Abstract
Mycobacterium bovis bacille Calmette–Guérin (BCG) is the most widely used live bacterial vaccine. However, limited information is available correlating route and dose of vaccination and induction of specific T cell responses with protection against tuberculosis. We compared efficacy of oral and systemic vaccination and correlated vaccine-induced T cell responses with protection in experimental tuberculosis of mice. After oral and systemic vaccination, we observed profound differences in persistence and dissemination of BCG and frequencies and location of specific IFN-γ-secreting CD4+ and CD8+ T cells. Yet, both vaccination routes caused comparable levels of protection against aerosol challenge with Mycobacterium tuberculosis. Protection correlated best with rapid accumulation of specific CD8+ T cells in infected tissues of challenged mice. In contrast, specific IFN-γ production by CD4+ T cells reflected the load of M. tuberculosis rather than the strength of protection. Our data question the measurement of IFN-γ secretion by CD4+ T cells and emphasize the need for new biomarkers for evaluation of tuberculosis vaccine efficacies.
Keywords: Mycobacterium tuberculosis, protective immunity
Tuberculosis causes high morbidity and mortality and is responsible for 2 million deaths annually (1). The only vaccine available is Mycobacterium bovis bacille Calmette-Guérin (BCG), an attenuated strain of M. bovis (2). BCG is widely used, with 4 billion doses administered so far, and is valued as a safe vaccine, which can be administered directly after birth (3). BCG was developed by Calmette and Guérin originally as an oral vaccine. Gradually, the oral route was replaced by intradermal administration. Oral vaccines target the mucosal immune system directly, thus potentially improving protection to aerogenic infection. Moreover, they are needle-free and thus safer and cheaper. BCG prevents severe forms of childhood tuberculosis only and fails to protect against pulmonary tuberculosis in adults. Accordingly, recent years have witnessed attempts to develop new vaccine candidates, many of which have entered clinical trials. Despite extensive use of BCG as a vaccine, persistence and dissemination of mycobacteria after different routes of application and induction of specific immunity at the mucosal and the systemic level are still ill defined.
Protection against Mycobacterium tuberculosis is dominated by CD4+ and CD8+ T cells (4). T cells restrict exponential growth of M. tuberculosis and are responsible for establishment of a plateau level of bacterial load in infected tissues, which usually develops 3–4 weeks after infection and remains at a constant magnitude for months (5). CD4+ T cells are involved in all phases of the acquired immune response and are pivotal for control of M. tuberculosis during the plateau phase. In the absence of CD4+ T cells, M. tuberculosis maintains exponential growth without formation of a stationary phase. Bacterial replication exacerbates pathology and reduces survival time (5–7). The majority of specific CD4+ T cells are Th1 cells, and IFN-γ and TNF-α produced by these cells are considered central for protection (5, 6). In the absence of CD8+ T cells, mice still restrict growth of M. tuberculosis and generate a plateau phase of infection yet at a 10-fold-higher mycobacterial load (5, 8, 9). Protective mechanisms mediated by CD8+ T cells include cytotoxicity against infected cells as well as production of Th1 cytokines such as TNF-α and IFN-γ (10, 11).
Vaccination-induced protection against M. tuberculosis relies on the generation of specific T cells, and current and future vaccination regimes aim at the generation of high frequencies of specific CD4+ Th1 and CD8+ T cells (4). After challenge infection, the secondary immune response in vaccinated mice or mice previously cured of M. tuberculosis infection is characterized by accelerated accumulation of effector T cells in infected tissues and early production of Th1 cytokines (12–18). Consequently, mycobacterial growth is restricted more efficiently in the initial phase of infection, and growth reaches a plateau at a 10-fold-lower level as compared with nonvaccinated animals. The mechanisms responsible for early T cell-mediated restriction of bacterial growth probably involve Th1 cytokines and chemokines as well as thus far unknown mechanisms. Determination of frequencies of mycobacteria-specific T cells secreting Th1 cytokines, in particular IFN-γ, is therefore widely used as an indicator for vaccine efficacy. However, recent studies failed to reveal a direct correlation between frequencies of IFN-γ secreting T cells and the degree of protection against M. tuberculosis (19). Thus, the IFN-γ readout as a sole correlate of protection requires critical reevaluation. This issue gains particular relevance with several novel vaccine candidates entering phase II trials soon. For these trials, reliable biomarkers that indicate immunogenicity of tuberculosis vaccine candidates are urgently needed (4).
We used experimental infection of mice to determine the efficacy of oral and systemic vaccination. We determined persistence and dissemination of BCG after i.v. or intragastric (i.g.) administration and compared the degree of vaccine-induced protection against subsequent aerosol challenge with M. tuberculosis. In parallel, frequencies of specific IFN-γ secreting CD4+ and CD8+ T cells were analyzed during vaccination and challenge and correlated with dissemination of BCG as well as strength of protection against tuberculosis.
Results
Persistence and Dissemination of BCG After Systemic and Oral Vaccination.
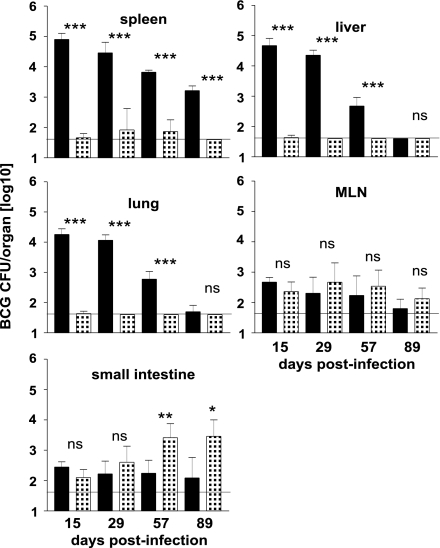
Mice received 106 BCG organisms via i.v. injection or 109 BCG organisms via i.g. gavage, and BCG titers were determined in spleen, liver, lung, mesenteric lymph nodes (MLN), and small intestine (Fig. 1). Systemic vaccination caused a high mycobacterial load in spleen, liver, and lung. In these organs, titers slowly declined, but mycobacteria were still detectable in spleen 3 months after infection. In MLN and small intestine, few mycobacteria were detected throughout the experiment. After oral vaccination, low numbers of mycobacteria were present in small intestine and MLN at all time points of analysis. In small intestine, titers increased 2 months after vaccination. Mycobacteria were infrequently detected in spleen, liver, and lung, indicating that BCG was efficiently contained in the intestine and intestine-associated lymphoid tissues and only sporadically disseminated to distant organs.
Fig. 1.
Dissemination and persistence of BCG after different routes of vaccination. C57BL/6 mice were vaccinated with 106 or 109 BCG organisms via the i.v. (black bars) or the i.g. route (patterned bars), respectively. Titers were determined in spleen, liver, lung, MLN, and small intestine (tissue plus luminal content). Bars represent means ± SD of titers of five individually analyzed animals per group and time point. The horizontal line indicates the limit of detection (40 CFU per tissue). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05.
Induction of Specific CD4+ T Cell Responses After Systemic and Oral Vaccination.
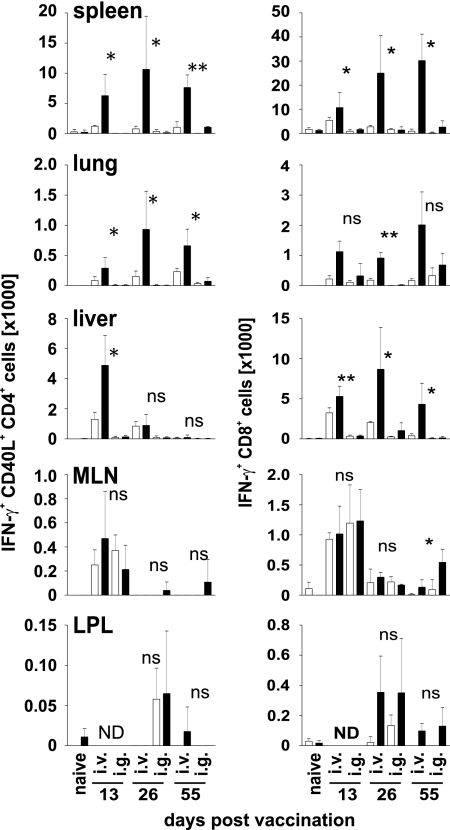
Lymphocytes were isolated from different tissues and incubated for 5 h with a combination of the peptides Ag85A241–260 and Ag85B240–260 or with Mtb32309–318 to stimulate mycobacteria-specific CD4+ and CD8+ T cells, respectively (13, 20). T cell responses were determined by intracellular IFN-γ staining. In samples with high mycobacterial titers, we regularly observed high frequencies of spontaneous IFN-γ+ T cells, particularly among CD4+ T cells. Therefore, cells were intracellularly stained for CD40L/CD154, in addition. After TCR stimulation, CD4+ T cells transiently up-regulate expression of CD40L (21). CD40L expression is therefore highly indicative for peptide-specific CD4+ T cell stimulation during the 5 h of incubation. Representative results for IFN-γ and CD40L expression of CD4+ and CD8+ T cells after peptide stimulation are shown in supporting information (SI) Fig. 6.
After systemic vaccination, high frequencies of CD4+ T cells spontaneously producing IFN-γ were isolated from spleen, liver, and lung at 2 and 4 weeks after vaccination (Fig. 2 and SI Fig. 7). Ag85A241–260- and Ag85B240–260-specific CD4+ T cells, as indicated by IFN-γ and CD40L costaining after peptide stimulation, were observed in spleen and lung at all three time points. In these tissues, CD4+ T cell responses peaked 4 weeks after systemic vaccination. In the liver, the peak of response was already seen after 2 weeks. In the intestinal tract, T cell responses were determined in MLN. In other mouse infection models, T cells primed in gut-associated lymphoid tissues rapidly accumulate in the intestinal mucosa, leading to high frequencies of effector and memory T cells in the small intestine lamina propria (22, 23). In both MLN and lamina propria, only marginal frequencies of IFN-γ secreting CD4+ T cells were detected after systemic vaccination. We observed a small increase in spontaneous IFN-γ secretion in MLN and lamina propria but did not identify Ag85A241–260- and Ag85B240–260-specific CD4+ T cells at levels significantly above background.
Fig. 2.
Specific T cell responses in BCG-vaccinated mice. C57BL/6 mice were i.v. or i.g. vaccinated, and cells isolated from indicated tissues were restimulated in vitro with Ag85A241–260 and Ag85B240–260 or with Mtb32309–318 to stimulate mycobacteria-specific CD4+ and CD8+ T cells, respectively. Cells were analyzed as described in Materials and Methods. Graphs show total numbers of IFN-γ+ CD40L+ CD4+ T cells (Left) or total numbers of IFN-γ+ CD8+ T cells (Right) isolated from different tissues and days after infection, as indicated. Age-matched naïve mice were included in the analyses at each time point. Because we never detected mycobacteria-specific T cell responses in naïve mice, only one representative set of data is shown (indicated as naïve). Open and filled bars indicate incubation without or with peptide, respectively. Bars give mean ± SD for cells from three individually analyzed mice. ND, not determined. *, P < 0.05; **, P < 0.01; ns, P > 0.05.
After oral vaccination, spontaneous as well as Ag85A241–260- and Ag85B240–260-specific IFN-γ production by CD4+ T cells was marginal in spleen, liver, and lung (Fig. 2 and SI Fig. 7). There was a low level of spontaneous IFN-γ production in the MLN, and at 2 months after oral vaccination, a small number of Ag85A241–260- and Ag85B240–260-specific CD4+ T cells were detected in this tissue. Similar to systemic vaccination, specific CD4+ T cells exceeding background levels were not identified in the lamina propria of orally vaccinated mice.
Independent from the route of vaccination, tissue, and time point of analysis, we never detected an increase of the CD40L+ IFN-γ- T cell population after peptide restimulation of CD4+ T cells, indicating that Ag85A241–260- and Ag85B240–260-specific CD4+ T cells induced by vaccination were largely IFN-γ secreting Th1 cells (data not shown).
Induction of Specific CD8+ T Cell Responses After Systemic and Oral Vaccination.
After systemic vaccination, high total numbers and frequencies of Mtb32309–318-specific CD8+ T cells were detected in spleen, liver, and lung (Fig. 2 and SI Fig. 8). Specific CD8+ T cells were detectable 2 weeks after vaccination and remained at high levels over the next months. We observed some spontaneous IFN-γ production by CD8+ T cells in the MLN and only marginal frequencies of Mtb32309–318-specific CD8+ T cells. However, we consistently identified Mtb32309–318-specific CD8+ T cells in the lamina propria after systemic vaccination, although frequencies varied between individual mice and accounted for only a minute number of cells as compared with cell numbers identified in spleen, liver, or lung.
After oral vaccination, marginal frequencies and total numbers of Mtb32309–318-specific CD8+ T cells were detected in spleen and liver. In lungs, these cells formed a distinct population, although at reduced frequencies and numbers as compared with lungs after systemic vaccination. In MLN, we observed some spontaneous IFN-γ production by CD8+ T cells, and only 2 months after oral vaccination, significant numbers of Mtb32309–318-specific CD8+ T cells were detected. Similar to systemic vaccination, we consistently detected a low number of Mtb32309–318-specific CD8+ T cells in the lamina propria after oral BCG vaccination.
Vaccination-Induced Protection Against M. tuberculosis.
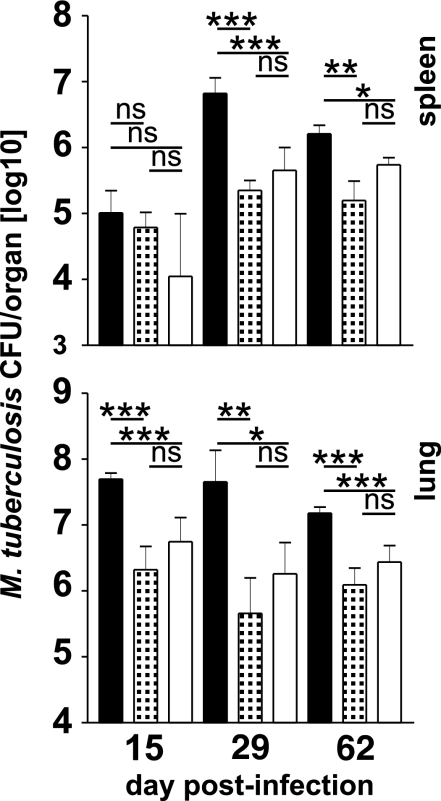
Vaccinated mice were challenged with M. tuberculosis by low-dose aerosol infection 3 months after vaccination. Mycobacterial titers in spleens and lungs were determined 2, 4, and 9 weeks later (Fig. 3). In lungs of both groups of vaccinated mice, the mycobacterial load was significantly reduced at all time points as compared with nonvaccinated controls, but protection was independent from vaccination routes. In spleens, the M. tuberculosis load was slightly reduced in vaccinated mice 2 weeks after challenge. One month after challenge, mice of both vaccinated groups were significantly protected, and protection in spleens decreased after 2 months. Again, titers in spleens did not differ significantly in mice after systemic or oral vaccination.
Fig. 3.
Protection against tuberculosis in BCG-vaccinated mice. C57BL6 were vaccinated with BCG via the i.v. (patterned bars) or the i.g. (white bars) route as described in the legend of Fig. 1. After 99 days, vaccinated and age-matched naïve mice (black bars) were aerosol infected with ≈200 CFU of M. tuberculosis. After 15, 29, and 62 days, mycobacterial titers in spleen and lung of animals were determined. Bars represent mean ± SD of titers of five individually analyzed animals per group and time point. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05.
Specific CD4+ T Cell Responses in BCG-Vaccinated and M. tuberculosis-Challenged Mice.
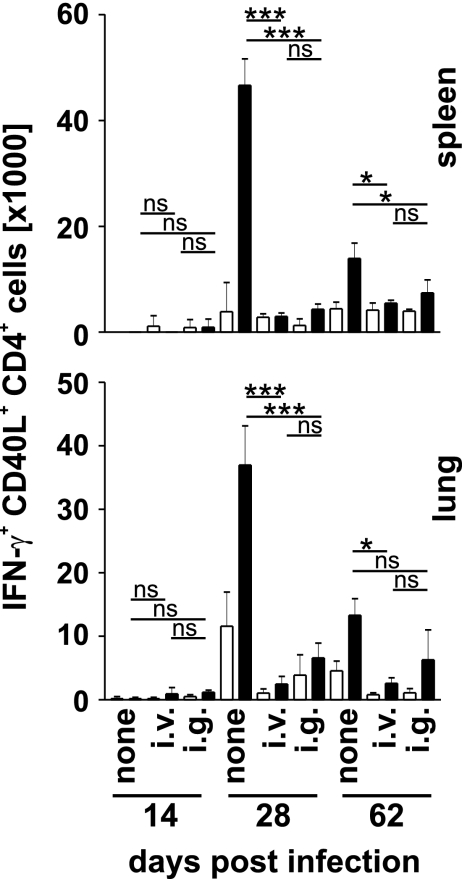
Two, 4, and 9 weeks after M. tuberculosis challenge, specific CD4+ and CD8+ T cell responses were determined in lungs and spleens by peptide restimulation and intracellular IFN-γ and CD40L staining (SI Fig. 9). Two weeks after challenge, background frequencies of Ag85A241–260- and Ag85B240–260-specific CD4+ T cells were detected in spleens and lungs of vaccinated and nonvaccinated mice, and spontaneous IFN-γ production in these tissues was marginal (Fig. 4 and SI Fig. 10). One and 2 months after infection, specific CD4+ T cell responses were detected in lungs and spleens of vaccinated and nonvaccinated mice. Compared with vaccinated mice, nonvaccinated controls showed considerably stronger specific CD4+ T responses and a higher level of spontaneous IFN-γ production. In nonvaccinated mice, CD4+ T cell responses peaked after 1 month and then declined, whereas responses in vaccinated mice remained at a similar level as long as 2 months after challenge. Overall, frequencies of Ag85-specific IFN-γ-secreting CD4+ T cells correlated with bacterial load rather than with degree of protection, and oral and systemic vaccination caused similar specific responses to M. tuberculosis-challenge.
Fig. 4.
Specific CD4+ T cell responses in BCG-vaccinated and M. tuberculosis-challenged mice. C57BL/6 mice were treated and analyzed as described in the legends of Figs. 2 and 4. Graphs show results total numbers of Ag85A241–260- and Ag85B240–260-specific IFN-γ+ CD40L+ CD4+ T cells in spleen and lung at the indicated day after M. tuberculosis infection. Open and filled bars indicate incubation without and with peptide, respectively. Bars give mean ± SD for cells from three individually analyzed mice. *, P < 0.05; ***, P < 0.001; ns, P > 0.05.
Specific CD8+ T Cell Responses in BCG-Vaccinated and M. tuberculosis-Challenged Mice.
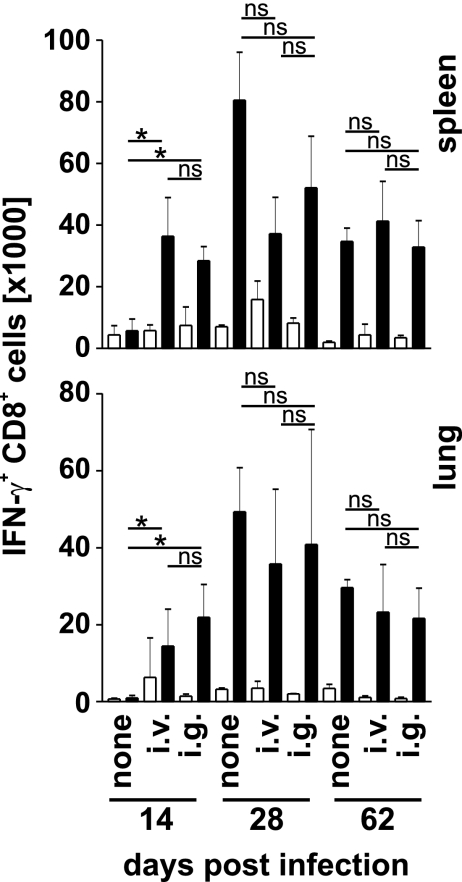
Two weeks after infection, similar frequencies and numbers of Mtb32309–318-specific CD8+ T cells were detected in spleens and lungs from i.v.- and i.g.-vaccinated mice (Fig. 5 and SI Figs. 9 and 11). In contrast, we did not identify specific CD8+ T cells in tissues of nonvaccinated mice. After 1 and 2 months, comparable frequencies and numbers of these cells were observed in spleens and lungs of all vaccinated and nonvaccinated mice. Thus, in contrast to the CD4+ T cell response, frequencies and absolute numbers of specific CD8+T cells were largely independent from the severity of disease, and early accumulation of specific CD8+ T cells and protection were correlated.
Fig. 5.
Specific CD8+ T cell responses in BCG-vaccinated and M. tuberculosis-challenged mice. C57BL/6 mice were treated and analyzed as described in the legends of Figs. 2 and 4. Graphs show results total numbers of Mtb32309–3180-specific IFN-γ+ CD8+ T cells in spleen and lung at the indicated day after M. tuberculosis infection. Open and filled bars indicate incubation without and with peptide, respectively. Bars give mean ± SD for cells from three individually analyzed mice. *, P < 0.05; ns, P > 0.05.
Discussion
After systemic vaccination, BCG persistently infected spleen, liver, and lung at relatively high levels, whereas low numbers of BCG were found in MLN and small intestinal tissue. In contrast, after oral vaccination, BCG microorganisms were consistently detected in MLN and small intestine but only rarely disseminated into deeper tissues. Thus, the intestinal mucosa and the associated lymphoid tissues formed a tight barrier for orally administered mycobacteria. Similar results have been reported by Lagranderie et al. (24). Shortly after oral vaccination with BCG, but not at later time points, minute numbers of mycobacteria were detected in Peyer's patches. BCG later appeared in the MLN but failed to disseminate any further (24). Compared with the peak of infection after systemic BCG vaccination, orally vaccinated mice contained a significantly lower total load of BCG. Although a reservoir in tissues not analyzed cannot be excluded formally, our results indicate that, at least in the first 2 months, orally vaccinated mice contained 100- to 1,000-fold less BCG than systemically vaccinated mice. Despite limited colonization and dissemination, oral vaccination induced similar protection against subsequent M. tuberculosis infection. Hence, there was no direct correlation between localization and extent of BCG infection and level of vaccine-induced protection. This situation is similar when other routes of BCG vaccination are compared. In different studies, i.v., s.c., aerosol, or rectal BCG application caused similar protection against M. tuberculosis (24–26). In line with these results is the observation that systemic vaccination with BCG using a wide range of inocula generates similar levels of protection against tuberculosis (27–29). Finally, intradermal vaccination of guinea pigs with as few as 10 BCG microorganisms induced similar protection to that of a high dose (106 BCG microorganisms) vaccination, despite marginal dissemination (30). Thus, it appears that dose and route of application and consequently the dissemination and tissue localization of BCG have minor impact on protective immunity against tuberculosis. Our results also advocate attempts to develop oral vaccine candidates. Oral application forms for BCG vaccines should combine equal or higher vaccine efficacy with a high safety level because of the limited dissemination. However, in contrast to previous application of BCG in liquid, which sometimes led to colonization of the lymphatic ring surrounding the upper respiratory tract, novel vaccine formulations should be applied as encapsulated preparations.
High levels of CD4+ T cells spontaneously secreting IFN-γ were detected in spleen, liver, and lung after systemic vaccination. Because these tissues contained large numbers of BCG microorganisms, profound IFN-γ production could simply reflect contact of CD4+ T cells with infected cells. However, these IFN-γ+ cells failed to express CD40L. It is therefore possible that IFN-γ secretion was, at least in part, induced by TCR-independent mechanisms. Inflammatory cytokines such as IL-12 and IL-18 can induce TCR-independent IFN-γ production in conventional effector and memory T cells as well as in natural killer T cells (31–33). Because inflammatory cytokines should be abundant in heavily infected tissues, such a scenario is likely, and it would be interesting to determine the extent to which TCR-independent IFN-γ production by T cells contributes to protective immunity against tuberculosis.
Staining of T cells for IFN-γ and CD40L allowed identification of Ag85-specific CD4+ T cells even in samples with high levels of “unspecific” IFN-γ production. We detected specific CD4+ T cells in spleen, liver, and lung but not in MLN and intestinal mucosa after systemic vaccination. In contrast, frequencies of Ag85-specific CD4+ T cells were at or below detection limits in all tissues after oral vaccination. CD8+ T cells do not express CD40L after TCR stimulation. However, frequencies of IFN-γ+ CD8+ T cells after incubation without peptide were generally lower than those observed for CD4+ T cells, allowing identification of peptide-specific T cells even without additional markers. For the immunodominant peptide Mtb32309–318, profound CD8+ T cell responses were detected in spleen, liver, and lung and weak responses in intestinal mucosa after systemic vaccination. We identified CD8+ T cells specific for this peptide in lungs and small intestine after oral vaccination.
After aerosol challenge with M. tuberculosis, Mtb32309–318-specific CD8+ T cells rapidly accumulated in spleens and lungs of mice from both vaccinated groups, consistent with marked expansion of specific CD8+ T cells already present in lung and recruitment of further CD8+ T cells from mucosal sites to spleen and lung. Rapid accumulation of specific IFN-γ-secreting CD8+ T cells is in line with previous observations of accelerated accumulation of CD8+ T cells and early IFN-γ production in tissues of vaccinated or infected and cured mice (13–15, 18). At later time points after challenge, nonvaccinated mice showed similar numbers of specific IFN-γ secreting CD8+ T cells when compared with vaccinated mice. Thus, accelerated CD8+ T cell accumulation rather than the magnitude of CD8+ T cell responses at the plateau phase of M. tuberculosis infection correlated with vaccine-induced protection.
In contrast to CD8+ T cells, we observed only marginal accumulation of Ag85A241–260 and Ag85B240–260-specific CD4+ T cells in lung and spleen of vaccinated mice, 2 weeks after M. tuberculosis challenge, and this was also the case after systemic vaccination, where these cells were clearly induced by vaccination. At the plateau phase of M. tuberculosis infection, frequencies of Ag85-specific IFN-γ secreting CD4+ T cells correlated with mycobacterial load in tissues rather than with protection. This lack of correlation of specific CD4+ T cells with protection was unexpected, because CD4+ T cell responses, in particular, IFN-γ secretion, are considered pivotal for control of tuberculosis (14).
By simply correlating specific T cell responses after vaccination and challenge with protection, it could be argued that induction of CD8+ T cells during vaccination, particularly in intestine and lung, is decisive for the quality of vaccination and that vaccination-induced CD4+ T cells play only a minor role. However, limitations in the analysis of CD4+ T cells need to be considered. Although we used two CD4+ T cell epitopes, Ag85A241–260 and Ag85B240–260, the combined response was still weaker than that observed for the CD8+ T cell response against Mtb32309–318. Thus, we could miss Ag85-specific CD4+ T cells during vaccination because of detection limits of our assay. However, our assay should be sufficiently sensitive to detect early accumulation of these T cells after M. tuberculosis infection even at moderate levels, which was not the case even after systemic vaccination. Another possible limitation could be the nature of the antigen used to detect specific CD4+ T cell responses. Ag85A and Ag85B could have a restricted expression window during M. tuberculosis infection, with limited expression in the early phase of infection. The strong response observed at 4 weeks after infection is difficult to reconcile with such an assumption. Furthermore, although others have observed accelerated accumulation of CD4+ T cells in M. tuberculosis-infected lungs of vaccinated or infected and cured mice (14, 17, 18), we observed only moderate, <2-fold-higher total CD4+ T cell counts in lungs of vaccinated mice when compared with nonvaccinated controls 2 weeks after infection (data not shown). This observation argues against a profoundly accelerated CD4+ T cell response missed by the epitopes used here. A final solution of the limited correlation of specific- CD4+ T cell responses will await analysis of further MHC class II epitopes, particularly epitopes expressed during different phases of M. tuberculosis infection.
In general terms, our results question the determination of frequencies of vaccination-induced specific T cells as a sufficient correlate of protection. Frequencies of Ag85-specific CD4+ T cells did not correlate with protection. For CD8+ T cells, only determination of Mtb32309–318-specific CD8+ T cells in mucosal sites, such as lung and intestine, allowed tentative prediction of protection, and, even in these tissues, linear correlation between frequencies of vaccination-induced CD8+ T cells and protection was not revealed. A possible explanation for the lack of linear correlation is given by the slow growth kinetics of M. tuberculosis and the high plasticity of the T cell response. As long as frequencies of vaccination-induced T cells reach a certain threshold, the slow growth of M. tuberculosis could leave sufficient time for the immune system to compensate for lower frequencies of T cells by accelerated T cell proliferation. The quality of vaccination-induced T cells is equally decisive. In our study, we used IFN-γ production as a readout for specific T cells. For CD4+ T cells, application of anti-CD40L mAb indicated that the majority of peptide-specific T cells secreted IFN-γ. However, protective T cells could require additional qualities, which could be restricted to a subpopulation of specific IFN-γ+ T cells. Protective attributes of T cells could include expression of multiple cytokines and surface molecules, changes in migration behavior, and the capability of long-term survival or of proliferation in response to antigen (34). The biological activities of T cells, which protect against tuberculosis, need to be defined more precisely (35, 36). For subunit vaccines or recombinant vaccines, where the immune response is focused on few dominant epitopes, analysis of epitope-specific IFN-γ production allows determination of individual responses to vaccination. However, even in this situation, IFN-γ production is most likely only a poor correlate of protection, and analysis of further protective attributes will allow better predictions regarding the quality of vaccination.
Several new vaccine candidates have entered clinical trials (4). Identification of biomarkers which can serve as correlates of protection and hence of vaccine efficacy is urgently needed for the decision-making process during phase II clinical trials. Thus far, specific IFN-γ production, mostly by CD4+ T cells from the peripheral blood, has been used most widely as measure of protective immunity and vaccine efficacy. Our findings argue that this parameter is insufficient and needs to be extended to a more comprehensive biosignature.
Materials and Methods
Mycobacteria, Vaccination, and Challenge of Mice.
M. bovis BCG strain Pasteur and M. tuberculosis H37Rv were cultured in Dubos broth base (Difco; BD Diagnostics, Sparks, MD) supplemented with 10% Dubos medium albumin (Difco; BD Diagnostics) at 37°C. Midlogarithmic cultures were aliquoted and stored at −80°C until use. Female C57BL/6 mice were purchased from the Bundesinstitut für Risikobewertung (BfR) (Berlin, Germany). Experiments were conducted in our animal facility according to the German animal protection law. Mice were vaccinated i.v. with 106 CFU of BCG. For oral vaccination, mice received 200 μl of a NaHCO3 solution (3% NaHCO3 in H2O) via i.g. gavage. Five minutes later, mice received 109 CFU of BCG via i.g. gavage. At 90–100 days after i.g. or i.v. vaccination, animals were aerosol-challenged with ≈200 CFU of M. tuberculosis H37Rv by using an aerosol chamber as described (9). At the indicated days after vaccination or infection, mice were killed, and serial dilutions of tissue homogenates were plated onto Middlebrook 7H11 ampicillin plates. Colonies on plates were counted after 3–4 weeks of incubation at 37°C.
Purification of Cells from Different Tissues.
Cells from spleen, liver, MLN, and small intestine lamina propria were prepared as described (22). Lungs were perfused with PBS via the right ventricle and homogenized by using an iron mesh sieve. Cells were washed, and remaining erythrocytes were lysed. Cells were purified by a 40%/70% Percoll gradient.
In Vitro Restimulation of Cells and Flow Cytometric Determination of Cytokine Expression.
Cells (1–3 × 106) were cultured in a volume of 1 ml of RPMI medium 1640 supplemented with glutamine, Na-pyruvate, 2-mercaptoethanol, penicillin, streptomycin, and 10% heat-inactivated FCS and stimulated for 5 h with 10−5 M of a combination of the peptides Ag85A241–260 (QDAYNAGGGH NGVFDFPDSG) and Ag85B240–260 (FQDAYNAAGG HNAVFNFPPN G) or with 10−6 M of the peptide Mtb32/RV0125309–318 (GAPINSATAM) (13, 20). During the final 4 h of culture, 10 μg/ml brefeldin A were added. Cultured cells were washed and incubated for 5 min with rat serum and anti-CD16/CD32 mAb (2.4G2) to block unspecific antibody binding. Subsequently, cells were stained with phycoerythrin-, PerCP- or PECy7-conjugated anti-CD8α mAb and anti-CD4 mAb (all BD Pharmingen, Franklin Lakes, NJ), and, after 15 min on ice, cells were washed with PBS and fixed for 15 min at room temperature with PBS 4% paraformaldehyde. Cells were washed with PBS 0.1% BSA, permeabilized with PBS 0.1% BSA 0.5% saponin, and incubated in this buffer with rat serum and anti-CD16/CD32 mAb. After 5 min, FITC-conjugated anti-IFN-γ mAb (R4–6A2) and Cy5-conjugated anti-CD154/CD40L mAb (MR1) were added. After a further 15 min at room temperature, cells were washed with PBS and fixed with PBS 1% paraformaldehyde. Cells were analyzed by using a BD FACSCanto and the BD FACSDiva software (Becton Dickinson, Mountain View, CA).
Statistical Analysis.
All results are representative of at least two independent experiments with similar results. Bacterial titers were analyzed with the Mann–Whitney test or the Kruskal–Wallis test and Dunn's multiple comparison test. Frequencies and numbers of IFN-γ-positive T cells were compared with the unpaired Student t test or by ANOVA and Bonferroni's multiple comparison test (in all figures, *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05).
Supplementary Material
Acknowledgments
We thank Manuela Stäber for technical assistance and Mary Louise Grossman for editing of the manuscript. H.-W.M., U.S., and S.H.E.K. were supported by the European Sixth Framework Program, Mucosal Vaccines for Poverty-Related Diseases (MUVAPRED).
Abbreviations
- i.g.
intragastric
- LPL
lamina propria lymphocytes
- MLN
mesenteric lymph nodes
- BCG
Mycobacterium bovis bacille Calmette-Guérin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703510104/DC1.
References
- 1.World Health Organization. 2006 Fact Sheet No 104, Rev March 2006, www.who.int/mediacentre/factsheets/fs104/en.
- 2.Calmette A, Guérin C. Ann Inst Pasteur. 1924;38:371–398. [Google Scholar]
- 3.Fine PE. Scand J Infect. 2001;33:243–245. doi: 10.1080/003655401300077144. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann SHE. Nat Rev Immunol. 2006;6:699–704. doi: 10.1038/nri1920. [DOI] [PubMed] [Google Scholar]
- 5.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chackerian AA, Perera TV, Behar SM. Infect Immun. 2001;69:2666–2674. doi: 10.1128/IAI.69.4.2666-2674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders BM, Frank AA, Orme IA, Cooper AM. Cell Immunol. 2002;216:65–72. doi: 10.1016/s0008-8749(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 8.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Proc Natl Acad Sci USA. 1992;89:12013–712017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolph MS, Raupach B, Kobernick HH, Collins HL, Perarnau B, Lemonnier FA, Kaufmann SHE. Eur J Immunol. 2001;31:1944–1949. doi: 10.1002/1521-4141(200106)31:6<1944::aid-immu1944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar SM. J Exp Med. 2004;200:1479–1489. doi: 10.1084/jem.20041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serbina NV, Flynn JL. Infect Immun. 1999;67:3980–3988. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goter-Robinson C, Derrick SC, Yang AL, Jeon BY, Morris SL. Vaccine. 2006;24:3522–3529. doi: 10.1016/j.vaccine.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Irwin SM, Izzo AA, Dow SW, Skeiky YA, Reed SG, Alderson MR, Orme IM. Infect Immun. 2005;73:5809–5816. doi: 10.1128/IAI.73.9.5809-5816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung YJ, Ryan L, LaCourse L, North RJ. J Exp Med. 2005;201:1915–1924. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath A, Woodworth JS, Behar SM. J Immunol. 2006;177:6361–6369. doi: 10.4049/jimmunol.177.9.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamath AT, Groat NL, Bean AG, Britton WJ. Clin Exp Immunol. 2000;120:476–482. doi: 10.1046/j.1365-2249.2000.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarevic V, Yankura DJ, DiVito SJ, Flynn JL. Infect Immun. 2005;73:2910–2922. doi: 10.1128/IAI.73.5.2910-2922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serbina NV, Flynn JL. Infect Immun. 2001;69:4320–4328. doi: 10.1128/IAI.69.7.4320-4328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, Nouze C, Ladant D, Cole ST, Sebo P, Leclerc C. Infect Immun. 2006;74:2128–2137. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Souza S, Rosseels V, Romano M, Tanghe A, Denis O, Jurion F, Castiglione N, Vanonckelen A, Palfliet K, Huygen K. Infect Immun. 2003;71:483–493. doi: 10.1128/IAI.71.1.483-493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 22.Kursar M, Bonhagen K, Köhler A, Kamradt T, Kaufmann SHE, Mittrücker H-W. J Immunol. 2002;168:6382–6387. doi: 10.4049/jimmunol.168.12.6382. [DOI] [PubMed] [Google Scholar]
- 23.Masopust D, Vezys V, Marzo AL, Lefrancois L. Science. 2001;291:2413–2417. [PubMed] [Google Scholar]
- 24.Lagranderie M, Chavarot P, Balazuc AM, Marchal G. Vaccine. 2000;18:1186–1195. doi: 10.1016/s0264-410x(99)00386-2. [DOI] [PubMed] [Google Scholar]
- 25.Abolhassani M, Lagranderie M, Chavarot P, Balazuc AM, Marchal G. Infect Immun. 2000;68:5657–5662. doi: 10.1128/iai.68.10.5657-5662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palendira UA, Bean G, Feng CG, Britton WJ. Infect Immun. 2002;70:1410–1416. doi: 10.1128/IAI.70.3.1410-1416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruppo V, Orme IM. Tuberculosis. 2002;82:267–273. doi: 10.1054/tube.2002.0340. [DOI] [PubMed] [Google Scholar]
- 28.Izumi T, Costello R. J Exp Med. 1971;133:376–388. doi: 10.1084/jem.133.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefford MJ. Infect Immun. 1980;28:508–515. doi: 10.1128/iai.28.2.508-515.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz MA. Vaccine. 2005;24:443–451. doi: 10.1016/j.vaccine.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Berg RE, Crossley E, Murray S, Forman J. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brigl M, Bry L, Kent AM, Gumperz JE, Brenner MB. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 33.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. J Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 34.Foulds KE, Wu CY, Seder RA. Immunol Rev. 2006;211:58–66. doi: 10.1111/j.0105-2896.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen M, Repsilber D, Gutschmidt A, Neher A, Feldmann K, Mollenkopf HJ, Ziegler A, Kaufmann SHK. J Mol Med. 2007;85:613–621. doi: 10.1007/s00109-007-0157-6. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann SHK. Immunity. 2006;24:351–357. doi: 10.1016/j.immuni.2006.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.