Abstract
Th1 and Th2 cells represent the two main functional subsets of CD4+ T helper cell, and are defined by their cytokine expression. Human Th1 cells express IFNγ, whilst Th2 cells express IL-4, IL-5, and IL-13. Th1 and Th2 cells have distinct immunological functions, and can drive different immunopathologies. Here, we show that in vitro-differentiated human Th2 cells highly selectively express the gene for pro-melanin-concentrating hormone (PMCH), using real-time RT-PCR, enzyme immunoassay, and Western blot analysis. PMCH encodes the prohormone, promelanin-concentrating hormone (PMCH), which is proteolytically processed to produce several peptides, including the orexigenic hormone melanin-concentrating hormone (MCH). PMCH expression by Th2 cells was activation responsive and increased throughout the 28-day differentiation in parallel with the expression of the Th2 cytokine genes. MCH immunoreactivity was detected in the differentiated Th2 but not Th1 cell culture supernatants after activation, and contained the entire PMCH protein, in addition to several smaller peptides. Human Th1 and Th2 cells were isolated by their expression of IFNγ and CRTH2, respectively, and the ex vivo Th2 cells expressed PMCH upon activation, in contrast to the Th1 cells. Because Th2 cells are central to the pathogenesis of allergic diseases including asthma, expression of PMCH by activated Th2 cells in vivo may directly link allergic inflammation to energy homeostasis and may contribute to the association between asthma and obesity.
Keywords: appetite regulation, asthma, T cell differentiation, Th1/Th2 cells
Th1 and Th2 cells are the two main subsets of CD4+ T helper cell and are defined by their cytokine expression patterns (1). The subsets have different roles in the clearance of pathogens, and their unregulated activation leads to distinct immune pathologies. Th1 cells express IFNγ and are crucial for the phagocytic immune response against intracellular pathogens, such as Mycobacterium tuberculosis, but their aberrant activation is implicated in autoimmunity (2). Human Th2 cells express IL-4, IL-5, and IL-13 and orchestrate an immune response characterized by eosinophilia and IgE class-switching to fight extracellular pathogens (2). Th2 cell activation is also central to the pathogenesis of allergic diseases, such as asthma, in which the Th2 cytokines play important roles (3–5).
PMCH encodes a prohormone, pro-melanin-concentrating-hormone (PMCH) of 165 aa which is proteolytically processed to form several peptides including the orexigenic peptide melanin concentrating hormone (MCH) (6). PMCH was first implicated in the regulation of appetite by the finding that the gene is up-regulated in obese, leptin-deficient mice (7). Further studies demonstrated that intracerebroventricular administration of MCH into rats increases feeding (8) and that weight gain occurs after chronic infusion of the peptide into the lateral ventricle (9), strongly suggesting that the peptide stimulates appetite. In addition, mice deficient in MCH are lean and hypophagic (10), and animals overexpressing the gene are obese (11). Several groups cloned the G protein-coupled receptor for MCH (MCHR1) (12–15), and MCHR1-deficient mice are resistant to diet-induced obesity (16, 17). A second receptor, MCHR2, was later identified in humans by its homology to MCHR1 (18–23). MCHR2 is not present in the rodent genomes, but orthologs have been identified in ferret, dog and rhesus monkey, in addition to the human gene (24). PMCH therefore has an important role in increasing appetite (25), and a small molecule antagonist of MCHR1 has been shown to reduce feeding and weight gain in rats fed a high fat diet ad libitum (26).
The prevalence of both asthma and obesity are increasing in the western world, and a link between the two conditions has been proposed and debated (reviewed in ref. 27). Epidemiological evidence from several studies suggests a correlation between body mass index and asthma (28–32). Using quantitative real time RT-PCR, Western blot analysis, and enzyme immunoassay, we found that activated Th2 cells selectively expressed the gene PMCH. Activated Th2 cells secreted MCH-containing proteins, and ex vivo Th2 but not Th1 cells also expressed the gene. We hypothesize that expression of PMCH by Th2 cells in vivo in the asthmatic lung may link asthma and obesity.
Results
In Vitro-Differentiated Th2 Cells Selectively Express PMCH.
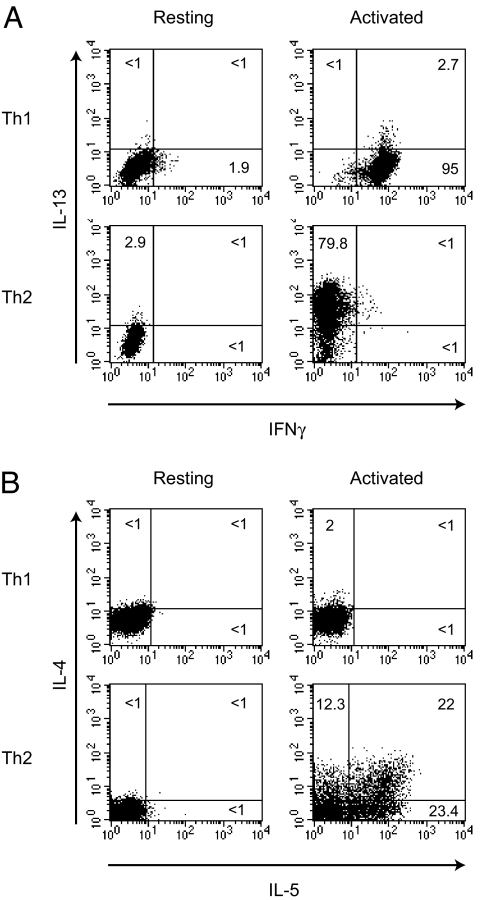
Naïve human CD4+ T cells were cultured in either Th1 (IL-12 and anti-IL-4) or Th2 (IL-4 and anti-IFNγ), inducing conditions for up to 28 days with weekly restimulations (33). After differentiation the cells were highly polarized as shown by intracellular cytokine staining (Fig. 1 A and B), with 95% of the Th1 cells producing IFNγ on stimulation, but few expressing any of the Th2 cytokines. Less than 1% of the activated Th2 cells produced IFNγ, but 80% of the cells expressed IL-13 with large numbers also producing IL-4 and IL-5. Preliminary microarray analyses of these human Th1 and Th2 cells showed that the PMCH gene was selectively expressed by Th2 cells (data not shown). Other genes with roles in the regulation of appetite, including leptin, ghrelin, and orexin, were not expressed by the Th1 and Th2 cells (data not shown). In addition, the genes AROM (antisense RNA overlapping MCH) and IGF1 (insulin-like growth factor 1), which flank PMCH on chromosome 12, were not selectively expressed by Th2 cells, suggesting that the Th2-specific expression of PMCH is not simply due to location in an adventitious chromosomal position. The MCH receptors, MCHR1 and MCHR2, were not expressed by either Th1 or Th2 cells.
Fig. 1.
Intracellular cytokine staining of in vitro-differentiated Th1 and Th2 cells. Naïve T cells were cultured under either Th1- or Th2-inducing conditions for 28 days, and intracellular cytokine staining was carried out on resting cells or cells that had been activated for 4 h with PMA/ionomycin as indicated, for IL-13 and IFNγ (A) and IL-4 and IL-5 (B). Results shown are representative of six independent experiments.
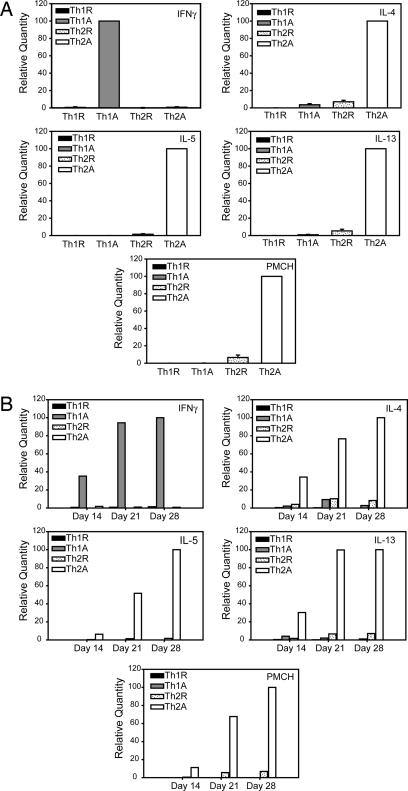
We performed real-time RT-PCR, and the levels of gene expression mirrored the intracellular cytokine staining data. IFNγ mRNA was expressed primarily in activated Th1 cells, and the Th2 cytokines, IL-4, IL-5, and IL-13 were expressed by activated Th2 cells (Fig. 2A). PMCH mRNA was also detected at high levels in the activated Th2 cells, with lower levels in the resting Th2 cells, and no expression by the Th1 cells (Fig. 2A). Expression of IFNγ by the Th1 cells increased over the 28-day differentiation, with negligible levels in the Th2 cells throughout the time course (Fig. 2B). Similarly, expression of IL-4, IL-5, and IL-13 by the Th2 cells increased until day 28, and we observed the same pattern of expression for PMCH (Fig. 2B). Levels of PMCH mRNA remained very low in the Th1 cells at all time points, whereas PMCH expression in the Th2 cells increased over the four weeks (Fig. 2B), indicating that the differential expression is due to an up-regulation of PMCH expression during Th2 cell differentiation, rather than the gene being down-regulated in Th1 cells.
Fig. 2.
Quantitative RT-PCR on in vitro-differentiated Th1 and Th2 cells. (A) Naïve T cells were cultured under either Th1- or Th2-inducing conditions for 28 days. Total RNA was isolated from resting cells (Th1R or Th2R) or cells that had been activated for 4 h with PMA/ionomycin (Th1A or Th2A), and quantitative RT-PCR was carried out with primers to IFNγ, IL-4, IL-5, IL-13, or PMCH. Results are the mean ± SEM of four independent experiments. (B) Total RNA was isolated from resting cells (Th1R or Th2R) or cells that had been activated for 4 h with PMA/ionomycin (Th1A or Th2A) after 14, 21, or 28 days of differentiation, and quantitative RT-PCR was carried out with primers to IFNγ, IL-4, IL-5, IL-13, or PMCH. Results shown are representative of two independent experiments.
In Vitro-Differentiated Th2 Cells Express PMCH Protein.
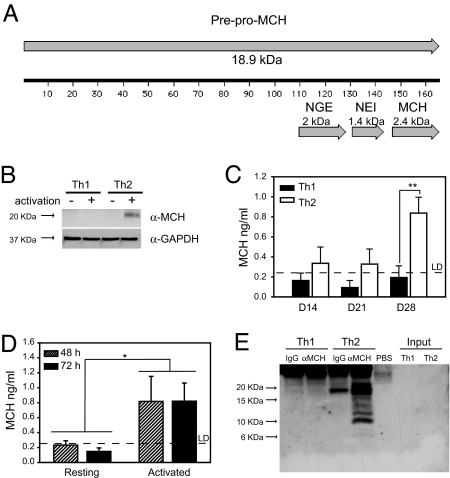
Because Th2 cells selectively expressed the PMCH gene, we determined whether the cells also produced PMCH protein. PMCH is a prohormone that is proteolytically cleaved to produce several peptides, including MCH (as represented in Fig. 3A). A single band of MCH-immunoreactive protein was detected in cytoplasmic extracts from activated Th2 cells but not from resting cells or resting or activated Th1 cells (Fig. 3B). The protein was ≈20 kDa in size, which corresponds to the whole PMCH prohormone (Fig. 3A). An enzyme immunoassay (EIA) for MCH identified MCH-containing protein in the Th2 but not Th1 cell culture supernatants, which was greatest after 28 days of differentiation (Fig. 3C), indicating that Th2 cells secreted PMCH gene products. Because the PMCH gene was activation responsive in Th2 cells (Fig. 2) we assayed Th2 cell supernatants after activation. Significant levels of MCH immunoreactivity were detected after 48 and 72 h of activation with plate bound anti-CD3 and anti-CD28 (Fig. 3D).
Fig. 3.
PMCH protein expression by the Th2 cells. (A) Diagram of the PMCH protein, showing the location and length of the peptides, MCH, NEI, and NGE. (B) Cytoplasmic extracts were prepared from resting (−) Th1 and Th2 cells or cells which had been stimulated for 4 h with PMA/ionomycin (+). Western blots were carried out on these extracts with an anti-MCH antibody. Membranes were then reprobed with an antibody to GAPDH. Results shown are representative of three independent experiments. (C) The concentration of MCH in Th1 or Th2 cell culture supernatants (as indicated) was assayed after 14, 21, and 28 days of differentiation. Data shown are the mean ± SEM of three independent experiments. **, P = 0.004 by Tukey two-way ANOVA. (D) After 28 days of differentiation, Th2 cells were removed to fresh media and the concentrations of MCH in supernatants from resting cells, or cells stimulated for 48 or 72 h with plate bound anti-CD3 and anti-CD28 were measured by EIA. By Tukey two-way ANOVA, there is a significant difference between resting and activated cells (*, P = 0.026). (E) An antibody against MCH (α-MCH) or a control antibody (IgG) was used to immunoprecipitate protein from Th1 and Th2 cell culture supernatants or from PBS as indicated. Western blots were carried out on the precipitated material and on untreated supernatant (Input). Data shown are representative of three independent experiments.
Western blot analysis demonstrated that Th2 cells produced the whole PMCH protein, but smaller peptides were not detected in the Th2 cytoplasmic extracts (Fig. 3B). Western blot analysis was not sensitive enough to detect any PMCH products in the Th2 cell supernatant (Fig. 3E, Input), so we used an anti-MCH antibody to immunoprecipitate protein from the supernatants (Fig. 3E). A range of specific protein bands were detected in the protein precipitated from the Th2 cell culture supernatants, from 20 kDa (the size of the whole prohormone) to <10 kDa (Fig. 3E). The blot shown in Fig. 3E is representative of three independent experiments. Similar results were obtained in each replicate, although the intensity of the smaller bands was variable.
PMCH Is Selectively Expressed by ex Vivo Th2 Cells.
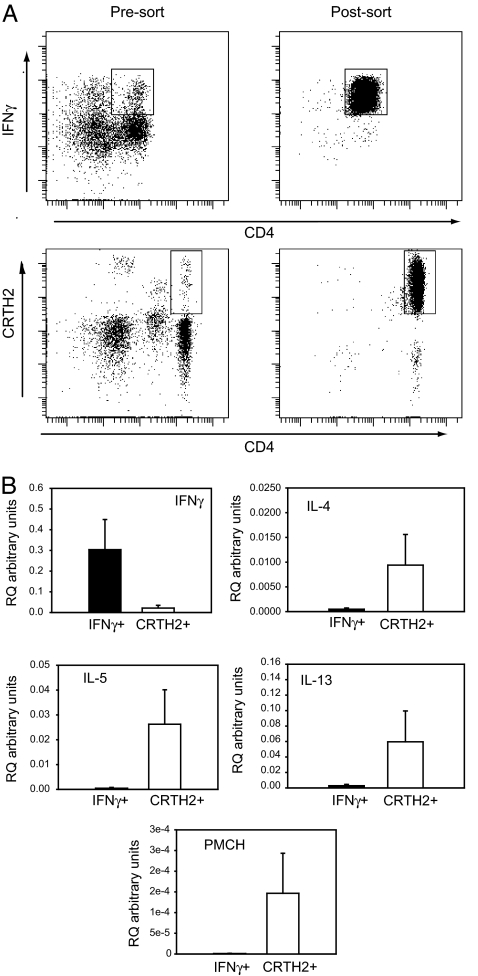
Th1 and Th2 cells were isolated from peripheral blood by cell sorting. CD4+ Th1 cells were identified by IFNγ secretion, whereas CD4+ Th2 cells were identified by their expression of the Th2 specific receptor, CRTH2 (34) (Fig. 4A). The ex vivo Th1 cells expressed greater levels of IFNγ than the ex vivo Th2 cells, and the Th2 cells expressed the Th2 cytokines on activation (Fig. 4B). In support of our data from the in vitro-differentiated Th1 and Th2 cells, ex vivo Th2 cells also expressed far greater levels of PMCH than Th1 cells (Fig. 4B).
Fig. 4.
Quantitative RT-PCR on ex vivo Th1 and Th2 cells. (A) CD4+ IFNγ secreting T cells were identified in human PBMCs by using an IFNγ secretion assay, and isolated by FACS. CD4+CRTH2+ T cells were isolated from PBMCs by FACS or with magnetic beads and activated overnight with PMA/ionomycin. (B) Total RNA was isolated, and quantitative RT-PCR was carried out to determine the expression of IFNγ, IL-4, IL-5, IL-13, and PMCH. Data shown are the mean ± SEM from three independent experiments.
Discussion
We have demonstrated that human Th2 cells selectively express the gene PMCH and secrete protein containing the orexigenic peptide, MCH, using several techniques. The Th2 cells expressed PMCH mRNA and protein after activation in a similar manner to the activation responsive Th2 cytokine genes. MCH-immunoreactive protein of 20 kDa was identified in Th2 cell supernatants, representing the whole prohormone, and smaller proteins were also detected. The smaller peptides may be the result of intracellular proteolytic processing before secretion, or degradation products of the secreted PMCH produced by proteolytic activity in cell supernatants. The smallest MCH-containing peptides identified were ≈6 kDa, hence larger than the 2.4-kDa MCH peptide. Both Th1 and Th2 cells express the prohormone convertases, PC7 and furin (data not shown), two enzymes that have been previously shown to process PMCH most efficiently from a panel of prohormone convertases (35). It therefore seems likely that the PMCH produced is processed to MCH, but that the Western blot was not able to detect peptides <6 kDa.
MCH has a well defined function in the brain where it acts in the hypothalamus to stimulate appetite (25). There are numerous examples of hormones acting in the hypothalamus after their production at distal sites. For example, the orexigenic hormone, ghrelin, and the anorectic peptide, leptin, also act in the hypothalamus, despite being synthesized at sites remote from the central nervous system, in adipose tissue and the gut, respectively (36). These proteins reach the brain by diffusing through the blood–brain barrier, or via the circumventricular organs, which lack blood–brain barrier function (36). Thus PMCH products released into the circulation by activated Th2 cells in the asthmatic lung could stimulate the hypothalamus and provide an important biochemical link between airway inflammation and appetite enhancement. There are clear examples of proteins produced locally by Th2 cells exerting systemic effects. For instance, IL-5 produced in the airways following allergen inhalation promotes eosinophil differentiation and release from the bone marrow (37). Th2 cells secrete MCH-containing protein of up to 20 kDa, which is notable, because PMCH products longer than the MCH peptide have been shown to have greater activity because of their increased stability (38). Hence the PMCH secreted by Th2 cells may persist for longer in vivo. Furthermore, PMCH products secreted from activated Th2 cells at sites of helminth infection may increase appetite during the resolution phase of infection, thus promoting recovery. It would have been interesting to analyze the levels of MCH circulating in the blood of asthmatic patients and people infected with helminth parasites, but problems of specificity have been reported in the measurement of serum MCH (39), making such studies currently impossible.
PMCH is located on chromosome 12q23.1, a region consistently shown to have linkage to asthma (39, 40), and our finding that the gene is selectively expressed by human Th2 cells may relate to the proposed link between asthma and obesity (27). Although the mechanism for the epidemiological association is not known, obesity is thought to result in low-grade systemic inflammation, and inflammatory markers are increased in the obese (reviewed in ref. 41). A recent study identified increased circulating levels of the inflammatory chemokine CCL11 (eotaxin) in obese mice and humans (42), which are findings particularly pertinent to asthma, because CCL11 recruits cell types with important roles in allergic inflammation and asthma (3). Although Th2-derived PMCH may be involved in appetite, we cannot rule out the possibility that the protein products of the PMCH gene have as-yet unidentified roles in the immune system, acting in a similar manner to the Th2 cytokines.
Our data demonstrate that the orexigenic protein, PMCH, is expressed selectively by human Th2 cells and may provide a mechanistic link between allergic inflammation, asthma and obesity. Asthmatic individuals are not necessarily obese, however; therefore future work will address whether there are genetic polymorphisms of PMCH that are associated with both asthma and obesity.
Materials and Methods
Isolation and Differentiation of Naïve CD4+ T Cells.
The isolation of naïve CD4+ T cells and their differentiation to Th1 or Th2 cells was carried out as described in ref. 33. Briefly, venous blood was taken from healthy, nonatopic volunteers and anticoagulated with heparin. PBMCs were isolated by using Lymphoprep (Axis-shield, Kimbolton, U.K.), and CD4+ T cells were isolated from PBMCs by using a CD4 Positive Isolation Kit (Invitrogen, Carlsbad, CA). Naïve CD45RA+ T cells were purified from CD4+ T cells by depletion of CD45RO+ cells, using mouse anti-human CD45RO (UCHL1; BD Biosciences, San Jose, CA) (0.5 μg/1 × 106 cells) and rat anti-mouse IgG Dynabeads (Invitrogen) according to the manufacturer's instructions. The purity of fractionated cell populations was determined by FACS analysis. Samples were analyzed on a FACSCalibur (BD Biosciences) and were >95% pure. CD4+CD45RA+ T cells were cultured at 1 × 106 cells/ml in RPMI medium 1640 (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine (Invitrogen), 100 units/ml penicillin (Invitrogen), and 100 μg/ml streptomycin (Invitrogen). Cells were stimulated with plate-bound anti-CD3 (1 μg/ml; clone OKT3) and anti-CD28 (2 μg/ml; clone 15E8; Sanquin Reagents, Amsterdam, The Netherlands) and rIL-2 (50 units/ml; Novartis, Horsham, U.K.). For Th1 differentiation, rIL-12 (2.5 ng/ml; R&D Systems, Abingdon, Oxfordshire, U.K.), anti-IL-4 (5 μg/ml; clone MP4–25D2; BD Biosciences), and anti-IL-10 (5 μg/ml; clone JES3–9D7; Invitrogen) were added. For Th2 differentiation, rIL-4 (12.5 ng/ml; R&D Systems), anti-IFNγ (5 μg/ml; clone B-B1; Invitrogen), and anti-IL-10 (5 μg/ml; clone JES3–9D7; Invitrogen) were added. After 4 days, the cells were expanded under the same conditions in the absence of anti-CD3 or anti-CD28. Cells were then restimulated every 7 days. When required, cells were activated with PMA (5 ng/ml; Sigma–Aldrich, St. Louis, MO) and ionomycin (500 ng/ml; Calbiochem, San Jose, CA) at 37°C for 4 h.
Intracellular Cytokine Staining.
Cells were activated with PMA (5 ng/ml) and ionomycin (500 ng/ml) for 4 h, and monensin (2 μM; Sigma) was added for the final 2 h. Cells were harvested, 7-amino-actinomycin D (4 mg/ml; Sigma) was added, and cells were incubated for 10 min on ice. Cells were washed with FACSFlow (BD Biosciences) and processed for intracellular cytokine staining with the Cytofix/Cytoperm kit (BD Biosciences), according to the manufacturer's instructions. Antibodies used were (BD Biosciences, unless stated otherwise): anti-IL-4-PE (BD FastImmune); anti-IL-5-Allophycocyanin (TRFK5); anti-IL-13-PE (JES10–5A2); and anti-IFNγ-FITC (B27). Samples were analyzed on a FACSCalibur (BD Biosciences). Live cells were analyzed based on forward and side scatter and exclusion of 7-amino-actinomycin D. At least 10,000 live cells were analyzed for each sample. Quadrant markers were set based on background staining of matched control antibodies (also from BD Biosciences) and on unactivated cells treated in parallel.
RNA Isolation and Quantitative RT-PCR.
RNA isolation and real time RT-PCR were carried out as described in ref. 43. Briefly, total RNA was isolated by using the RNeasy miniprep kit (Qiagen, Crawley, U.K.) according to the manufacturer's instructions, and RNA was treated with TURBO DNase (Ambion, Austin, TX) according to the manufacturer's instructions to remove contaminating DNA. RNA was reverse-transcribed in the presence of RNAguard (GE Healthcare, Chalfont St. Giles, U.K.) by using random hexamers and revert-aid reverse transcriptase (Fermentas International, York, U.K.) according to the manufacturer's instructions. Quantitative RT-PCR was carried out by using Taqman MGB Gene expression assays (Applied Biosystems, Foster City, CA). Probe sets used were: IL-4, Hs00929862_m1; IL-5, Hs00174200_m1; IL-13, Hs00174379_m1; IFNγ, Hs00174143_m1; PMCH, Hs00173595_m1; 18s rRNA, Hs99999901_s1.
Western Blot Analysis.
Cytoplasmic extracts were produced from T cells by using the NE-PER nuclear and cytoplasmic extract kit (Pierce, Cramlington, Northumberland, U.K.) according to the manufacturer's instructions. Fifty micrograms of protein was run on precast 16% tricine reducing gels (Invitrogen), and the proteins were transferred to PVDF (GE Healthcare). Membranes were blocked overnight with 4% milk in TBS-Tween and probed with anti-MCH (5.7 μg/ml; Sigma–Aldrich) and a goat anti-rabbit HRP-conjugated antibody (20 ng/ml; sc-2004; Santa Cruz Biotechnology, Santa Cruz, CA), or mouse monoclonal anti-GAPDH (20 ng/ml; 6C5; Abcam, Cambridge, U.K.) with a goat anti-mouse HRP-conjugated antibody (80 ng/ml; sc-2005; Santa Cruz Biotechnology). Signals were detected with ECL (GE Healthcare).
Immunoprecipitation.
Ten milliliters of cell culture supernatant were incubated with 3 μg of anti-MCH antibody (Sigma) or control IgG (sc-2027; Santa Cruz Biotechnology) overnight at 4°C. As an additional control, anti-MCH was incubated in PBS. Protein A bead slurry (120 μl; Upstate Biotechnology, Lake Placid, NY) was added, and supernatants were incubated overnight at 4°C. Beads were washed with PBS and resuspended in loading buffer (Invitrogen). Samples were run on precast 16% tricine nonreducing gels (Invitrogen) with input supernatant samples. Proteins were transferred to PVDF and probed with anti-MCH as described above.
MCH EIA.
MCH EIA was carried out by using the MCH EIA kit (Phoenix Pharmaceuticals Inc., Burlingame, CA) according to the manufacturer's instructions. The limit of detection of the assay was 0.23 ng/ml.
Isolation ex Vivo Human Th1 and Th2 Cells.
To isolate Th1 cells, PBMCs were stained with the IFNγ-PE secretion kit (Miltenyi Biotech, Auburn, CA) and anti-CD4-FITC (Miltenyi Biotech). IFNγ+CD4+ T cells were then sorted in comparison with unstimulated control cells treated in the same way, by using a FACSAria (BD Biosciences). Sorted cells were >85% pure. To isolate Th2 cells, PBMCs were stained with rat anti-human CRTH2, goat anti-rat APC, and anti-CD4-FITC (BD Biosciences). Cells were sorted on a FACSAria (BD Biosciences) by their CD4 expression and CRTH2 expression in comparison with the relevant isotype control (BD Biosciences). Sorted cells were >85% pure. Alternatively, CD4+ T cells were isolated from PBMCs by using a negative isolation kit (Miltenyi Biotech), and CRTH2+ cells were isolated from this population by using a CRTH2+ positive isolation kit (Miltenyi Biotech). Isolated CRTH2+CD4+ T cells were stimulated at 1 × 106 cells/ml in RPMI medium 1640 (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine (Invitrogen), 100 units/ml penicillin (Invitrogen), and 100 μg/ml streptomycin (Invitrogen), with PMA (5 ng/ml) and ionomycin (500 ng/ml) at 37°C overnight.
Acknowledgments
We thank David Richards for assistance with cell sorting; the research nurses, Kheem Jones and Cherylin Reinholtz, for phlebotomy; and Cecilia Soh, Paul Lavender, and other members of the laboratory for helpful discussions. This work was funded by Medical Research Council Grant G9536930; grants from the Friends of Guy's, The Guy's and St. Thomas's Charitable Foundation, and Asthma U.K.; and a Medical Research Council Ph.D. Studentship (J.M.).
Abbreviations
- EIA
enzyme immunoassay
- MCH
melanin concentrating hormone
- MCHR
G protein-coupled receptor for MCH
- PMCH
prohormone, pro-melanin-concentrating hormone.
Footnotes
The authors declare no conflict of interest.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Cohn L, Elias JA, Chupp GL. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 4.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. Endocrinology. 1989;125:2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- 7.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 8.Rossi M, Choi SJ, O'Shea D, Miyoshi T, Ghatei MA, Bloom SR. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- 9.Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Int J Obes Relat Metab Disord. 2002;26:1289–1295. doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- 10.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachner D, Kreienkamp H, Weise C, Buck F, Richter D. FEBS Lett. 1999;457:522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- 13.Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, Dytko GM, Foley JJ, Martin J, Liu WS, et al. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- 14.Lembo PM, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C, St-Onge S, Pou C, Labrecque J, Groblewski T, et al. Nat Cell Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- 16.Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, et al. Proc Natl Acad Sci USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Hu C, Hsu CK, Zhang Q, Bi C, Asnicar M, Hsiung HM, Fox N, Slieker LJ, Yang DD, et al. Endocrinology. 2002;143:2469–2477. doi: 10.1210/endo.143.7.8903. [DOI] [PubMed] [Google Scholar]
- 18.An S, Cutler G, Zhao JJ, Huang SG, Tian H, Li W, Liang L, Rich M, Bakleh A, Du J, et al. Proc Natl Acad Sci USA. 2001;98:7576–7581. doi: 10.1073/pnas.131200698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sailer AW, Sano H, Zeng Z, McDonald TP, Pan J, Pong SS, Feighner SD, Tan CP, Fukami T, Iwaasa H, et al. Proc Natl Acad Sci USA. 2001;98:7564–7569. doi: 10.1073/pnas.121170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez M, Beauverger P, Naime I, Rique H, Ouvry C, Souchaud S, Dromaint S, Nagel N, Suply T, Audinot V, et al. Mol Pharmacol. 2001;60:632–639. [PubMed] [Google Scholar]
- 21.Mori M, Harada M, Terao Y, Sugo T, Watanabe T, Shimomura Y, Abe M, Shintani Y, Onda H, Nishimura O, Fujino M. Biochem Biophys Res Commun. 2001;283:1013–1018. doi: 10.1006/bbrc.2001.4893. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Behan J, O'Neill K, Weig B, Fried S, Laz T, Bayne M, Gustafson E, Hawes BE. J Biol Chem. 2001;276:34664–34670. doi: 10.1074/jbc.M102601200. [DOI] [PubMed] [Google Scholar]
- 23.Hill J, Duckworth M, Murdock P, Rennie G, Sabido-David C, Ames RS, Szekeres P, Wilson S, Bergsma DJ, Gloger IS, et al. J Biol Chem. 2001;276:20125–20129. doi: 10.1074/jbc.M102068200. [DOI] [PubMed] [Google Scholar]
- 24.Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, et al. Genomics. 2002;79:785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- 25.Coll AP, Farooqi IS, O'Rahilly S. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, et al. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 27.Beuther DA, Weiss ST, Sutherland ER. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Thorax. 2001;56:835–838. doi: 10.1136/thorax.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Figueroa-Munoz JI, Chinn S, Rona RJ. Thorax. 2001;56:133–137. doi: 10.1136/thorax.56.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinn S, Jarvis D, Burney P. Thorax. 2002;57:1028–1033. doi: 10.1136/thorax.57.12.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 32.Hasler G, Gergen PJ, Ajdacic V, Gamma A, Eich D, Rossler W, Angst J. Int J Obes (London) 2006;30:1111–1118. doi: 10.1038/sj.ijo.0803215. [DOI] [PubMed] [Google Scholar]
- 33.Cousins DJ, Lee TH, Staynov DZ. J Immunol. 2002;169:2498–2506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- 34.Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, Abe H, Tada K, Nakamura M, Sugamura K, Takano S. J Immunol. 1999;162:1278–1286. [PubMed] [Google Scholar]
- 35.Viale A, Ortola C, Hervieu G, Furuta M, Barbero P, Steiner DF, Seidah NG, Nahon JL. J Biol Chem. 1999;274:6536–6545. doi: 10.1074/jbc.274.10.6536. [DOI] [PubMed] [Google Scholar]
- 36.Klok MD, Jakobsdottir S, Drent ML. Obes Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 37.Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, Kay AB, Denburg J. J Allergy Clin Immunol. 2003;111:714–719. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 38.Maulon-Feraille L, Della Zuana O, Suply T, Rovere-Jovene C, Audinot V, Levens N, Boutin JA, Duhault J, Nahon JL. J Pharmacol Exp Ther. 2002;302:766–773. doi: 10.1124/jpet.302.2.766. [DOI] [PubMed] [Google Scholar]
- 39.Waters SM, Krause JE. J Clin Endocrinol Metab. 2005;90:6337. doi: 10.1210/jc.2005-1367. author reply 6337. [DOI] [PubMed] [Google Scholar]
- 40.Raby BA, Silverman EK, Lazarus R, Lange C, Kwiatkowski DJ, Weiss ST. Hum Mol Genet. 2003;12:1973–1979. doi: 10.1093/hmg/ddg208. [DOI] [PubMed] [Google Scholar]
- 41.Fantuzzi G. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 42.Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. J Clin Endocrinol Metab. 2006;91:256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- 43.Gilmour J, Cousins DJ, Richards DF, Sattar Z, Lee TH, Lavender P. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.03.033. [DOI] [PubMed] [Google Scholar]