Abstract
Recent studies have suggested that, in ES cells, inactive genes encoding early developmental regulators possess bivalent histone modification domains and are therefore poised for activation. However, bivalent domains were not observed at typical tissue-specific genes. Here, we show that windows of unmethylated CpG dinucleotides and putative pioneer factor interactions mark enhancers for at least some tissue-specific genes in ES cells. The unmethylated windows expand in cells that express the gene and contract, disappear, or remain unchanged in nonexpressing tissues. However, in ES cells, they do not always coincide with common histone modifications. Genomic footprinting and chromatin immunoprecipitation demonstrated that transcription factor binding underlies the unmethylated windows at enhancers for the Ptcra and Alb1 genes. After stable integration of premethylated Ptcra enhancer constructs into the ES cell genome, the unmethylated windows readily appeared. In contrast, the premethylated constructs remained fully methylated and silent after introduction into Ptcra-expressing thymocytes. These findings provide initial functional support for a model in which pioneer factor interactions in ES cells promote the assembly of a chromatin structure that is permissive for subsequent activation, and in which differentiated tissues lack the machinery required for gene activation when these ES cell marks are absent. The enhancer marks may therefore represent important features of the pluripotent state.
Keywords: chromatin, hematopoiesis
Epigenetic properties responsible for the pluripotency of ES cells are of interest because of the therapeutic potential of stem cell-derived tissues. Pluripotency is established during early embryogenesis because somatic cell nuclei can be converted to a pluripotent state upon injection into oocytes (1). An initial view was that epigenetic reprogramming involves broad demethylation of DNA during the earliest stages of embryogenesis, followed by extensive methylation (2–4). Studies using methylation-sensitive restriction enzymes contributed to a model in which methylation promotes the assembly of condensed silent chromatin at tissue-specific genes, with subsequent transcriptional activation requiring chromatin decondensation (2–4). Support for this model was provided by studies of the Alb1 (albumin) gene. FoxA1 appears to act as the pioneer factor at Alb1 by binding an Alb1 enhancer in definitive gut endoderm and initiating the events that culminate in transcription (5, 6).
In contrast to the above model, genome-wide analyses of histone modifications have suggested that, in ES cells, genes encoding regulators of early development are associated with bivalent chromatin domains that are poised for activation (7–10). In one model that is consistent with the accumulated data, genes encoding regulators of lineage commitment are poised in ES cells, but more typical tissue-specific genes are unmarked and assembled into silent chromatin structures as they await chromatin decondensation catalyzed by pioneer factors. Nevertheless, active histone modifications were found within the B-lineage-specific λ5 locus in ES cells, leading to the hypothesis that these modifications may be necessary for transcription competence (11, 12).
The current study began as an effort to understand the developmental regulation of a thymocyte-specific gene, Ptcra, with DNA methylation analyses used as one method for monitoring the epigenetic state of the locus. To our surprise, a selectively unmethylated window was found within a well studied thymocyte-specific enhancer in ES cells and hematopoietic stem cells (HSCs). An analysis of enhancers for other lineage-restricted genes suggested that the presence of unmethylated windows in ES cells is quite common and results from transcription factor binding. Most importantly, functional studies provided evidence that factor binding in ES cells may be essential for gene transcription in differentiated cell types.
Results and Discussion
DNA Methylation at the Ptcra Locus.
To study events that regulate a typical tissue-specific gene during embryogenesis, we used bisulfite sequencing to monitor DNA methylation at the mouse Ptcra locus. Ptcra, which encodes a subunit of the pre-T cell receptor complex, is expressed almost exclusively in immature thymocytes (13). The Ptcra locus is located between two constitutively expressed genes that contain cytidine phosphate guanosine (CpG)-island promoters. Between the CpG islands, the Ptcra enhancer and promoter are the only noncoding DNA regions that exhibit evolutionary conservation. Studies of transgenic mice have shown that these regions are sufficient for proper Ptcra regulation (13–16).
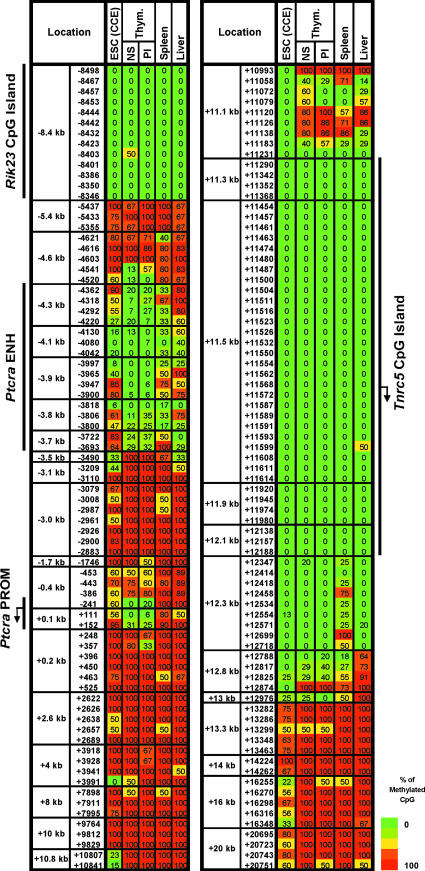
In primary mouse thymocytes, most CpGs within the Ptcra locus were heavily methylated. However, low methylation was observed at 18 CpGs within an 848-bp region spanning the Ptcra enhancer and at three CpGs close to the promoter (Fig. 1). Upon transcriptional inactivation after thymocyte maturation, the methylation pattern was unchanged.
Fig. 1.
Ptcra methylation in ES cells. Ptcra DNA methylation profiles are shown for the CCE ES cell line, nonstimulated (NS) and PMA/ionomycin-stimulated (PI) thymocytes, spleen, and liver cells. Methylation levels are represented in a gradation of colors: dark green (0–20%), light green (21–40%), yellow (41–60%), orange (61–80%), and red (81–100%). The same data with the ratio values are shown in SI Fig. 16.
Surprisingly, when the mouse CCE ES cell line was examined, a clear unmethylated window was observed at the Ptcra enhancer (Fig. 1), despite the absence of transcription [supporting information (SI) Fig. 6]. One of the unmethylated CpGs (−4,080) is within an Myb binding site shown to be critical for enhancer function (13, 14). As additional controls, DNA methylation was examined in total spleen and liver, revealing reduced methylation at the Ptcra enhancer in both tissues.
Ptcra Methylation in Hematopoietic and Nonhematopoietic Populations.
To characterize Ptcra methylation further, 16 additional cell populations were examined (Fig. 2), including an independent ES cell line (J1). In both ES cell lines, low methylation (40% or less) was found within the Ptcra enhancer at several CpGs. Although variability was observed, similar variability was observed within the CCE line in independent experiments (SI Fig. 7).
Fig. 2.
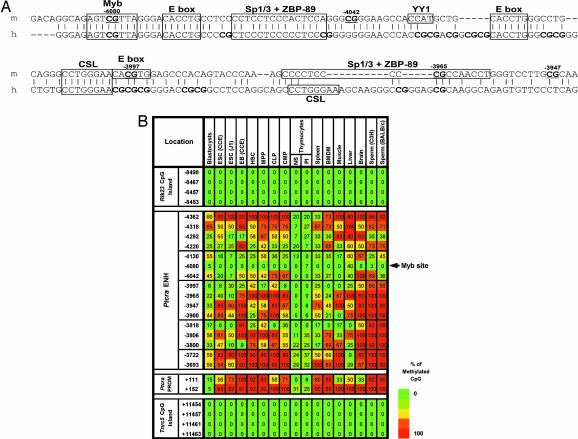
The Ptcra enhancer contains unmethylated windows in numerous tissues. (A) An alignment of the mouse (m) and human (h) Ptcra enhancer core sequences. Previously identified transcription factor binding sites are boxes, and CpG locations are indicated. (B) Methylation profiles for the Ptcra enhancer and promoter in various cells and tissues. Results from a minimum of two experiments are shown for blastocysts, CCE ES cells, thymocytes, BMDM, and sperm cells. Results obtained with the HSC, MPP, CLP, and CMP populations are also described in Attema et al. (40). The same data with the ratio values are shown in SI Fig. 17.
After differentiation of the CCE ES cells into embryoid bodies (EB) (17), methylation increased at some CpGs within the Ptcra enhancer, but others remained largely unmethylated. In primary blastocysts, low methylation was found at a subset of the CpGs that exhibited low methylation in the ES cell lines (Fig. 2B). Overall, methylation in blastocyst DNA was low, consistent with previous reports (2).
Methylation was also examined in purified HSCs (18). In HSCs, which do not express Ptcra (SI Fig. 6), low methylation (40% or less) was observed at several CpGs in the Ptcra enhancer (Fig. 2B). We also analyzed methylation in purified multipotent progenitors (18), common lymphoid progenitors (H. Karsunky and I.L.W., unpublished data, and ref. 19), and common myeloid progenitors (20) and observed profiles similar to the HSC profile. Finally, in bone marrow-derived macrophages (BMDM), muscle, brain, and sperm, methylation was absent at the CpG overlapping the Myb site (−4,080), with low methylation at a few other CpGs in most tissues. These results demonstrate that the tissue-specific Ptcra enhancer possesses an unmethylated window in ES cells, HSCs, and all other tissues examined.
DNA Methylation at an Inducible, Tissue-Specific Il12b Enhancer.
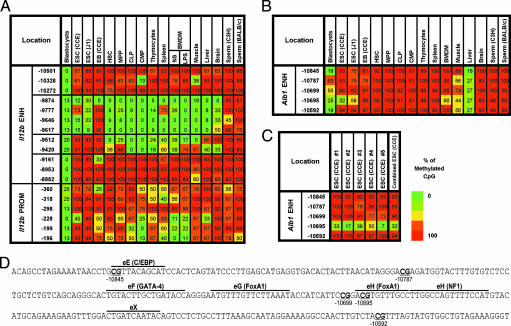
We next examined an enhancer for Il12b, which encodes the p40 subunit of IL-12 and IL-23 (21, 22). The Il12b enhancer coincides with a 545-bp conserved region 10 kb upstream of the start site (Fig. 3A; −9,437 to −9,981) (22). Il12b is expressed only in differentiated macrophages and dendritic cells after stimulation (SI Fig. 6) (21). In fact, the Il12b enhancer was discovered as a DNase I hypersensitive site detectable only in macrophages stimulated with LPS (22). Furthermore, ChIP experiments revealed that Oct-1, Oct-2, C/EBPβ, and BRG1 associate with the enhancer only after LPS stimulation (22, 23). Thus, the Il12b enhancer appears to possess an unperturbed chromatin structure in resting macrophages, with substantial alterations only after microbial stimulation.
Fig. 3.
The Il12b and Alb1 enhancers contain unmethylated windows. (A) Methylation of the Il12 enhancer and promoter in numerous cell types. Results are diagrammed as in Fig. 1. The ratio values are shown in SI Fig. 8. (B) CpG methylation in the 300-bp Alb1 enhancer in numerous cell types. (C) Results from five bisulfite sequencing experiments in CCE ES cells. The ratio values are shown in SI Fig. 9. (D) DNA sequence of the Alb1 enhancer (−10,566 to −10,865). Transcription factor binding sites are marked above the sequences and CpGs are underlined.
Despite the above, all six CpGs within the enhancer were selectively unmethylated in resting BMDM (Fig. 3A and SI Fig. 8; −9,420 through −9,874). Remarkably, in the two ES cell lines, most of these CpGs were also unmethylated (Fig. 3A). The three CpGs closest to the promoter were unmethylated in BMDM, suggesting that the gene is poised for activation. However, the promoter CpGs were mostly methylated in ES cells, consistent with the absence of Il12b expression.
DNA methylation at Il12b was examined in several other cell populations (Fig. 3A). The regions examined were largely unmethylated in total blastocysts, consistent with previous evidence of low overall methylation in blastocysts (2). It is not clear why methylation in blastocysts is lower at the Il12b locus than the Ptcra locus. In EB, the unmethylated window at the Il12b enhancer narrowed, reminiscent of the Ptcra results. The unmethylated window at the Il12b enhancer was also apparent in HSCs and the other populations examined, with brain exhibiting somewhat higher methylation than the other tissues. No CpGs in the Il12b enhancer were unmethylated in sperm.
An Unmethylated CpG at the Alb1 Enhancer.
We next examined the liver-specific Alb1 (albumin) enhancer (5, 6, 24). FoxA1 binding sites in this enhancer (Fig. 3D) are occupied in gut endoderm, leading to the hypothesis that FoxA1 acts as a pioneer transcription factor that promotes opening of the locus. However, the Alb1 enhancer had not been examined in ES cells.
Five CpGs spanning the Alb1 enhancer exhibited low methylation in liver, consistent with Alb1 expression in these cells (Fig. 3B and SI Fig. 6). The partial methylation probably reflects the fact that some cells in the liver preparation were not hepatocytes. Interestingly, in the two ES cell lines, one CpG in the Alb1 enhancer was extensively unmethylated. This CpG, at −10,695, is located within one of two FoxA1 binding sites (Fig. 3D). An examination of the ES cells used for the bisulfite sequencing analysis revealed that the cells uniformly expressed two markers of pluripotent ES cells, Oct-4 and SSEA-1, ruling out the possibility that the low methylation was due to spontaneous differentiation (SI Fig. 9). In addition, comparable results were obtained in five independent analyses of CCE ES cells (Fig. 3C). It is noteworthy that the methylation profile of this enhancer differs from those of the Ptcra and Il12b enhancers, because the Alb1 enhancer was fully methylated in most other tissues examined.
Protein–DNA Interactions at the Ptcra and Alb1 Enhancers.
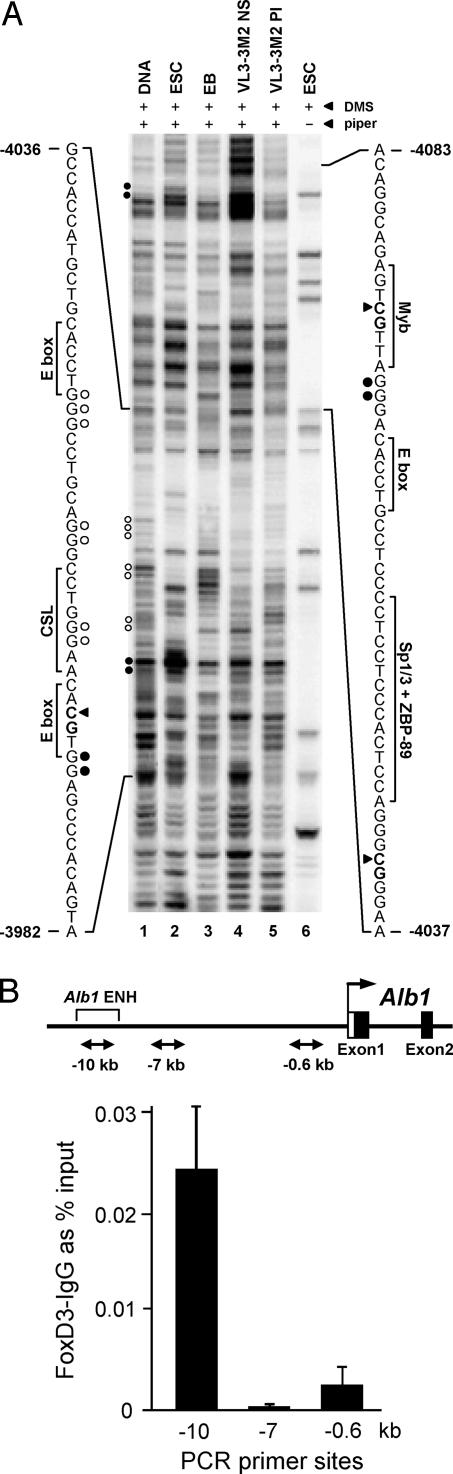
A likely explanation for the presence of the unmethylated windows is that sequence-specific DNA binding proteins occupy the tissue-specific enhancers in ES cells. Dimethyl sulfate genomic footprinting experiments with the Ptcra enhancer confirmed this prediction because clear regions of protection from modification were observed in ES cells, with a few nucleotides hypersensitive to modification (Fig. 4A; see also SI Fig. 10). The nucleotides that were most clearly protected in ES cells are in close proximity to two E boxes and a CSL site [CBF-1, Su(H), Lag-1] (15). These results demonstrate that proteins occupy the Ptcra enhancer in ES cells, although the identities of the proteins remain unknown.
Fig. 4.
Binding of transcription factors to the Ptcra and Alb1 enhancers in ES cells. (A) In vivo protein–DNA interactions at the Ptcra enhancer region. DMS footprinting was performed with purified CCE ES cell DNA (lane 1), CCE ES cells (lane 2), EB cells (lane 3), Ptcra+ VL3-3M2 thymocytes (lane 4), Ptcra− PMA/ionomycin-treated VL3-3M2 cells (lane 5), and CCE ES cells without piperidine cleavage of methylated DNA (lane 6). Nucleotides consistently protected from methylation in ES cells (open circles) and nucleotides exhibiting enhanced methylation (filled circles) are shown. Potential transcription factor binding sites are shown. Filled triangles mark the positions of CpGs (−3,997, −4,042, and −4,080). SI Fig. 10 shows the results of two additional experiments. (B) Binding of FoxD3 to the Alb1 enhancer in ES cells. ChIP assays were performed with FoxD3 antibodies. Convergent arrows beneath the Alb1 map indicate PCR priming sites. Real-time PCR signals from control IgG ChIPs were subtracted, and the data were expressed as percent input values. Standard deviations from two replicate experiments are shown.
At the Alb1 enhancer, the CpG that is unmethylated in ES cells coincides with a binding site for FoxA1 (5, 6). FoxA1 is not expressed in ES cells (data not shown), but bioinformatics studies suggested that another Fox family member, FoxD3, may bind the enhancer. Furthermore, previous studies had suggested that FoxD3 contributes to a pluripotency network (25–27). Indeed, ChIP experiments demonstrated that FoxD3 interacts with the Alb1 enhancer in ES cells (Fig. 4B). FoxD3 binding may therefore be responsible for the unmethylated window, and pioneer interactions with the Alb1 enhancer may first occur in pluripotent cells rather than committed endoderm.
Histone Modifications at the Ptcra and Il12b Enhancers.
To determine whether the unmethylated windows coincide with histone modifications, ChIP experiments were performed. Peaks of histone H3-K9 acetylation and histone H3-K4 di- and trimethylation were observed at the Ptcra enhancer in ES cells (SI Fig. 11), but these modifications were not observed at the Il12b or Alb1 enhancers (SI Fig. 12 and data not shown). Peaks of histone H3-K27 trimethylation were not observed at any of the enhancers in ES cells (SI Figs. 11 and 12), despite the successful use of these antibodies in positive control experiments (SI Fig. 13). These results suggest that the unmethylated windows in ES cells coincide with common histone modifications at some, but not all, enhancers.
Enhancer Marks in ES Cells May Be Essential for Subsequent Transcription.
The above results provide compelling evidence that at least some silent tissue-specific genes in ES cells are not assembled into chromatin structures that render them inaccessible to transcription factor binding. However, the functional significance of the marks remains unknown. The ES cell marks could be necessary for the subsequent expression of the genes, could help keep the genes silent in pluripotent cells, or could have no functional relevance.
To distinguish among these possibilities, we stably transfected Ptcra enhancer-promoter-GFP-insulator reporter plasmids into ES cells after premethylation with the SssI CpG methylase to eliminate the unmethylated windows (Fig. 5A and SI Fig. 14 A and D). The initial hope was that the plasmids would remain fully methylated, allowing us to differentiate the cells into thymocytes to determine whether the absence of the unmethylated window prevented transcription. However, analysis of seven independent stably transfected ES cell clones revealed that methylation was lost at CpGs within the enhancer (Fig. 5B and SI Fig. 14C). The loss of methylation was most efficient at −4,080, which exhibits the lowest methylation in a broad range of tissues. Methylation was not lost at two other regions of the plasmid (data not shown). These results suggest that transcription factors in ES cells can gain access to the premethylated enhancer and promote the loss of methylation by preventing maintenance methylation or by promoting active demethylation. This transition to the unmethylated state occurs even though the GFP reporter gene remains inactive (SI Fig. 15).
Fig. 5.
ES cells but not thymocytes promote the loss of methylation at a premethylated plasmid. This diagram provides a summary of the results in SI Fig. 14. (A) Methylation profile of the premethylated plasmid before transfection. Colors indicate methylation levels as described in the legend to Fig. 1. (B) Methylation profiles for five ES cell clones transfected with the unmethylated Ptcra enhancer-promoter-reporter plasmid and seven ES cell clones transfected with the premethylated plasmid. (C) Methylation profiles for eight VL3-3M2 clones transfected with the unmethylated plasmid and 20 VL3-3M2 clones transfected with the premethylated plasmid. (D) Methylation profiles for four EL4 clones transfected with the unmethylated plasmid and six EL4 clones transfected with the premethylated plasmid.
Although the loss of methylation prevented us from performing the experiment that was originally envisioned, a surprising result was obtained when the experiment was repeated in the Ptcra-expressing VL3-3M2 thymocyte line. Although GFP reporter activity was readily detected when the unmethylated plasmid was stably transfected into this line (SI Fig. 15), the premethylated plasmid was resistant both to transcription and loss of enhancer methylation (Fig. 5C and SI Fig. 15). That is, although pluripotent ES cells do not express the full complement of factors required for Ptcra transcription, they express the factors and/or machinery required for the efficient loss of methylation from a premethylated Ptcra enhancer. In contrast, differentiated thymocytes express the factors required for Ptcra transcription, but they lack factors or machinery required for activation of a reporter plasmid in which the enhancer mark has been eliminated by in vitro methylation. Efficient methylation remained intact in all VL3-3M2 clones from two transfection experiments and in all clones derived from transfection of a second thymocyte line (Fig. 5D; see also SI Fig. 14 G and H). These results provide initial evidence that the enhancer marks in ES cells may be essential for subsequent expression of tissue-specific genes; these marks may be necessary because differentiated tissues may lack specific factors or machinery needed to activate an unmarked enhancer.
Conclusions
Our results show that a feature of pluripotent ES cell lines is the existence of unmethylated windows within the enhancers of typical tissue-specific genes. The binding of transcription factors appears to be responsible for the unmethylated windows. A central unanswered question is whether the unmethylated windows and underlying protein–DNA interactions observed in ES cells are important for the proper regulation of tissue-specific genes. The protein–DNA interactions could be essential for subsequent transcription in differentiated tissues, or they could contribute to the silent state in ES cells. Alternatively, the marks may serve simply as diagnostic marks that could be useful for predicting the developmental potential of stem and progenitor cells.
The stable transfection results provide initial evidence that the enhancer marks may indeed be essential for subsequent transcription. ES cells, but not thymocytes, appear to possess factors or machinery required for the loss of methylation from a premethylated plasmid. The thymocytes may lack this capacity because the DNA binding proteins required for endogenous Ptcra transcription in thymocytes may differ from the enhancer binding proteins present in ES cells. Alternatively, protein complexes that modulate chromatin structure may be fundamentally different in pluripotent versus differentiated cells. We favor this latter possibility based on evidence that ES cell chromatin is generally less compact than chromatin in differentiated tissues (12).
Although the data support a model in which differentiated tissues may not be competent to activate a locus in the absence of preexisting enhancer marks, further studies are needed to establish the precise reason these marks exist. Previous studies have shown that differentiated cell lines are indeed capable of promoting the loss of methylation at some premethylated tissue-specific enhancers (28, 29). Therefore, premethylation does not always induce the formation of an activation-resistant chromatin structure in differentiated cells. One notable difference between our studies and the previous studies, however, was the existence of flanking β-globin insulator sequences in our plasmids, which may further restrict the loss of methylation. In the future, it will be necessary to devise a strategy that can erase enhancer marks at endogenous loci in differentiated tissues. At this time, we can only conclude that an enhancer capable of promoting the loss of DNA methylation in ES cells cannot promote the loss of methylation in cells that express the corresponding endogenous gene.
We must also consider the possibility that the unmethylated windows and underlying protein–DNA interactions at tissue-specific enhancers in ES cells help keep the genes silent. The histone modification results seem inconsistent with this hypothesis. However, it is noteworthy that the CSL site in the Ptcra enhancer appeared to be occupied in the genomic footprinting experiments. CSL proteins are repressors in the absence of Notch signaling and activators when associated with the Notch intracellular domain (15, 30). Although this finding raises the possibility that the interactions observed in ES cells contribute to repression, the histone acetylation observed at the Ptcra enhancer is inconsistent with this hypothesis.
Although several previous studies of DNA methylation in early embryos have been performed, the existence of unmethylated windows in enhancers for tissue-specific genes was not observed for multiple reasons. First, the earliest studies relied on methylation-sensitive restriction enzymes, which provided information about only one or two CpGs in the vicinity of a gene. Another limitation is that most previous studies focused on primary embryos rather than ES cell lines. An examination of primary ES cells derived from blastocysts, without in vitro expansion, would clearly be preferable to ES cell lines. However, previous studies of DNA methylation during embryogenesis focused on total blastocysts, although pluripotent cells within blastocysts are restricted to the inner cell mass and the number of pluripotent cells within the inner cell mass remains unknown. The extent to which the methylation properties of ES cell lines are representative of primary ES cells will therefore remain unknown until primary ES cells are purified in sufficient quantities for methylation analysis.
Finally, it is important to consider the possible relevance of these enhancer marks to the pluripotency of ES cell lines. If the unmethylated windows and protein–DNA interactions observed at the Ptcra, Il12b, and Alb1 enhancers are truly essential for transcription in differentiated cells, the factors responsible for these marks may be required for full ES cell pluripotency. Because different DNA binding proteins are likely to be associated with different tissue-specific enhancers in ES cells, a large number of DNA binding proteins may contribute to pluripotency, although loss of any one factor may preclude the subsequent expression of a relatively small number of tissue-specific genes.
Materials and Methods
Cells.
Thymocytes and tissues were isolated from C3H mice (4–8 weeks old) as described in ref. 31. The VL3-3M2 and EL4 thymocyte lines and J774 macrophage line were maintained as described in refs. 32 and 33. Thymocytes were stimulated with PMA (7.5 ng/ml) and ionomycin (180 ng/ml) for 16–18 h. BMDM were prepared as described in ref. 34. Mature sperm and blastocysts were collected by using standard methods. Hematopoietic progenitors were purified from C57BL/6 bone marrow cells as described (refs. 18–20; H. Karsunky and I.L.W., unpublished data).
CCE ES cell lines and J1 were maintained on gelatin-coated Petri dishes in DMEM (Invitrogen, Carlsbad, CA) with 15% FBS (Gemini Bio-Products, West Sacramento, CA), 150 μM monothioglycerol (MTG; Sigma–Aldrich, St. Louis, MO), and 1,000 units/ml leukemia inhibitory factor (Chemicon, Temecula, CA). ES cells were differentiated into EB for 6 days (17).
Bisulfite Sequencing.
DNA from preimplantation embryos was isolated as described in ref. 35. DNA from hematopoietic progenitor cells (118,000–460,000 cells) was isolated by using a modified SDS/proteinase K protocol (36). DNA from cultured ES cells, EB, and tissues was isolated by using the DNeasy tissue kit (Qiagen, Valencia, CA). Preimplantation embryos were treated with bisulfite as described in ref. 35. DNA from adult tissues or cultured cells was treated with bisulfite as described in refs. 37 and 38 with modest modifications (see SI Materials and Methods). PCR of the bisulfite-treated DNA was performed by using specific primers (see SI Materials and Methods).
Plasmids and Stable Transfection.
A 0.37-kb Ptcra core enhancer fragment and a 0.5-kb promoter fragment were subcloned into the NheI and BstBI sites of the reporter vector pd2EGFP (Clontech, Mountain View, CA), using PCR methods. A 1.2-kb chicken β-globin insulator was subcloned before the Ptcra enhancer and after the EGFP polyA site. Twenty-five to forty micrograms of the plasmid was linearized by XmnI digestion, followed by incubation with 40–100 units of SssI (CpG) methylase (New England BioLabs, Ipswich, MA). Incubation was carried out overnight at 37°C in 400 μl of NEBuffer 2 (50 mM NaCl/10 mM Tris·HCl, pH 7.9/10 mM MgCl2/1 mM DTT) and 160 μM S-adenosylmethionine, which was added every 4 h. The methylated DNA was extracted with phenol/chloroform, precipitated with ethanol, and dissolved in 50 mM Tris·HCl (pH 7.4). Efficient methylation was confirmed by bisulfite sequencing.
Linearized unmethylated or methylated plasmid (25–40 μg), together with 2 μg of a vector containing a neomycin-resistant gene (pQCIXN; Clontech), was used for each stable transfection. CCE ES cells were electroporated by using a Bio-Rad (Hercules, CA) GenePulser II at 500 μFD, 0.24 kV. ES cell stable clones were selected in G418 (150 μg/ml) for 7–10 days; 20–40 colonies were transferred to 24-well plates for further culture. Positive clones containing the transgenes were verified by PCR and Southern blot. For stable transfection of VL3-3M2 and EL4 cells, 107 cells were electroporated by using a pulse of 960 μFD, 0.27 kV. After 48 h, cells were selected with puromycin (1 μg/ml), expanded, and screened as above.
Genomic Footprinting and ChIP.
Footprinting experiments were performed as described in ref. 39 with minor modifications (see SI Materials and Methods). ChIP of the Alb1 locus was performed by quantitative real-time PCR as described in the legend to SI Fig. 11, using FoxD3 antibody (Chemicon; catalog no. AB5687) and an IgG control (Upstate Biotechnology, Lake Placid, NY; catalog no. 12-370).
Supplementary Material
Acknowledgments
We thank Meisheng Jiang for isolation of blastocysts; Joseph Chan, Abe Chang, Kevin Doty, Wiam Turki-Judeh, and Wei Wu for technical assistance; Guoping Fan, Steve Jacobsen, and Boris Reizis for valuable discussions; and Caiyi Li, Rupa Sridharan, and Hilde Schjerven for critical reading of the manuscript. This work was supported by National Institutes of Health Grants P01 DK53074 (to I.L.W.), R01 GM47903 (to K.S.Z.), and T32-AI07323 (to S.D.P.); a Mathers Foundation grant (to K.S.Z.); a California Institute of Regenerative Medicine Postdoctoral Fellowship (to J.L.A.); a C. J. Martin Postdoctoral Fellowship (to P.P.); and National Institutes of Health and National Research Service Award GM066367-05 (to J.A.W.). S.T.S. is a Howard Hughes Medical Institute Investigator.
Abbreviations
- BMDM
bone marrow-derived macrophages
- EB
embryoid bodies
- HSC
hematopoietic stem cell.
Footnotes
Conflict of interest statement: I.L.W. was a member of the scientific advisory board of Amgen, cofounded and is a director of Stem Cells, Inc., and cofounded Cellerant, Inc.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704579104/DC1.
References
- 1.Hochedlinger K, Jaenisch R. Nature. 2006;441:1061–1067. doi: 10.1038/nature04955. [DOI] [PubMed] [Google Scholar]
- 2.Kafri T, Ariel M, Brandeis M, Shemer R, Urven L, McCarrey J, Cedar H, Razin A. Genes Dev. 1992;6:705–714. doi: 10.1101/gad.6.5.705. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Takai D. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 4.Reik W, Dean W, Walter J. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 5.Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- 6.Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Mol Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 7.Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, Fisher AG. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 10.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono KI, et al. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szutorisz H, Canzonetta C, Georgiou A, Chow CM, Tora L, Dillon N. Mol Cell Biol. 2005;25:1804–1820. doi: 10.1128/MCB.25.5.1804-1820.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meshorer E, Misteli T. Nat Rev Mol Cell Biol. 2006;7:540–546. doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- 13.Reizis B, Leder P. J Exp Med. 1999;189:1669–1678. doi: 10.1084/jem.189.10.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reizis B, Leder P. J Exp Med. 2001;194:979–990. doi: 10.1084/jem.194.7.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reizis B, Leder P. Genes Dev. 2002;16:295–300. doi: 10.1101/gad.960702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gounari F, Aifantis I, Martin C, Fehling HJ, Hoeflinger S, Leder P, von Boehmer H, Reizis B. Nat Immunol. 2002;3:489–496. doi: 10.1038/ni778. [DOI] [PubMed] [Google Scholar]
- 17.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen JL, Weissman IL. Proc Natl Acad Sci. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo M, Weissman IL, Akashi K. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 20.Akashi K, Traver D, Miyamoto T, Weissman IL. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri G. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Nazarian AA, Xu J, Tantin D, Corcoran LM, Smale ST. Mol Cell Biol. 2007;27:2698–2712. doi: 10.1128/MCB.00788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaya D, Hayamizu T, Bustin M, Zaret KS. J Biol Chem. 2001;276:44385–44389. doi: 10.1074/jbc.M108214200. [DOI] [PubMed] [Google Scholar]
- 25.Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, Fletcher CF, Jenkins NA, Copeland NG, Klemsz M, Hromas R. J Biol Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- 26.Tompers DM, Foreman RK, Wang Q, Kumanova M, Labosky PA. Dev Biol. 2005;285:126–137. doi: 10.1016/j.ydbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Pan G, Li J, Zhou Y, Zheng H, Pei D. FASEB J. 2006;20:1730–1732. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
- 28.Kirillov A, Kistler B, Mostoslavsky R, Cedar H, Wirth T, Bergman Y. Nat Genet. 1996;13:435–441. doi: 10.1038/ng0895-435. [DOI] [PubMed] [Google Scholar]
- 29.Forrester WC, Fernandez LA, Grosschedl R. Genes Dev. 1999;13:3003–3014. doi: 10.1101/gad.13.22.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai EC. EMBO Rep. 2002;3:840–845. doi: 10.1093/embo-reports/kvf170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su RC, Brown KE, Saaber S, Fisher AG, Merkenschlager M, Smale ST. Nat Genet. 2004;36:502–506. doi: 10.1038/ng1351. [DOI] [PubMed] [Google Scholar]
- 32.Groves T, Katis P, Madden Z, Manickam K, Ramsden D, Wu G, Guidos CJ. J Immunol. 1995;154:5011–5022. [PubMed] [Google Scholar]
- 33.Weinmann AS, Plevy SE, Smale ST. Immunity. 1999;11:665–675. doi: 10.1016/s1074-7613(00)80141-7. [DOI] [PubMed] [Google Scholar]
- 34.Sanjabi S, Williams KJ, Saccani S, Zhou L, Hoffmann A, Ghosh G, Gerondakis S, Natoli G, Smale ST. Genes Dev. 2005;19:2138–2151. doi: 10.1101/gad.1329805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millar DS, Warnecke PM, Melki JR, Clark SJ. Methods. 2002;27:108–113. doi: 10.1016/s1046-2023(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 36.Warnecke PM, Mann JR, Frommer M, Clark SJ. Genomics. 1998;51:182–190. doi: 10.1006/geno.1998.5371. [DOI] [PubMed] [Google Scholar]
- 37.Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. Proc Natl Acad Sci. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark SJ, Harrison J, Paul CL, Frommer M. Nucleic Acids Res. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carey M, Smale ST. Transcriptional Regulation in Eukaryotes: Concepts, Strategies, and Techniques. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2000. [Google Scholar]
- 40.Attema JL, Papathanasiou P, Forsberg EC, Xu J, Smale ST, Weissman IL. Proc Natl Acad Sci USA. 2007;104:12371–12376. doi: 10.1073/pnas.0704468104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.