Abstract
The angiotensin-converting enzyme (ACE; EC 3.4.15.1) gene (Ace) encodes both a somatic isozyme found in blood and several other tissues, including the epididymis, and a testis-specific isozyme (testis ACE) found only in developing spermatids and mature sperm. We recently used gene targeting to disrupt the gene coding for both ACE isozymes in mice and reported that male homozygous mutants mate normally but have reduced fertility; the mutant females are fertile. Here we explore the male fertility defect. We demonstrate that ACE is important for achieving in vivo fertilization and that sperm from mice lacking both ACE isozymes show defects in transport within the oviducts and in binding to zonae pellucidae. Males generated by gene targeting that lack somatic ACE but retain testis ACE are normally fertile, establishing that somatic ACE in males is not essential for their fertility. Furthermore, male and female mice lacking angiotensinogen have normal fertility, indicating that angiotensin I is not a necessary substrate for testis ACE. Males heterozygous for the mutation inactivating both ACE isozymes sire wild-type and heterozygous offspring at an indistinguishable frequency, indicating no selection against sperm carrying the mutation.
Keywords: gene targeting, oviduct, sperm function, zona pellucida
Angiotensin-converting enzyme (ACE; EC 3.4.15.1) catalyzes the cleavage of C-terminal dipeptides from several substrates including angiotensin I and bradykinin (1). The gene for ACE (ACE in humans, Ace in mice) codes for both a somatic and a smaller testis-specific isozyme. Somatic ACE is anchored to the plasma membranes of vascular endothelial cells and several epithelia, including cells in the epididymis, and a soluble form is present in blood. The testis isozyme is found only in developing spermatids and in mature sperm (2–5).
Somatic ACE encoded by the entire gene is composed of two homologous amino acid domains (6, 7). The testis isozyme is encoded by the second half of the gene under the control of a testis-specific promoter located within intron 12 (8). The testis isozyme has a unique N-terminal sequence determined by a testis-specific exon; its remaining sequence is identical to the C-terminal domain of somatic ACE (9–11). Transcription of testis ACE in mouse spermatogenic cells begins in late pachytene spermatocytes (3) or after meiosis (4), and ACE protein is first detected in haploid spermatids (2–4). The tissue specificity of testis ACE is achieved with a promoter sequence of 91 base pairs (12). The functions of the ACE isozymes in male reproduction are unknown.
We recently generated mice carrying an insertional disruption of exon 14 of the murine Ace gene, which prevents the synthesis of both testis and somatic ACE (13). Intercrossing of heterozygous mice gave ≈11% homozygous mutant mice compared with the expected 25%. Compared with wild-type mice (which we designate AceST/AceST, hereafter abbreviated STST to indicate the presence of both the somatic and testis isozymes), the homozygous mutant (stst) mice lacking both isozymes have blood pressures reduced about 34 mm Hg, abnormally thickened arteries in the kidneys, and atrophy of the renal cortex. Female stst homozygotes show similar blood pressure and kidney abnormalities but are normally fertile. The stst males mate normally and have normal sperm counts, testis histology, and sperm morphology by light microscopy (13), but they sire very few pups. This finding led to the current work exploring the role of ACE in reproductive function. We here test the hypothesis that ACE is important for achieving in vivo fertilization and examine several parameters of sperm function to identify specific defects. In addition, we have generated sTsT males lacking somatic ACE but having normal testis ACE to determine whether somatic ACE is required for male fertility. To determine whether angiotensin substrates play a role in male reproduction, we have also examined the fertility of mice lacking angiotensinogen. Finally, we have determined whether sperm from heterozygous (STst) mice carrying the mutant st allele are at a disadvantage relative to sperm carrying the wild-type ST allele.
METHODS
Mice Deficient in Somatic and Testis ACE.
The generation of mice lacking somatic and testis ACE by the targeted disruption of the Ace gene in strain 129/Ola embryonic stem cells has been described previously (13). Chimeras derived from targeted embryonic stem cells were mated to C57BL/6J females to generate strain 129/Ola × C57BL/6J F1 wild-type and heterozygous offspring. The F1 heterozygous mice were intercrossed to give wild-type (STST), heterozygous (STst), and homozygous mutant (stst) F2 offspring. Mice in the current experiments were from F2 through F4 generations.
Fertilization Assay.
Six- to eight-week-old CD1 females (Charles River Breeding Laboratories) were superovulated by intraperitoneal injection of 5 units of pregnant mare’s serum (Sigma) followed by 5 units of human chorionic gonadotropin (Sigma) 46–48 hr later. Eggs from females having a copulatory plug the day after being placed with stst or STST males were collected into M2 medium (Sigma). The eggs were treated with M2 containing hyaluronidase (300 μg/ml, Sigma) to remove the cumulus, washed in M2, and then maintained in M2 for ≈1 hr for assessment of the presence of male and female pronuclei. The eggs were then cultured in 50-μl drops of KSOM medium (Specialty Media, Lavallette, NJ) covered with dimethylpolysiloxane oil (DMPS-24, Sigma), which had been equilibrated overnight in a 5% CO2 37°C incubator. The cultured eggs were checked for progressive development over 4 days. Successful fertilization was scored as observation of male and female pronuclei at 0.5 days and/or progression to at least the 8-cell stage during days 1–4 of development.
Examination of Sperm Function.
To evaluate parameters of sperm function, stst (or wild-type) sperm were flushed from the uteri of superovulated females 1 hr after mating. This strategy avoids sacrificing the stst animals and allows collection of sperm from available animals on multiple occasions. All parameters were assessed by investigators who did not know the genotypes of the mice.
Sperm viability was assessed by using the live/dead sperm viability kit (Molecular Probes) according to the manufacturer’s protocol; at least 200 sperm from each animal were counted. Percent motility was determined by recording samples of sperm on videotape and scoring for forward motility (14); 100 sperm per group were assessed. Additional parameters of sperm motility were determined by computer-assisted sperm analysis as described (15).
To evaluate sperm capacitation, uterine sperm were incubated for 1 hr in Whitten’s medium (Specialty Media) and stained with chlortetracycline (16). The presence of a dark band just anterior to the tail was taken as indicating capacitation.
To assay the binding of sperm to zonae pellucidae, oocytes from CD1 superovulated females were collected 14–16 hr after human chorionic gonadotropin administration, and the cumulus was removed as described above. Zonae pellucidae were isolated from ova by forcing oocytes through a micropipette with a 30- to 50-μm internal diameter and were washed through three drops of fresh medium. Uterine sperm (1 × 105) were added to the washed zonae in 50-μl drops of Whitten’s medium under oil and incubated for 1 hr at 37°C in 5% CO2. Zonae were then moved to M2 medium and gently washed to remove unbound sperm by using a large-bore micropipet. The number of sperm bound to each zona was counted. In otherwise identical experiments, the binding of sperm to zonae pellucidae was also determined in the presence of rotation on an orbital shaker at 80–100 rpm (17).
To assay the acrosome reaction, uterine sperm were incubated for 1 hr at 37°C in a 5% CO2 incubator in Whitten’s medium alone, in medium plus the calcium ionophore A23187 (20 μM, Sigma), or in medium plus heat-solubilized zonae pellucidae (3–4 zona eq/μl) (18) made from zonae that had been solubilized by incubating at 60°C for 1 hr (19). To determine the percentage of sperm that had undergone the acrosome reaction, sperm were fixed and stained with Coomassie brilliant blue G250 as previously described (20). At least 200 sperm from each ejaculate were assayed for the presence or absence of the characteristic dark blue acrosomal crescent.
To examine sperm transport in the female reproductive tract, males were mated with superovulated females 13–17 hr after human chorionic gonadotropin injection, and 1 hr after mating, oviducts were fixed in Bouin’s solution, embedded in paraffin, and serially sectioned at 7 μm. One section every 50 μm was stained with Gill’s hematoxylin and eosin, sperm were counted, and oviduct regions were identified according to Suarez (21). An average of 369 sections/female was counted, and the number of sperm located in each region of the oviduct was then calculated.
Mean values for each parameter of sperm function were determined for individual mice, and group means ± SEM were calculated. Statistical analysis of sperm number/oviduct region was performed by ANOVA following log transformation and confirmed by using the nonparametric Wilcoxon rank sum test. Other parameters were compared by using Student’s two-tailed t tests.
Generation of sTsT Mice.
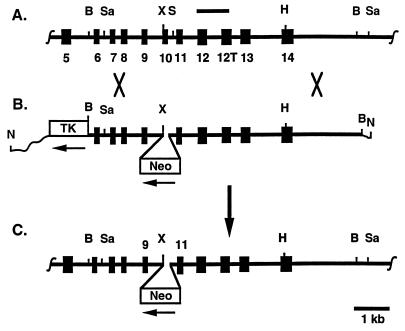
Mice (sTsT) lacking somatic ACE but having testis ACE were generated by gene targeting. Ace DNA was cloned as described (13). By using the pPNT vector (22), a targeting construct (Fig. 1B) was made in which the sequence from the XbaI site of exon 10 to the SnaBI site in intron 10 of the Ace gene (Fig. 1A) was replaced by sequences coding for the neomycin resistance gene (Neo). The construct was linearized with NotI, and targeted cells were produced as described (23). The targeted locus (Fig. 1C) contains the exon 10 splice acceptor site and the first nucleotide of the exon; the remainder of exon 10 is replaced by the Neo gene oriented oppositely from the testis Ace promoter. Correctly targeted embryonic stem cells, identified by Southern analysis with BamHI- and SacI-digested genomic DNA, were injected into blastocysts to generate chimeras. The chimeras were mated to C57BL/6J females to produce F1 offspring. F1 mice heterozygous for the mutation were identified by Southern analysis and were intercrossed to obtain STST, STsT, and sTsT F2 mice.
Figure 1.
Targeting of the Ace gene to eliminate somatic ACE but leave testis ACE intact. (A) The endogenous Ace locus from exon 5 to 14 is shown; the horizontal bar indicates the probe used for cloning and Southern analysis; the numbered black boxes indicate exons, with 12T indicating the testis-specific exon; B, Sa, X, S, and H indicate BamHI, SacI, XbaI, SnaBI, and HindIII. (B) The targeting construct is shown. Sequences from the XbaI site to the SnaBI site have been replaced with the neomycin resistance gene (Neo) oriented oppositely from the Ace promoters. TK indicates the thymidine kinase gene oriented as indicated; wavy lines indicate plasmid sequences (not drawn to scale). (C) The targeted Ace locus with most of exon 10 replaced by an inserted copy of Neo is shown.
In Situ Hybridization and Immunohistochemistry.
Testes were fixed either in 4% paraformaldehyde in phosphate-buffered saline for in situ hybridization or in Bouin’s solution for immunohistochemistry, embedded in paraffin, and processed by using standard techniques. A labeled riboprobe made from a 1.9-kb fragment of the rat cDNA (a generous gift from J. Allegrini) was used for in situ mRNA hybridization. Polyclonal antibody C28, directed against a C-terminal sequence of human ACE common to both somatic and testis isozymes, was used for immunohistochemistry (2, 4). Normal rabbit serum was used as a negative control. In situ hybridization used standard procedures except for a microwave heating step for signal enhancement (24). The intensity of the RNA hybridization signal and the level of immunostaining were evaluated by two independent investigators who did not know the genotypes of the mice.
Analysis of ACE Activity.
Serum was obtained by retroorbital bleeding and stored at −70°C. ACE activities were assayed by using a scaled-down reaction with a commercially available kit (Sigma) with activities expressed as units/liter.
Fertility Tests.
Male mice were placed with females for 6 weeks and then removed. Cages were checked at least once daily during this period and for 1 month thereafter, and the numbers of litters and pups were recorded.
RESULTS
Sperm from stst Males Achieve in Vivo Fertilization at a Reduced Frequency.
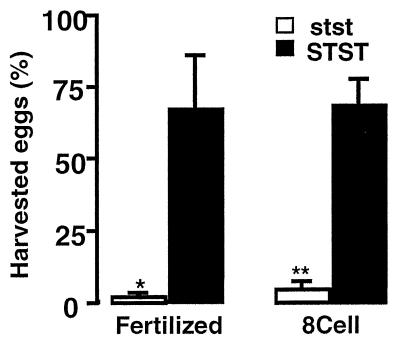
Our previous studies demonstrated that stst males lacking both somatic and testis ACE produce very few offspring although stst females are normally fertile (13). To determine whether this reduced fertility results from lower levels of fertilization, wild-type female mice were mated with stst or STST males, and eggs were collected and allowed to develop in vitro. In three experiments we compared the progress of 70 eggs from females (n = 5) inseminated by STST males (n = 2) with the progress of 113 eggs from females (n = 6) inseminated by age-matched stst males (n = 2). More than 65% of the eggs from the STST matings were fertilized and developed to the 8-cell stage or beyond compared with less than 5% of the eggs from the stst matings (Fig. 2; P < 0.001).
Figure 2.
Percentage of harvested eggs from females inseminated by stst males (2 males, 6 females) or STST males (2 males, 5 females) that were fertilized and/or progressed to the 8-cell stage or beyond. Error bars show standard errors of the means. ∗, P < 0.02; ∗∗, P < 0.001 for stst vs. STST.
Sperm from stst Males Show Defects in Oviduct Transport.
The testis histology, sperm counts, and sperm morphology of stst males have been reported previously by us (13) and others (25) to be indistinguishable from those in STST males by light microscopy. In the present experiments, we examined sperm collected from the uterus 1 hr after mating. Although the number of sperm collected in this fashion was variable (0.01–13.8 × 106 sperm per collection), there were no significant differences in the mean number of sperm collected from stst and STST mice. Sperm from mice of both genotypes did not differ significantly in assays of viability, motility, capacitation, or acrosome reaction in the presence of calcium ionophore or solubilized zonae pellucidae (Table 1). In addition to curvilinear velocity and straightness (Table 1), other parameters measured by computer-assisted sperm analysis, including straight line velocity, average path velocity, and amplitude of lateral head displacement, were indistinguishable (not shown). When incubated in Whitten’s medium with 20 mg/ml BSA for 1 hr, sperm from both stst and STST males also displayed changes in motility consistent with hyperactivation (not shown).
Table 1.
Parameters of sperm function
| Experiments | STST (n) | stst (n) | P* | |
|---|---|---|---|---|
| Sperm, ×10−6 | 26 | 4.2 ± 0.4 (10) | 4.0 ± 0.4 (10) | 0.67 |
| Live, % | 2 | 72 ± 3 (4) | 73 ± 1 (2) | 0.78 |
| Motility | ||||
| Motile, % | 4 | 57 ± 19 (4) | 50 ± 11 (3) | 0.77 |
| Curvilinear velocity | 3 | 236.4 ± 24.1 (4) | 261.8 ± 42.7 (3) | 0.64 |
| Straightness | 3 | 66.7 ± 4.6 (4) | 68.5 ± 3.3 (3) | 0.76 |
| Capacitated, % | 3 | 49 ± 7 (5) | 45 ± 4 (3) | 0.71 |
| Acrosome reacted, % | ||||
| Medium only | 6 | 22.5 ± 3.1 (9) | 21.5 ± 1.2 (7) | 0.78 |
| Ionophore | 6 | 76.4 ± 4.6 (9) | 81.9 ± 2.5 (7) | 0.39 |
| Solubilized zonae | 3 | 65.8 ± 6.3 (4) | 61.8 ± 4.9 (4) | 0.63 |
P values determined by Student’s t test.
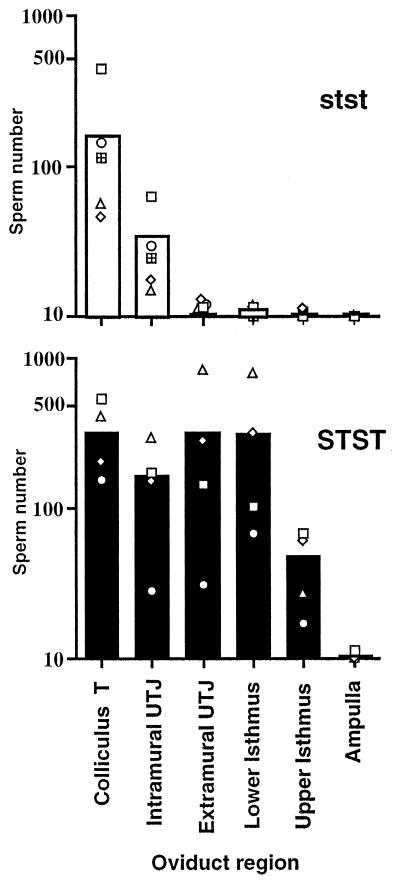
Although the mutant sperm show no motility defects, the mean number of sperm from stst males counted in the oviducts 1 hr after mating (175 ± 86) was much lower than the mean number of sperm from STST males counted in the oviducts under identical conditions (1135 ± 428, P < 0.001 by ANOVA). This difference was most pronounced in the extramural uterotubal junction and in the lower and upper isthmus regions of the oviducts (Fig. 3). Very few sperm from either genotype reach the ampulla in 1 hr.
Figure 3.
Location of sperm from stst (n = 5) and STST males (n = 4) in oviducts 1 hr after mating. Sperm in each oviduct region were counted (one section every 50 μm) in six experiments. The open symbols represent means for individual males; the open (stst) and solid (STST) bars indicate group means for each oviduct region. Very few sperm from stst males progress beyond the two oviduct regions nearest the uterus [colliculus tubaris (T) and intramural uterotubal junction (UTJ)]. Significantly more sperm from STST males reach the extramural uterotubal junction and the lower and upper isthmus regions of the oviducts (P < 0.001 by ANOVA; P < 0.02 by Wilcoxon rank sum tests).
Sperm from stst Males Show Defects in Binding to Zonae Pellucidae.
By using an in vitro assay, the mean number of sperm from stst males (n = 9) bound per zona pellucida (5.7 ± 1.0) was found to be significantly lower (P < 0.02 by Student’s t test) than the mean number of sperm from STST males (n = 10) bound per zona (>14.1 ± 2.9) in 12 experiments. The mean number of sperm bound per zona for STST males is a minimum estimate, because individual zona frequently had >20 sperm bound and could not be counted accurately. Orbital shaking did not affect the binding of either stst or STST sperm to zonae, in agreement with the absence of motility defects in the stst sperm.
Generation of sTsT Mice Having Testis ACE but Lacking Somatic ACE.
Animals (sTsT) were generated by using gene targeting as shown in Fig. 1. This targeting disrupts the sequence encoding somatic ACE but leaves the sequence encoding testis ACE intact. The genotypes of 23 F2 litters were assessed at 3 weeks of age and included 49 STST (29 males, 20 females), 95 STsT (60 males, 35 females), and 7 sTsT (5 males, 2 females) animals. These proportions differ significantly from Mendelian 1:2:1 ratios (P < 0.001 by χ2) because of a deficiency of sTsT mice.
Various tests demonstrate that the sT mutation eliminated somatic ACE while leaving testis ACE unchanged. The serum ACE activity in sTsT mice (23 ± 23 units/liter, n = 2) was not significantly different from zero, as in stst mice (13). Serum ACE activities in mice heterozygous for the sT mutation (177 ± 9 units/liter; n = 12) or for the st mutation, which inactivates both the somatic and testis functions of the Ace gene (176 ± 8 units/liter; n = 21), were reduced to 64% of wild-type values (273 ± 8 units/liter; n = 22). Immunohistochemistry with a somatic ACE-specific antibody showed that somatic ACE is absent in the testes of sTsT mice (data not shown). The kidneys of two of two sTsT mice showed vascular hyperplasia and cortical atrophy (not shown) indistinguishable from the pathology previously reported in stst mice (13) and in mice lacking a functional angiotensinogen gene (26).
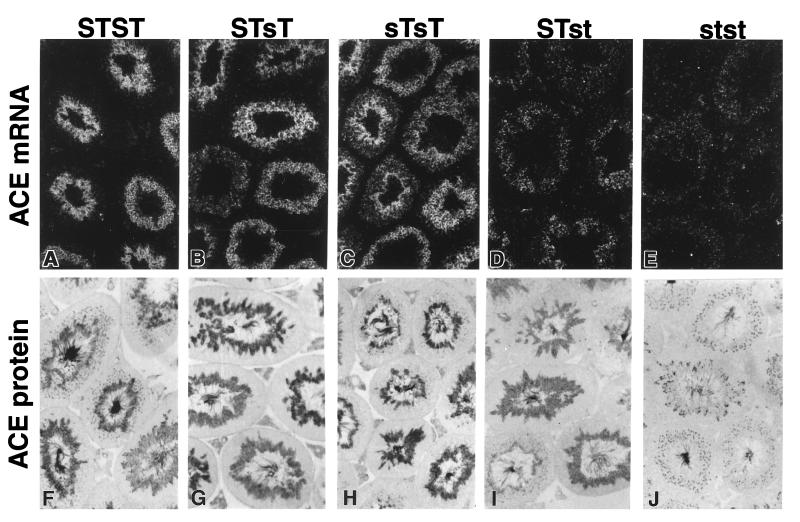
The sT mutation does not change the expression of testis ACE. The levels and distribution of testis ACE mRNA and protein were indistinguishable in comparisons of testes from STST mice (Fig. 4 A and F) with those from STsT mice (Fig. 4 B and G) or sTsT mice (Fig. 4 C and H). In contrast, the levels of testis ACE mRNA and protein at identical stages of spermiogenesis were reduced in STst mice (Fig. 4 D and I). Testis ACE mRNA and protein in stst (Fig. 4 E and J) were indistinguishable from controls (sense probe or normal rabbit serum, respectively.)
Figure 4.
ACE mRNA (A–E) and protein (F–J) expression in testis from wild-type and mutant mice (genotypes indicated at top) as shown by in situ hybridization to a rat cDNA riboprobe (dark field, upper panel) and immunohistochemistry (lower panel) by using a polyconal antibody against a C-terminal sequence common to human somatic and testis ACE with no counterstaining. Both mRNA and protein were detected only in spermatids, adjacent to the lumen of each seminiferous tubule. Magnification, ×80.
Somatic ACE Is Not Essential for Normal Male Fertility.
To test whether testis ACE alone is sufficient to restore normal fertility, we examined the fertility of sTsT males, which lack somatic ACE but have normal levels of testis ACE in developing spermatids. The numbers of pups per wild-type female in a 6-week mating period with sTsT and STST males did not differ significantly (P > 0.2; Table 2). The number of pups per wild-type female sired by the sTsT males was 37 times higher than the number sired by stst males (P < 0.001; Table 2). As an additional confirmation of normal sTsT sperm function, we tested sperm from an sTsT male in the above described zona binding assay. In two experiments with sperm from the sTsT male, 45 of 45 zonae pellucidae had >20 sperm bound. These results establish that the absence of somatic ACE in males does not impair their fertility and that testis ACE is sufficient for normal fertility. As expected, female sTsT mice were also fertile, with 3 tested females giving 6 litters and 29 pups.
Table 2.
Testing of male fertility
| Male genotype | n, males | n, females* | Litters | Pups | Pups/female |
|---|---|---|---|---|---|
| stst† | 7 | 12 | 2 | 4 | 0.33‡ |
| sTsT | 5 | 10 | 15 | 123 | 12.3 |
| STST | 2 | 4 | 7 | 56 | 14.0 |
All females were wild type, STST.
Includes some previously reported data (13).
P < 0.001 vs. sTsT.
Angiotensins in the Male Are Not Essential for Normal Fertility.
ACE catalyzes cleavage of the C-terminal dipeptide from several substrates including angiotensin I. We have previously reported (26) that one male lacking angiotensinogen sired 3 litters with an average of 8.3 pups per litter. In the present 6-week fertility test, 2 additional males lacking angiotensinogen were tested with 2 wild-type females and gave 4 litters yielding 35 pups. These results confirm that male-derived angiotensins are not essential for male fertility.
Heterozygous STst Mice Transmit Mutant Sperm as Frequently as Wild-Type Sperm.
To test whether haploid mutant (st) sperm from STst mice are transmitted to offspring at a reduced frequency compared with nonmutant (ST) sperm, we mated F1 and F2 STst males with wild-type females and genotyped the offspring at 3 weeks of age. The observed proportions of offspring (F1: 57 STST, 60 STst; and F2: 17 STST, 18 STst) did not differ from 1:1 (P > 0.5). Thus, st sperm from STst males are functionally equivalent to ST sperm. In situ hybridization and immunohistochemistry results are relevant in this context (Fig. 4). In STst animals the levels of ACE mRNA and protein are indistinguishable between individual spermatids, although they differ in their Ace haplotypes.
DISCUSSION
We (13) and others (25) have observed previously that male but not female mice lacking both ACE isozymes (stst) have markedly reduced fertility. Likewise, some combinations of mutations in the Drosophila ACE gene result in about 10% survival and male sterility (27), indicating the long standing evolutionary importance of ACE in male fertility. We here demonstrate that the decreased fertility of the mice can be fully accounted for by the reduced capacity of their sperm to achieve fertilization in vivo. Those few oocytes that are fertilized by sperm from stst mice develop normally to the 8-cell stage, and occasional pups are sired by these males (13).
Mice that are stst completely lack somatic ACE, have low blood pressures, and develop a severe kidney pathology. Any of these factors could contribute to the low male fertility. To assess whether absence of somatic ACE is relevant, we generated mice with an insertional disruption of the somatic but not the testis portion of the Ace gene. Males homozygous for this mutation (sTsT) have normal amounts of testis ACE mRNA and protein but completely lack somatic ACE and like the stst mice have severe kidney pathology. Nevertheless, sTsT males have normal fertility, proving conclusively that somatic ACE in males is not essential for their fertility.
We infer from these findings that testis ACE is necessary and sufficient for sperm function. Nevertheless, stst males produce normal numbers of sperm that are indistinguishable from wild-type sperm in assays of viability, motility, capacitation, and induction of the acrosome reaction. Despite these normal functional parameters, very few sperm from stst males are found in oviduct regions above the intramural uterotubal junction 1 hr after mating. In the same time period many sperm from STST males (P of mutant vs. wild type < 0.001) reach the isthmus, where sperm adhere to the oviductal epithelium and are retained until near the time of ovulation (21, 28). Additionally, even those stst sperm that reach the oviductal ampulla are less likely than normal sperm to fertilize eggs because of their reduced capacity for zona binding.
Other investigators have proposed that changes in the sperm surface at capacitation mediate the release of sperm from the oviductal epithelium (29, 30). This raises the possibility that sperm from stst males adhere to the oviductal epithelium and/or to secreted material in the female reproductive tract but are not efficiently released because they lack testis ACE. Consistent with this hypothesis, an ACE-related protein in equine sperm was recently localized to the periacrosomal plasma membrane (31), which is the same membrane domain that binds to the oviduct epithelium (21). It is also noteworthy in this context that testis ACE, like the somatic isozyme, appears to be membrane-bound (32, 33), and a proportion of the enzyme is released during capacitation (34). This release is likely a consequence of proteolysis at a specific site comparable with that occurring with somatic ACE and would leave behind a specific remnant on the sperm surface (32, 33). Possibly the release of testis ACE is important for the detachment of sperm from the oviduct epithelium at capacitation, or the specific remnant remaining in the membrane may serve important functions.
ACE is important in the catalytic conversion of angiotensin I to angiotensin II and in the inactivation of bradykinin. Other peptides are hydrolyzed by ACE in vitro, but the functional consequences of these enzymatic actions in vivo are not clear. The substrate for testis ACE, if it is acting enzymatically, has not been identified. To address this question, we tested the fertility of males that lack angiotensinogen (26), the initial substrate from which angiotensin I is generated. These mice had normal fertility, showing that male-derived angiotensin I is not an essential substrate for testis ACE. Our own experience in other contexts indicates that females lacking angiotensinogen have no obvious fertility problems, and Nagata et al. (35) have established a mouse line lacking angiotensinogen, implying that female-derived angiotensin I is also not an essential substrate for testis ACE. However, we stress that the possibility remains that sperm ACE is required to modify a different substrate in the female reproductive tract.
We also asked whether gametes from individuals heterozygous for mutations affecting testis ACE have any functional differences that might affect gamete function. We found that STst males transmit the wild-type and mutant Ace alleles to their offspring with equal frequency even though testis ACE is expressed postmeiotically. Our results from in situ hybridization and immunohistochemical studies bear on this result in showing that all late spermatids in STst mice contain ACE mRNA and protein, probably because of the intercellular bridges that are normally present between developing spermatogenic cells. Sharing between genetically distinct spermatids of a postmeiotically expressed protein has been reported previously (36). Our studies suggest that a similar sharing of ACE mRNA and protein occurs and enables the transmission of wild-type and mutant Ace alleles to offspring at indistinguishable frequencies. This equality of segregation opens up the possibility that male infertility in humans could occur as a consequence of homozygous defects of testis ACE. Thus nonfunctional alleles carried by heterozygous males and females could both be transmitted to their offspring.
Matings between mice heterozygous for mutations in the somatic portion of the Ace gene (stST and sTST) produce only small numbers of homozygous mutant animals (stst and sTsT). Both homozygotes show severe kidney pathology indistinguishable from that observed in mice lacking angiotensinogen (26) but not present in mice lacking the angiotensin type 1a (37) or type 2 receptors (38, 39). The difficulty of generating sufficient numbers of these homozygous mutant animals for further study was overcome in our present work by evaluating sperm function with sperm collected from female uteri, because this method does not require sacrifice of the males. Future studies to determine the detailed molecular mechanism behind the low fertility of the mice lacking testis ACE are likely to be impeded by this problem. We are currently generating StSt mice, which will lack testis ACE but should have normal amounts of somatic ACE and therefore be produced in greater number.
Overall, we conclude that ACE plays an important functional role in fertilization because the absence of both ACE isozymes causes defects in sperm transport in the oviducts and in binding to zonae pellucidae. However, because the absence of somatic ACE does not impair male fertility, we also conclude that it is the testis ACE isozyme that is important for reproductive function. Furthermore, because the absence of angiotensin I does not impair fertility, we conclude that angiotensin I is unlikely to be the substrate for testis ACE. Our results should help to focus further studies aimed at characterizing the molecular events by which testis ACE influences sperm function and may suggest new avenues for human infertility and contraception research.
Acknowledgments
We thank H.-S. Kim, L. L. Tuning, G. Masquelier, M. T. Morin, L. Peng, S. D. Perreault, G. Luta, and R. E. Hicks for their help. This work was funded by Grants GM20069 and HL49277 (to O.S.), HL03470 (to J.H.K.), HD26485 (to D.A.O.), U54-HD35041 (Laboratories for Reproductive Biology), and CA16086 (Lineberger Comprehensive Cancer Center) from the National Institutes of Health. J.H.K. was a Howard Hughes Medical Institute Physician Research Fellow; J.S.M. was a Howard Hughes Medical Institute Medical Student Research Fellow. We are grateful to the W. M. Keck Foundation for a grant to the University of North Carolina at Chapel Hill to support work with animals.
ABBREVIATIONS
- ACE
angiotensin-converting enzyme
- ACE
human ACE gene
- Ace
mouse ACE gene
- ST
Ace allele encoding wild-type somatic and testis ACE isozymes
- st
Ace allele encoding neither isozyme
- sT
Ace allele encoding only the testis ACE isozyme
References
- 1.Corvol P, Williams T A, Soubrier F. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- 2.Sibony M, Gasc J-M, Soubrier F, Alhenc-Gelas F, Corvol P. Hypertension. 1993;21:827–835. doi: 10.1161/01.hyp.21.6.827. [DOI] [PubMed] [Google Scholar]
- 3.Langford K G, Zhou Y, Russell L D, Wilcox J N, Bernstein K E. Biol Reprod. 1993;48:1210–1218. doi: 10.1095/biolreprod48.6.1210. [DOI] [PubMed] [Google Scholar]
- 4.Sibony M, Segretain D, Gasc J-M. Biol Reprod. 1994;50:1015–1026. doi: 10.1095/biolreprod50.5.1015. [DOI] [PubMed] [Google Scholar]
- 5.Williams T A, Villard E, Prigent Y, Dadoune J-P, Soubrier F. Mol Cell Endocrinol. 1995;107:215–219. doi: 10.1016/0303-7207(94)03446-z. [DOI] [PubMed] [Google Scholar]
- 6.Soubrier F, Alhenc-Gelas F, Hubert C, Allegrini J, John M, Tregear G, Corvol P. Proc Natl Acad Sci USA. 1988;85:9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein K E, Martin B M, Edwards A S, Bernstein E A. J Biol Chem. 1989;264:11945–11951. [PubMed] [Google Scholar]
- 8.Howard T E, Shai S-Y, Langford K G, Martin B M, Bernstein K E. Mol Cell Biol. 1990;10:4294–4302. doi: 10.1128/mcb.10.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehlers M R, Fox E A, Strydom D J, Riordan J F. Proc Natl Acad Sci USA. 1989;86:7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R S, Kusari J, Roy S N, Soffer R L, Sen G C. J Biol Chem. 1989;264:16754–16758. [PubMed] [Google Scholar]
- 11.Lattion A-L, Soubrier F, Allegrini J, Hubert C, Corvol P, Alhenc-Gelas F. FEBS Lett. 1989;252:99–104. doi: 10.1016/0014-5793(89)80897-x. [DOI] [PubMed] [Google Scholar]
- 12.Howard T, Balogh R, Overbeek P, Bernstein K E. Mol Cell Biol. 1993;13:18–27. doi: 10.1128/mcb.13.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krege J H, John S W M, Langenbach L L, Hodgin J B, Hagaman J R, Bachman E S, Jennette J C, O’Brien D A, Smithies O. Nature (London) 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 14.Katz D F, Overstreet J W. Fertil Steril. 1981;35:188–193. doi: 10.1016/s0015-0282(16)45320-3. [DOI] [PubMed] [Google Scholar]
- 15.Slott V L, Suarez J D, Poss P M, Linder R E, Strader L F, Perreault S D. Fundam Appl Toxicol. 1993;21:298–307. doi: 10.1006/faat.1993.1102. [DOI] [PubMed] [Google Scholar]
- 16.Ward C R, Storey B T. Dev Biol. 1984;104:287–296. doi: 10.1016/0012-1606(84)90084-8. [DOI] [PubMed] [Google Scholar]
- 17.Saling P M. Biol Reprod. 1982;26:429–436. doi: 10.1095/biolreprod26.3.429. [DOI] [PubMed] [Google Scholar]
- 18.Bleil J D, Wassarman P M. Dev Biol. 1983;95:317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- 19.Ward C R, Storey B T, Kopf G S. J Biol Chem. 1994;269:13254–13258. [PubMed] [Google Scholar]
- 20.Thaler C D, Cardullo R A. Biochemistry. 1995;34:7788–7795. doi: 10.1021/bi00024a002. [DOI] [PubMed] [Google Scholar]
- 21.Suarez S S. Biol Reprod. 1987;36:203–210. doi: 10.1095/biolreprod36.1.203. [DOI] [PubMed] [Google Scholar]
- 22.Tybulewicz V L J, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 23.Smithies O, Kim H S. Proc Natl Acad Sci USA. 1994;91:3612–3615. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sibony M, Commo F, Callard P, Gasc J-M. Lab Invest. 1995;73:586–591. [PubMed] [Google Scholar]
- 25.Esther C R, Howard T E, Marino E M, Goddard J M, Capecchi M R, Bernstein K E. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 26.Kim H-S, Krege J H, Kluckman K D, Hagaman J R, Hodgin J B, Best C F, Jennette J C, Coffman T M, Maeda N, Smithies O. Proc Natl Acad Sci USA. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatei K, Cai H, Ip Y T, Levine M. Mech Dev. 1995;51:157–168. doi: 10.1016/0925-4773(95)00349-5. [DOI] [PubMed] [Google Scholar]
- 28.Smith T T, Nothnick W B. Biol Reprod. 1997;56:83–89. doi: 10.1095/biolreprod56.1.83. [DOI] [PubMed] [Google Scholar]
- 29.Smith T T, Yanagimachi R. J Reprod Fertil. 1991;91:567–573. doi: 10.1530/jrf.0.0910567. [DOI] [PubMed] [Google Scholar]
- 30.Demott R P, Lefebvre R, Suarez S S. Biol Reprod. 1995;52:1395–1403. doi: 10.1095/biolreprod52.6.1395. [DOI] [PubMed] [Google Scholar]
- 31.Dobrinski I, Ignotz G G, Fagnan M S, Yudin A I, Ball B A. Mol Reprod Dev. 1997;48:251–260. doi: 10.1002/(SICI)1098-2795(199710)48:2<251::AID-MRD13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Ramchandran R, Sen G C, Misono K, Sen I. J Biol Chem. 1994;269:2125–2130. [PubMed] [Google Scholar]
- 33.Ehlers M R W, Schwager S L U, Scholle R R, Manji G A, Brandt W F, Riordan J F. Biochemistry. 1996;35:9549–9559. doi: 10.1021/bi9602425. [DOI] [PubMed] [Google Scholar]
- 34.Köhn F-M, Miska W, Schill W-B. J Androl. 1995;16:259–265. [PubMed] [Google Scholar]
- 35.Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T. Lab Invest. 1996;75:745–753. [PubMed] [Google Scholar]
- 36.Braun R E, Behringer R R, Peschon J J, Brinster R L, Palmiter R D. Nature (London) 1989;337:373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- 37.Ito M, Oliverio M I, Mannon P J, Best C F, Maeda N, Smithies O, Coffman T M. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hein L, Barsh G S, Pratt R E, Dzau V J, Kobilka B K. Nature (London) 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 39.Ichiki T, Labosky P A, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan B L M, Inagami T. Nature (London) 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]