Abstract
Amyloid protein A (AA) amyloidosis is a consequence of some long-standing inflammatory conditions, and subsequently, an N-terminal fragment of the acute phase protein serum AA forms β-sheet fibrils that are deposited in different tissues. It is unknown why only some individuals develop AA amyloidosis. In the mouse model, AA amyloidosis develops after ≈25 days of inflammatory challenge. This lag phase can be shortened dramatically by administration of a small amount of amyloid extract containing an as yet undefined amyloid-enhancing factor. In the present study, we show that preformed amyloid-like fibrils made from short synthetic peptides corresponding to parts of several different amyloid fibril proteins exert amyloidogenic enhancing activity when given i.v. to mice at the induction of inflammation. We followed i.v. administered, radiolabeled, heterologous, synthetic fibrils to the lung and to the perifollicular area in the spleen and found that new AA–amyloid fibrils developed on these preformed fibrils. Our findings thus show that preformed, synthetic, amyloid-like fibrils have an in vivo nidus activity and that amyloid-enhancing activity may occur, at least in part, through this mechanism. Our findings also show that fibrils of a heterologous chemical nature exert amyloid-enhancing activity.
Secondary or amyloid protein A (AA) amyloidosis is a life-threatening systemic disease in which deposits of amyloid can be found in most tissues of the body. The main constituent of the amyloid deposits is a fibril formed by β-pleated sheets of protein AA, the latter of which is an N-terminal 44- to ≈100- amino acid cleavage product of the 104 amino acid precursor serum AA (SAA) (for review, see ref. 1). In humans, there are at least three different SAA genes coding for SAA1, SAA2 and SAA4, respectively (2). SAA1 and SAA2 are acute phase reactant high density apolipoproteins. These are expressed mainly by the liver, but SAA4 is expressed in several different tissues (3, 4). N-terminal fragments of SAA1 and SAA2 give rise to protein AA and polymerize to amyloid fibrils in humans. SAA is a conserved protein, and AA amyloidosis occurs, with varying frequency, in many mammalian species and in some birds (for review, see refs. 5 and 6). The mouse (Mus musculus) is prone to develop AA amyloidosis and is the most commonly used animal model for AA amyloidosis. The two mouse SAA isoforms of high density lipoprotein, SAA1 and SAA2, are both acute phase reactants, but only SAA2 is amyloidogenic and is found as protein AA in the fibrils (7, 8).
The plasma concentration of SAA is normally low both in humans and in mice, but the SAA concentration rises quickly at an acute inflammation as a response to cytokines, especially interleukin-1, interleukin-6, and tumor necrosis factor α (for review, see ref. 9), to levels as high as 1 mg/ml. When inflammation subsides, the SAA concentration gradually returns to normal. In humans, AA amyloidosis most commonly occurs in individuals with long term active inflammatory disease, which, in developed countries, usually is rheumatoid arthritis. In developing countries, the common cause of AA amyloidosis is a chronic infectious disease among which tuberculosis, leprosy, and malaria predominate (10). However, most individuals with these chronic inflammatory diseases never develop AA amyloidosis despite the high plasma concentration of SAA. It is still unclear why only a subset of individuals develops AA amyloidosis. In contrast to the disease in the mouse, there is so far no definite proof of a specific amyloid-prone SAA variant in humans, although some SAA isotypes may be over represented as amyloid fibrils (11, 12). Therefore, in addition to high concentrations of an amyloidogenic protein, other factors are important in the pathogenesis of AA amyloidosis.
AA amyloidosis can be easily induced experimentally in many strains of mice by a prolonged inflammatory challenge, e.g. s.c. injections of silver nitrate or casein (13). It also was observed several decades ago that an extract of mouse amyloid tissue, given i.v. with the simultaneous induction of inflammation, dramatically shortens the time for AA amyloid to develop (14). The same effect can be achieved by injection of cells from mice with AA amyloidosis (15). Despite many efforts, the active component [called “amyloid enhancing factor” (AEF)] has never been isolated or defined. It has, however, been suggested to act like the infectious protein in scrapie-related cerebral diseases (16). Because a general consensus concerning how amyloid deposits develop includes the role of a nidus, we previously tested (17) the effect of synthetic amyloid-like fibrils made from short peptides on the time necessary for amyloid induction and found an AEF-like effect of these fibrils. We now present strong evidence that AEF-like activity is exerted by synthetic amyloid-like fibrils by serving as a nidus on which new AA amyloid fibrils form.
MATERIALS AND METHODS
Mice.
Outbred female NMRI mice were purchased from Bomholtgard Breeding and Research Center (Ry, Denmark). The mice were 10–16 weeks of age and had free access to water and pellets (Type R 36; Lactamin, Vadstena, Sweden).
Peptides.
C-terminally amidated peptides were synthesized as described (18). The following peptides, previously shown to be highly fibrillogenic in vitro, were used: a peptide corresponding to positions 20–29 of human islet amyloid polypeptide with an added N-terminal tyrosine residue [Tyr-islet amyloid polypeptide(20-29)] (19) and the following regions of human transthyretin (TTR): TTR(10–20), TTR(24–35), TTR(105–115) and TTR(115–124) (20).
In Vitro Fibril Formation.
Fibrils were made in vitro as described elsewhere (19, 20). In brief, synthetic peptides were dissolved in 10% acetic acid at a concentration of 10 mg/ml and were left for 24 hr at room temperature. The peptide solutions then were neutralized to pH 7 with 25% NH4OH. After 30 min, samples from each of the solutions were dried on glass slides, stained with Congo red, and evaluated for green birefringence in polarized light, which was found with all materials. Negatively stained samples were also studied by electron microscopy to verify presence of amyloid-like fibrils. The fibril preparations were diluted with distilled water to a concentration of 1 mg/ml and were stored at +4°C.
Labeling of Synthetic Fibrils with 125I.
Because TTR contains a tyrosine residue at position 116, the peptide TTR(115–124) was chosen for labeling. Labeling with Na125I (100 mCi/ml; Amersham) was performed with the chloramine-T method (21). Na125I (5 μl) was dispensed into an Eppendorf plastic tube placed within a lead cylinder. HCl (5 μl of 10 mM) then was added to the iodide solution, immediately followed by 150 μl of freshly made TTR(115–124) peptide solution (10 mg/ml in 10% acetic acid) and 25 μl of chloramine T-solution (5.2 mg/ml in distilled water). The solutions were mixed, and after 10 min, 40 μl sodium metabisulfphite solution (4.8 mg/ml in distilled water) was added, followed 30 sec later by the addition of 20 μl of solium iodide (10 mg/ml in distilled water).
For formation of labeled fibrils, the above peptide solution was neutralized with ammonia and left for 30 min at room temperature. Bound and free 125I were separated by dilution of the fibril suspension with distilled water followed by centrifugation at 100,000 × g for 15 min. This latter procedure was repeated once. The presence of birefringent Congophilic material was verified as described above. The pelleted material was diluted with distilled water to the volume of 1.5 ml (1 mg/ml; specific activity 1.4 mCi/mg) and was used in the experiments.
Preparation of AA Fibril Supernatants Used for AEF Activity.
AEF was prepared from the livers of mice with severe systemic AA amyloidosis as described (17). In brief, liver tissue was homogenized in 0.15 M NaCl, followed by centrifugation at 15,000 × g for 30 min at 4°C. Homogenization and centrifugation of the pellet were repeated 10 times. Amyloid fibrils subsequently were extracted with distilled water (22), and pooled supernatants from the second and third water extractions were used as AEF. The total protein concentration was 1.32 mg/ml as determined by a protein assay kit (Bio-Rad).
Induction of Amyloidosis.
Mice were divided into six groups, with 10 animals in each group to determine the AEF effect of the synthetic fibril preparations. Each mouse in the experimental groups received a single injection of 0.1 ml of AEF or 0.1 ml of the synthetic fibril suspension into the lateral tail vein. Both types of fibril suspensions were sonicated briefly before injection to reduce possible aggregates. A control group of 30 animals received an i.v. injection of 0.1 ml of vehicle (10% acetic acid neutralized with 25% NH4OH and diluted 10 times with distilled water). These i.v. injections were followed immediately by an s.c. dorsal injection of 0.5 ml of 1% AgNO3 as an inflammatory stimulus. AgNO3 injections (0.1 ml) were repeated on day 7 and, for remaining animals, on day 14. Seven animals from each of the experimental groups and the control groups were killed on day 10, and the remaining animals of each group were killed on day 16. Tissue samples from spleen were fixed in 10% neutral buffered formalin and embedded in paraffin. In another experiment, 12 mice received TTR(24-35) fibrils i.v., and another 13 mice received the same amount of the peptide dissolved in dimethyl sulfoxide. In both groups of animals, the injections were followed by dorsal injections of 0.1 ml of 1% AgNO3 exactly as above, and all mice were killed on day 16.
Fate of Synthetic Fibrils in Vivo.
The fate of injected fibrils was followed by using a group of 11 mice injected i.v. with 0.1 ml of 125I-iodinated TTR(115-124) fibrils, followed immediately by an s.c. injection of 0.5 ml of 1% AgNO3. The mice subsequently were given 0.1 ml of 1% AgNO3 s.c. on days 7 and 14. Three mice died immediately after the i.v. injection of iodinated TTR fibrils. Of the remaining mice, one mouse was killed on each of days 1, 6, 11 and 20, and four mice were killed on day 22. Samples from spleen, liver, kidneys, lungs, and thyroid were fixed in 10% neutral buffered formalin for 24 hr and embedded in paraffin or were fixed in 10% neutral buffered formalin for 24 hr and subsequently infiltrated in 5% sucrose in 0.05 M Tris⋅Cl buffer (pH 8.0) containing 0.15 M NaCl (Tris-buffered saline), and then snap-frozen in Tissue-Tek OCT Compound embedding medium (Histolab, Västra Frölunda, Sweden). Small samples of lung tissue from mice killed on days 20 and 22 were fixed for 2 hr at room temperature in 4% paraformaldehyde and 0.5% glutaraldehyde in 0.1% sodium cacodylate buffer (pH 7.4) containing 0.1% sucrose. Thereafter, the samples were dehydrated for electron microscopy and embedded in Epon (Agar Aids, Essex, UK) polymerized at 60°C or Unicryl or Lowicryl K4M (Polysciences) photopolymerized at −20°C.
Antisera.
Antiserum against the synthetic peptide TTR(115–124) was raised in a rabbit as described elsewhere (23). Antiserum to TTR(115–124) is amyloid-specific, i.e., it reacts only with TTR in, or purified from, amyloid deposits (23). Antiserum to mouse protein AA was raised in rabbits as described elsewhere (17). Goat antiserum to mouse amyloid P component kindly was provided by M. B. Pepys, London, U.K.
Light Microscopy.
Paraffin sections (10 μm) and cryostat sections (10 μm) were stained with alkaline Congo red and hematoxylin (24) and subsequently were examined for the presence of amyloid, indicated by green birefringence with polarized light. The amount of amyloid in the paraffin sections of spleen was graded as described (17).
Macroautoradiography.
Air-dried cryostat sections (10 μm) of thyroid, spleen, liver, kidney and lung from the mice injected with 125I-labeled TTR(115–124) fibrils were placed on Kodak X-OmatAR x-ray film in an x-ray cassette with x-ray intensifying screens at −70°C for 7 days.
Microautoradiography.
Air-dried cryostat sections (10 μm) were coated with Hypercoat LM-1 autoradiographic emulsion (Amersham), air dried, and exposed in light proof boxes at +4°C for 6, 10, or 18 weeks. Slides without sections were coated with autoradiographic emulsion, air dried, and subsequently exposed together with the coated sections to serve as control autoradiographs. The slides were developed in Kodak D-19 developer, fixed in Kodak Unifix fixer for 4 min, and washed in two changes of distilled water. The sections were immediately post-stained with hematoxylin. To investigate whether silver injected as AgNO3 reduced the autoradiographic emulsion, sections from one of the experimental mice that had received silver nitrate s.c. but no labeled fibrils were coated and exposed for 2 hr and then were processed as described above.
ImmunoGold Electron Microscopy.
Semithin sections were cut from blocks embedded in Unicryl, Lowicryl, and Epon. The sections were exposed on Kodak X-OmatAR x-ray film in the same way as described above. Ultrathin sections were cut thereafter from the blocks containing radioactive deposits as determined by macroautoradiography. Consecutive sections from Unicryl- or Lowicryl- embedded blocks were mounted on Formvar-coated (Ladd Research Industries, Burlington, VT) nickel grids and used for single indirect ImmunoGold labeling. The sections from Epon-embedded blocks were placed on uncoated nickel grids and used for double indirect ImmunoGold labeling.
Before immunolabeling, all of the sections were incubated with 5% BSA in Tris-buffered saline for 30 min to block nonspecific binding sites. For single immunolabeling, the sections then were transferred onto a drop of rabbit antiserum against mouse AA, TTR(115–124), or mouse serum amyloid P-component (all diluted 1:200) and incubated overnight at room temperature in a humidity chamber. After washing in 0.05 sodium phosphate buffer (pH 7.4) containing 0.15 M NaCl (PBS) and blocking in 5% BSA in Tris-buffered saline for 10 min, the sections were incubated with 10 nm of colloidal gold-labeled goat anti-rabbit IgG (Biocell Laboratories) for 1 hr at room temperature. Thereafter, the sections were washed in PBS, rinsed in distilled water, and counterstained with 5% uranyl acetate and lead citrate.
For double immunolabeling, one face of the grid was performed as described above, applying rabbit anti-AA antiserum followed by 10 nm of colloidal gold labeled goat anti-rabbit IgG. The other face of the grid then was labeled with rabbit anti-TTR(115–124) antiserum, and the reaction was visualized with 20 nm of protein A gold particles (Biocell Laboratories). Care was taken not to wet the opposite face during the incubations. The sections were counterstained only on one face with uranyl acetate and lead citrate. The sections were viewed in a JEOL 1200 electron microscope at 80 kV.
Statistical Analysis.
For statistical comparison between groups, Fisher’s exact test was used with instat 2.01 software.
RESULTS
Fibrils.
All synthetic peptides formed Congophilic aggregates in vitro that exhibited green birefringence in polarized light. The extracted murine AA amyloid fibrils used as AEF showed strong green birefringence after Congo red staining.
In Vivo Effects of Injected Synthetic Peptide Fibrils and AEF.
Injection (i.v.) of fibrils made from TTR(10–20), TTR(24–35), TTR(105–115), TTR(115–124), or islet amyloid polypeptide(20–29) induced accelerated amyloid deposition in mice given AgNO3 as detected with Congo red staining (Table 2). Of 50 mice that received synthetic fibrils, 13 developed amyloidosis, whereas 1 of 28 surviving control mice that received vehicle had only minute amyloid deposits (P = 0.01). In all animals, the amyloid deposits were localized in the perifollicular areas of the spleen. Amyloid deposits also were detected perifollicularly in the spleen of all mice treated with AEF and AgNO3. The deposits in the latter group were moderate in six mice and extensive in four mice.
Table 2.
Splenic amyloid deposits induced by treatment with AgNO3 and different synthetic peptide fibrils compared with controls treated with AgNO3 and vehicle or AEF
| Treatment | Mice, n | Mice with amyloid | Amyloid grade |
|---|---|---|---|
| TTR(10–20) | 10 | 1 | 1+ |
| TTR(24–35) | 10 | 3 | 1+-3+ |
| TTR(105–115) | 10 | 2 | 1+-3+ |
| TTR(115–124) | 10 | 5 | 2+ |
| IAPP(20–29) | 10 | 2 | 2+ |
| AEF | 10 | 10 | 3+-4+ |
| Vehicle | 28 | 1 | 1+ |
In the experiment in which the same peptide [TTR(24–35)] was injected in a fibrillar or nonfibrillar form, none of the 13 mice given nonfibrillar peptide showed amyloid on day 16, whereas 4 of 12 mice injected with fibrils had splenic amyloid deposits (P < 0.04).
Effects of TTR(115–124) Fibrils Labeled with 125I.
No splenic amyloid was detected in mice that died immediately after injections or in mice killed on days 1, 6, or 11, as determined by Congo red staining. All of the mice killed on days 20 (n = 1) and 22 (n = 4) had small to extensive amyloid deposits perifollicularly in the spleen. Three of the mice had hepatic amyloid deposits that were localized to the central vein areas. One mouse showed severe generalized amyloidosis with amyloid deposition not only in the spleen and liver but also in the kidneys, lungs, and thyroid.
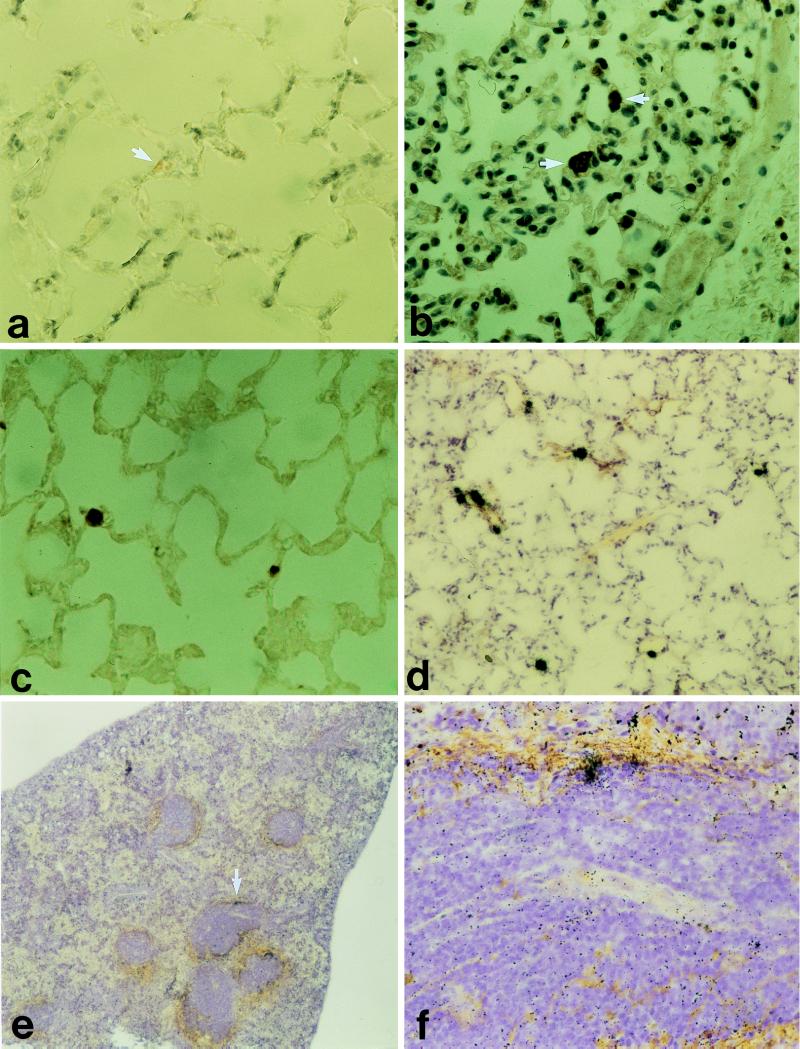
Small congophilic deposits were seen in some sections of lungs of animals (killed on days 20 and 22) that had received TTR(115–124) fibrils (Fig. 1a). These amyloid deposits reacted strongly with antiserum to TTR(115–124) (Fig. 1c) immunohistochemically, and somewhat weaker immunoreactivity was observed with antiserum to amyloid P component (not shown). There was also a strong reaction with antiprotein AA antiserum (Fig. lb). No staining was detected with normal rabbit serum, and the tissues from an untreated control mouse showed no reaction with the antisera used.
Figure 1.
(a–d): Lung tissue from a mouse given synthetic TTR(115–124) fibrils i.v. Small amyloid deposits (arrows) occasionally were found in vascular lumina stained with Congo red and viewed in polarized light (a) and labeled with antiserum to mouse protein AA and to the synthetic peptide TTR(115–124) (b and c). (d–f) Tissues taken from a mouse that had received 125I-labeled TTR(115–124) fibrils i.v. 22 days before it was killed. (d) Lung tissue with several radioactivity labeled spots corresponding to the minute amyloid deposits. e A section of the spleen and silver grains occur perifollicularly at the amyloid deposits. (f) Close-up of e at the arrow.
Distribution of 125I-Labeled TTR(115–124) Fibrils.
In the mouse killed on day 1, macroautoradiography showed strong focal radioactivity in the lungs and liver and moderate multifocal areas of radioactivity in the spleen. The mice killed on days 6, 11, 20, and 22 showed strong focal radioactivity in the lungs. Only weak multifocal radioactivity was detected in spleen from mice killed on days 6 and 11, and very weak radioactivity was present in the spleen sections from three of five mice killed on days 20 and 22. No radioactivity was detected in the livers of the mice killed on days 6, 11, 20, or 22.
Microautoradiography verified the results obtained by macroautoradiography. Microautoradiography showed varying amounts of silver grains distributed in diffuse perifollicular patterns in spleen sections from the three mice killed on days 20 and 22 (Fig. 1 e and f). The radioactivity was localized to the areas of spleen in which amyloid deposits were detected by Congo red staining and by immunohistochemistry. Varying numbers of focally distributed heavy accumulations of silver grains were present in the lungs of all animals (Fig. ld). The thyroid glands had very strong concentration of silver grains confined to the follicles. No silver reduction was observed in the autoradiographs of the livers. The silver precipitate in the tissues originating from the injected AgNO3 easily was discriminated from the silver grains in the film.
Immunoelectron Microscopy of Lung Tissue.
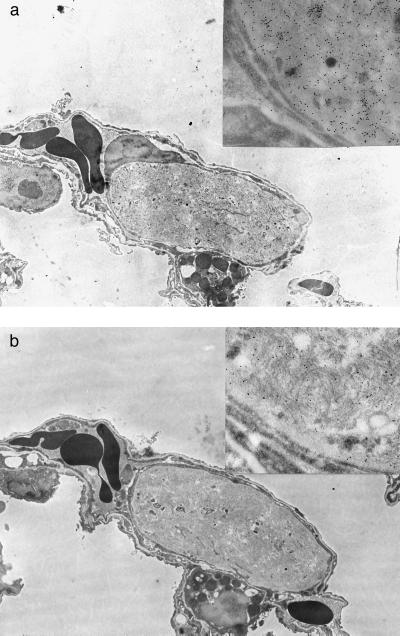
Lung tissue was chosen for the immunoelectron microscopic study because the strongly radioactivity-labeled and well demarcated amyloid deposits were easy to find. Such deposits were found at the electron microscopic level in the lungs from three of five mice receiving 125I-labeled TTR(115–124) fibrils i.v. The small deposits were rounded and almost always were surrounded by basal membrane-like material and appeared to be located within lumina of small blood vessels (Fig. 2). The amyloid fibrils had no definite organization and varied somewhat in morphology. In some deposits, thicker fibrils were evident, whereas in others the fibrils were uniformly thin (not shown).
Figure 2.
Electron microscopical picture of an amyloid deposit in the lung of a mouse that had received TTR(115–124) fibrils i.v. at the induction of amyloidosis and was killed on day 22. The fibrils are labeled with antiserum to TTR(115–124) (a) and mouse protein AA (b). Both antisera label the fibrils specifically. (×5,000.) (Insets) Parts of the deposits. (×37,500.)
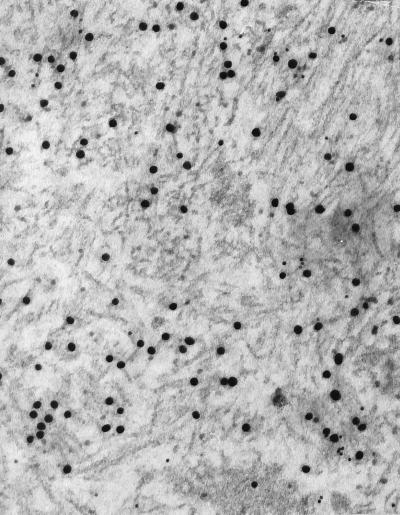
Immunolabeling with antiserum to TTR(115–124) varied. Thicker fibrils showed a very strong labeling with antiserum to TTR(115–124) (Fig. 2a) and only slight labeling with antiserum to mouse protein AA (Fig. 2b). The opposite phenomenon was observed with thin fibrils (not shown). In double immunolabeling experiments [in which one face of the section was incubated with antiserum to TTR(115–124) and the other with antiserum to mouse protein AA], labeling with both antisera was clearly evident in the amyloid deposits (Fig. 3). However, the proportion of labeling with the different antisera varied in that a strong labeling with anti TTR(115–124) was followed by a weaker protein AA labeling and vice versa. Thus, of 19 different deposits studied, protein AA immunoreactivity predominated in 7, and TTR immunoreactivity predominated in 12. All deposits were labeled with antiserum to amyloid P-component.
Figure 3.
Double immunolabeling of a lung amyloid deposit with antiserum to TTR(115–124) (20 nm of gold particles) and to mouse protein AA (10-nm gold particles). This deposit seems to consist mainly of synthetic TTR fibrils, but specific protein AA-labeling also is seen. (×80,000.)
DISCUSSION
In this study, we show that preformed fibrils made from synthetic peptides and given in small doses i.v. in mice shorten the lag phase between an inflammatory stimulus and development of AA amyloidosis and, consequently, have amyloid-enhancing effects. By the use of radioactivity-labeled synthetic fibrils, we followed injected synthetic fibrils to the lung and spleen. Some fibril aggregates were trapped in lung capillaries and formed small distinctive amyloid foci that could be identified by Congo red staining. By double ImmunoGold labeling, we were able to show that these amyloid foci not only contained the synthetic fibrils but also murine protein AA fibrils. Likewise, we found that early perifollicular splenic amyloid deposits contained radioactivity labeled material, indicating that synthetic fibrils not only were trapped in the lung but also were present at an area known to be a target for the first deposits in murine experimental AA amyloidosis (25). Sections of kidney and liver, organs in which amyloid deposits occur later (25), exhibited no radioactivity.
Formation of amyloid fibrils is supposed to occur as an off-pathway event from specific near-native protein intermediates at the folding–unfolding pathway (26). A nucleation mechanism is believed to be of importance because seeding a solution of amyloid fibril proteins like β-protein (27) or islet amyloid polypeptide (28) with preformed fibrils made from the homologous proteins strongly enhances the speed by which new fibrils are formed. This fibril growth follows first-order kinetics (29, 30). The nucleation mechanism is similar to that implicated in the prion model of “infectivity”; the protein with abnormal tertiary structure serves as template for other protein molecules (16, 31). However, in prion diseases, the infectivity may be dissociated from the formation of amyloid fibrils (32). The formation of the nucleus in the amyloidogenesis in general is, however, poorly understood, but it should be noted that in the present experiments only peptides in their fibrillar form exerted amyloid-enhancing effects.
The finding that amyloid-like fibrils synthetically made from heterologous proteins enhanced the development of AA amyloidosis presumably by acting as nidi is of great interest. This finding raises the provocative question of whether other components in addition to amyloid fibrils can act as nidi in the amyloidogenesis. One of the major and unsolved questions in the pathogenesis of human AA amyloidosis asks why only some individuals with longstanding inflammatory conditions and high SAA plasma concentration develop amyloid deposits. It is known from the mouse (33) and the mink (1, 34) that several SAA isoforms exist and that they vary strongly in amyloidogenicity. Such a mechanism may exist also in humans (11, 12) but is probably less important because in most instances, human AA amyloid is derived from common SAA isotypes also present in individuals who do not develop amyloidosis. Therefore, other factors in addition to a suitable SAA must be of importance in the human amyloidogenesis. The mechanism found in the present study in which exogenous substances enhance AA-amyloidogenesis in an experimental mouse model may be true also in the human situation. Hypothetically, an exogenous substance may form a nidus on which the first AA fibrils form. Such a mechanism could explain why only a fraction of individuals with longstanding high plasma SAA concentration get AA amyloidosis. Furthermore, similar mechanisms may be important not only in AA amyloidosis but also in other types of amyloid deposits.
Table 1.
C-terminally amidated synthetic peptides used for in vitro formation of amyloid-like fibrils
| Peptide | Amino acid sequence |
|---|---|
| IAPP(20–29) | YSNNFGAILSS-NH2 |
| TTR(10–20) | CPLMVKVLDAV-NH2 |
| TTR(24–35) | PAINVAVHVFRK-NH2 |
| TTR(105–115) | YTIAALLSPYS-NH2 |
| TTR(115–124) | SYSTTAVVTN-NH2 |
Acknowledgments
We thank Marie-Louise Eskilsson and Christer Bergman for skilled technical assistance. This work was supported by the Swedish Medical Research Council (Project 5941) and the Amyl Foundation.
ABBREVIATIONS
- AA
amyloid protein A
- AEF
amyloid enhancing factor
- SAA
serum amyloid A
- TTR
transthyretin
References
- 1.Husby G, Marhaug G, Dowton B, Sletten K, Sipe J D. Amyloid. 1994;1:119–137. [Google Scholar]
- 2.Whitehead A S, de Beer M C, Steel D M, Rits M, Lelias J M, Lane W S, de Beer F C. J Biol Chem. 1992;267:3862–3867. [PubMed] [Google Scholar]
- 3.Benditt E P, Meek R L. J Exp Med. 1989;169:1841–1846. doi: 10.1084/jem.169.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meek R L, Urieli-Shoval S, Benditt E P. Proc Natl Acad Sci USA. 1994;91:3186–3190. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zschiesche W, Jakob W. Pharmacol Ther. 1989;41:49–83. doi: 10.1016/0163-7258(89)90102-2. [DOI] [PubMed] [Google Scholar]
- 6.Johnson K H, Westermark P, Sletten K, O’Brien T D. Amyloid. 1996;3:270–289. [Google Scholar]
- 7.Hoffman J S, Ericsson L H, Eriksen N, Walsh K A, Benditt E P. J Exp Med. 1984;159:641–646. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiroo M, Kawahara E, Nakanishi I, Migita S. Scand J Immunol. 1987;26:709–716. doi: 10.1111/j.1365-3083.1987.tb02307.x. [DOI] [PubMed] [Google Scholar]
- 9.McAdam, K. P. W. J., Raynes, J. G., Alpers, M. P., Westermark, G. T. & Westermark, P. (1998) Papua New Guinea Med. J., in press. [PubMed]
- 10.McAdam K P W J. Papua New Guinea Med J. 1978;21:69–78. [PubMed] [Google Scholar]
- 11.Baba S, Masago S A, Takahashi T, Kasama T, Sugimura H, Tsugane S, Tsutsui Y, Shirasawa H. Hum Mol Genet. 1995;4:1083–1087. doi: 10.1093/hmg/4.6.1083. [DOI] [PubMed] [Google Scholar]
- 12.Westermark P, Sletten K, Westermark G T, Raynes J, McAdam K P. Biochem Biophys Res Commun. 1996;223:320–323. doi: 10.1006/bbrc.1996.0892. [DOI] [PubMed] [Google Scholar]
- 13.Skinner M, Shirahama T, Benson M D, Cohen A S. Lab Invest. 1977;36:420–427. [PubMed] [Google Scholar]
- 14.Willerson J T, Gordon J K, Talal N, Barth W F. Arthritis Rheum. 1969;12:232–240. doi: 10.1002/art.1780120311. [DOI] [PubMed] [Google Scholar]
- 15.Werdelin O, Ranløv P. Acta Pathol Microbiol Scand. 1966;68:1–18. doi: 10.1111/apm.1966.68.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Gajdusek D C. Ann N Y Acad Sci. 1994;724:173–190. doi: 10.1111/j.1749-6632.1994.tb38909.x. [DOI] [PubMed] [Google Scholar]
- 17.Ganowiak K, Hultman P, Engström U, Gustavsson Å, Westermark P. Biochem Biophys Res Commun. 1994;199:306–312. doi: 10.1006/bbrc.1994.1229. [DOI] [PubMed] [Google Scholar]
- 18.Gustavsson Å, Engström U, Westermark P. Biochem Biophys Res Commun. 1991;175:1159–1164. doi: 10.1016/0006-291x(91)91687-8. [DOI] [PubMed] [Google Scholar]
- 19.Westermark P, Engström U, Johnson K H, Westermark G T, Betsholtz C. Proc Natl Acad Sci USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustavsson Å, Engström U, Westermark P. Amyloid. 1997;4:1–12. [Google Scholar]
- 21.Hunter, W. M. (1978) Handb. Exp. Immunol. Immunochem. 14.1–14.40.
- 22.Pras M, Schubert M, Zucker-Franklin D, Rimon A, Franklin E C. J Clin Invest. 1968;47:924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustavsson Å, Engström U, Westermark P. Am J Pathol. 1994;144:1301–1311. [PMC free article] [PubMed] [Google Scholar]
- 24.Puchtler H, Sweat F, Levine M. J Histochem Cytochem. 1962;10:355–364. [Google Scholar]
- 25.Shirahama T, Cohen A S. Am J Pathol. 1980;99:539–550. [PMC free article] [PubMed] [Google Scholar]
- 26.Wetzel R. Trends Biotechnol. 1994;12:193–198. doi: 10.1016/0167-7799(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 27.Jarrett J T, Lansbury P T. Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 28.Ashburn T T, Lansbury P T. J Am Chem Soc. 1993;115:11012–11013. [Google Scholar]
- 29.Naiki H, Higuchi K, Nakakuki K, Takeda T. Lab Invest. 1991;65:104–110. [PubMed] [Google Scholar]
- 30.Esler W P, Stimson E R, Ghilardi J R, Vinters H V, Lee J P, Mantyh P W, Maggio J E. Biochemistry. 1996;35:749–757. doi: 10.1021/bi951685w. [DOI] [PubMed] [Google Scholar]
- 31.Cohen F E, Pan K-M, Huang Z, Baldwin M, Fletterick R J, Prusiner S B. Science. 1994;264:530–531. doi: 10.1126/science.7909169. [DOI] [PubMed] [Google Scholar]
- 32.Wille H, Baldwin M A, Cohen F E, DeArmond S J, Prusiner S B. In: The Nature and Origin of Amyloid Fibrils. Bock G R, Goode J A, editors. New York: Wiley; 1996. pp. 181–201. [Google Scholar]
- 33.Meek R L, Hoffman J S, Benditt E P. J Exp Med. 1986;163:499–510. doi: 10.1084/jem.163.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foyn Bruun C, Rygg M, Nordstoga K, Sletten K, Marhaug G. Scand J Immunol. 1994;40:337–344. doi: 10.1111/j.1365-3083.1994.tb03470.x. [DOI] [PubMed] [Google Scholar]