Abstract
Genes of the Polycomb group maintain long-term, segment-specific repression of the homeotic genes in Drosophila. DNA targets of Polycomb group proteins, called Polycomb response elements (PREs), have been defined by several assays, but they have not been dissected in their original chromosomal context. An enhanced method of gene conversion was developed to generate a series of small, targeted deletions encompassing the best-studied PRE, upstream of the Ultrabithorax (Ubx) transcription unit in the bithorax complex. Deletions that removed an essential 185-bp core of the PRE caused anterior misexpression of Ubx and posterior segmental transformations, including the conversion of the third thoracic segment toward a duplicate first abdominal segment. These phenotypes were variable, suggesting some cooperation between this PRE and others in the bithorax complex. Larger deletions up to 3 kb were also created, which removed DNA sites reportedly needed for Ubx activation, including putative trithorax response elements. These deletions resulted in neither loss of Ubx expression nor loss-of-function phenotypes. Thus, the 3-kb region including the PRE is required for repression, but not for activation, of Ubx.
Keywords: bithorax, Ultrabithorax, gene conversion
The genes of the Polycomb group (PcG) (1, 2) were first identified in Drosophila by their mutant phenotypes of segmental transformations, caused by derepression of the homeotic genes of the Antennapedia and bithorax complexes. Homologous genes have been identified in mammals, where they appear to have analogous functions (3). In early Drosophila embryos, the homeotic genes are initially repressed in specific segments by the gap and pair rule genes. When the gap and pair rule gene products disappear, the PcG genes maintain that repressed state. The PcG genes are thought to act at specific sites (4), called Polycomb response elements (PREs) (5).
PRE sites have been studied primarily in transgene constructs inserted at random chromosomal locations. PcG products act at the PREs in such constructs to repress or restrict expression of certain reporter genes (5–8), and PRE transgenes can recruit the POLYCOMB protein to new chromosomal locations (6, 9–11). The binding of PcG proteins to PREs can also be assayed by formaldehyde cross-linking of nuclei from cultured cells, whole embryos, or imaginal discs (12–15). These assays may define where PREs lie on the DNA sequence, but do not reveal how they function in their original context.
Because of technical limitations, PREs have not been recognized or studied by deletions or point mutations in their normal chromosomal positions. There are a few deletions that remove PREs, but these are typically several kb in extent, deleting enhancers or other DNA elements in addition to the PRE. One exception is a deletion of a PRE (or a partial PRE) immediately adjacent to the Fab7 boundary element (16); its properties are described in Discussion. It is not clear what phenotype to expect from the loss of a PRE. Ectopic expression of one of the homeotics might be predicted, although it is not obvious which segments might be affected. There have been several reports that PRE regions are also bound by transcriptional activators, notably those assigned to the trithorax group, and that PREs are necessary for activation of homeotic genes, not only for their repression (17, 18). Indeed, trithorax response elements (TREs) have been positioned within PREs or very close to them. A recent report suggested that TREs interspersed within a well studied PRE are templates for small, noncoding RNAs, which, in turn, recruit activators for the homeotic genes (19). Thus, a PRE/TRE might serve dual functions, repressing in some segments and activating in others. To address these issues, we have attempted a deletion analysis of the most extensively studied PRE and its neighboring TREs. The dissected region spans 3 kb; it lies in the bxd regulatory region of the bithorax complex, ≈25 kb upstream of the Ultrabithorax (Ubx) homeotic transcription unit.
Results
An Enhanced Method for Gene Conversion.
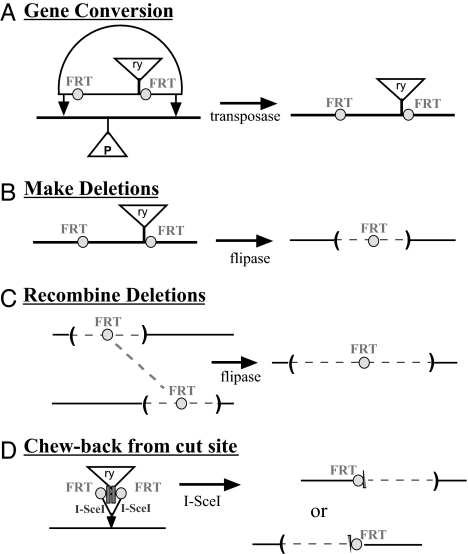
The homeotic transcription units and their regulatory regions are so large that it has been difficult to dissect their functions by using transgenes. Fortunately, we have recovered several insertions of P mobile elements in the region of the bxd PRE (20), which can be used to initiate targeted gene conversion. We worried that deletion of the PRE might cause potentially lethal misexpression of Ubx, making it impossible to recover animals carrying the deletion. Therefore, we separated the gene conversion and the deletion generation into two steps. First, pairs of flipase recombination target (FRT) recombination sites were introduced by P-element-mediated gene conversion (21) to flank the genomic sequences to be removed (Fig. 1A). Then convertant animals were treated with the yeast flipase protein to produce many progeny with the intended deletion (Fig. 1B). We followed both steps with marker genes (rosy+ or GAL4-VP16) in the conversion constructs; these marker genes were also flanked by the FRTs. The GAL4–VP16 expression patterns could also be used to monitor the activity of the PRE and local enhancers (G.K. and L.S., unpublished work). The deletions produced by flipase retained a single FRT site at the position of the deletion, making further manipulations possible. Any two viable deletion chromosomes could be induced to recombine with each other, resulting in larger deletions (Fig. 1C), or tandem duplications for the DNA segments between the FRT sites. In some cases, the conversion constructs included cut sites for the rare-cutting endonuclease I-SceI. These I-SceI sites were used to induce randomly sized deletions associated with repair of double-strand breaks (Fig. 1D) (G.K. and L.S., unpublished work).
Fig. 1.
Genetic methods for creation of deletions. (A) A plasmid is constructed containing the genomic region of interest, with a pair of FRT recombination sites flanking the segment to be deleted. A selectable marker, such as the rosy (ry) gene, may also be inserted between the FRTs. The plasmid is injected into embryos of a strain with a P-element insertion near the homologous region of interest. Coinjection of a source of the P-element transposase will excise the P element and make possible the integration of the genomic sequences from the plasmid. (B) If the FRT sites in the successful convertant are in the same orientation, they can be induced to recombine in cis by supplying a source of flipase. The rosy selectable marker is also deleted in the recombination event, but a single FRT site remains at the site of the deletion. (C) Any two deletions with residual FRT sites can be induced to recombine in trans, thus creating a larger deletion. Note that the reciprocal recombinant chromosome will contain a smaller deletion, or a duplication, depending on whether the two initial deletions overlap. (D) Some of the conversion constructs carried sites for the homing endonuclease I-Sce I. When I-Sce I is supplied, the chromosome is cut, and some repaired products result from resection, followed by nonhomologous end joining. A series of deletions can be generated extending in either direction from the initial site of the conversion construct.
Initial Deletions in the Central PRE Region.
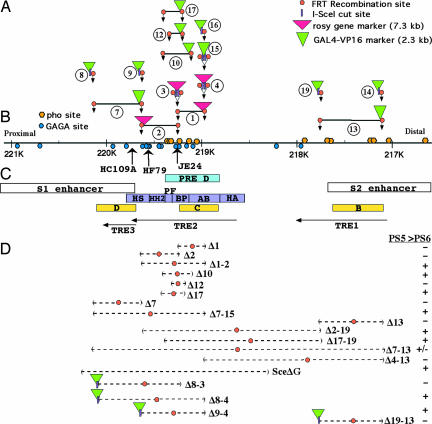
Fig. 2A shows 15 separate gene conversion constructs (from a total of 33) that were integrated into the bithorax complex, after excision of the P elements HC109A, HF79, or JE24 (Fig. 2B). The first conversion constructs targeted the DNA segments that showed the strongest activity in a transgene assay (23) and the highest levels of POLYCOMB cross-linking (15). This region contains clusters of binding sites for the pleiohomeotic protein (called PHO sites) and for the GAGA factor or the pipsqueak protein (called GAGA sites) (Fig. 2B). Both PHO and GAGA sites are associated with several PREs (24–26). Our first two conversion constructs (① and ② in Fig. 2A) were used to generate deletions of 269 and 396 bp, respectively (Δ1 and Δ2 in Fig. 2D). Neither deletion gave any phenotype discernible in adult flies, whether heterozygous with a wild-type chromosome, heterozygous with a deletion (“hemizygous”), or homozygous. However, when these two deletions were merged by flipase recombination (Δ1-2, 665 bp; Fig. 2D), we saw dramatic posteriorly directed segmental transformations.
Fig. 2.
Map of the bxd PRE region dissected with deletions. (A) Fifteen conversion constructs are diagramed, with vertical arrows showing the map positions where the constructs are inserted. The constructs with horizontal bars contained genomic sequences, initially flanked by FRT sites, which were then deleted by flipase-mediated recombination. (B) This DNA sequence map covers 4.5 kb, marked in the coordinates of Seq89E (37). Binding sites for pleiohomeotic protein (GCCAT) and GAGA factor (GAGAG) are indicated by orange and blue balls, respectively. Three vertical arrows mark the insertion sites of P elements used for gene conversion. (C) The white boxes indicate the positions of embryonic enhancers, and blue and violet boxes show subfragments with PRE activity in transgene assays (7, 23). Yellow boxes indicate TREs (17). The TRE1, TRE2, and TRE3 arrows mark short RNA transcripts reported to recruit activators for Ubx (19). (D) The dashed lines indicate the extents of the deletions generated. Orange balls on the deletion lines indicate an FRT site remaining at the position of the deletion. The bottom four deletions also retain a GAL4-VP16 marker gene and an I-SceI cut site. The presence or absence of a posterior transformation phenotype (PS5 to PS6) is indicated for each deletion.
Phenotypes of the Δ1-2 Deletion.
Flies heterozygous for Δ1-2 often showed partial transformation of posterior wing toward haltere, resembling the phenotypes of Contrabithorax mutations (Fig. 3A). Less frequently, the anterior wing margin was also transformed toward haltere tissue (Fig. 3B). Rarely, flies also had dark pigment and (very rarely) large bristles on the posterior edge of the first abdominal tergite, evidence of a partial transformation of the first abdominal segment toward the second abdominal segment. These transformations are likely caused by occasional misexpression of ABD-A (not tested), although we did not observe any corresponding reduction in UBX expression in the first abdominal segment (see Fig. 4). Occasionally, small clones of foreign tissue with small bristles appeared between the thorax and the abdomen. The origin of this tissue was made clear in homozygotes, which showed a variable, but sometimes complete, transformation of the third thoracic segment into a copy of the first abdominal segment (Fig. 3C), a phenotype never described before among mutations of the bithorax complex. In general, all transformations seen in heterozygotes looked stronger and/or more penetrant in homozygotes. The penetrance of transformation phenotypes approached 100% for Δ1-2 homozygotes and remained stable through several generations. However, when the Δ1-2 chromosome is maintained over a balancer chromosome, the penetrance drops from 64% to ≈10% in a few generations. A quantitative listing of adult mutant phenotypes is included in supporting information (SI) Table 1. Posterior transformations were also seen in larvae (Fig. 3D).
Fig. 3.
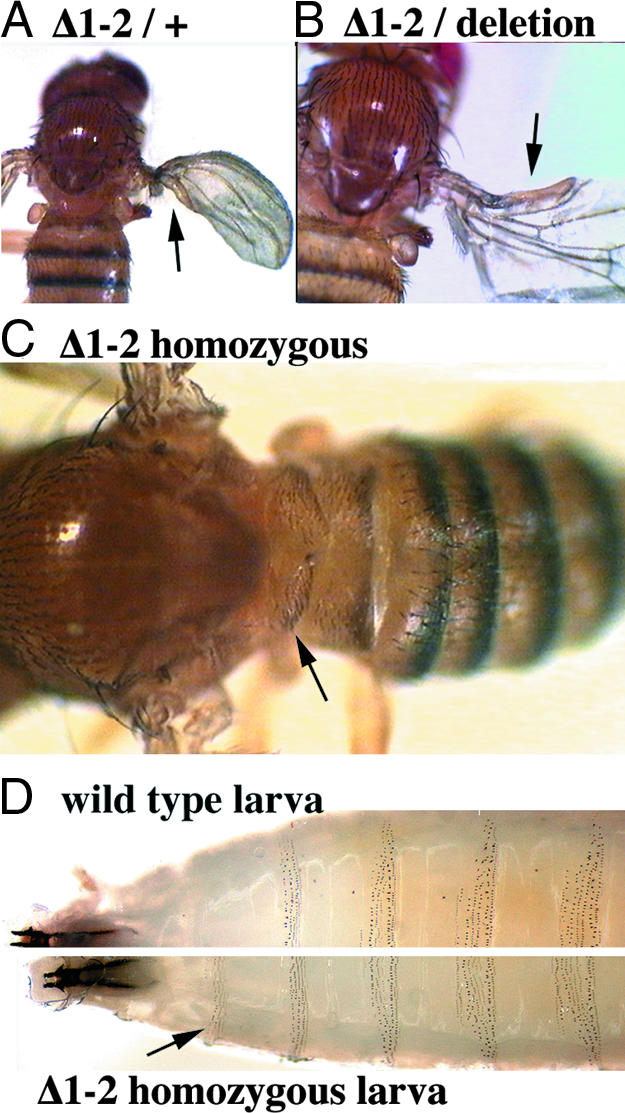
Phenotypes of bxd PRE deletion. (A) Transformation of posterior wing to haltere tissue, shown for a Δ1-2 heterozygote. (B) A transformation of anterior wing to haltere tissue, shown in a Δ1-2 hemizygote [heterozygous with Df(3R)Ubx109]. (C) Transformations of the third thoracic segment toward the first abdominal segment, shown in a Δ1-2 homozygote. (D) Unstained cuticles of third-instar larvae. The denticles of the third thoracic segment are not pigmented in wild type, but are black in the Δ1-2 larva (arrow).
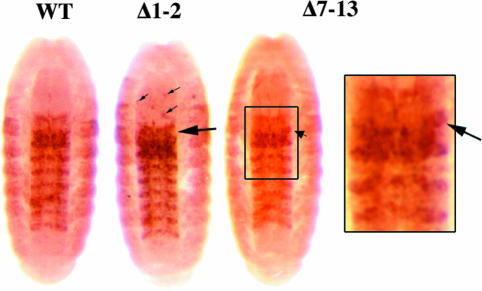
Fig. 4.
Ubx expression in PRE deletion animals. Embryos, ≈16 h after fertilization, were immunostained for the Ubx protein. Overexpression of UBX is most apparent in the developing central nervous system of a homozygous Δ1-2 embryo. Ectopic staining is strong and widespread in the third thoracic segment (large arrow), but occasional cells turn on UBX in more anterior segments (small arrows). UBX in the abdominal segments resembles that in the wild type (WT). A homozygous Δ7-13 embryo also shows no apparent reduction in UBX relative to the wild type, and there is some UBX misexpression in the third thoracic segment (arrow). The enlargement shows the third thoracic through third abdominal segments in the nerve chord of the Δ7-13 embryo.
Δ1-2 heterozygotes and homozygotes never showed any opposite, anteriorly directed transformations, which would have indicated a loss of function of the Ubx gene. In a more sensitive assay, we generated Δ1-2 hemizygotes (heterozygous with the deletions pbx2, Ubx109, and P9). These hemizygotes also showed no enhanced or new anteriorly directed transformations as compared with Df Ubx109 or Df P9 heterozygotes, but the posterior transformations were almost as severe and frequent as in homozygotes. The posteriorly directed segmental transformations caused by Δ1-2 were still detectable when it was heterozygous with the tandem duplication of bithorax complex, DpP5, confirming that the transformations are caused by a gain of function (GOF), not a loss.
These adult phenotypes were consistent with misexpression of the Ubx gene in parasegment 5 (PS5, the posterior edge of the second thoracic segment plus most of the third thoracic segment), resembling the wild-type expression in PS6 (posterior third thoracic segment plus most of the first abdominal segment). In embryos homozygous for Δ1-2, UBX expression patterns were most often only slightly elevated in PS5, but some embryos showed dramatic misexpression of UBX in PS5, and occasional cells misexpressed UBX in more anterior segments (Fig. 4). Similarly variable UBX misexpression could also be seen in the PS5 region of the central nervous system of third-instar larvae, and, rarely, spots of UBX expression were seen in third-instar wing discs (data not shown). No abnormal UBX expression was seen in the brain lobes, the eye-antennal discs, or the anterior leg discs.
Smaller Deletions with GOF Phenotypes.
The 665-bp Δ1-2 deletion removed most of the clustered GAGA and PHO sites (Fig. 2B), but it was not clear whether all of those sites were needed. We designed conversion constructs to define better the minimal DNA segment, which, when removed, results in segmental transformations. Construct ⑩ (Fig. 2A) was used to delete 280 bases straddling the junction between deletions Δ1 and Δ2. Δ10 removes the central four GAGA and four PHO sites; it also matches closely the two most active PRE subfragments defined by transgene studies [fragments BP and PF (23)]. Animals with this 280-bp deletion show posterior transformation phenotypes similar to those of the 665-bp Δ1-2. ⑫ was designed to remove 127 bp internal to the segment defined by Δ10; it covered only one PHO and two GAGA sites. This deletion caused no transformations. Finally, Construct ⑰ was designed to enlarge Δ12 in the proximal direction to include three more PHO sites. The resulting 185-bp deletion gave transformations similar in strength and character to those from Δ10, but with a reduced penetrance (78% of heterozygotes for Δ10, 22% for Δ17). We call this 185-bp sequence the bxd PRE core. The penetrance of these smaller deletions also declined in successive generations, when maintained in heterozygous stocks. The penetrance could be partially restored by outcrossing to wild-type animals, although this was not analyzed quantitatively. In Δ10 homozygotes, the penetrance was near 100% and stable (similar to Δ1-2 homozygotes), whereas, uniquely, it still declined in Δ17 homozygotes. Moreover, the strength of transformations weakened in homozygous stocks of Δ17 and Δ10, but it remained constant in Δ1-2 homozygotes.
Dissection of the Flanking Regions.
Previous analyses of DNA fragments from the bxd PRE region in transgenes and by immunoprecipitations suggested that there might be multiple redundant PRE/TRE elements spread over ≈3 kb (17, 23). Two of these DNA regions contain only a few GAGA or PHO sites, and they partially overlap regions defined as embryonic enhancers (S1 and S2; Fig. 2C) (27). However, our deletions to the left (Δ7 of 529 bp, Δ8-3 of 925 bp) and right (Δ13 of 646 bp, Δ4-13 of 1,843 bp) of the central region did not cause any phenotype as heterozygotes or homozygotes (Fig. 2D). The result for Δ4-13 was unexpected, because this deletion removes the most conserved region near the PRE (28).
In contrast, deletions taking out the essential 185 bp plus large segments either to the left (Δ8-4, SceΔG) or right (Δ17-19, Δ2-19) all cause the GOF phenotypes seen with deletion Δ10, but neither is more severe (Fig. 2D). This finding was surprising, because one might expect that larger deletions would result in more severe phenotypes or higher penetrance, as in the case of deletions Δ17 and Δ10 (see above). Finally, Δ7-13, a deletion of 3,036 bp, removes the whole region, including all three putative TREs (and the TRE RNA templates, except for the first 40 bases of the TRE 1 transcript), and parts of both the S1 and S2 enhancer regions (Fig. 2). Δ7-13/+ heterozygotes and hemizygotes (Δ7-13/pbx2) look wild type; they fail to give the posteriorly directed transformations seen in Δ1-2 and other smaller deletions. Homozygous Δ7-13 adults are viable and show similar posterior transformations to those seen in Δ1-2 flies, except that there are no transformations in the wing. Despite the lack of all three TREs, no anterior transformations were seen. Δ7-13 homozygous embryos resemble Δ1-2 homozygotes, with occasional UBX protein misexpression in the third thoracic segment (PS5), but without any apparent reduction or loss of the wild-type UBX pattern (Fig. 4). We have no simple explanation for the milder GOF phenotype of Δ7-13, compared with smaller deletions, but the partial removal of the S2 enhancer may be responsible (see Discussion).
PRE Duplications.
Multimerized PRE fragments in transgenic constructs can induce silencing of a neighboring marker gene (23). We used the FRT recombination strategy to generate tandem duplications of the DNA segments between convertants ④ and ③, between convertants ④ and ⑨, and between convertants ④ and ⑧ (duplications of 268, 664, and 1,193 bp, respectively). All three duplications were recovered free of marker genes. Flies either heterozygous or homozygous for any of these duplications looked wild type. These duplications caused no phenotypes even in a sensitized background, in the presence of only one copy of functional Ubx gene. However, a tandem duplication for the bithorax complex, DpP5, seems to partially suppress the dominant, GOF phenotype of Δ1-2. This partial suppression may be caused by an interaction of the deletion chromosome with the two copies of the bxd PRE on the DpP5 chromosome or with additional PREs from other regions of the bithorax complex (see Discussion).
Discussion
We have developed an enhanced method of gene conversion for the generation of small, targeted deletions in the bxd PRE region. These enhancements of P-element-mediated gene conversion were used to generate deletions with random or predetermined sizes. The insertion of selectable markers with the conversion events eliminated the need for PCR screening of all of the chromosomes from which the P elements were excised.
One prior report of a PRE deletion (16) described the loss of a 450-bp segment in the iab-7 region of the bithorax complex, immediately adjacent to the Front abdominal-7 (Fab-7) boundary element, but thought to be separate from that element. Most animals homozygous for this deletion had no visible phenotype, but rare exceptions showed weak posterior transformations in the sixth abdominal segment. Our deletion of the core bxd PRE (185 bp), even as a heterozygote, is ≈10 times more penetrant. The iab-7 PRE might not have a single core, but may include multiple elements that overlap the Fab-7 boundary region or extend distally into iab-7. The 450-bp iab-7 deletion might remove only some of these elements, which could explain its very weak phenotype. Alternatively, it is possible that there are other separable, but redundant, PREs in the iab-7 region, or that the iab-7 PRE cooperates with PREs in the iab-6 or iab-8 regions (29).
PRE Cooperation.
In contrast to the 450-bp iab-7 PRE deletion, our 665-bp deletion, Δ1-2, seems to eliminate most of the PRE functions in the bxd region. This notion is suggested by the stable and strong phenotype and by the nearly 100% penetrance in Δ1-2 homozygotes. But it is still not clear why the transformation phenotypes of our deletions are partial and variable. It is possible that the bxd PRE on the wild-type homolog in heterozygotes (or cryptic PRE elements on both homologues) can variably compensate for the impaired bxd PRE. The possibility of cooperation between homologs is suggested by our observation that the hemizygous phenotype of Δ1-2 is more severe than the heterozygous phenotype. In addition, a PRE in an adjacent regulatory region of Ubx (the region of bithorax mutations) might fill in when the bxd PRE is lacking. Both of these PREs have POLYCOMB bound to them in cells of a segment where Ubx is repressed (30); perhaps the two sites interact. The transformations observed in Δ1-2 heterozygotes are more penetrant and more severe in a Polycomb heterozygous mutant background (unpublished observation), which also suggests such interactions.
It was not expected that the transformations caused by our PRE deletions would be largely limited to the third thoracic segment (PS5). Again, the predicted PRE in the bithorax region might repress Ubx in more anterior segments, or perhaps the homeotic proteins from the Antennapedia complex supersede Ubx function. The occasional GOF transformations seen in anterior wing (PS4) and first abdominal tergite (PS6) suggest that, in wild type, there is cooperation between the bxd PRE and the PREs in adjacent segmental domains. The disruption of such interactions by chromosomal rearrangements could be responsible for the “Cis-overexpression” (COE effect) described by Lewis (6, 31). To test these ideas, it will be important to construct a chromosome simultaneously deleted for the bithorax and the bxd PREs.
It is more difficult to explain the gradual reduction in penetrance with successive generations in deletion heterozygotes (or in Δ17 homozygotes), although such a gradual reduction in phenotype is common for GOF mutations in the HOX complexes. There may be allele variants or spontaneous mutations at other loci that are selected as suppressors. A less conventional idea is that there are spontaneous, but heritable, epigenetic changes in the region of the PRE deletion. In any case, these effects implicate the regions flanking the 185-bp PRE core. The difference in the stability of phenotypes between Δ17, Δ10, and Δ1-2 homozygotes suggests that these regions are necessary for the gradual restoration of the bxd PRE function.
Modular Structure of the bxd PRE.
Our 185-bp (Δ17) deletion designates the minimal region that must be deleted to get dominant, posteriorly directed transformations. The 185 bp region must have a specific function not replaceable by neighboring sequences. However, the absence of transformations in flies carrying deletions that remove only parts of the 185-bp PRE core (Δ1, Δ2 and Δ12) argue that the structure of the 185-bp “PRE core” is modular, and the functions encoded by these modules are redundant.
All but one of our deletions removing the 185-bp PRE core (Fig. 2) cause posterior transformations in heterozygotes. The only exception, Δ7-13, is reminiscent of the 16-kb deletion, pbx2 (32, 33), the only previously isolated mutation removing the bxd PRE. Both pbx2 heterozygotes and Δ7-13 heterozygotes look wild type. Thus, the GOF seen with the PRE deletions described here must depend on other sequences covered by both the pbx2 and the Δ7-13 deletions, but not by our smaller deletions. All of our deletions causing posterior transformations in heterozygotes leave intact the 1-kb Sau3A fragment that harbors the S2 embryonic enhancer (Fig. 2). We noted that the Gal4VP16 expression levels seen in Δ19-13 embryos and larvae were dramatically reduced, relative to the levels seen in convertant ⑱ (unpublished data). An intact S2 enhancer region may be important for the ectopic expression caused by deletions in the central PRE region. Posterior transformations do appear in Δ7-13 homozygotes, suggesting that the sequences needed for the misexpressed functions are not completely removed by this deletion. Modularity of PREs is also suggested by previous biochemical studies (34, 35).
Function of the TREs and PREs.
The Δ7-13 deletion removes all three reported TREs in the region, but animals homozygous for this 3-kb deletion show no apparent reduction of Ubx expression in embryos or larvae. The PRE deletion larvae and adults show no anterior transformations (such as transformation of haltere to wing, third leg to second, or first abdominal segment to thoracic), which, for mutations in the bithorax complex, would indicate a loss of function. Thus, there is no evidence that PREs are required in situ for activation and repression. It is possible that the TREs are redundant with other activation sites outside the region covered by our deletions, or that the only function of the TREs is to prevent the repression by POLYCOMB in segments where the bxd region should be active. It is more difficult to reconcile our results with the report of TRE transcripts required for activation of Ubx. Targeting these TRE transcripts with antisense RNA or siRNAs was reported to attenuate Ubx expression in wing and leg discs (19). The Δ7-13 deletion removes templates for all three TRE transcripts, and there are no obvious homologs of these sequences elsewhere in the genome; yet UBX is not reduced in level or pattern in Δ7-13 animals. TRE transcription could be required to “switch off” the PRE in PS6, as has been suggested for larger noncoding RNAs in the region (36), although this could not account for the reported action of TRE transcripts in trans (19). In any case, the consequence of the simultaneous removal of all PREs/TREs included in the dissected 3-kb region is derepression, not inactivation of Ubx.
Materials and Methods
Stocks and Crosses.
Stocks were maintained at 18°C or 25°C using a standard cornmeal fly food. Crosses were set up at 25°C en masse, unless otherwise noted. Descriptions of mutations and balancer chromosomes can be found in FlyBase (http://flybase.bio.indiana.edu) or The Genome of Drosophila melanogaster (37).
Nucleic Acid Procedures.
PCRs were performed by using gradient thermal cyclers [Robocycler (Stratagene, La Jolla, CA) and PTC-200 (MJ Research, Cambridge, MA)]. A mixture of Taq and Pfu polymerases (20:1, in units) was used, sold separately by Promega (Madison, WI) and Fermentas (Vilnius, Lithuania). Detailed descriptions of the primers and PCR programs used are available on request. For whole-genome Southern blots, DNA preparation from adult flies used the detergent lysis method (38).
Source of DNA Fragments.
To eliminate DNA mismatches that might reduce the frequency of gene conversion, we generated genomic fragments by PCR from ry502 homozygous flies, the strain used as the background for the P-element insertions. One or both of the PCR primers for each fragment had a half FRT sequence at its 5′ end, including the endogenous XbaI site in the center of the FRT site. Amplified genomic fragments were cloned into the Bluescript II KS+ plasmid. Two 76-bp oligos (complementary to each other) were synthesized to produce a half-FRT/I-SceI/HindIII/I-Sce/half-FRT cassette, which was inserted between the genomic fragments. Marker genes were inserted at the HindIII site of this cassette. A 7.3-kb genomic HindIII fragment containing the rosy+ gene was used in constructs ①–④. All other constructs contained a 2.3-kb HindIII fragment of Gal4-VP16, driven by the P-element promoter and followed by three copies of HSP-70 termination signal. More detailed descriptions of cloning steps are available on request.
The DNA endpoints of the deletions diagramed in Fig. 2 are listed below. The DNA coordinates follow the Seq89E numbering (ref. 39; GenBank accession no. U31961) and indicate the bases removed by the deletion.
Simple “flip-out” deletions were: Δ1, 219230–218962 (269 bp); Δ2, 219626–219231 (396 bp); Δ7, 220155–219627 (529 bp); Δ10, 219374–219095 (280 bp); Δ12, 219316–219190 (127 bp); Δ13, 217765–217120 (646 bp); and Δ17, 219374–219190 (185 bp).
Synthetic deletions were: Δ1-2, 219626–218962 (665 bp); Δ9-4, 219626–218962 (665 bp; carries Gal4VP16); Δ7-15, 220155–218962 (1,194 bp); Δ8-4, 220155–218962 (1,194 bp; carries Gal4VP16); Δ8-3, 220155–219231 (925 bp; carries Gal4VP16); Δ2-19, 219626–217765 (1,862 bp); Δ17-19, 219374–217765 (1,610 bp); Δ4-13, 218962–217120 (1,843 bp); and Δ7-13: 220155–217120 (3,036 bp).
I-SceI induced deletion was SceΔG, 220277–218212 (2,066 bp).
Description of Conversion Experiments.
To induce double-strand breaks in the proximity of the region designated for conversion we used fly stocks carrying the flipped-out versions of P-element inserts of JE24, HF79, or HC109A (20). For the conversion of constructs ①, ③, and ④, ry JE24/MKRS flies were crossed to ry pbx2/MKRS flies. Embryos were injected with the appropriate construct (1 μg/μl) together with the helper plasmid, Δ2-3 (250 ng/μl). The hatching adult ry JE24/ry pbx2 injectees were pair-crossed to cn1; ry502 Fab-7 flies. ry+ individuals were used to establish stocks balanced over MKRS or TM2 ry2101. Conversion events were confirmed by using whole-genome Southern blotting. For the conversion of construct ②, we used homozygous ry HF79 flies instead of ry JE24/MKRS. For constructs ⑤ and ⑥, ry JE24/MKRS flies were crossed to ry pbx2 Δ2-3 99B/TM3 ry Δ2-3 99B, and the resulting embryos were injected with only the conversion construct. The hatching adult ry JE24/ry pbx2 Δ2-3 99B injectees were crossed en masse to flies homozygous for a P{UAS-eGFP} transgene on the second chromosome. Embryos were collected on apple-juice plates made black with charcoal, and the hatching larvae were screened for GFP fluorescence with a MZ FLIII stereo microscope (Leica, Heerbrugg, Switzerland). Larvae with a segmentally restricted pattern of GFP in the epidermis and nervous system were collected individually and used to set up stocks. Conversion events were confirmed by PCR. For the conversion of constructs ⑦–⑨, the injected embryos had the ry pbx2 Δ2-3 99B chromosome heterozygous with a genomic deletion-bearing ry− chromosome, generated by FRT recombination between inserts HF79 and HC109A. For the conversion of all other constructs, homozygous ry JE24 Fab-7 males were crossed to ry pbx2 Δ2-3/TM3 ry, Δ2-3 99B females.
FLP Recombination To Make Deletions.
Males carrying conversions ① or ② (marked with the ry+ gene) were crossed to homozygous y1 w1118, P{ry+, hsflp}; ry502 Fab-7 females. The progeny were heat-shocked as first-instar larvae (1 h, 37°C). The hatching males were crossed to MKRS/TM2 ry2101 females, and their sons were screened for ry−, non-Fab-7 individuals. These males, carrying the presumptive deletion, were used to set up stocks. Deletion events were confirmed by PCR, and the PCR products were occasionally sequenced to verify the deletion endpoints. Similarly, males carrying convertant ⑦ (marked with Gal4-VP16) were crossed to females, as above. Their heat-shocked sons were crossed to w1118, P{UAS-eGFP}; MKRS/TM2 ry2101 females. The non-Fab-7 sons lacking GFP fluorescence were presumed to carry the deletion. Males carrying convertants ⑩, ⑫, ⑬, and ⑰ were crossed to y1 w1118, P{ry+, hsflp}, ry502 females. The heat-shocked sons were crossed to w1118, P{UAS-eGFP}, ry502 females. The Fab-7 sons lacking GFP fluorescence were presumed to carry the deletion.
FLP Recombination Between Two Conversion Chromosomes.
Synthetic deletions or duplications were generated by recombination between two FRT sequences (FRT1 and FRT2) localized in trans on two different conversion chromosomes. Flanking markers were added to each conversion chromosome to make it possible to recognize the recombinants. In a generic scheme, y1 C (1)/Y; FRT2 Dr1/TM6B, Sb, Tb females were crossed to y1 w1118, P{ry+, hsflp}; ry502/Ki1 FRT1 males. The produced first-instar larvae were heat-shocked and the hatching y1 w1118, P{ry+, hsflp}; Ki1 FRT1/FRT2 Dr1 males were crossed to cn1; ry502 or cn1; ry502 Fab-7 females. Selection for or against the flanking markers marked the direction of cross-overs in the recombinants, resulting in deletions or duplications. Recombinants were tested by PCR.
Immunostaining of Embryos and Larvae.
Wild-type embryos and homozygotes for deletions Δ1-2 and Δ7-13 were collected during ≈17 h egg laying at 25°C. Embryos of different genotypes were treated in parallel as described (22). The primary antibody against UBX was a kind gift of Robert White (Cambridge University, Cambridge, U.K.). Photos of whole embryos were taken in PBS (137 mM NaCl, 5 mM KCl, 10 mM sodium phosphate, pH 7.0) in a depression slide.
Wandering third-instar larvae homozygous for deletions Δ1-2 and Δ7-13 and wild-type larvae carrying the Bc larval marker were collected at 25°C, washed in distilled water, and cut in half in chilled (4°C) PBS. Anterior halves of larvae were turned inside out with tweezers, and tissues (other than imaginal discs and larval brain) were removed from the epidermis. Four larval halves (a pair of wild-type and a pair of deletion homozygotes) were treated in the same microcentrifuge tube following the protocol for immunostaining of embryos (22).
Preparation of Larval Cuticles and Photography.
Larvae were overanesthetized with ether and boiled in PBS until their muscles relaxed. For taking pictures, they were put on a glass slide and covered with immersion oil and a coverslip. The coverslips were gently pressed to flatten the boiled larvae. Photographs were taken with a M420 Makroscop (Wild Heerbrugg, Heerbrugg, Switzerland) and a KP-C550 CCD camera (Hitachi, Tokyo) or a Leica MZ FLIII stereo microscope and a Leica DC300F digital camera. Contrast and brightness was adjusted by using Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Supplementary Material
Acknowledgments
We thank József Mihaly and Henrik Gyurkovics for discussions and critical reading of the manuscript and Anna Rehák, Edit Gyányi, Edina Ördög, Anikó Berente, and Ildikó Krausz for technical help. This work was supported by the National Institutes of Health (W.B.) and a Fogarty International Award (to L.S. and W.B.).
Abbreviations
- PRE
Polycomb response element
- TRE
trithorax response element
- PcG
Polycomb group
- FRT
flipase recombination target
- PS
parasegment
- GOF
gain of function.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703144104/DC1.
References
- 1.Jürgens G. Nature. 1985;316:153–155. [Google Scholar]
- 2.Simon J. Curr Opin Cell Biol. 1995;7:376–385. doi: 10.1016/0955-0674(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 3.Akasaka T, van Lohuizen M, van der Lugt N, Mizutqani-Koseki Y, Kanno M, Taniguchi M, Vidal M, Alkema M, Berns A, Koseki H. Development (Cambridge, UK) 2001;128:1587–1597. doi: 10.1242/dev.128.9.1587. [DOI] [PubMed] [Google Scholar]
- 4.Pirrotta V. Trends Genet. 1997;13:314–318. doi: 10.1016/s0168-9525(97)01178-5. [DOI] [PubMed] [Google Scholar]
- 5.Simon J, Chiang A, Bender W, Shimell MJ, O'Connor M. Dev Biol. 1993;158:131–144. doi: 10.1006/dbio.1993.1174. [DOI] [PubMed] [Google Scholar]
- 6.Chiang A, O'Connor MB, Paro R, Simon J, Bender W. Development (Cambridge, UK) 1995;121:1681–1689. doi: 10.1242/dev.121.6.1681. [DOI] [PubMed] [Google Scholar]
- 7.Fritsch C, Brown JL, Kassis JA, Müller J. Development (Cambridge, UK) 1999;126:3905–3913. doi: 10.1242/dev.126.17.3905. [DOI] [PubMed] [Google Scholar]
- 8.Fauvarque M-O, Dura J-M. Genes Dev. 1993;7:1508–1520. doi: 10.1101/gad.7.8.1508. [DOI] [PubMed] [Google Scholar]
- 9.Zink B, Paro R. Nature. 1989;337:468–471. doi: 10.1038/337468a0. [DOI] [PubMed] [Google Scholar]
- 10.DeCamillis M, Cheng N, Pierre D, Brock HW. Genes Dev. 1992;6:223–232. doi: 10.1101/gad.6.2.223. [DOI] [PubMed] [Google Scholar]
- 11.Chan C-S, Rastelli L, Pirrotta V. EMBO J. 1994;13:2553–2564. doi: 10.1002/j.1460-2075.1994.tb06545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strutt H, Cavalli G, Paro R. EMBO J. 1997;16:3621–3632. doi: 10.1093/emboj/16.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlando V, Jane EP, Chinwalla V, Harte P, Paro R. EMBO J. 1998;17:5141–5150. doi: 10.1093/emboj/17.17.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 15.Kahn TG, Schwartz YB, Dellino GI, Pirrotta V. J Biol Chem. 2006;281:29064–29075. doi: 10.1074/jbc.M605430200. [DOI] [PubMed] [Google Scholar]
- 16.Mihaly J, Hogga I, Gausz J, Gyurkovics H, Karch F. Development (Cambridge, UK) 1997;124:1809–1820. doi: 10.1242/dev.124.9.1809. [DOI] [PubMed] [Google Scholar]
- 17.Tillib S, Petruk S, Sedkov Y, Kuzin A, Fujioka M, Goto T, Mazo A. Mol Cell Biol. 1999;19:5189–5202. doi: 10.1128/mcb.19.7.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poux S, Horard B, Sigrist CJ, Pirrotta P. Development (Cambridge, UK) 2002;129:2483–2493. doi: 10.1242/dev.129.10.2483. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Elsner T, Gou D, Kremmer E, Sauer F. Science. 2006;311:1118–1123. doi: 10.1126/science.1117705. [DOI] [PubMed] [Google Scholar]
- 20.Bender W, Hudson A. Development (Cambridge, UK) 2000;127:3981–3992. doi: 10.1242/dev.127.18.3981. [DOI] [PubMed] [Google Scholar]
- 21.Nassif N, Penny J, Pal S, Engels WR, Gloor GB. Mol Cell Biol. 1994;14:1613–1625. doi: 10.1128/mcb.14.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sipos L, Mihaly J, Karch F, Schedl P, Gausz J, Gyurkovics H. Genetics. 1998;149:1031–1050. doi: 10.1093/genetics/149.2.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horard B, Tatout C, Poux S, Pirrotta V. Mol Cell Biol. 2000;20:3187–3197. doi: 10.1128/mcb.20.9.3187-3197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagstrom K, Muller M, Schedl P. Genetics. 1997;146:1365–1380. doi: 10.1093/genetics/146.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mihaly J, Mishra RK, Karch F. Mol Cell. 1998;1:1065–1066. doi: 10.1016/s1097-2765(00)80107-0. [DOI] [PubMed] [Google Scholar]
- 26.Brown JL, Mucci D, Whiteley M, Dirksen ML, Kassis JA. Mol Cell. 1998;1:1057–1064. doi: 10.1016/s1097-2765(00)80106-9. [DOI] [PubMed] [Google Scholar]
- 27.Pirrotta V, Chan CS, McCabe D, Qian S. Genetics. 1995;141:1439–1450. doi: 10.1093/genetics/141.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dellino G, Tatout C, Pirrotta V. Int J Dev Biol. 2002;46:133–141. [PubMed] [Google Scholar]
- 29.Mihaly J, Barges S, Sipos L, Maeda R, Cléard F, Hogga I, Bender W, Gyurkovics H, Karch F. Development (Cambridge, UK) 2006;133:2983–2993. doi: 10.1242/dev.02451. [DOI] [PubMed] [Google Scholar]
- 30.Papp B, Müller J. Genes Dev. 2007;20:2041–2054. doi: 10.1101/gad.388706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis EB. Cold Spring Harbor Symp Quant Biol. 1985;50:155–164. doi: 10.1101/sqb.1985.050.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Lewis EB. In: Developmental Biology Using Purified Genes. Brown DD, Fox CF, editors. New York: Academic; 1981. pp. 189–208. [Google Scholar]
- 33.Peifer M. Boston, MA: Harvard University; 1988. PhD thesis. [Google Scholar]
- 34.Blastyák A, Mishra RK, Karch F, Gyurkovics H. Mol Cell Biol. 2006;26:1434–1444. doi: 10.1128/MCB.26.4.1434-1444.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgson J, Argiropoulos B, Brock HW. Mol Cell Biol. 2001;21:4528–4543. doi: 10.1128/MCB.21.14.4528-4543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt S, Prestel M, Paro R. Genes Dev. 2005;19:697–708. doi: 10.1101/gad.326205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsley DL, Zimm GG. The Genome of Drosophila melanogaster. San Diego: Academic; 1992. [Google Scholar]
- 38.Bender W, Spierer P, Hogness DS. J Mol Biol. 1983;168:17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- 39.Martin CH, Mayeda CA, Davis CA, Ericsson CL, Knafels JD, Mathog DR, Celniker SE, Lewis EB, Palazzolo MJ. Proc Natl Acad Sci USA. 1995;92:8398–8402. doi: 10.1073/pnas.92.18.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.