Abstract
Leptin, an adipocyte hormone, is a trophic factor for the reproductive system; however, it is still unknown whether there is a dynamic relation between fluctuations in circulating leptin and hypothalamic—pituitary–ovarian (HPO) axis hormones. To test the hypothesis that fluctuations in plasma leptin concentrations are related to the levels of luteinizing hormone (LH) and estradiol, we sampled plasma from six healthy women every 7 min for 24 h during days 8–11 of the menstrual cycle. Cross-correlation analysis throughout the 24-h cycle revealed a relation between release patterns of leptin and LH, with a lag of 42–84 min but no significant cross-correlation between LH and estradiol. The ultradian fluctuations in leptin levels showed pattern synchrony with those of both LH and estradiol as determined by cross-approximate entropy (cross-ApEn). At night, as leptin levels rose to their peak, the pulsatility profiles of LH changed significantly and became synchronous with those of leptin. LH pulses were fewer, of longer duration, higher amplitude, and larger area than during the day. Moreover, the synchronicity of LH and leptin occurred late at night, at which time estradiol and leptin also exhibited significantly stronger pattern coupling than during the day. We propose that leptin may regulate the minute-to-minute oscillations in the levels of LH and estradiol, and that the nocturnal rise in leptin may determine the change in nocturnal LH profile in the mid-to-late follicular phase that precedes ovulation. This may explain the disruption of hypothalamic—pituitary–ovarian function that is characteristic of states of low leptin release, such as anorexia nervosa and cachexia.
Leptin, the hormonal product of the OB gene (1), is a signal of nutritional status. High leptin levels in mice not only cause weight loss, by suppressing food intake and increasing motor activity, energy metabolism, oxygen consumption, and body temperature (2–4), but also stimulate reproduction. Administration of exogenous leptin to immature animals accelerates the onset of puberty (5, 6). In adult animals, leptin administration corrects the sterility defect in homozygous obese female mice (7) and corrects hypogonadism in mice starved for 2 days, without affecting body weight (8). In ad libitum-fed, but not in food-deprived, animals, leptin facilitates female sexual behavior (9). Leptin treatment of ob/ob female mice for 2 weeks causes elevations of serum levels of LH, increases in ovarian and uterine weights and in total ovarian follicle number as well as primary and Graafian follicles, and increases in uterine cross-sectional area, epithelial height, endometrial area, and glandular areas; in ob/ob males, 2 weeks of leptin treatment results in a rise in serum levels of follicle-stimulating hormone, as well as augmentation in the weights of the testes and seminal vesicles, associated with increases in the epithelial height of the seminal vesicles (10). In humans, increases in plasma leptin concentrations seem to be associated with the onset of puberty in boys. Mantzoros et al. (11) found that leptin levels rose by ≈50% before the onset of puberty in healthy boys and decreased to baseline values after the initiation of puberty, remaining stable for extended periods; those changes occurred despite constantly increasing body mass index (11). In persons with anorexia nervosa, low leptin levels may contribute to the amenorrhea that is characteristic of that disorder (12–14).
Leptin acts not only in the hypothalamus (15), where it can produce rapid synaptic modulatory effects (16) and increased luteinizing hormone-releasing hormone release from medial basal hypothalamic explants (17), but it also has direct actions on the rat pituitary gland and ovary; in the pituitary, it stimulates luteinizing hormone (LH) and follicle-stimulating hormone release (17), and in the ovary, it impairs the insulin-like growth factor I-mediated augmentation of follicle-stimulating hormone-stimulated estradiol-17β synthesis by granulosa cells (18). Thus, leptin can affect the hypothalamic–pituitary–gonadal axis by direct actions in the hypothalamus, the pituitary gland, and the gonads.
Even though it is well established that leptin affects reproductive function, studies have not yet elucidated whether leptin is only a necessary trophic factor for the reproductive system or whether the minute-to-minute dynamics of leptin concentrations are associated with hypothalamic–pituitary–ovarian (HPO) function. Our group and others have demonstrated that leptin concentrations in human plasma have ultradian and circadian fluctuations (19–21). Because the hormones of the HPO axis exhibit striking pulsatility, we designed a study to examine the hypothesis that pulsatile release of leptin, LH, and estradiol exhibit pattern coupling or synchronicity by measuring plasma concentrations of the three hormones every 7 min throughout the 24 h.
METHODS
Clinical Research Protocol.
Six healthy women were studied on days 8–11 of the menstrual cycle (mid-to-late follicular phase) after giving informed consent for a clinical research protocol approved by the National Institute of Mental Health Institutional Review Board. Partial results of leptin, but not LH or estradiol, concentrations have been reported (21). Subjects had an average age of 25.5 ± 1.6 years and an average body mass index (calculated as the weight in kilograms divided by the square of the height in meters) of 21.6 ± 1.1 kg/m2. Women with history of psychiatric or medical illness, obesity, smoking, or substance abuse were excluded, as reported. For the 30-day period preceding each study, as well as during the study, no subject was taking prescribed or over-the-counter medications, hormones, or dietary supplements.
Subjects were acclimated to a research bed in the National Institutes of Health Clinical Center for 48 h before the study. We collected blood via an indwelling catheter that was inserted during the previous evening; samples were withdrawn every 7 min for 1442 min, yielding a total of 207 samples per subject, starting at 08:00 h. Clinical research protocol and diet are described in ref. 21. Subjects were exposed to light from 07:00–23:00 h and were studied in bed, in the dark from 23:00–07:00 h, during which time they slept. Sleep was monitored by the NightCap apparatus (Healthdyne Technologies, Marietta, GA) (22, 23). To maintain their level of physical activity at a comparable baseline, on the day of blood collection, subjects were allowed only to walk from their beds to the bathroom and to an adjacent hospital dayroom.
Hormone Assays.
Total human leptin was measured by radioimmunoassay as described (21). LH was measured by radioimmunoassay (Nichols Institute, San Juan Capistrano, CA), and the sensitivity of the assay was 0.1 units/liter (Second International Reference Preparation); intra-assay CV was 2.6% and inter-assay CV was 5.4%. Estradiol was measured by radioimmunoassay (Diagnostic Products Corporation, Los Angeles). The sensitivity of the assay was 8 pg/ml; intra-assay CV was 7.0% and inter-assay CV was 8.1%.
To estimate an index of experimental within-assay variation, leptin was measured from one pool of blood 207 times, providing a series of replicates with the same length as the experimental series obtained from the study subjects.
Pulse Analysis: cluster.
To assess possible changes in plasma LH, leptin, and estradiol concentration pulse parameters during daytime (08:00–17:00 h, which is characterized by lower leptin levels) and nighttime (23:00–08:00 h, which is characterized by higher leptin levels), we used cluster (24), a computerized pulse analysis algorithm to identify statistically significant pulses in relation to measurement error in each hormone time series. The use of this program has been described (21). Measurement error (within sample variance) for the 207 replicated samples in each series was modeled as a power function of sample concentration. Significantly increased or decreased hormone concentrations (peak margins) were judged by pooled t statistics, which were applied to moving test nadirs and peak clusters that began with the onset of the experimental series and traversed all points. We identified the following mean properties of pulsatile hormone concentrations: pulse frequency (number of significant peaks/24 h), interpeak interval (time separating consecutive peak maxima), pulse duration in min, pulse height (maximal hormone concentration in a peak), pulse height as percentage increase over preceding baseline (100% corresponds to preceding baseline), pulse area, and largest increase (increment) above pre-peak basal or nadir.
Cross-Correlation Analysis.
Cross-correlation was calculated after lagging the concentration time series of one hormone relative to the concentration time series of another hormone. Cross-correlation was carried out at variable lags, which are the times in minutes separating consecutive samples in the paired hormone series of interest. Significant cross-correlation values for the group of six subjects at any particular lag were tested against the null hypothesis of purely random associations via the one-sample Kolmogorov–Smirnov statistic applied to the z-score-transformed r values, assuming that uncorrelated data show a unit normal z-score distribution with 0 mean.
Approximate Entropy (ApEn) and Cross-ApEn.
ApEn is a scale- and translation-invariant and model-independent regularity statistic developed to quantify the orderliness of sequential measures (25, 26), such as hormonal time series. Larger ApEn values correspond to greater randomness (irregularity). Technically, ApEn measures the logarithmic likelihood that runs of patterns that are similar remain so on next incremental comparison. The basic derivation and calculation of ApEn have been presented (27, 28). Two input parameters, m (window or vector length) and r (tolerance or threshold), must be specified to compute ApEn. For this study, we calculated ApEn values for each hormone profile with window length m = 1 and tolerance parameter r = 20% of the average SD of the individual subject’s hormone time series. Thus, this calculated ApEn is denoted as ApEn (1, 20%). Previous theoretical analyses and clinical applications have demonstrated that these input values produce good statistical validity of ApEn for time series of 50 or more data points (28–31). Mathematically stated, the ApEn application with m = 1 is said to estimate the rate of entropy for a first-order (m = 1) approximating Markov chain to the underlying true process.
In choosing the r input parameter (tolerance) in ApEn as a fixed percentage of the SD of each data set, we normalized ApEn for each profile. This so-called normalized ApEn is both translation- and scale-invariant (27). This point is important when different absolute hormone levels are expected, as they were here.
ApEn is stable to small changes in noise characteristics and infrequent albeit significant outliers (25, 31). This statistic evaluates a variety of dominant and subordinate patterns in the data; for example, ApEn can detect and quantify changes in underlying regularity of hormone release that are not necessarily reflected in changes in peak frequency or amplitude (31). ApEn identifies consistency of point-by-point variations in the data, rather than macroscopic patterns or diurnal trends. Indeed, the latter are removed by first-differencing of the data. Additionally, ApEn provides a barometer of feedback changes in many coupled systems (31, 32).
ApEn is a family of statistics, which individually provides a relative, not absolute, measure of process regularity. ApEn thus can show significant variation in absolute value with changing m or r input parameters, N (data series length), and/or noise characteristics (experimental variability) (28). Because ApEn generally will increase with increasing N and noise (and, hence, increasing intra-assay CV), it is important to compare data sets with similar Ns and assay CVs, as we do here. Thus, day–night ApEn comparisons were both restricted to fixed windows of time (08:00–17:00 h and 23:00–08:00 h). Technical and statistical properties of ApEn, including so-called mesh interplay, relative consistency of (m, r) pair choices, asymptotic normality under general assumptions, statistical bias, and error estimation for general processes are discussed elsewhere (33). To compare observed ApEn measures with those expected on the basis of purely chance patterns occurring within the estradiol, LH, or leptin profiles, each hormone time series was shuffled 1,000 times to produce randomly assigned sample sequences. The resultant so-called random ApEn values were assumed to reflect assay and sampling noise without true biological rhythmicity. Hence, the ratio of random-to-observed ApEn, given the calculated SD of this ratio for 1,000 simulations in each series, monitors the extent of significantly nonrandom regularity structure or patterning in the data.
To evaluate relative regularity of LH and estradiol, LH and leptin, and estradiol and leptin concentrations, we used the cross-approximate entropy statistic (cross-ApEn). This measure quantifies the conditional regularity or synchrony of point-by-point variations across two time series. It is distinct from cross-correlation analysis because cross-ApEn is independent of lag. This issue is discussed in detail by Pincus et al. (34). In particular, cross-ApEn measures the relative pattern orderliness of two data series, with lower absolute cross-ApEn values denoting greater conditional regularity or synchronicity. Cross-ApEn is calculated following z-score transformation of both data series but otherwise captures the same combinatorial structure as ApEn. The detailed technical description of cross-ApEn is given further elsewhere (28, 35).
Comparisons were made by using Student’s t test or ANOVA. When multiple comparisons were made a protected value of P < 0.01 was used to determine statistical significance.
RESULTS
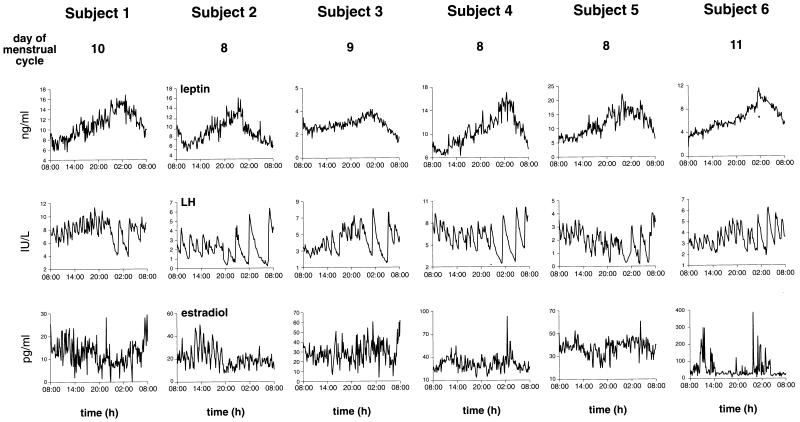
The pattern of plasma leptin over the 24 h was similar in all six subjects (Fig. 1) and is illustrated in more detail in one subject (subject 2) (Fig. 2). The nadir was reached either at the beginning or just after the onset of sampling at 08:00 h (Fig. 1; Table 1). After this point, values rose to a peak in all subjects, which was reached between 01:00 and 02:00 h. In the case of LH (Figs. 1 and 2), the patterns were different in that a rise occurred in subjects 1, 3, and 6, reaching a peak at 20:00–21:00 h. In the other three subjects, LH values either did not rise or actually decreased over this time (in subject 5). All subjects showed a change in the pattern of pulsatile LH release from a rapid, low pulse amplitude pattern to a slow, high pulse amplitude pattern, which commenced between 01:00 and 02:00 h at the time that leptin values reached the peak in each subject.
Figure 1.
Frequently sampled (every 7 min), 24-h profiles of plasma leptin, LH, and estradiol concentrations in six healthy women. Note in all subjects a nocturnal rise in plasma leptin concentrations and a change in LH pulse patterns from high frequency–low amplitude during the day to low frequency–high amplitude at night.
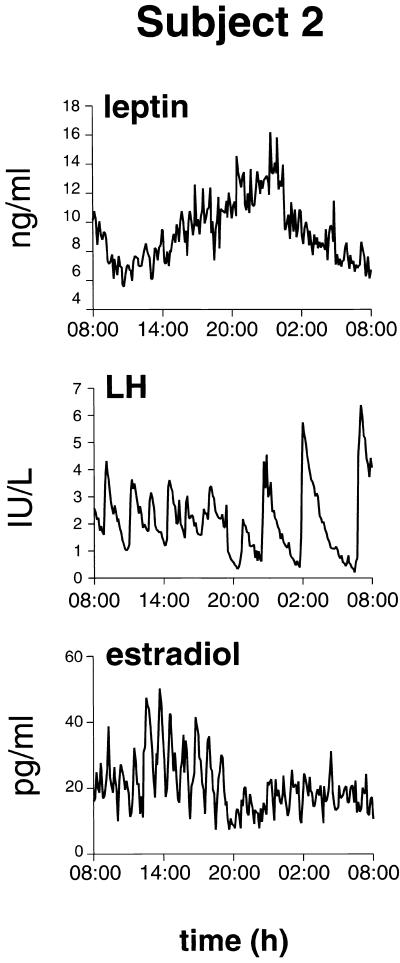
Figure 2.
Detail of leptin, LH, and estradiol profiles for subject 2. Note a change in LH pulse patterns and in estradiol concentrations during the nocturnal rise in plasma leptin concentrations.
Table 1.
Daytime and nighttime parameters of leptin, LH, and estradiol pulsatility and cross-ApEn
| Parameter | Day (08:00–17:00 h) | Night (23:00–08:00 h) | Significance |
|---|---|---|---|
| Average leptin concentrations, ng/mL | 7.63 ± 1.20 | 10.2 ± 1.7 | P < 0.03 |
| LH pulse number/h | 0.73 ± 0.06 | 0.39 ± 0.06 | P < 0.0004 |
| LH inter-Pulse interval, min | 75 ± 7 | 118 ± 15 | P < 0.03 |
| LH pulse width, min | 58 ± 6 | 104 ± 13 | P < 0.005 |
| LH pulse height, unit/liter | 5.93 ± 1.34 | 6.43 ± 1.24 | NS |
| LH pulse height, % increase | 160 ± 20 | 370 ± 74 | P < 0.02 |
| LH average pulse area, unit/liter × mis | 57 ± 11 | 227 ± 53 | P < 0.02 |
| LH mean largest value as increase above basal | 1.57 ± 0.16 | 3.77 ± 0.74 | P < 0.03 |
| E2 pulse number/h | 0.32 ± 0.08 | 0.28 ± 0.08 | NS |
| E2 inter-pulse interval, min | 92 ± 16 | 88 ± 8 | NS |
| E2 pulse width, min | 88 ± 11 | 87 ± 28 | NS |
| E2 pulse height, pg/mL | 54.7 ± 16.6 | 70.8 ± 31.3 | NS |
| E2 pulse height, % increase | 263 ± 45 | 304 ± 68 | NS |
| E2 average pulse area, pg/ml × min | 1288 ± 504 | 1063 ± 349 | NS |
| E2 mean largest value as increase above basal | 34.7 ± 14.8 | 50.3 ± 27.0 | NS |
| Significant AM vs. PM differences in cross-ApEn | Observed | Random | Ratio |
| Leptin vs. LH | P < 0.05 | P = NS | P < 0.05 |
| Leptin vs. E2 | P = NS | P = NS | P = NS |
| E2 vs. LH | P = NS | P = NS | P = NS |
NS, not significant.
Further statistical analysis revealed that LH, but not estradiol, pulse parameters were significantly different during daytime (08:00–17:00 h) when leptin levels were lower, compared with late at night (23:00–08:00 h) when leptin levels were higher (Table 1; Fig. 1). In all subjects, the nocturnal rise in leptin was associated with significant changes in the profile of LH pulses from more frequent, shorter pulses of smaller area during the day to pulses during the night that were half as frequent and twice as wide (representing twice as large a percentage increase in LH levels) and that had a fourfold increase in pulse area. This change in LH pulse patterns near the nocturnal peak in leptin levels was observed in all subjects studied (Table 1).
There were no consistent changes in estradiol pulsatility parameters during the nighttime compared with daytime in this group of six subjects. However, when differences in daytime or the nighttime mean concentrations of estradiol were compared within each subject, we found highly significant differences in day vs. night estradiol concentrations in four of six subjects (Table 2). Of interest, two of those four subjects had decreases in estradiol levels at night and two had increases in estradiol levels at night. The individuals whose estradiol concentrations decreased at night had daytime levels of the steroid under 25 pg/ml, and those whose estradiol levels increased at night had day estradiol levels over 25 pg/ml.
Table 2.
Daytime and nighttime leptin and estradiol concentrations
| Hormone concentrations | Day (08:00–20:00 h) | Night (20:00–08:00 h) | Significance |
|---|---|---|---|
| Leptin, ng/ml | |||
| Subject 1 | 9.08 ± 0.16 | ↑ 12.60 ± 0.22 | P < 10−6 |
| Subject 2 | 8.76 ± 0.16 | ↑ 9.93 ± 0.24 | P < 10−4 |
| Subject 3 | 2.72 ± 0.04 | ↑ 3.02 ± 0.07 | P < 10−3 |
| Subject 4 | 8.66 ± 0.15 | ↑ 12.37 ± 0.22 | P < 10−6 |
| Subject 5 | 8.53 ± 0.22 | ↑ 14.5 ± 0.32 | P < 10−6 |
| Subject 6 | 4.71 ± 1.0 | ↑ 7.90 ± 0.15 | P < 10−6 |
| Estradiol, pg/mL | |||
| Subject 1 | 13.9 ± 0.5 | ↓ 11.3 ± 0.6 | P < 10−3 |
| Subject 2 | 24.7 ± 0.9 | ↓ 16.8 ± 0.4 | P < 10−6 |
| Subject 3 | 26.7 ± 0.8 | ↑ 31.2 ± 1.2 | P < 2 × 10−3 |
| Subject 4 | 31.6 ± 0.8 | 31.5 ± 1.0 | NS |
| Subject 5 | 35.9 ± 0.6 | ↑ 39.3 ± 0.6 | P < 10−3 |
| Subject 6 | 57.9 ± 5.4 | 46.7 ± 5.4 | NS |
↑, significantly increased; ↓, significantly decreased; NS, not significant.
Synchronicity among leptin, LH, and estradiol time series was assessed independently by cross-correlation analysis and by cross-ApEn. We found statistically significant cross-correlation values between leptin and LH for this group of six female subjects, with a group P < 0.02. For the cross-correlation of LH and leptin, the association between LH and leptin was inverse at a lag of −42–84 min; namely, LH increases tended to follow significantly leptin decreases by 42–84 min (and conversely, LH decreases tended to follow leptin increases by 42–84 min), to a significantly nonrandom degree in the group of six subjects considered as a whole. An analysis by time of day showed that the cross-correlation between the leptin and LH hormone series could be explained solely by the nighttime correlation between these two hormones. Thus, as leptin levels rose at night, leptin pulsatility changed from high frequency–low amplitude to low frequency–high amplitude and became statistically significantly correlated with LH. The cross-correlation between plasma leptin and estradiol and between plasma LH and estradiol concentrations was not significant at any lag across an interval of −150 through +150 min for the group of six subjects studied at nighttime, daytime, or throughout the 24-h period.
In our subjects, all individual leptin, LH, and estradiol release profiles had a statistical structure that was significantly different from random. ApEn calculated from random time series obtained after shuffling the three types of data series 1,000 times was 1.900 ± 0.038: In the 6 subjects studied, with a total of 1,242 measurements per hormone, the mean ApEn of leptin was 1.362 ± 0.078; the ApEn of LH was 1.204 ± 0.098, and the ApEn of estradiol was 1.661 ± 0.111. When we estimated ApEn for these three hormones, LH varied significantly over the course of the day, with the highest organization at night. Leptin tended to follow this pattern, but with less pronounced differences between different times of the day. There were no differences in the level of organization of estradiol throughout the 24-h period.
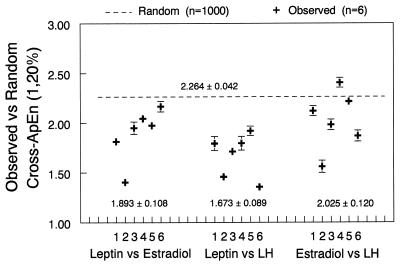
To independently assess pattern synchronicity between leptin, LH, and estradiol, we calculated cross-ApEn for leptin–LH, leptin–estradiol, and LH–estradiol (Fig. 3). The mean random cross-ApEn had a value of 2.264 ± 0.042, which is a global random cross-ApEn that is anticipated when hormone pairs are completely unrelated by way of patterned consistency or synchrony. The cross-ApEn of LH–estradiol was not different from the mean random cross-ApEn, supporting the lack of cross-correlation in these women at this phase of the menstrual cycle (days 8–11). In contrast, the significantly reduced (nonrandom) cross-ApEn values between leptin and LH (1.673 ± 0.089) and between leptin and estradiol (1.893 ± 0.108) indicate that there is synchronicity or pattern coupling between leptin and LH and between leptin and estradiol. When we compared daytime and nighttime patterns, we found that the cross-ApEn of LH and leptin exhibited significantly higher pattern coupling at nighttime compared with daytime (1.83 ± 0.03 vs. 1.52 ± 0.06, P < 0.05) and that the correlation observed between these two hormones occurred only at night. This was confirmed further by a separate analysis of cross-ApEn in four 6-h intervals (08:00–14:00 h, 14:00–20:00 h, 20:00–02:00 h, and 02:00–08:00 h), showing that the cross-ApEn for leptin vs. LH varies significantly (P = 0.024 by ANOVA), with significantly increased orderliness in the 20:00–02:00 h and 02:00–08:00 h periods. This pattern was similar to that seen for cross-ApEn of leptin/estradiol (P = 0.0039) across the four windows of time, with a significantly lower cross-ApEn (more orderly pattern synchrony) at night. There were no significant group differences between the synchronicity of leptin and estradiol at night compared with daytime.
Figure 3.
Individual values of cross-ApEn in six individual women; blood was sampled at 7-min intervals for 24 h. Cross-ApEn quantifies the conditional regularity or relative synchrony between hormone pairs noted. Each woman’s observed cross-ApEn value is plotted, with a SD denoting the Monte Carlo-predicted experimental uncertainty in the cross-ApEn calculation, given the dose-dependent within-assay errors. Below each set of cross-ApEn values is a mean ± SEM for the group of six individuals. Above the observed data, the interrupted line denotes the mean global random cross-ApEn obtained by shuffling data in each of the 18 pairs of hormones 1,000 times and recalculating ApEn on the shuffled series. Hence, this value (2.264 ± 0.042) is a random cross-ApEn anticipated when hormone pairs are completely unrelated by way of patterned consistency or relative synchrony. Lower cross-ApEn values indicate greater pattern repetition or conditional synchrony in the point-by-point release profiles of the two hormones.
DISCUSSION
In an intensively sampled group of six healthy women, studied in the mid-to-late follicular phase of the menstrual cycle (days 8–11), we found by determination of ApEn that rapidly sampled plasma concentrations of leptin and LH have ultradian release patterns with quantifiable levels of orderliness that are significantly different from random. We demonstrated previously that frequent sampling at 7-min intervals best characterizes the ultradian pattern of leptin fluctuations (21). By using two independent methods to assess temporal linkages, cross-correlation analysis and determination of cross-ApEn, we demonstrated in our subjects synchronicity or pattern coupling between leptin and LH. Additionally, by cross-ApEn, we showed lag- and scale-independent pattern coupling of leptin and estradiol that was also significantly lower (more orderly synchrony pattern) at night. In the phase of the menstrual cycle in which this group of healthy young women was studied, we could not in contrast detect significant nonrandom cross-correlation or cross-ApEn values for LH and estradiol. Moreover, we found that, in this phase of the menstrual cycle, in all subjects, the nocturnal rise in plasma leptin levels was associated with a profound change in the pattern of LH pulses from rapid and smaller pulses during the day to fewer, longer, and incrementally higher pulses of a fourfold larger area at the time of the nocturnal rise of leptin.
Even though some individuals, such as subjects 2 and 5, exhibited clear changes in estradiol pulsatility patterns at nighttime (Fig. 1), there were no consistent changes in estradiol pulsatility parameters at nighttime for this group of women as a whole. This might be due to individual variability, limited sample size, or phase of the menstrual cycle. However, when we looked at daytime vs. nighttime differences in mean estradiol concentration with each individual subject, we found highly significant changes in nighttime estradiol concentrations in four of six subjects. Of interest, the subjects whose estradiol concentrations decreased at night had mean daytime estradiol concentrations under 25 pg/ml, and those whose estradiol concentrations increased at night had mean estradiol daytime concentrations over 25 pg/ml. The hypothesis that the effects of the nocturnal rise of leptin on estradiol depend on a threshold effect of baseline estradiol concentrations should be tested. Further studies are warranted to determine whether the nocturnal rise in leptin levels is associated temporally with alterations in estradiol concentration pulsatility parameters. A key point must be addressed in future studies: In addition to group analyses, data must be assessed individually in each patient. In this study, because two patients had highly significant decreased and two subjects had highly significant increased nighttime estradiol, averaging out the values for the whole group would mask completely these differences.
It long has been known that a certain level of nutritional status is required for normal functioning of the reproductive axis. Frisch (36, 37) proposed the critical weight hypothesis, according to which a certain level of body weight is required for the onset of puberty. However, when starved rats are rapidly fed, they reach puberty before attaining their “critical” weight (38). Amenorrhea and loss of reproductive function are such prominent features of conditions characterized by low body weight that amenorrhea is the only neuroendocrine defect that is required for the diagnosis of a psychiatric disorder, namely anorexia nervosa (39). More recently, the studies of Chehab et al. (6) and Ahima et al. (5) have shown that leptin acts as a hormonal signal that conveys biologic information from fat tissue to the reproductive axis; those investigators showed that leptin treatment accelerates the onset of puberty in mice.
In humans, during conditions of starvation, such as anorexia nervosa, leptin levels fall and rise during the refeeding process (12, 13). Moreover, Köpp et al. (40) have found that fasting leptin levels are a better predictor of menstrual function than body mass index, fat mass, or percentage body fat. In that study, the critical level of leptin that predicted lifetime occurrence of amenorrhea was in the range of 1.85 μg L−1. The authors concluded that a critical blood leptin concentration was needed to maintain menstruation. Low leptin concentrations are associated with specific behaviors. In underweight women, low leptin levels are correlated with restrained eating behavior (41, 42). On the other hand, genetically determined leptin deficiency in humans results in increased food intake and marked obesity (43).
Leptin seems to affect reproduction at various levels by modulating reproductive behavior (9) and acting directly in the hypothalamus (16, 17), pituitary gland (17), and ovary (18). Besides serving as a signal of nutritional status to the reproductive system, leptin also is synthesized in the placenta at comparable or greater levels than in adipose tissue; it has been hypothesized that the placenta serves as a source of leptin for the growing fetus, where it may function as a growth factor, possibly signaling nutritional status from mother to fetus (44–46).
The role of altered leptin concentrations in the pathophysiology of ovarian disease is currently unclear. There has been controversy in the literature regarding the effects of leptin in polycystic ovary syndrome (PCOS) (47–50).
The nocturnal change in follicular phase LH levels was first observed by Kapen et al. (51) who noted a change of LH levels at nighttime, which they subsequently attributed to the sleep cycle (52). Additional studies by other investigators further characterized the changes in LH pulsatility that occur at night during the follicular phase of the menstrual cycle (53–55). Because naloxone infusion has been shown to prevent the sleep-associated decrease in LH pulse frequency, a role for an opioidergic mechanism has been proposed for that decrease in LH pulse frequency (56).
In the present study, nocturnal changes in LH pulse parameters were associated temporally with the rise of plasma concentrations of leptin at night. The fluctuations of plasma leptin concentrations were synchronous with those of LH and estradiol, and the pattern of synchrony of leptin/LH and leptin/estradiol was more orderly at night. As leptin levels rose at night, LH pulsatility significantly changed from low amplitude and high frequency to high amplitude and low frequency and became synchronous with leptin. We suggest that the nocturnal rise in leptin concentrations may contribute to the nighttime changes in LH pulsatility patterns that we observe in the mid-to-late follicular phase of the menstrual cycle, preceding ovulation.
In our study using frequently sampled, 24-h leptin release profiles, we showed that leptin levels exhibit synchronicity or pattern coupling with LH, as assessed by two independent methods. The synchronicity of leptin and estradiol was observed only by assessment of cross-ApEn. This result highlights the need for scale- and lag-independent assessments of hormonal time series that can reveal pattern coupling that might not be evidenced by lag-dependent cross correlation analysis (34). The mechanisms for such pattern coupling of leptin, LH, and estradiol are not yet elucidated and might involve direct actions of leptin in the central nervous system, pituitary, and/or ovary. Indeed, in the ovariectomized rat, the response to intraventricular leptin is related to the plasma estradiol concentration. A rapid stimulation of LH release only occurred when the animals had received an injection of 10 μg of estradiol benzoate 72 h before, whereas after a 50-μg dose, LH release was inhibited. These effects probably were mediated by altered luteinizing hormone-releasing hormone release (ref. 17; A. Walczewska and S.M.M., unpublished data).
Additionally, the nocturnal increase in leptin levels was associated temporally with a profound change in LH pulse parameters. We propose that leptin is not only a trophic factor for the reproductive system but that the pulse patterns as well as absolute concentrations of leptin may organize the optimal minute-to-minute functioning of the HPO axis. Such a close association among leptin, LH, and estradiol may be of importance for reproduction and provides an additional level of communication between nutritional status and episodic activity of the reproductive axis. Our findings might contribute to explain the disruption of HPO function that is present in states of low leptin production, such as anorexia nervosa and cachexia. Further studies using agonists and antagonists to leptin receptors should help to test the mechanisms by which alterations in the dynamics of leptin concentrations may affect various levels of HPO axis functioning and reproduction. Comparable studies also will be required in males to evaluate leptin–LH–testosterone synchronicity within the hypothalamic—pituitary–testicular axis, a component of which (LH–testosterone) exhibits altered pattern synchronicity in healthy older males (34).
Acknowledgments
We are very grateful to Anna Esposito, Jonathan Gold, Pooja Khatri, Ramesh Kumar, Rachel Lisman, Zeba Qaadri, Amer Al-Shekhlee, and Fuyiu A. Yip for their outstanding technical assistance. This study could not have been conducted without the expertise of the 4W nursing staff, National Institutes of Health Clinical Center. This work was supported by the National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases (to J.S.F. and Grant R01 43900 to S.M.M.), the National Institutes of Health-National Institute on Aging (1R01 147991 to J.D.V.), the National Institutes of Health-National Institute of Child Health and Human Development (P-30 Reproduction Center Grant HD 28934 to J.D.V.), the National Institutes of Health-National Institute of Mental Health (R37 51853 to S.M.M.), and the National Alliance for Research on Schizophrenia and Depression (M.-L.W.). C.M. is the Clinical Associate Physician, Beth Israel Deaconess Medical Center General Clinical Research Center, supported by National Institutes of Health Grant RR 01032-22S1.
ABBREVIATIONS
- LH
luteinizing hormone
- ApEn
approximate entropy
- HPO
hypothalamic-pituitary-ovarian
- CV
coefficient of variation
- PCOS
polycystic ovary syndrome
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 3.Hwa J J, Ghibaudi L, Compton D, Fawzi A B, Strader C D. Horm Metab Res. 1996;28:659–663. doi: 10.1055/s-2007-979873. [DOI] [PubMed] [Google Scholar]
- 4.Hwa J J, Fawzi A B, Graziano M P, Ghibaudi L, Williams P, Van H M, Davis H, Rudinski M, Sybertz E, Strader C D. Am J Physiol. 1997;272:R1204–R1209. doi: 10.1152/ajpregu.1997.272.4.R1204. [DOI] [PubMed] [Google Scholar]
- 5.Ahima R S, Dushay J, Flier S N, Prabakaran D, Flier J S. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chehab F F, Mounzih K, Lu R, Lim M E. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- 7.Chehab F F, Lim M E, Lu R. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 8.Ahima R S, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier J S. Nature (London) 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 9.Wade G N, Lempicki R L, Panicker A K, Frisbee R M, Blaustein J D. Am J Physiol. 1997;272:R1354–R1358. doi: 10.1152/ajpregu.1997.272.4.R1354. [DOI] [PubMed] [Google Scholar]
- 10.Barash I A, Cheung C C, Weigle D S, Ren H, Kabigting E B, Kuijper J L, Clifton D K, Steiner R A. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 11.Mantzoros C S, Flier J S, Rogol A D. J Clin Endocrinol Metab. 1997;82:1066–1070. doi: 10.1210/jcem.82.4.3878. [DOI] [PubMed] [Google Scholar]
- 12.Mantzoros C, Flier J S, Lesem M D, Brewerton T D, Jimerson D C. J Clin Endocrinol Metab. 1997;82:1845–1851. doi: 10.1210/jcem.82.6.4006. [DOI] [PubMed] [Google Scholar]
- 13.Hebebrand J, Blum W F, Barth N, Coners H, Englaro P, Juul A, Ziegler A, Warnke A, Rascher W, Remschmidt H. Mol Psychiatry. 1997;2:330–334. doi: 10.1038/sj.mp.4000282. [DOI] [PubMed] [Google Scholar]
- 14.Licinio J. Mol Psychiatry. 1997;2:267–269. doi: 10.1038/sj.mp.4000298. [DOI] [PubMed] [Google Scholar]
- 15.Flier J S. Proc Natl Acad Sci USA. 1997;94:4242–4245. doi: 10.1073/pnas.94.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glaum S R, Hara M, Bindokas V P, Lee C C, Polonsky K S, Bell G I, Miller R J. Mol Pharmacol. 1996;50:230–235. [PubMed] [Google Scholar]
- 17.Yu W H, Kimura M, Walczewska A, Karanth S, McCann S M. Proc Natl Acad Sci USA. 1997;94:1023–1028. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zachow R J, Magoffin D A. Endocrinology. 1997;138:847–850. doi: 10.1210/endo.138.2.5035. [DOI] [PubMed] [Google Scholar]
- 19.Sinha M K, Ohannesian J P, Heiman M L, Kriauciunas A, Stephens T W, Magosin S, Marco C, Caro J F. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha M K, Sturis J, Ohannesian J, Magosin S, Stephens T, Heiman M L, Polonsky K S, Caro J F. Biochem Biophys Res Commun. 1996;228:733–738. doi: 10.1006/bbrc.1996.1724. [DOI] [PubMed] [Google Scholar]
- 21.Licinio J, Mantzoros C, Negrao A B, Cizza G, Wong M-L, Bongiorno P B, Chrousos G P, Karp B, Allen C, Flier J S, et al. Nat Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 22.Pace-Schott E F, Kaji J, Stickgold R, Hobson A. Sleep. 1994;8:688–692. doi: 10.1093/sleep/17.8.688. [DOI] [PubMed] [Google Scholar]
- 23.Ajilore O, Stickgold R, Rittenhouse C D, Hobson A. Psychophysiology. 1995;32:92–98. doi: 10.1111/j.1469-8986.1995.tb03410.x. [DOI] [PubMed] [Google Scholar]
- 24.Veldhuis J D, Johnson M L. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 25.Pincus S M. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao E, Xia-Zhang L, Thornell D, Ferin M. J Clin Endocrinol Metab. 1996;81:2136–2141. doi: 10.1210/jcem.81.6.8964841. [DOI] [PubMed] [Google Scholar]
- 27.Pincus S M, Huang W M. Commun Stat Theor Methods. 1992;21:3061–3077. [Google Scholar]
- 28.Pincus S M, Singer B H. Proc Natl Acad Sci USA. 1996;93:2083–2088. doi: 10.1073/pnas.93.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman M L, Pincus S M, Johnson M L, Matthews D H, Faunt L M, Vance M L, Thorner M O, Veldhuis J D. J Clin Invest. 1994;94:1277–1288. doi: 10.1172/JCI117446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pincus S M, Gevers E, Robinson I C A, Roelfsema F, Hartman M L, Veldhuis J D. Am J Physiol. 1996;270:E107–E115. doi: 10.1152/ajpendo.1996.270.1.E107. [DOI] [PubMed] [Google Scholar]
- 31.Pincus S M, Keefe D L. Am J Physiol. 1992;262:E741–E754. doi: 10.1152/ajpendo.1992.262.5.E741. [DOI] [PubMed] [Google Scholar]
- 32.Pincus S M. Math Biosci. 1994;122:161–181. doi: 10.1016/0025-5564(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 33.Pincus S M, Goldberger A. Am J Physiol. 1994;266:H1643–H1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- 34.Pincus S M, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis J D. Proc Natl Acad Sci USA. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pincus S M, Kalman R E. Proc Natl Acad Sci USA. 1997;94:3513–3518. doi: 10.1073/pnas.94.8.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frisch R E, Revelle R. Science. 1970;169:397–379. doi: 10.1126/science.169.3943.397. [DOI] [PubMed] [Google Scholar]
- 37.Frisch R E, McArthur J W. Science. 1974;185:949–951. doi: 10.1126/science.185.4155.949. [DOI] [PubMed] [Google Scholar]
- 38.Ronnekleiv O K, Ojeda S R, McCann S M. Biol Reprod. 1978;19:414–424. doi: 10.1095/biolreprod19.2.414. [DOI] [PubMed] [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 40.Köpp W, Blum W, von Prittwitz S, Ziegler A, Låbbert H, Emons G, Herzog W, Herpertz S, Deter H-C, Remschmidt H, et al. Mol Psychiatry. 1997;2:335–340. doi: 10.1038/sj.mp.4000287. [DOI] [PubMed] [Google Scholar]
- 41.von Prittwitz S, Blum W F, Ziegler A, Scharmann S, Remschmidt H, Hebebrand J. Mol Psychiatry. 1997;2:420–422. doi: 10.1038/sj.mp.4000300. [DOI] [PubMed] [Google Scholar]
- 42.Mantzoros C. Mol Psychiatry. 1997;2:377–380. doi: 10.1038/sj.mp.4000323. [DOI] [PubMed] [Google Scholar]
- 43.Montague C T, Farooqi I S, Whitehead J P, Soos M A, Rau H, Wareham N J, Sewter C P, Digby J E, Mohammed S N, Hurst J A, et al. Nature (London) 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 44.Hassink S G, de Lancey E, Sheslow D V, Smith-Kirwin S M, O’Connor D M, Considine R V, Opentanova I, Dostal K, Spear M L, Leef K, et al. Pediatrics. 1997;100:e1. doi: 10.1542/peds.100.1.e1. [DOI] [PubMed] [Google Scholar]
- 45.Hoggard N, Hunter L, Duncan J S, Williams L M, Trayhurn P, Mercer J G. Proc Natl Acad Sci USA. 1997;94:11073–11078. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, et al. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 47.Brzechffa P R, Jakimiuk A J, Agarwal S K, Weitsman S R, Buyalos R P, Magoffin D A. J Clin Endocrinol Metab. 1996;81:4166–4169. doi: 10.1210/jcem.81.11.8923878. [DOI] [PubMed] [Google Scholar]
- 48.Mantzoros C S, Dunaif A, Flier J S. J Clin Endocrinol Metab. 1997;82:1687–1691. doi: 10.1210/jcem.82.6.4017. [DOI] [PubMed] [Google Scholar]
- 49.Laughlin G A, Morales A J, Yen S S. J Clin Endocrinol Metab. 1997;82:1692–1696. doi: 10.1210/jcem.82.6.4028. [DOI] [PubMed] [Google Scholar]
- 50.Rouru J, Anttila L, Koskinen P, Penttila T A, Irjala K, Huupponen R, Koulu M. J Clin Endocrinol Metab. 1997;82:1697–1700. doi: 10.1210/jcem.82.6.3996. [DOI] [PubMed] [Google Scholar]
- 51.Kapen S, Boyar R, Perlow M, Hellman L, Weitzman E D. Life Sci. 1973;13:693–701. [Google Scholar]
- 52.Kapen S, Boyar R, Hellman L, Weitzman E D. J Clin Endocrinol Metab. 1976;42:1031–1040. doi: 10.1210/jcem-42-6-1031. [DOI] [PubMed] [Google Scholar]
- 53.Soules M R, Steiner R A, Cohen N L, Bremner W J, Clifton D K. J Clin Endocrinol Metab. 1985;61:43–49. doi: 10.1210/jcem-61-1-43. [DOI] [PubMed] [Google Scholar]
- 54.Filicori M, Santoro N, Merriam G R, Crowley W J. J Clin Endocrinol Metab. 1986;62:1136–1144. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- 55.Sollenberger M J, Carlsen E C, Booth R J, Johnson M L, Veldhuis J D, Evans W S. Am J Obstet Gynecol. 1990;163:1529–1534. doi: 10.1016/0002-9378(90)90620-m. [DOI] [PubMed] [Google Scholar]
- 56.Rossmanith W G, Yen S S. J Clin Endocrinol Metab. 1987;65:715–718. doi: 10.1210/jcem-65-4-715. [DOI] [PubMed] [Google Scholar]