Abstract
Bipedal walking is evident in the earliest hominins [Zollikofer CPE, Ponce de Leon MS, Lieberman DE, Guy F, Pilbeam D, et al. (2005) Nature 434:755–759], but why our unique two-legged gait evolved remains unknown. Here, we analyze walking energetics and biomechanics for adult chimpanzees and humans to investigate the long-standing hypothesis that bipedalism reduced the energy cost of walking compared with our ape-like ancestors [Rodman PS, McHenry HM (1980) Am J Phys Anthropol 52:103–106]. Consistent with previous work on juvenile chimpanzees [Taylor CR, Rowntree VJ (1973) Science 179:186–187], we find that bipedal and quadrupedal walking costs are not significantly different in our sample of adult chimpanzees. However, a more detailed analysis reveals significant differences in bipedal and quadrupedal cost in most individuals, which are masked when subjects are examined as a group. Furthermore, human walking is ≈75% less costly than both quadrupedal and bipedal walking in chimpanzees. Variation in cost between bipedal and quadrupedal walking, as well as between chimpanzees and humans, is well explained by biomechanical differences in anatomy and gait, with the decreased cost of human walking attributable to our more extended hip and a longer hindlimb. Analyses of these features in early fossil hominins, coupled with analyses of bipedal walking in chimpanzees, indicate that bipedalism in early, ape-like hominins could indeed have been less costly than quadrupedal knucklewalking.
Keywords: biomechanics, human evolution, locomotion, limb length, inverse dynamics
As predicted by Darwin (1), bipedalism is the defining feature of the earliest hominins (2) and thus marks a critical divergence of the human lineage from the other apes. One enduring hypothesis for this transition is that bipedalism evolved to reduce locomotor costs in early hominins, relative to the ape-like last common ancestor (LCA) of chimpanzees and humans (3). Under this scenario, reducing the energy cost of walking provided early hominins with an evolutionary advantage over other apes by reducing the cost of foraging. Such an advantage may have been especially important given the cooler, drier climate that prevailed at the end of the Miocene (4, 5) and that would have increased the distance between food patches.
Testing this hypothesis requires comparative data not only on the cost of locomotion (COL) in humans and chimpanzees but also on the biomechanical determinants of these costs. The only previous study of chimpanzee locomotor cost used juvenile chimpanzees and indicated that bipedalism and quadrupedalism were equally costly in chimpanzees and that both were more costly than human locomotion (6). Although this study has been central to the debate over energetics and the evolution of bipedalism (3, 7), the reliability of these data has been questioned because adult and juvenile locomotor mechanics and costs can differ substantially (7) and because of recent evidence that bipedalism is more costly than quadrupedalism in other primates (8). Furthermore, the study by Taylor and Rowntree (6) did not include a biomechanical analysis of the determinants of chimpanzee locomotor costs, limiting the potential application of the study to the hominin fossil record.
Here, we compare human and adult chimpanzee locomotor energetics and biomechanics to determine links among anatomy, gait, and cost. Our study focuses on two primary questions. First, do adult chimpanzees follow the pattern of costs found previously for juveniles (6)? Second, do differences in anatomy and gait between bipedal and quadrupedal walking, as well as between chimpanzees and humans, explain observed differences in cost? Using this biomechanical approach to link differences in anatomy and gait to cost, we then examine what changes, if any, would lower the cost of bipedalism for an early hominin, such that bipedalism would be more economical than the ape-like quadrupedalism of the last common ancestor.
We focused on walking speeds because walking is the gait commonly used during terrestrial travel in wild chimpanzees (9). We tested two sets of predictions; first, based on recent studies of primate mechanics and energetics (8, 10), we predicted that bipedal and quadrupedal (i.e., “knucklewalking”) costs will differ in adult chimpanzees and that both bipedal and quadrupedal walking in chimpanzees will be energetically more costly relative to other quadrupeds and humans. Second, following previous work (11, 12), we predicted that these differences in cost would be explained by corresponding differences in (i) the force required to support bodyweight during each step and (ii) the volume of muscle activated to generate one unit of ground force.
To test these predictions, we collected metabolic, kinematic, and kinetic data during walking from five chimpanzees, aged 6–33 years, and four adult humans (see Table 1 and Methods). The magnitude of ground force was estimated as the inverse of the duration of foot–ground contact time, tc, per step (11, 13), and the volume of muscle activated per unit of ground force, Vmusc, was estimated by using inverse dynamics (14) (see Methods). Following Roberts et al. (12), we predicted that the COL (ml of O2 kg−1 s−1), varies as the ratio of Vmusc and tc, Vmusc/tc. Thus, any difference in Vmusc/tc, either between species or between gaits, should lead to a proportional difference in COL.
Table 1.
Chimpanzee and human costs of transport
| Subject | Mass, kg | Hip height, cm | Speed, m/s | Froude number | Cost of transport, ml of O2 kg−1 m−1 |
||
|---|---|---|---|---|---|---|---|
| Bipedal | Quadrupedal | P | |||||
| C1, 6-year-old male | 33.9 | 45.0 | 1.0 | 0.2 | 0.28 (0.033) | 0.18 (0.012) | 0.03 |
| C2, 9-year-old male | 51.6 | 52.5 | 1.0 | 0.2 | 0.26 (0.017) | 0.18 (0.007) | 0.01 |
| C3, 19-year-old female | 63.9 | 51.0 | 1.0 | 0.2 | 0.20 (0.011) | 0.14 (0.014) | 0.02 |
| C4, 33-year-old female | 67.3 | 41.3 | 1.0 | 0.2 | 0.16 (0.020) | 0.29 (0.021) | 0.02 |
| C5, 27-year-old female | 82.3 | 40.5 | 1.0 | 0.3 | 0.15 (0.011) | 0.16 (0.006) | 0.39 |
| Chimpanzees, n = 5 | 59.8 | 46.1 | 1.0 | 0.2 | 0.21 (0.014) | 0.19 (0.013) | 0.16 |
| Humans, n = 4 | 69.3 | 92.2 | 1.3 | 0.2 | 0.05 (0.004) | ||
Individual means (with SEs in parentheses) were calculated from four speeds in each gait for each subject. Species means were calculated from individual means. Froude number was calculated from hip height by following Alexander and Jayes (34). P values are one-tailed, for paired-samples Student's t tests.
Results and Discussion
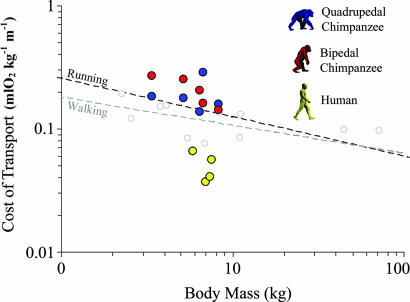
The mass-specific cost of transport (ml of O2 kg−1 m−1) for chimpanzees was greater than expected for their body size (15) (Fig. 1). In contrast, human walking was less expensive than expected for their body size and substantially (≈75%) less expensive than chimpanzee locomotion (Fig. 1). Within the entire chimpanzee sample, bipedal walking was modestly, but not significantly, more costly (≈10%) than quadrupedal walking (Fig. 1). This finding is consistent with previous work on juvenile chimpanzees (6), which indicated that bipedal and quadrupedal locomotion were equally costly for chimpanzees. However, differences in bipedal and quadrupedal cost varied among individuals (Fig. 1), and in contrast to the study by Taylor and Rowntree (6), most subjects exhibited significant differences between gaits. For three chimpanzees (C1-C3) bipedalism was 32.2% more expensive (P < 0.001, Student's paired t test), but for two other chimpanzees, bipedal costs were similar (P = 0.39; C5) or even less than quadrupedal costs (P < 0.05; C4).
Fig. 1.
Net cost of transport (ml of O2 kg−1 m−1) for chimpanzee quadrupedal walking (blue), chimpanzee bipedal walking (red), and human walking (yellow). Dashed lines indicate trendlines for running and walking in birds and mammals. The running trendline is for 65 terrestrial species (15). Walking data (open symbols) were collected from the literature [see supporting information (SI) Table 2].
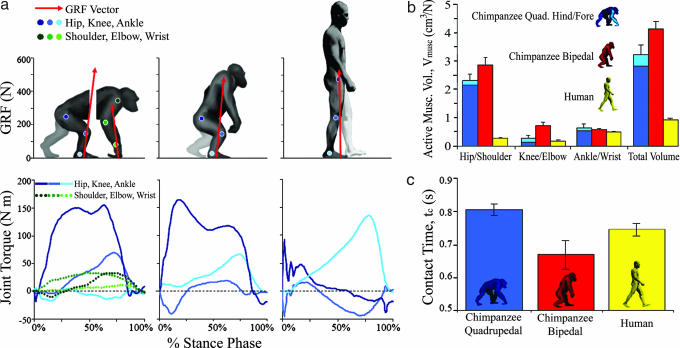
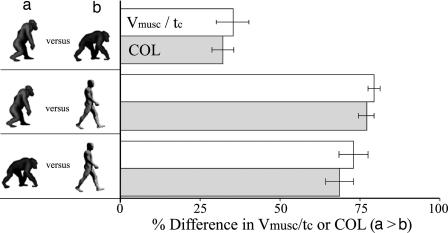
Given these results, we investigated potential biomechanical sources of the observed differences in cost among chimpanzees and between chimpanzees and humans. As predicted, differences in kinematics and estimated muscle activation explained observed differences in cost between bipedal and quadrupedal walking as well as between humans and chimpanzees. In the three chimpanzees for which Vmusc were estimated (subjects C1-C3; see Methods), an increase in Vmusc and shorter tc increased Vmusc/tc by 35.2% (±5.2%) during bipedal walking compared with quadrupedal walking. This difference corresponds closely to the observed 32.2% (±3.2%) increase in COL during bipedal walking for these subjects (Figs. 2 and 3). When human walking was compared with chimpanzee bipedal walking, humans activated smaller muscle volumes per unit of ground force and used longer tc than bipedal chimpanzees (Fig. 2). These differences caused a 79.4% (±1.6%) lower ratio of Vmusc/tc, which corresponded closely to the observed 76.8% (±2.6%) decrease in locomotor cost (Fig. 3). Similarly, although tc for quadrupedal chimpanzees were slightly longer than for humans, they activated so much more muscle per unit of ground force that Vmusc/tc was 72.8% (±4.6%) lower for humans than for quadrupedal chimpanzees, matching the 68.5% (±4.3%) difference in COL (Figs. 2 and 3).
Fig. 2.
Comparison of walking mechanics in chimpanzees and humans. (a) GRF vectors and joint torque for humans and chimpanzees. Figures show joint positions at 50% stance (forelimb and hindlimb shown separately for quadrupedal chimpanzees). Positive torque values indicate flexion, whereas negative values indicate extension. Note the large hip flexion moments in chimpanzees relative to humans. Horizontal dashed lines indicate zero joint torque. (b) Vmusc per newton of bodyweight (cm3/N) at each joint and in the whole limb for chimpanzee hindlimbs (dark blue) and forelimbs (light blue) during quadrupedal walking, chimpanzee hindlimbs during bipedalism (red), and human hindlimbs (yellow). (c) Mean tc (in seconds) during walking in chimpanzees and humans. Note that Froude numbers are similar for all groups (Fr = 0.2; Table 1), but absolute speeds are slightly higher for humans (Table 1).
Fig. 3.
Comparison of differences in Vmusc/tc (white bars) and COL (gray bars) between gaits and species. Error bars indicate ±1 SEM percent difference.
Interspecific differences in tc and Vmusc point directly to anatomical and kinematic sources for the observed differences in cost between chimpanzees and humans. First, the shorter legs of chimpanzees (Table 1) lead to shorter tc for a given speed (16) during bipedal walking (Fig. 2c), which increases the magnitude of the ground reaction force (GRF) impulse for each step. That is, because of their shorter hindlimbs, bipedal chimpanzees must generate greater ground forces at a faster rate than humans, thereby increasing bipedal costs (11, 13). Conversely, the long forelimbs of chimpanzees increase tc and decrease ground force impulses during quadrupedal walking. Second, the bent-hip, bent-knee gait of chimpanzees positions their body's center of mass anterior to the hip joint and increases the moment arm of the GRF. This posture generates large external flexion moments (Fig. 2a) that, when combined with chimpanzees' long muscle fibers (17), must be opposed by activating a correspondingly large volume of hip extensor muscle (Fig. 2b). Additionally, the long muscle fibers (17) and crouched posture of chimpanzees result in large Vmusc at the knee (Fig. 2b). In contrast, humans decrease Vmusc by adopting an upright posture, which orients the GRF vector nearer to the hip and knee joints and confines large moments to the ankle, where muscle fibers are short (Fig. 2a). Thus, although the long forelimbs of chimpanzees enable them to knucklewalk using longer tc than humans at dynamically similar speeds (Froude number ≈0.2), walking costs are lower in humans than in chimpanzees.
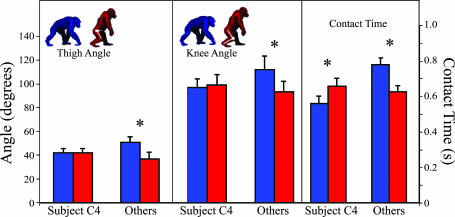
The influence of tc and joint angle on locomotor cost is further supported by subject C4. Only this chimpanzee used longer tc during bipedal walking and flexed her knee and hip to a similar degree during bipedal and quadrupedal walking (Fig. 4). As expected, C4 was also the only subject whose costs were lower during bipedal versus quadrupedal walking, although not as low as in humans (Fig. 1). These results highlight how slight kinematic changes can lead to large variations in locomotor cost and are consistent with previous work demonstrating that differences in posture can affect cost (18–20). Note, however, that chimpanzees cannot employ the full hip and knee extension typical of humans because of their distally oriented ischia, which reduce the ability of the hamstrings to produce an extensor moment when the femur is extended relative to the pelvis (21, 22). Chimpanzee pelvic anatomy thus requires them to walk with a flexed hip and knee throughout their stride. In contrast, human ischia are oriented dorsally, allowing large hamstrings extensor moments when the femur is fully extended (21).
Fig. 4.
Comparison of thigh angle, knee flexion, and tc for C4 versus other chimpanzees (n = 4). Thigh angles is measured as the angle between the thigh segment and the horizontal (e.g., thigh angle is 90° when the thigh is perpendicular to the ground). Knee angle is measured as the angle between the thigh and leg where full knee extension is 180°. tc is the time elapsed from touchdown to toe-off. Asterisks indicate significant differences between quadrupedal (blue) and bipedal (red) and strides (P < 0.05, Student's paired t test).
Results of our biomechanical analyses linking anatomy and gait to cost generate two testable predictions for the hominin fossil record. If locomotor economy was a selective force behind hominin bipedalism, then early hominin lower limbs should be longer than those of apes, and the ischia of early hominin pelves should be more dorsally projecting. The fossil record does not yet allow us to test these predictions in the earliest hominins, but an increase in leg length is apparent in partially complete specimens of Australopithecus afarensis (AL-288) and Australopithecus africanus (22–24), consistent with selection to increase tc and thereby lower locomotor cost. Furthermore, A. afarensis (AL 288-1) and A. africanus (STS-14) both have a more dorsally oriented ischium compared with chimpanzees (21, 22, 25). These modifications would have increased the mechanical advantage of the hamstrings when the hip was extended beyond that of chimpanzees, thereby reducing Vmusc and thus lowering walking costs.
However, previous morphological investigations have suggested that australopithecines walked with greater knee and hip flexion, and therefore greater cost, than modern humans (25, 26), although this issue remains intensely debated (27). Nonetheless, even if early hominins used a bent-hip, bent-knee form of bipedalism (25), our results suggest that early transitional forms would have reaped some energy savings with minor increases in hip extension and leg length. Given the evidence that the last common ancestor of humans and chimpanzees was a chimpanzee-like knucklewalker (28–30), the variation within our chimpanzee sample (Fig. 4) demonstrates that some members of the last common ancestor population likely had the ability to extend their hindlimb more fully and to use longer tc during bipedal locomotion. These kinematics would have decreased the cost of bipedal walking below that of quadrupedal knucklewalking in these individuals (Fig. 4, Table 1). Even small increases in energy savings from slight increases in hindlimb extension or length may have provided critical selection pressure for early hominins (31, 32).
Our results, therefore, support the hypothesis that energetics played an important role in the evolution of bipedalism. Unfortunately, a lack of postcranial evidence from the earliest hominins and their immediate forebears prevents us from testing the hypothesis that locomotor economy provided the initial evolutionary advantage for hominin bipedalism. However, regardless of the context under which bipedalism evolved, our biomechanical analysis of adult chimpanzee costs, coupled with previous analyses of early hominin pelvic and hindlimb morphology, suggests that improved locomotor economy may have accrued very early within the hominin lineage. Future fossil discoveries from the earliest hominins will resolve whether this energetic advantage was in fact the key factor in the evolution of hominin bipedalism.
Methods
Five chimpanzees (two males and three females; mean age, 18.2 years; range, 6–33 years) were trained over the course of 4 months to walk quadrupedally (i.e., knucklewalk) and bipedally on a treadmill (Smooth 9.15; Smooth Fitness, Sparks, NV). Three of these subjects (C1-C3) were also trained to walk down a force-plate-equipped track. All subjects were socially housed in large, outdoor enclosures at a United States Department of Agriculture registered and approved facility. Institutional Animal Care and Use Committee approval was obtained before the beginning of the study, and institutional animal care guidelines were followed throughout.
During treadmill trials, subjects wore loose-fitting masks that collected expired air, and the mass-specific COL was measured via established open-flow methods (15). COL was measured at a range of speeds for each individual. Only trials lasting a minimum of 3 min, and in which the oxygen-consumption rate visibly plateaued, were included for analysis. Multiple COL measurements were taken at each speed for each subject, and means were used for subsequent analyses. For a subset of treadmill trials, a set of kinematic variables, including tc (i.e., duration of stance for one foot or hand) was collected via high-speed video (125 frames/s; Redlake, Tucson, AZ).
During force-plate trials, subjects walked down a 10-m track equipped with an embedded force-plate (Kistler, Amherst, NY) recording at 4 kHz, providing vertical and fore-aft GRFs. Simultaneous kinematic data were collected via high-speed video (125 frames/s; Redlake), with joint centers for forelimbs and hindlimbs (shoulder, elbow, wrist, hip, knee, and ankle) marked on each subject by using nontoxic water-based white paint. Because flexion and extension of the limb joints occur primarily in the sagittal plane during walking and because mediolateral forces were smaller than anteroposterior ground forces and generally <10% of vertical ground forces, we restricted our analyses to the sagittal plane. Force-plate trials were accepted only if one limb (fore or hind) contacted the force-plate cleanly and if fore-aft GRF traces indicated constant forward speed.
Body mass and external measurements for each subject were used to calculate segment inertial properties by following Raichlen (33). Inverse dynamics were then used to calculate joint moments by following Winter (14), by using force and kinematic data. Joint moments were combined with published data on chimpanzee muscle moment arms (17) to calculate the opposing extensor muscle forces generated for each muscle group. The volume of muscle activated for each step was then calculated by following Roberts et al. (12), by using published muscle-fiber lengths (17).
Previous work (12) has shown that the mass-specific energy COL for terrestrial animals is a function of tc and Vmusc (cm3 N−1) activated to apply a unit of ground force, such that COL = k × (Vmusc/tc), where k is a constant relating oxygen consumption and force production (ml of O2 N−1). This relationship holds because the energy cost of terrestrial locomotion derives primarily from muscle forces generated to support bodyweight.
For comparison with humans, a similar dataset of locomotor cost, kinematics, and muscle activation was collected for a sample of four humans (one female and three males). Subjects were recreationally fit adults with no gait abnormalities and gave informed consent for this study. Human subjects committee approval was obtained before this study, and institutional guidelines were followed throughout. Methods for obtaining locomotor cost and kinetic data were identical to those used for chimpanzees, with the following exceptions: kinematics were measured via a high-speed infrared motion analysis system (Qualisys, Gothenburg, Sweden), data for muscle-fiber lengths and joint mechanical advantage were calculated by following Biewener et al. (20), and segment inertial properties were calculated by following Winter (14).
Supplementary Material
Acknowledgments
We thank D. E. Lieberman, A. A. Biewener, P. S. Rodman, H. M. McHenry, D. M. Bramble, and two anonymous reviewers for useful comments on earlier versions of this manuscript. A. A. Biewener, D. E. Lieberman, J. Jones, and P. Rodman generously provided necessary equipment. This project was supported by grants from the National Science Foundation (Grant BCS-0424092) and the L. S. B. Leakey Foundation.
Abbreviations
- COL
cost of locomotion (measured as the mass-specific rate of oxygen consumption, ml of O2 kg−1 s−1)
- GRF
ground reaction force
- tc
contact time (the duration of foot–ground contact for one step)
- Vmusc
volume of muscle activated per unit of ground force during locomotion.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703267104/DC1.
References
- 1.Darwin C. The Decent of Man, and Selection in Relation to Sex. London: John Murray; 1871. reprinted (1981) (Princeton Univ, Princeton) [Google Scholar]
- 2.Zollikofer CPE, Ponce de Leon MS, Lieberman DE, Guy F, Pilbeam D, Likius A, Mackaye HT, Vignaud P, Brunet M. Nature. 2005;434:755–759. doi: 10.1038/nature03397. [DOI] [PubMed] [Google Scholar]
- 3.Rodman PS, McHenry HM. Am J Phys Anthropol. 1980;52:103–106. doi: 10.1002/ajpa.1330520113. [DOI] [PubMed] [Google Scholar]
- 4.Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR. Nature. 1997;389:153–158. [Google Scholar]
- 5.Cerling TE, Ehleringer JR, Harris JM. Philos Trans R Soc London B. 1998;353:159–170. doi: 10.1098/rstb.1998.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor CR, Rowntree VJ. Science. 1973;179:186–187. doi: 10.1126/science.179.4069.186. [DOI] [PubMed] [Google Scholar]
- 7.Steudel-Numbers KL. J Hum Evol. 2003;44:255–262. doi: 10.1016/s0047-2484(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 8.Nakatsukasa M, Ogihara N, Hamada Y, Goto Y, Yamada M, Hirakawa T, Hirakasi E. Am J Phys Anthropol. 2004;124:248–256. doi: 10.1002/ajpa.10352. [DOI] [PubMed] [Google Scholar]
- 9.Hunt KD. Am J Phys Anthropol. 1992;87:83–105. doi: 10.1002/ajpa.1330870108. [DOI] [PubMed] [Google Scholar]
- 10.Thorpe SKS, Crompton RH, Wang WJ. Folia Primatol (Basel) 2004;75:253–265. doi: 10.1159/000078937. [DOI] [PubMed] [Google Scholar]
- 11.Kram R, Taylor CR. Nature. 1990;346:265–267. doi: 10.1038/346265a0. [DOI] [PubMed] [Google Scholar]
- 12.Roberts TJ, Chen MS, Taylor CR. J Exp Biol. 1998;201:2753–2762. doi: 10.1242/jeb.201.19.2753. [DOI] [PubMed] [Google Scholar]
- 13.Pontzer H. J Exp Biol. 2007;210:484–494. doi: 10.1242/jeb.02662. [DOI] [PubMed] [Google Scholar]
- 14.Winter DA. Biomechanics and Motor Control of Human Movement. New York: Wiley; 1990. [Google Scholar]
- 15.Taylor CR, Heglund NC, Maloiy GMO. J Exp Biol. 1982;97:1–21. doi: 10.1242/jeb.97.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Hoyt DF, Wickler SJ, Cogger EA. J Exp Biol. 2000;203:221–227. doi: 10.1242/jeb.203.2.221. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe SKS, Crompton RH, Gunther MM, Ker RF, Alexander RM. Am J Phys Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Carey TS, Crompton RH. J Hum Evol. 2005;48:25–44. doi: 10.1016/j.jhevol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Crompton RH, Li Y, Wang WJ, Gunther MM, Savage R. J Hum Evol. 1998;35:55–74. doi: 10.1006/jhev.1998.0222. [DOI] [PubMed] [Google Scholar]
- 20.Biewener AA, Farley CT, Roberts TJ, Temaner M. J Appl Physiol. 2004;97:2266–2274. doi: 10.1152/japplphysiol.00003.2004. [DOI] [PubMed] [Google Scholar]
- 21.Robinson JT. Early Hominid Posture and Locomotion. Chicago: Chicago Univ Press; 1972. [Google Scholar]
- 22.Fleagle J, Anapol FC. J Hum Evol. 1992;22:285–305. [Google Scholar]
- 23.Jungers WL. Nature. 1982;297:676–678. [Google Scholar]
- 24.McHenry HM. Am J Phys Anthropol. 1991;85:149–158. doi: 10.1002/ajpa.1330850204. [DOI] [PubMed] [Google Scholar]
- 25.Stern JT, Susman RL. Am J Phys Anthropol. 1983;60:279–317. doi: 10.1002/ajpa.1330600302. [DOI] [PubMed] [Google Scholar]
- 26.Susman RL, Stern JT, Jungers WL. Folia Primatol (Basel) 1984;43:113–156. doi: 10.1159/000156176. [DOI] [PubMed] [Google Scholar]
- 27.Ward CV. Yearb Phys Anthropol. 2002;45:185–215. doi: 10.1002/ajpa.10185. [DOI] [PubMed] [Google Scholar]
- 28.Washburn SL. Proc R Anthropol Inst Gr Br Ireland. 1967;3:21–27. [Google Scholar]
- 29.Pilbeam DR. Mol Phylogenet Evol. 1996;5:155–168. doi: 10.1006/mpev.1996.0010. [DOI] [PubMed] [Google Scholar]
- 30.Richmond BG, Strait DS. Nature. 2000;404:382–385. doi: 10.1038/35006045. [DOI] [PubMed] [Google Scholar]
- 31.Steudel KL. Evol Anthropol. 1994;3:42–48. [Google Scholar]
- 32.Leonard WR, Robertson ML. Curr Anthropol. 1997;38:304–309. [Google Scholar]
- 33.Raichlen DA. J Hum Evol. 2004;46:719–738. doi: 10.1016/j.jhevol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Alexander RM, Jayes AS. J Zool. 1983;201:135–152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.