BACKGROUND
Approximately 6–10% of persons with HIV infection are co-infected with HBV. [1, 2] Currently, three licensed anti-HIV drugs, lamivudine, emtricitabine, and tenofovir, also possess activity against HBV, and many experts advocate their use as part of highly active anti-retroviral therapy (ART) in persons with both HIV and HBV.[2, 3] Despite the overall effectiveness of ART, a subset of patients may undergo paradoxical worsening, an entity termed immune reconstitution inflammatory syndrome (IRIS).[4, 5] Though more commonly associated with opportunistic infections, IRIS has been associated with HBV.[5–8] Immune-mediated anti-HBV hepatocyte toxicity is often attenuated during HIV infection[2], but HBV-specific immune responses can be restored following ART initiation, resulting in acute hepatitis.[6] We describe a case of immune reconstitution hepatitis following initiation of therapy active against both HIV and HBV. We suggest that the high HBV antigen burden present during immune reconstitution led to this complication, and that treatment of his HBV prior to initiating HIV therapy may have prevented this syndrome.
CASE
A 43 year old male with HIV and chronic HBV infection, a CD4 count of 85 cells/μL (10%) and HIV viral load of 41,800 copies/mL, presented after being off ART therapy for 4 years. Initial laboratory evaluation revealed: HBV surface antigen (HBsAg) positive, HBV core antibody (HBcAb) positive, HBV early antibody (HBeAb) positive, albumin 4.3 g/dL, bilirubin level 0.3 mg/dL, Aspartate aminotransferase (AST) 59 U/L, Alanine transaminase (ALT) 82 U/L, creatinine 0.95 mg/dL, and a plasma HBV DNA level of >1,000,000,000 copies/mL (Roche COBAS TaqMan HBV Analyte Specific Reagent). Previous ART included exposure to zidovudine, zalcitabine, nelfinavir, saquinavir, stavudine, lamivudine, and efavirenz. In 2005, upon reinitiating care, he restarted ART with tenofovir DF, emtricitabine, abacavir, and atazanavir with ritonavir boosting.
Four weeks after re-starting ART, he was doing well clinically, HIV RNA had dropped (193 copies/mL), transaminases were unchanged (AST 57 U/L, ALT 84 U/L), and bilirubin was 3.3 mg/dL (attributed to atazanavir use). At eight weeks of ART, he presented to clinic with jaundice, dark urine and abdominal pain for one week. The laboratory evaluation revealed acute hepatitis (AST 1289 U/L, ALT 2409 U/L, bilirubin 2.2 mg/dL, alkaline phosphatase 292 U/L, gamma-glutamyl transferase 137 U/L, and prothrombin time international normalized ratio (INR) of 1.2). He had not taken other medications (acetaminophen level <1 mg/L) or consumed alcohol. He had a response to HBV therapy with a ≥3.5 log drop in his HBV DNA to 334,000 copies/mL, his HIV RNA was undetectable (<50 copies/mL), and CD4 count had nearly doubled to 152 cells/mL (14%). Additional testing for other viral hepatitis etiologies was negative (hepatitis A, hepatitis C, hepatitis D, cytomegalovirus, Epstein-Barr, herpes simplex). A summary of the laboratory results over time is presented (Figure 1).
FIGURE 1.
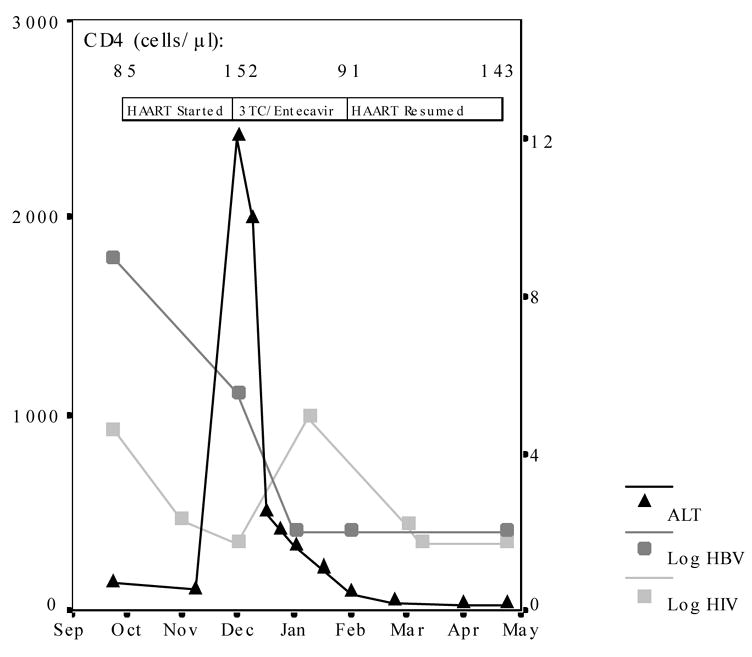
Alanine aminotransferase levels (ALT; left axis), log HBV DNA level (right axis), and log HIV RNA level (right axis) are presented over time. Treatment regimen and CD4 T-cell count at the time therapy was changed is also indicated.
HIV ART medications were discontinued, and lamivudine and entecavir were started for treatment of his HBV. He was thought to have already acquired HIV resistnace to lamivudine (based on ART history) and was also at high risk of harboring HBV resistance,[9] so entecavir was added to optimize viral suppression of HBV.[10] His transaminases normalized over three subsequent months (AST and ALT both 43 U/L), and complete suppression of his HBV occurred (<200 copies/mL). At this point, lamivudine and entecavir were stopped, and he resumed the prior ART regimen of tenofovir DF, emtricitabine, abacavir, and atazanavir with ritonavir boosting. Over the next 3 months, back on ART, his HIV RNA rapidly returned to undetectable (<50 copies/mL) with a CD4+ T-cell rise to 143 cells/mL (12%), and his HBV DNA remained suppressed (<200 copies/mL) with persistently normal liver function tests (AST and ALT both 30 U/L).
DISCUSSION
This patient with HIV/HBV co-infection suffered an acute exacerbation of hepatitis 8 weeks after starting potent therapy against HIV and HBV, despite a rapid virologic response. After his HBV DNA was fully suppressed (<200 copies/mL), he had no further complications upon re-initiation of the identical ART regimen. The steady decline in HBV DNA, lack of evidence for drug toxicity, and timing with immune reconstitution all strongly suggest IRIS as the overriding explanation for his clinical course. It seems that his persistent HBV antigen burden in the setting of improved immunity was responsible for the development of the clinical hepatitis. Perhaps, if his HBV infection was fully treated prior to the initiation of ART this clinical syndrome could have been prevented.
Risk factors for the development of IRIS after the initiation of ART include a decrease in HIV RNA load and a low pretreatment CD4 count with a rise on therapy. [4, 11] IRIS is thought to arise from the restoration of the host’s pathogen-specific immune response, so it follows that the antigen burden (at the time ART is started) may predict risk for IRIS. This is clearly the case with other opportunistic infections, such as TB and cryptococcus. [12, 13] In fact, where HBV DNA has been reported with IRIS, levels have been ≥80th percentile among persons with HBV, and our patient fell in the 90th percentile. [2, 6, 7, 14] Entecavir, a new and effective anti-HBV therapy without any overlapping HIV activity, was useful for decreasing the HBV-related antigen burden without treating HIV. Cases such as this suggest that we may prevent IRIS by controlling HBV infection with agents such as entecavir prior to initiating anti-HIV therapy.
Acknowledgments
The authors would like to thank Dr. Winston Cavert, Alice Medley, Margaret Thornton, and Matt Larson for their assistance in the care of this patient.
References
- 1.Brook MG, Gilson R, Wilkins EL. BHIVA Guidelines: coinfection with HIV and chronic hepatitis B virus. HIV Med. 2003;4 (Suppl 1):42–51. doi: 10.1046/j.1468-1293.4.s1.1.x. [DOI] [PubMed] [Google Scholar]
- 2.Soriano V, Puoti M, Bonacini M, et al. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV International Panel. Aids. 2005;19:221–40. [PubMed] [Google Scholar]
- 3.Thio CL, Sulkowski MS, Thomas DL. Treatment of chronic hepatitis B in HIV-infected persons: thinking outside the black box. Clin Infect Dis. 2005;41:1035–40. doi: 10.1086/496921. [DOI] [PubMed] [Google Scholar]
- 4.Boulware DR, Bohjanen PR. Chapter 19: Immune Reconstitution Inflammatory Syndrome. In: MA S, P V, J L, W G, editors. Global HIV/AIDS Medicine. Philadelphia: Elsevier; 2006. [Google Scholar]
- 5.Shelburne SA, 3rd, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev. 2003;5:67–79. [PubMed] [Google Scholar]
- 6.Carr A, Cooper DA. Restoration of immunity to chronic hepatitis B infection in HIV-infected patient on protease inhibitor. Lancet. 1997;349:995–6. doi: 10.1016/S0140-6736(05)62892-9. [DOI] [PubMed] [Google Scholar]
- 7.Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis. 2004;39:129–32. doi: 10.1086/421386. [DOI] [PubMed] [Google Scholar]
- 8.Mastroianni CM, Trinchieri V, Santopadre P, et al. Acute clinical hepatitis in an HIV-seropositive hepatitis B carrier receiving protease inhibitor therapy. Aids. 1998;12:1939–40. [PubMed] [Google Scholar]
- 9.Zoulim F, Poynard T, Degos F, et al. A prospective study of the evolution of lamivudine resistance mutations in patients with chronic hepatitis B treated with lamivudine. J Viral Hepat. 2006;13:278–88. doi: 10.1111/j.1365-2893.2005.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opio CK, Lee WM, Kirkpatrick P. Entecavir. Nat Rev Drug Discov. 2005;4:535–6. doi: 10.1038/nrd1780. [DOI] [PubMed] [Google Scholar]
- 11.Shelburne SA, Visnegarwala F, Darcourt J, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. Aids. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 12.Wendel KA, Alwood KS, Gachuhi R, Chaisson RE, Bishai WR, Sterling TR. Paradoxical worsening of tuberculosis in HIV-infected persons. Chest. 2001;120:193–7. doi: 10.1378/chest.120.1.193. [DOI] [PubMed] [Google Scholar]
- 13.Lortholary O, Fontanet A, Memain N, Martin A, Sitbon K, Dromer F. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. Aids. 2005;19:1043–9. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 14.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]


