used, although we also could have used the lowest NRS score while the block yielded any “yes” response to “is the nerve block providing pain relief?” Similarly, we ultimately used the highest NRS score in the 12 hours after a “no” response; although we could have used the NRS score at the time of the first “no” response. With 2 “Yes” NRS scores and 2 “No” NRS pain scores available to us, we made 4 total RPS calculations, and these computations led to very high correlation with each other (Pearson’s r ≥ 0.886, P<0.001). Our recommendation for future studies would be to use consistent duration and RPS (Table 2) parameters, such that future studies have a better chance of being meaningfully comparable.
Table 2.
Proposal for the Calculation of Rebound Pain Scores (RPS) after Peripheral Nerve Block Resolution in Outpatients
Premises for Measurement of Rebound Pain:
|
Measurement:
|
Our linear regression model identified predictors of RPS, and only nerve block duration (and no other factors) was a predictor. This seems important since nerve block duration as the primary influence of RPS (when the multimodal analgesic plan is held constant) may minimize the importance of factors outside of the anesthesiologist’s control (gender, physical status, baseline pain score), and allow the anesthesiologist to make a significant difference in patient outcome (e.g., providing a continuous perineural catheter instead of a single-injection nerve block).
In our opinion, the described methods seem to be a reasonable template for future studies when attempting to quantify Rebound Pain Scores after nerve block resolution. Of interest would be to study difference in Rebound Pain Scores as influenced by a myriad of multimodal analgesics (one-at-a-time, or in combinations), such as parenteral ketamine, various intra-articular analgesic combinations, and various oral analgesics such as non-steroidal anti-inflammatory drugs, and other novel analgesics such as gabapentin,8 pregabalin,9 and beta-blockers.10,11 Another consideration is that levobupivacaine was withdrawn from the United States marketplace by its manufacturer, despite the continuing availability of this local anesthetic worldwide. Ropivacaine single-injection blocks are well-documented to last only 12 hours,12–15 and studies are needed to examine patient-perceived ropivacaine nerve block duration (and RPS) when using a similar perioperative multimodal analgesic technique as we used in this study. Another option would be to study Rebound Pain Score differences as nerve blocks resolve based on intraoperative anesthetic technique (volatile agents for maintenance, propofol for maintenance, or spinal anesthesia, of which the former two have been shown to lead to increased postoperative pain when compared with spinal anesthesia).16,17 Studies incorporating RPS with versus without analgesic adjuncts would help better define risk-benefit ratios before routinely administering such adjuncts in routine practice of ambulatory regional anesthesia.
With respect to the methodology used to determine nerve block success rates, we acknowledge that our methodology (based on retrospective reviews of prospectively-collected pain diaries) is not likely comparable to nerve block success rates reported using more conventional methods. However, we thought that it was more important to maintain blinding as best as possible at the time of the nerve block procedure. Similarly, success of a single-injection block or perineural infusion, we believe, is based on whether the patient (at home, hours-to-days later) believes that the technique was successful in leading to a “less-than-moderate” postoperative pain score.
To summarize, increasing nerve block duration leads to a small but significant reduction in Rebound Pain after a nerve block resolves. Further study is needed to differentiate Rebound Pain reductions attributable to the nerve block duration in isolation, versus the effects of combination multimodal analgesic strategies that are employed, or intraoperative anesthetic that was used.
Acknowledgments
Financial support (for Dr. B. Williams): National Institutes of Health/National Institute of Arthritis, Musculoskeletal, and Skin Diseases K23 AR47631, Bethesda, Maryland, United States; and International Anesthesia Research Society Clinical Scholar Research Award (2001), Cleveland, Ohio, United States. Additional support from the University of Pittsburgh Department of Anesthesiology.
The lead author would like to acknowledge the teamwork provided by enrolling anesthesiologists Raymond Schwartz, MD, and Steven L. Orebaugh, MD (both –University of Pittsburgh Department of Anesthesiology, UPMC South Side, Pittsburgh, Pennsylvania, United States). We also wish to thank the surgeons from the University of Pittsburgh Department of Orthopaedic Surgery (Pittsburgh, Pennsylvania, United States), Center for Sports Medicine, who allowed us to enroll their patients: Drs. Freddie H. Fu, Christopher D. Harner, Robin V. West, Patrick J. McMahon, and Craig H. Bennett. We also wish to acknowledge previous research coordinators for this study based at the University of Pittsburgh: Chiara M. Figallo, MLIS, and Kimberly A. Francis, MS, MPA; and offer special thanks to the former Director of Orthopaedic Clinical Research (University of Pittsburgh), Molly T. Vogt, Ph.D., Dr.P.H.
Nerve stimulation needles (Prolong PL-50) were provided by Spinal Specialties, inc., San Antonio, Texas, United States; Life-Tech®, inc., Stafford, Texas, United States; and I-Flow Corporation, Lake Forest, California, United States. Elastomeric nerve block infusion devices were provided by McKinley Medical, Wheat Ridge, Colorado, United States. Patient samples of rofecoxib were provided by Merck & Co., Inc., Whitehouse Station, New Jersey, United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mulroy MF, Larkin KL, Batra MS, Hodgson PS, Owens BD. Femoral nerve block with 0.25% or 0.5% bupivacaine improves postoperative analgesia following outpatient arthroscopic anterior cruciate ligament repair. Reg Anesth Pain Med. 2001;26:24–29. doi: 10.1053/rapm.2001.20773. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Boctor B, Verner J. The effect of single-injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Reg Anesth Pain Med. 2002;27:139–144. doi: 10.1053/rapm.2002.29253. [DOI] [PubMed] [Google Scholar]
- 3.Ilfeld BM, Enneking FK. Continuous peripheral nerve blocks at home: a review. Anesth Analg. 2005;100:1822–1833. doi: 10.1213/01.ANE.0000151719.26785.86. [DOI] [PubMed] [Google Scholar]
- 4.Klein SM, Evans H, Nielsen KC, Tucker MS, Warner DS, Steele SM. Peripheral nerve block techniques for ambulatory surgery. Anesth Analg. 2005;101:1663–1676. doi: 10.1213/01.ANE.0000184187.02887.24. [DOI] [PubMed] [Google Scholar]
- 5.Williams BA, Kentor ML, Vogt MT, Irrgang JJ, Bottegal MT, West RV, Harner CD, Fu FH, Williams JP. Reduction of verbal pain scores after anterior cruciate ligament reconstruction with two-day continuous femoral nerve block: A randomized clinical trial. Anesthesiology. 2006;104:315–327. doi: 10.1097/00000542-200602000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology. 1995;82:896–902. doi: 10.1097/00000542-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 7.White PF, Issioui T, Skrivanek GD, Early JS, Wakefield C. The use of a continuous popliteal sciatic nerve block after surgery involving the foot and ankle: does it improve the quality of recovery? Anesth Analg. 2003;97:1303–1309. doi: 10.1213/01.ANE.0000082242.84015.D4. [DOI] [PubMed] [Google Scholar]
- 8.Dahl JB, Mathiesen O, Møiniche S. ‘Protective premedication’: an option with gabapentin and related drugs? A review of gabapentin and pregabalin in the treatment of postoperative pain. Acta Anaesthesiol Scand. 2004;48:1130–1136. doi: 10.1111/j.1399-6576.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 9.Hill CM, Balkenohl M, Thomas DW, Walker R, Mathe H, Murray G. Pregabalin in patients with postoperative dental pain. Eur J Pain. 2001;5:119–124. doi: 10.1053/eujp.2001.0235. [DOI] [PubMed] [Google Scholar]
- 10.Chia YY, Chan MH, Ko NH, Liu K. Role of beta-blockade in anaesthesia and postoperative pain management after hysterectomy. Br J Anaesth. 2004;93:799–805. doi: 10.1093/bja/aeh268. [DOI] [PubMed] [Google Scholar]
- 11.Davidson EM, Doursout MF, Szmuk P, Chelly JE. Antinociceptive and cardiovascular properties of esmolol following formalin injection in rats. Can J Anaesth. 2001;48:59–64. doi: 10.1007/BF03019816. [DOI] [PubMed] [Google Scholar]
- 12.Klein SM, Greengrass RA, Steele SM, D’Ercole FJ, Speer KP, Gleason DH, DeLong ER, Warner DS. A comparison of 0.5% bupivacaine, 0.5% ropivacaine, and 0.75% ropivacaine for interscalene brachial plexus block. Anesth Analg. 1998;87:1316–1319. doi: 10.1097/00000539-199812000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Casati A, Magistris L, Fanelli G, Beccaria P, Cappelleri G, Aldegheri G, Torri G. Small-dose clonidine prolongs postoperative analgesia after sciatic-femoral nerve block with 0.75% ropivacaine for foot surgery. Anesth Analg. 2000;91:388–392. doi: 10.1097/00000539-200008000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Casati A, Fanelli G, Albertin A, Deni F, Anelati D, Antonino FA, Beccaria P. Interscalene brachial plexus anesthesia with either 0.5% ropivacaine or 0.5% bupivacaine. Minerva Anestesiol. 2000;66:39–44. [PubMed] [Google Scholar]
- 15.Casati A, Fanelli G, Cappelleri G, Beccaria P, Magistris L, Borghi B, Torri G. A clinical comparison of ropivacaine 0.75%, ropivacaine 1% or bupivacaine 0.5% for interscalene brachial plexus anaesthesia. Eur J Anaesthesiol. 1999;16:784–789. doi: 10.1046/j.1365-2346.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 16.Jankowski CJ, Hebl JR, Stuart MJ, Rock MG, Pagnano MW, Beighley CM, Schroeder DR, Horlocker TT. A comparison of psoas compartment block and spinal and general anesthesia for outpatient knee arthroscopy. Anesth Analg. 2003;97:1003–1009. doi: 10.1213/01.ANE.0000081798.89853.E7. [DOI] [PubMed] [Google Scholar]
- 17.Korhonen AM, Valanne JV, Jokela RM, Ravaska P, Korttila KT. A comparison of selective spinal anesthesia with hyperbaric bupivacaine and general anesthesia with desflurane for outpatient knee arthroscopy. Anesth Analg. 2004;99:1668–1673. doi: 10.1213/01.ANE.0000139351.40608.05. [DOI] [PubMed] [Google Scholar]


