Abstract
Activation of the natriuretic peptide system lowers blood pressure and causes the excretion of salt. Atrial natriuretic peptide and B-type natriuretic peptide are the humoral mediators of this effect; they act primarily by binding to membrane-bound natriuretic peptide receptor A (NPRA) and stimulating its intrinsic guanylate cyclase activity. To study whether genetically determined differences in NPRA expression affect blood pressure we have generated mice with one, two, three, or four copies of the gene encoding NPRA (Npr1 in the mouse). Atrial natriuretic peptide-dependent guanylate cyclase activity ranged progressively from approximately one-half normal in one-copy animals to twice normal in four-copy animals (P < 0.001). On different diets (0.05%, 2%, and 8% NaCl), the blood pressures of F1 male mice having only one copy of Npr1 averaged 9.1 mmHg (1 mmHg = 133 Pa) above those of wild-type two-copy males (P < 0.001), whereas males with three copies of the gene had blood pressures averaging 5.2 mmHg below normal (P < 0.01). The blood pressures of the one-copy F1 animals were significantly higher (by 6.2 mmHg; P < 0.01) on the high-salt than on the low-salt diet. The blood pressures of four-copy F3 males were significantly lower (by 7 mmHg; P < 0.05) on the high-salt than on the low-salt diet. These results demonstrate that below normal Npr1 expression leads to a salt-sensitive increase in blood pressure, whereas above normal Npr1 expression lowers blood pressures and protects against high dietary salt.
Keywords: guanylate cyclase-A, gene targeting, gene titration, salt sensitivity
Essential hypertension is a complex disease in which blood pressures are persistently high without identifiable cause. Both genetic and environmental factors are influential (1). Although a small number of individuals have been identified in which hypertension is the consequence of single gene defects that are inherited in a simple Mendelian fashion (2), the pattern of inheritance in the majority of individuals is not clear, suggesting that blood pressure, like many other quantitative traits, is determined by the action of many genes.
The natriuretic peptide system affects blood pressure directly through its vasodilatory and natriuretic activities (3); it also affects blood pressure indirectly, for example, by inhibiting the renin/angiotensin system whose action raises blood pressure (3). Genetic defects in the natriuretic peptide system are consequently candidate contributors to essential hypertension. Atrial natriuretic peptide (ANP), which induces a profound natriuresis, diuresis, and hypotension upon infusion into rats, was first discovered in 1981 by deBold et al. (4) in atrial extracts. Two other related peptides, B-type (brain-type) natriuretic peptide (BNP) and C-type natriuretic peptide (CNP), were subsequently isolated (5, 6). The major sites of synthesis of ANP and BNP (as precursors) are in the heart, and after release (as peptides) these hormones act primarily by binding to natriuretic peptide receptor A (NPRA), a membrane-bound form of guanylate cyclase (also known as guanylate cyclase-A or GC-A) (7). The binding of ligand stimulates production of cGMP, a second messenger, which relaxes smooth muscle, induces natriuresis and diuresis in the kidney, and inhibits aldosterone production in the adrenal glands. The third natriuretic peptide, CNP, mainly acts in the central nervous system through binding to natriuretic peptide receptor B (NPRB), also a guanylate cyclase type of receptor. All three peptides bind to a third receptor, natriuretic peptide type C receptor (NPRC), which lacks guanylate cyclase activity and is thought to function as a clearance receptor.
The study of genetic traits in living animals has been facilitated by gene targeting experiments in which desired genetic changes are made in the mouse germ line. When the trait of interest is quantitative, such as hypertension, rigorous investigations into how changes in expression of a relevant gene affect the phenotype of interest require great care in maintaining an otherwise uniform genetic background (8). The recently described method of gene titration (9, 10) provides a general strategy for implementing this type of investigation through analyses of mice that have an increased or decreased number of copies of a chosen target gene at its normal chromosomal location and controlled by its natural regulatory elements. This strategy typically produces heterozygous F1 offspring that are hybrids between the mouse strains 129 and C57BL/6 and have either one copy (1/0), two copies (1/1, wild type), or three copies (2/1) of the target gene. Because the resulting F1 animals are genetically identical except at the target locus, any phenotypic differences can be directly attributed to the changes in the target locus without the complications of genes, linked or unlinked, that may differ between the two strains of mice. By using this strategy, we have previously demonstrated that genetically determined changes in the level of expression of the angiotensinogen gene directly cause changes in the blood pressures of mice, and that these change are observable in animals that have all their normal homeostatic mechanisms intact (10).
Accordingly, to investigate the effects of quantitative changes in the natriuretic peptide system that are mediated by NPRA, we have used gene targeting to disrupt and duplicate the gene (Npr1) coding for NPRA in mice. The resulting F1 mice with one, two, or three copies of Npr1 and F3 mice with four copies establish a direct causative connection between Npr1 expression, ANP-stimulated guanylate cyclase activity, blood pressure, and its sensitivity to dietary salt.
MATERIALS AND METHODS
Gene Disruption and Gene Duplication by Homologous Recombination.
By using the published oligonucleotide sequence of the murine Npr1 cDNA (11) and the genomic organization of the rat Npr1 gene (12) as guides, two probes corresponding to exons 1 and 22 were generated by PCR with mouse genomic DNA as a template. DNA fragments that cover the entire Npr1 gene of a strain 129 mouse were isolated as six overlapping λ phage clones and mapped by restriction enzyme digests.
The targeting construct and procedures for disrupting the Npr1 gene have been described (13). The disruption replaced exon 1, intron 1, and a portion of exon 2 with a copy of the neomycin-resistance gene oriented oppositely from the Npr1 gene.
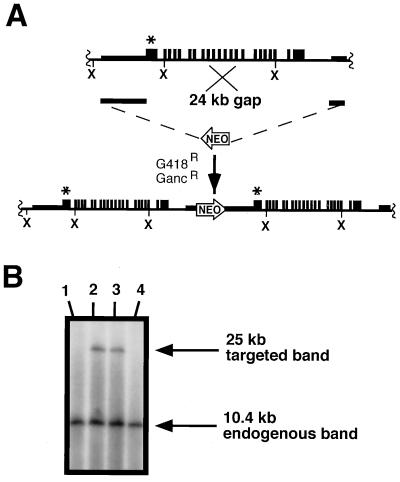
To duplicate the Npr1 gene, a targeting construct was made (see Fig. 1A) having a 6.5-kb fragment of strain 129 genomic DNA from immediately upstream of the 5′ coding region of the gene as the 5′ region of homology, with a 1.3-kb HindIII fragment from 6 kb downstream of the coding region as the 3′ region of homology. A neomycin-resistance cassette (NEO) from pMC1neopolA was placed between the 5′ and 3′ regions of homology, and the Herpes simplex thymidine kinase gene from pMC1TK was inserted downstream of the 1.3-kb fragment to allow for positive–negative selection (14). The embryonic stem (ES) cell line BK4 was electroporated with the linearized targeting construct, and the cells were cultured as described (15). G418/ganciclovir-resistant colonies were expanded, and their DNA was analyzed by Southern blots after digestion with XbaI and hybridization to a probe containing exon 1 sequences. Cells having the duplicated Npr1 gene give a 25-kb hybridizing fragment in addition to a 10.4-kb fragment corresponding to the wild-type gene.
Figure 1.
Targeted duplication of the murine Npr1 gene. (A) Homologous recombination and targeting construct. The endogenous Npr1 locus is shown schematically in the top line. The duplication targeting construct (middle line) uses a 6.5-kb 5′ region of homology from immediately upstream of the Npr1 coding region. The 3′ region of homology is a 1.3-kb fragment located approximately 6 kb downstream of exon 22. A 24-kb gap (indicated) is repaired during the recombination and a single crossover event leads to a 32-kb duplication, which includes the complete Npr1 gene and upstream and downstream flanking sequences. The bottom line shows the resultant locus with the two copies of Npr1 in tandem arrangement separated by the neomycin-resistant gene (NEO) (not drawn to scale). ×, XbaI sites; ∗, position of the probe. (B) Southern blots. Correctly targeted clones are identified after digesting ES cell DNA with XbaI and hybridizing with a probe corresponding to exon 1 of Npr1. The probe hybridizes to both 10.4- and 25-kb bands in the duplicated chromosome, but only to a 10.4-kb band in the unmodified chromosome.
Generation of Mice with Various Copies of the Npr1 Gene.
Chimeras for the duplication experiment were made by injecting targeted ES cells into blastocysts as described (16). Male chimeras transmitting the duplicated Npr1 gene to their offspring were mated to strain B6 female mice. The resulting F1 129 × B6 hybrid offspring, genotyped as described in the following section, have either three copies (2/1) of the Npr1 gene or two copies (1/1, i.e., wild type). Chimeras generated in the Npr1 disruption experiment (13) were mated to wild-type B6 females to provide F1 129 × B6 hybrid offspring with one gene copy (1/0). Because the 129 and B6 parental mouse strains are inbred, the F1 animals are genetically identical except for the targeted gene. Intercrossing the 2/1 F1 heterozygotes produced F2 offspring with two, three, or four copies (2/2) of the Npr1 gene. F3 animals having four copies of the Npr1 gene were also generated as described in Results.
Mouse Genotype Analysis.
Genotypes of the F1 animals were determined by the same Southern blot analyses used to identify targeted ES cells. Southern blot analysis cannot, however, reliably distinguish F2 or F3 three-copy and four-copy Npr1 offspring because both genotypes yield identical endogenous and targeted bands, which differ only in their relative amounts—an unreliable estimate in many Southern blots. Three- and four-copy offspring from matings between F1 or F2 animals were therefore distinguished by simple sequence length polymorphism analysis by using the Massachusetts Institute of Technology (MIT) markers D3MIT40 and D3MIT101 (17). These markers are located on opposite sides of the Npr1 gene at 2 and 3 centimorgans, respectively, and the corresponding PCR fragments differ in length in strain 129 and B6 animals. Typing for the markers allows determination of whether a specific animal has a copy of the Npr1 locus derived from strain 129 (and therefore has the genetically duplicated locus) accompanied or unaccompanied by a copy of the locus from strain B6 (the unaltered locus). PCR was performed on purified-tail DNA by using the published D3MIT40 and D3MIT101 primer sets and the PCR products were separated by polyacrylamide gel electrophoresis to distinguish band sizes characteristic of the two markers in strain 129 (110 bp and 124 bp) or in strain B6 (140 bp and 108 bp). For example, with D3MIT40, a single band of 110 bp indicates a strain 129-derived locus on both chromosomes, which in the F2 and F3 matings corresponds to a 2/2 (four-copy) genotype, whereas a single band of 140 bp indicates a B6-derived locus on both chromosomes, corresponding to a 1/1 (two-copy) mouse. Bands of 110 bp and 140 bp indicate a heterozygous 2/1 (three-copy) genotype. With D3MIT101, a single band of 124 bp corresponds to a 2/2 genotype, a single band of 108 bp corresponds to a 1/1 genotype, and bands of 108 bp and 124 bp indicate a 2/1 genotype. Animals having a discrepancy between the two flanking markers, indicating a crossover between them (about 5%), were not used for further analysis.
Guanylate Cyclase Assays.
Plasma membrane preparations were isolated as described (18) from tissues of 49–54-day-old animals having one, two, three, or four copies of Npr1. Animals were euthanized by anesthetic overdose. Dissected tissues were homogenized in ice-cold buffer and centrifuged to remove soluble cyclases. To determine cyclase activity, the membrane pellets were incubated at 37°C in the presence of 1 mM 3-isobutyl-1-methyl-xanthine, 2 mM ATP, 2 mM GTP, 4 mM MgCl2, 30 mM phosphocreatine, and 75 units/ml creatine kinase, with or without 1 μM ANP. At various times the incubated samples were treated with 6% trichloracetic acid, extracted with diethyl ether, and their cGMP content was determined by radioimmunoassay (19). The results are expressed as picomoles of cGMP synthesized per milligram of protein per minute, as determined by linear regression analysis of 0-, 5-, and 10-min time points. The basal rate of cGMP synthesis (with no ANP added before incubation) was subtracted from the rate of total cGMP synthesis (with ANP added immediately before incubation) to determine ANP-stimulated cGMP synthesis.
Blood Pressure Measurements.
All blood pressure measurements were made on conscious young adult male mice (ranging in age from 95–115 days) by a noninvasive computerized tail-cuff method (20). Blood pressures were first measured in animals that had been on low-salt chow (0.05% NaCl, TD94025; Teklad, Madison, WI) for 2 weeks. After 7 days of training, begun after approximately 1 week on the low-salt diet, the blood pressures for each animal were taken by an individual who did not know the genotypes of the animals. Animals were then placed on an intermediate-salt diet (2% NaCl, TD96037; Teklad) for an additional 2 weeks, during which the animals were retrained, and blood pressures were again measured. Finally, the animals were placed on a high-salt diet (8% NaCl, TD96038; Teklad) for 2 weeks including a retraining period and their blood pressures were measured. All blood pressures were calculated as the average of 6–10 sessions per day for 5 consecutive days. Statistical analyses were performed by using ANOVA for repeated measures with salt and genotype as variables.
Organ Weights and Hematocrit Measurements.
Tissues were removed and weighed from animals euthanized by anesthetic overdose. Organ weights were recorded as the percentage of total body weight. Hematocrits were the mean of the values obtained from each of three heparinized hematocrit tubes of blood taken from animals anesthetized with avertin (1.6 mg).
RESULTS
Gene Targeting.
Disruption of the Npr1 gene was achieved as described (13) by replacing exon 1, intron 1, and part of exon 2 of the gene with a neomycin resistance gene (NEO). Homozygotes for this disruption synthesize no NPRA (13). The principles of the method for duplicating genes by double-strand gap repair have been described previously (9). Double-strand gap repair uses the target gene as a template to fill in a gap between two regions of homology that span the gene, and a single crossover event completes the duplication event. The result is two identical copies of the gene arranged in tandem at the normal chromosomal locus. Fig. 1A illustrates the specific targeting construct and homologous recombination used with the Npr1 gene and the resultant duplicated locus. The entire coding region of the gene, together with 6.5 kb of sequence upstream of the Npr1 coding region and 7.3 kb of sequence downstream of the last coding exon, are included in the duplicated region, which totals 32 kb. This extensive duplication was aimed at ensuring that all regulatory regions are intact and duplicated. Correctly targeted ES cells, identified by Southern blot analysis of an XbaI digest of the cell DNA (Fig. 1B) by using a probe containing exon 1 sequences, occurred at a frequency of 1 in 9 doubly resistant colonies. Three male chimeras mated to B6 females produced F1 offspring; approximately 50% of the pups carried the duplicated locus as determined by Southern blot analysis of tail DNA.
The relative survival of pups with different numbers of functional copies of Npr1 was determined from 36 F1 × F1 matings (1/0 × 1/0 and 2/1 × 2/1), which together produced 228 F2 progeny. The numbers of progeny at weaning having zero, one, two, and three copies of the Npr1 gene conformed to normal Mendelian expectations, as did the numbers of four-copy females. However, there was a deficiency of male four-copy pups (12 expected, 2 observed; P < 0.05 by χ2). F2 males having three or four copies of Npr1 were therefore crossed with the four-copy F2 females to produce F3 offspring having three or four copies of the gene. These matings produced three-copy and four-copy progeny in essentially equal numbers (as expected) regardless of gender. Because of the difficulty of producing F2 male mice, F3 animals were used for studies of four-copy mice.
Copy Number-Dependent ANP-Stimulated cGMP Production in Mutant Mice.
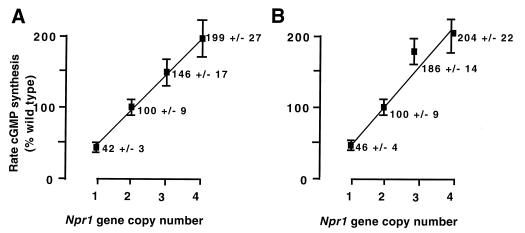
NPRA is a member of the guanylate cyclase family of receptors, which produce cGMP in response to ligand binding. NPRA elevates intracellular levels of cGMP when ANP binds to the extracellular domain of the receptor and allosterically activates its guanylate cyclase catalytic domain (21). To quantitate the biochemical consequences of altering the number of copies of the Npr1 gene, we measured ANP-dependent cGMP synthesis in membrane preparations from the various mutant animals and from controls. Fig. 2 shows the rates of ANP-dependent cGMP synthesis in lung and kidney membrane preparations from F1 animals with one, two, and three copies of the Npr1 gene and from F3 animals with four copies. The relative activities of one-, two-, three-, and four-copy animals, respectively 42%, 100% (by definition), 146%, and 199% in the lungs, and 46%, 100% (by definition), 186%, and 204% in the kidneys, demonstrate a linear and essentially direct proportionality between Npr1 gene copy number and ANP-dependent guanylate cyclase activity (P < 0.001 by ANOVA, r2 = 0.96 by linear regression analysis). Thus, the expression of NPRA protein as measured by the rate of ANP-stimulated cyclase activity is directly proportional to the Npr1 gene copy number. We infer that Npr1 gene expression at the mRNA level follows the same direct proportionality.
Figure 2.
Guanylate cyclase activity as a function of Npr1 gene copy number. Ordinate gives the rate of ANP-stimulated cGMP synthesis in lung (A) and kidney (B) membrane preparations from both male and female animals with one, two, three, and four copies of the Npr1 gene as a percent of the rate of normal two-copy animals. Solid squares represent the means of five animals for each genotype. Error bars represent the SEMs.
Blood Pressures in Npr1 Mutant Animals.
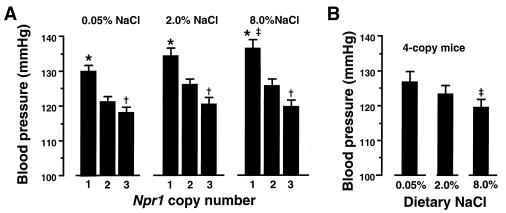
The natriuretic peptide/guanylate cyclase signaling system is generally thought to oppose the renin/angiotensin/aldosterone system by serving as a vasodilating system and as a regulator of blood volume and thence of blood pressure (22). To ascertain the effect of changes in expression of the Npr1 gene on blood pressures, and to determine whether animals with different steady-state levels of NPRA are able to maintain a stable blood pressure across a wide range of dietary sodium concentrations, animals were placed on low-(0.05% NaCl), intermediate-(2.0% NaCl), and high-(8% NaCl) salt diets, and their blood pressures were measured by a noninvasive computerized tail-cuff method (20). The tail-cuff system allows a longitudinal study of pressures to be made in the same animals while their dietary sodium intakes are changed. Fig. 3 presents tail-cuff blood pressure measurements on F1 and F3 males (12 per genotype) on each of the three diets. The data clearly establish that changes in the Npr1 gene copy number, and hence of NPRA expression, cause progressive changes in blood pressures at any of the three dietary salt concentrations (P < 0.0001 for effect of genotype by ANOVA for repeated measures). Blood pressures of the F1 generation mice having only one copy of Npr1 averaged 9.1 mmHg (1 mmHg = 133 Pa) above normal (P < 0.001); F1 mice with three functional copies of the gene had blood pressures that average 5.2 mmHg below normal (P < 0.01). There was a significant effect of salt on blood pressure in one-copy animals (P < 0.01, contrast of means, low salt versus high salt, by ANOVA for repeated measures), but not in animals having two or three copies of Npr1. Blood pressures in the F3 generation animals having four copies of Npr1 were negatively affected by increasing dietary salt (P < 0.05 for salt effect by ANOVA for repeated measures).
Figure 3.
(A) Tail-cuff blood pressures of male F1 animals having one, two, or three copies of the Npr1 gene. Twelve animals for each genotype were successively fed diets containing the indicated content of NaCl. (B) Tail-cuff blood pressures of male F3 mice having four copies of Npr1 (n = 12) and fed the same diets. (Results of the four-copy mice are graphed separately because the F3 mice are genetically distinct from the F1 animals.) Vertical bars represent mean values of each group of animals. Error bars represent SEMs. ∗, P < 0.001 compared with two-copy controls; †, P < 0.01 compared with two-copy controls; ‡, P < 0.05 compared with 0.05% salt.
General Features of the One- Through Four-Copy Mutant Animals.
Several general measurements were carried out with the one- through four-copy animals, including hematocrit, body weight, and weights of the kidney, lung, and heart. The resulting data (not shown) revealed no significant effects of this spectrum of Npr1 genotypes on any of the parameters. Histological evaluation of the hearts, lungs, and kidneys were also unremarkable (data not shown).
DISCUSSION
The chief observations made on the animals with one, two, three, and four copies of the Npr1 gene are: (i) ANP-stimulated cGMP synthesis increases essentially in direct proportion to the number of Npr1 genes, ranging from 42% and 46% normal in lungs and kidneys in animals with one copy of Npr1 to 199% and 204% normal in four-copy animals. (ii) Tail-cuff blood pressures decrease as Npr1 copy number and NPRA expression increases, with one-copy F1 males exhibiting blood pressures an average of 9.1 mmHg above wild-type controls, and three-copy F1 animals averaging 5.2 mmHg below controls; the average effect of Npr1 copy number is 7.2 ± 2.1 mmHg per copy (correlation coefficient 0.60; P < 0.01). (iii) No differences among the one-copy through four-copy genotypes were observed in hematocrits, organ weights, or histology of hearts, lungs, and kidneys. (iv) The blood pressures of the two- and three-copy F1 animals are not affected by changes in dietary salt over the range 0.05%, 2%, and 8%, but the one-copy F1 animals have blood pressures significantly higher when maintained on high-salt diet than on low-salt diet (by 6.2 mmHg), and the four-copy F3 animals show a significant decrease in blood pressure (6.9 mmHg) as dietary salt is increased from 0.05% to 8%.
Several conclusions follow from these observations. First, genetically determined quantitative changes in the expression of the Npr1 gene cause inverse changes in the blood pressures of mice that are otherwise genetically wild type and have all their normal homeostatic mechanisms intact. Thus, quantitative changes in the gene coding for NPRA, like that coding for angiotensinogen (10), directly affect blood pressures in otherwise normal mice. This is consistent with the previously published observation that mice completely lacking NPRA have elevated blood pressures (13, 23). The second conclusion is that NPRA expression affects the sensitivity of blood pressure to dietary salt: the one-copy F1 animals show small but statistically significant increases in pressure with increases in salt, whereas the four-copy F3 animals show small but also statistically significant decreases in pressure with increases in salt. Thus the Npr1 gene, like the gene coding for ANP, is a candidate for directly affecting the sensitivity of blood pressure to salt. The effects of decreased expression of Npr1 on the sensitivity of blood pressure to dietary salt in this study of heterozygous one-copy F1 mice differ from the absence of an effect reported by Lopez et al. (23) and by us (13) in F2 animals heterozygous (one-copy) for an Npr1 disruption. This difference is likely related to the greater difficulty of observing small genetic effects in F2 animals versus F1 animals (see ref. 8 for a discussion of this difficulty). Our conclusion that the ANP–NPRA system is a determinant of basal levels of blood pressure is in agreement with the findings from previous experiments by using genetically altered animals. Thus, transgenic mice with constitutive expression of the murine ANP gene in hepatocytes and a 5-fold normal plasma ANP level have blood pressures 25 mmHg lower than normal (24). A reduction of blood pressure in transgenic mice with 10- to 100-fold normal plasma levels of BNP has also been reported (25). Conversely, a genetic decrease in ANP production causes a salt-sensitive increase in the blood pressure of mice (26). In addition, Weidman et al. (27) have reported that the plasma ANP concentrations in children of two normotensive parents are higher than in children with a hypertensive parent, especially when the children ingest a high amount of salt, and Deng and Rapp (28) have demonstrated that specific alleles of the gene encoding NPRA in rats segregate with the blood pressure response to high salt intake in F2 hybrids between Milan salt-insensitive normotensive and Dahl salt-sensitive hypertensive rats.
Most importantly, the present “gene titration” experiments with the Npr1 gene in mice support two concepts. First, genetic alterations in the expression at the mRNA and/or protein level of the several protein-encoding genes that we have studied by gene titration [Agt (9), Ace (29), and Npr1], even in complex highly regulated biological systems like those controlling blood pressure, are not subject to direct feedback regulation by their products because the protein concentrations are essentially proportional to gene copy number. Second, genetically determined changes in the level of expression of an increasing variety of genes directly cause changes in blood pressure despite the existence of homeostatic systems. The degree to which these concepts are relevant to the genetic control of essential hypertension in humans depends on the existence and frequency in human populations of naturally occurring variants of the corresponding genes and on the degree to which the variants change expression. One such variant has been described for the human AGT gene (30, 31). Our present work strongly suggests that genetic polymorphisms affecting the level of expression of the human gene coding for NPRA, if they exist, would also be of importance in determining the inheritance of higher or lower than normal blood pressures, and perhaps of their sensitivity to dietary salt.
Acknowledgments
We thank Drs. Howard Rockman and Nobuyuki Takahashi for their review of our manuscript. This work was supported by U.S. Public Health Service Grants HL49277 and GM20069 (to O.S.), an American Heart Association Grant-in-Aid (to M.F.G.), a Howard Hughes Medical Institute Predoctoral Fellowship (to K.E.P), and a grant from the W. M. Keck Foundation for work with animal models.
ABBREVIATIONS
- ANP
atrial natriuretic peptide
- NPRA
natriuretic peptide receptor A
- BNP
B-type (“brain type”) natriuretic peptide
- ES
embryonic stem
References
- 1.Ward R. In: Hypertension, Pathophysiology, Diagnosis, and Management. Laragh J H, Brenner B M, editors. New York: Raven; 1990. pp. 81–100. [Google Scholar]
- 2.Lifton R P. Science. 1996;272:676–680. doi: 10.1126/science.272.5262.676. [DOI] [PubMed] [Google Scholar]
- 3.Espiner E A. In: Natriuretic Peptides in Health and Disease. Samson W K, Levin E R, editors. Totowa, NJ: Humana; 1997. pp. 123–146. [Google Scholar]
- 4.deBold A J, Borenstein H B, Veress A T, Sonnenberg H. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 5.Sudoh T, Kanagawa K, Minamino N, Matsuo H. Nature (London) 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 6.Sudoh T, Minamino N, Kanagawa K, Matsuo H. Biochem Biophys Res Commun. 1990;168:863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- 7.Garbers D L, Lowe D G. J Biol Chem. 1994;269:30741–30744. [PubMed] [Google Scholar]
- 8.Smithies O, Maeda N. Proc Natl Acad Sci USA. 1995;92:5266–5272. doi: 10.1073/pnas.92.12.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smithies O, Kim H-S. Proc Natl Acad Sci USA. 1994;91:3612–3615. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H-S, Krege J H, Kluckman K D, Hagaman J R, Hodgin J B, Best C F, Jennette J C, Coffman T M, Maeda N, Smithies O. Proc Natl Acad Sci USA. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey K L, Singh S. J Biol Chem. 1990;265:12342–12348. [PubMed] [Google Scholar]
- 12.Yamaguchi M, Rutledge J L, Garbers D L. J Biol Chem. 1990;265:20414–20420. [PubMed] [Google Scholar]
- 13.Oliver, P. M., Fox, J. E., Kim, R., Rockman, H. A., Kim, H.-S., Reddick, R. L., Pandey, K. N., Milgram, S. L., Smithies, O. & Maeda, N. (1998) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 14.Mansour S L, Thomas K R, Capecchi M R. Nature (London) 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 15.Koller B H, Kim H-S, Latour A M, Brigman K, Boucher R C, Scambler P, Wainwright B, Smithies O. Proc Natl Acad Sci USA. 1991;88:10730–10734. doi: 10.1073/pnas.88.23.10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koller B H, Hagemann L J, Doetschman T, Hagaman J R, Huang S, Williams P J, First N L, Maeda N, Smithies O. Proc Natl Acad Sci USA. 1989;86:8927–8931. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich W F, Miller J, Steen R, Stein L, Page D, Lander E S. Nature (London) 1996;380:149–153. [Google Scholar]
- 18.Goy M F. J Biol Chem. 1990;265:20220–20227. [PubMed] [Google Scholar]
- 19.Steiner A L, Parker C W, Kipnis D M. J Biol Chem. 1972;247:1106–1113. [PubMed] [Google Scholar]
- 20.Krege J H, Hodgin J H, Hagaman J R, Smithies O. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 21.Koller K J, Goedell D V. Circulation. 1992;86:1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- 22.Atlas S A, Maak T. Endocrinol Metab Clin N Am. 1987;16:107–143. [PubMed] [Google Scholar]
- 23.Lopez M J, Wong S K-F, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers D L, Beuve A. Nature (London) 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 24.Steinhelper M E, Cochrane K L, Field L J. Hypertension. 1990;16:301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimusa T, Uehira M, Matsuda S, Shino S, Nishimoto H, Nakao K. J Clin Invest. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John S W M, Krege J H, Oliver P M, Hagaman J R, Hodgin J H, Pang S C, Flynn G, Smithies O. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 27.Weidman P, Ferrari P, Alleman Y, Ferrari C, Shaw S G. Can J Physiol Pharmacol. 1991;69:1582–1585. doi: 10.1139/y91-235. [DOI] [PubMed] [Google Scholar]
- 28.Deng D, Rapp J P. Nat Genet. 1992;1:267–272. doi: 10.1038/ng0792-267. [DOI] [PubMed] [Google Scholar]
- 29.Krege J H, Kim H-S, Moyer J S, Jeanette J C, Peng L, Hiller S K, Smithies O. Hypertension. 1997;29:150–157. doi: 10.1161/01.hyp.29.1.150. [DOI] [PubMed] [Google Scholar]
- 30.Jeunemaitre X, Soubrier F, Kotelevtsev Y V, Lifton R P, Williams C S, Charru A, Hunt S C, Hopkins P N, Williams R R, Lalouel J-M, Corvol P. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 31.Inoue I, Nakajima T, Williams C S, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig E H, Sharma A M, Hata A, Jeunemaitre X, Lalouel J-M. J Clin Invest. 1997;99:1786–1797. doi: 10.1172/JCI119343. [DOI] [PMC free article] [PubMed] [Google Scholar]