Abstract
Amnesia produced by protein synthesis inhibitors such as anisomycin provides major support for the prevalent view that the formation of long-lasting memories requires de novo protein synthesis. However, inhibition of protein synthesis might disrupt other neural functions to interfere with memory formation. Intraamygdala injections of anisomycin before inhibitory avoidance training impaired memory in rats tested 48 h later. Release of norepinephrine (NE), dopamine (DA), and serotonin, measured at the site of anisomycin infusions, increased quickly by ≈1,000–17,000%, far above the levels seen under normal conditions. NE and DA release later decreased far below baseline for several hours before recovering at 48 h. Intraamygdala injections of a β-adrenergic receptor antagonist or agonist, each timed to blunt effects of increases and decreases in NE release after anisomycin, attenuated anisomycin-induced amnesia. In addition, similar to the effects on memory seen with anisomycin, intraamygdala injections of a high dose of NE before training impaired memory tested at 48 h after training. These findings suggest that altered release of neurotransmitters may mediate amnesia produced by anisomycin and, further, raise important questions about the empirical bases for many molecular theories of memory formation.
Keywords: anisomycin, protein synthesis-dependent memory, norepinephrine
A central tenet of contemporary neurobiological models of memory is that memory formation passes through two major phases, an early protein synthesis-independent phase and a later de novo protein synthesis-dependent phase (e.g., refs. 1–4). According to currently prevalent views, early or “short-term” memory depends on posttranslational modifications of proteins (5, 6), and late or “long-term” memory depends on de novo protein synthesis initiated by an experience that will later be remembered (1–4, 7, 8).
Support for the view that de novo protein synthesis is necessary for long-term memory formation comes largely from studies of anterograde and retrograde amnesia produced by inhibitors of protein synthesis administered near the time of training (1, 6, 9–12). Of particular importance are findings showing that, when a protein synthesis inhibitor is injected before training, memory remains intact during the first few hours after training but decays after that. A conventional interpretation of these findings is that the intact memory evident at short training-test intervals reflects early protein synthesis-independent memory, and impaired memory seen at tests a day or more after training reflects de novo protein synthesis-dependent memory.
In addition to application of this thinking to mechanisms of memory formation, these views have also been applied to the mechanisms underlying related synaptic plasticities including long-term potentiation and depression (e.g., refs. 1, 8, 13, and 14), as well as a wide range of other brain changes such as those underlying drug abuse and relapse (15, 16), epilepsy (17), and the organization and reorganization of motor cortex (18). In each of these contexts, the presumption is that protein synthesis inhibitors interfere with the establishment of enduring neural changes (i.e., those fundamental to memory, drug relapse, epilepsy, or motor cortex organization) by blocking mechanisms of neuronal and synaptic change that require de novo protein synthesis. Thus, evidence obtained with protein synthesis inhibitors has a pervasive influence on theories related to the fundamental mechanisms of not only memory formation but also a wide range of brain functions.
Although numerous reports describe the memory impairments obtained with protein synthesis inhibitors as direct evidence that new protein synthesis is necessary for long-term memory formation, there are many reasons to question this interpretation. These reasons include reports of memory formation that are not impaired by protein synthesis inhibitors. For example, amnesia for avoidance training does not appear if higher footshock levels, pretraining habituation trials, or multiple trials are used; similarly, some forms of long-term potentiation and depression are resistant to the effects of protein synthesis inhibitors (reviewed in ref. 6). Also, the time courses for decay of memory range from minutes to days across experiments (12), suggesting that the temporal properties for the onset of anterograde amnesia, like the temporal properties of retrograde amnesia gradients, do not directly reflect the time course of a memory consolidation process but instead reflect the efficacy of disruption of memory (12, 19). In addition, the findings of many experiments show that amnesias produced by protein synthesis inhibitors such as puromycin, cycloheximide, acetoxycycloheximide, and anisomycin can be blocked or rescued by administration near the time of training of many treatments that modulate memory (6, 9, 10). Importantly, treatments that reverse the effects of protein synthesis inhibitors on memory do so without concomitant attenuation of the extent of inhibition of protein synthesis (20–23). Thus, there are many experimental conditions in which memory formation proceeds in the presence of substantial inhibition of protein synthesis. If experience-induced de novo protein synthesis is a requirement for memory storage, these findings should not be seen.
In the face of a substantial body of evidence inconsistent with the general interpretation of studies of protein synthesis inhibitors and memory, the dominant view remains that impaired long-term memory after treatment with protein synthesis inhibitors means that new protein synthesis per se is necessary for long-term memory formation. In addition to the issue of findings that do not fit the theory, there is a logical problem as well. Most often, memory impairments after administration of the inhibitors are interpreted as showing that the absent mechanism, e.g., new protein synthesis, is necessary for memory formation. An alternative possibility is that the insult of protein synthesis inhibition introduces changes in neural functioning as a result of decreases in some proteins and superinduction of others (6), thereby interfering with normal neural processing needed for memory formation (11, 12).
The present experiments addressed possible interference with normal processing by examining changes in release of the biogenic amines norepinephrine (NE), dopamine (DA), and serotonin (5-HT) at the site of intraamygdala injections of anisomycin. There is a wealth of information showing that NE (24), DA (25), and 5-HT (26) act at the amygdala to modulate memory formation.
The findings described in the present article indicate that intraamygdala anisomycin injections result initially in extraordinarily large increases in release of biogenic amines near the site of injection, followed later by extensive and prolonged decreases in release of the amines. Additionally, blockade of amygdala β-adrenergic receptors at the time of anisomycin injections, i.e., at the time of high release of biogenic amines, attenuates the amnesia produced by anisomycin as tested 48 h after training. Similarly, activation of β2-adrenergic receptors during the time of amine depletion also attenuates anisomycin-induced amnesia assessed at 48 h after training. Moreover, a high dose of NE injected into the amygdala before training impairs memory to an extent similar to that seen after anisomycin injections. Together, these findings suggest that intraamygdala injections of anisomycin interfere with memory formation by inducing extraordinary changes in the release profiles of NE, DA, and 5-HT.
Results
Anisomycin-Impaired Memory and c-Fos Immunoreactivity After Training.
As shown in Fig. 1Left, latencies on a memory test 48 h after inhibitory avoidance training were significantly lower in rats treated with bilateral anisomycin infusions into the amygdala 20 min before training than in vehicle controls (P < 0.05). Thus, the anisomycin treatment successfully produced amnesia as tested 48 h after training.
Fig. 1.
Effects of intraamygdala injections of anisomycin (ANI) on memory and on c-Fos immunoreactivity. (Left) Memory assessed 48 h after training in rats pretreated with intraamygdala injections of anisomycin or vehicle. Anisomycin significantly impaired retention latencies (P < 0.05 vs. controls). (Center and Right) c-Fos immunoreactivity after intraamygdala infusions of either vehicle (Center) or anisomycin (Right). The sections shown were taken one to two sections posterior to the cannula tract, i.e., within 100 μm of the injection. Amygdala c-Fos immunoreactivity after anisomycin treatment was markedly reduced to nearly undetectable levels. n = 8, vehicle; n = 7, anisomycin.
In other rats, c-Fos immunoreactivity after training was used as a marker with which to monitor the efficacy of anisomycin in inhibiting protein synthesis (27, 28). Immunocytochemistry for c-Fos was performed on brains taken 60 min after footshock; the sections shown in Fig. 1 were taken immediately posterior to the cannulae tracts. Fig. 1 Center and Right shows representative photomicrographs of c-Fos immunoreactivity in the amygdala after vehicle (Center) or anisomycin (Right) injections. c-Fos immunoreactivity, apparent after footshock training, was essentially abolished by intraamygdala injections of anisomycin before training, providing evidence for effective inhibition of protein synthesis.
Neurotransmitter Release First Increased and Then Decreased Markedly After Anisomycin Injections.
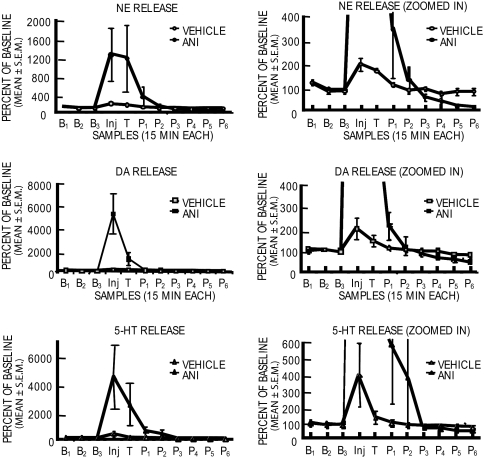
Fig. 2 shows the neurochemical results obtained for each of the biogenic amines tested in samples collected before, during, and after training from the rats for which the behavior was shown in Fig. 1 Left. Microdialysis ended 2 h after injection of anisomycin. Anisomycin injections into the amygdala produced extraordinary increases in release of NE (1,200%), DA (5,500%), and 5-HT (4,500%) soon after the injection (Fig. 2 Left). Sample by treatment interactions for each of the amines were statistically significant (P < 0.0001). The subsequent decreases in release are shown in the zoomed graphs (Fig. 2 Right) in which the y axis is expanded to show changes closer to baseline values. In later samples, both NE and DA release decreased significantly below that of controls (P < 0.05), apparently continuing to decrease at the end of the dialysis session. Although 5-HT levels increased substantially in initial samples, as did the other two amines, the levels declined from peak release to baseline but did not go significantly below the original baseline values (P > 0.4).
Fig. 2.
Effects of pretraining intraamygdala injections of anisomycin (ANI) (n = 5) or vehicle (n = 5) on release of NE, DA, and 5-HT near the site of injection. Microdialysis samples were collected for analyses every 15 min beginning 45 min before and ending 2 h after injections of anisomycin. (Left) Note that NE, DA, and 5-HT each exhibited very large increases in release in the samples collected soon after injection. (Right) The y axis was expanded to show, for the same data, the decreases in release of the neurotransmitters toward the end of the dialysis session. Note that the magnitude of release decreased with time after the initial large increase. Release levels were significantly below baseline for NE and DA at the time microdialysis was terminated. B, baseline; Inj., injection; T, training; P, posttraining.
Release of NE and DA Returns to Baseline Between 8 and 48 h After Anisomycin Injections.
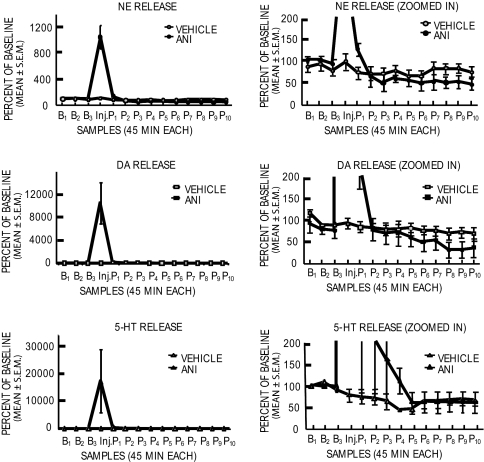
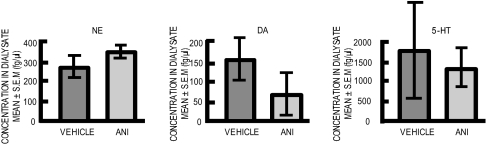
To examine whether and when recovery of release of NE and DA occurred, a second microdialysis experiment was conducted with a slower dialysis flow rate (0.6 μl/min vs. 2 μl/min in Fig. 2) for >8 h after an intraamygdala anisomycin injection. The dialysis was then discontinued and was started again, without additional treatment, 48 h later. Note first in Fig. 3 that the results are consistent with those in the first microdialysis experiment. Release of NE, DA, and 5-HT increased dramatically after anisomycin injections (vs. vehicle, all P values < 0.0001). The somewhat different mean percentage increases seen here compared with the values in the first experiment are likely due to the differences in flow rates and the times for each dialysis sample (15 min for data shown in Fig. 2 vs. 45 min here in Fig. 3). In comparison to the controls, the 5-HT levels were elevated for ≈3 h before returning to but not below control values. Release of NE and DA after anisomycin injection increased for ≈90 min before decreasing and crossing below control values at 2–3 h. These values then remained significantly below those of controls to the end of the dialysis session 8 h after anisomycin treatment [for NE and DA, values were significantly lower in anisomycin-treated rats at post-injection samples P8–P10 (P < 0.05), i.e., 6–8 h after anisomycin injection]. Even with the reduced flow rate, there was apparent rundown of release levels in controls, so all comparisons were made across rather than within groups. When reassessed in these rats 48 h later in a second microdialysis session, baseline release had recovered for all three neurotransmitters (P > 0.3) (Fig. 4).
Fig. 3.
Effects of intraamygdala anisomycin (ANI) (n = 4) and vehicle (n = 4) injections on release of NE, DA, and 5-HT. Microdialysis samples were collected every 45 min beginning 135 min before and ending 8 h after injections. As in Fig. 2, the y axis was expanded in Right to show, for the same data, the decreases in release of the neurotransmitters toward the end of the dialysis session. Note that 5-HT but not NE and DA levels had returned to baseline even 8+ h after anisomycin injections. B, baseline; Inj., injection; P, postinjection.
Fig. 4.
Levels of NE, DA, and 5-HT assessed 48 h after anisomycin (ANI) injections. All mean values were comparable with those of controls, showing recovery of neurotransmitter levels to baseline values at this time.
Timed Coadministration of an Adrenergic Receptor Antagonist or Agonist Attenuates Anisomycin-Induced Amnesia.
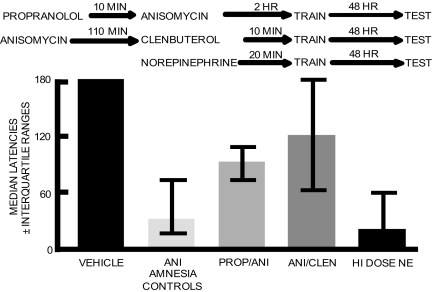
To begin an evaluation of the possibility that changes in release of NE contribute to anisomycin-induced amnesia, and to begin to determine whether it is the increase, decrease, or both in release of NE that might induce the amnesia, a β-adrenergic receptor antagonist, propranolol, and a β-adrenergic agonist, clenbuterol, were used to challenge the anisomycin effects on 48-h memory. The drugs were administered to coincide with the peak increases and decreases in NE release, respectively; the overall design is shown in Fig. 5Upper. Propranolol was injected into the amygdala just before anisomycin injections, i.e., just before the peak increase in release of NE; rats were trained 2 h later. In a separate group, the β-adrenergic receptor agonist, clenbuterol, was injected into the amygdala 110 min after anisomycin injection, i.e., during decreased NE release, 10 min before training.
Fig. 5.
Attenuation and induction of a amnesia with noradrenergic drugs. (Upper) Design for attenuation of anisomycin-induced amnesia with propranolol and clenbuterol. (Lower) Intraamygdala injections of anisomycin 2 h before training impaired memory tested 48 h after training. Propranolol (PROP) was administered 10 min before anisomycin (ANI) injections to reduce the effects on memory of increased release of NE soon after anisomycin treatment. Propranolol significantly attenuated amnesia on test trials 48 h after training administered 2 h after anisomycin treatment (P < 0.05 vs. anisomycin). In addition, clenbuterol (CLEN) was administered 10 min before training (110 min after anisomycin) to reduce the effects of decreased release of NE at this interval after anisomycin treatment. Clenbuterol also attenuated anisomycin-induced amnesia (P < 0.05 vs. anisomycin). To mimic the pulse of NE release after training, a high dose of NE was injected into the amygdala before training. The high dose of NE itself was sufficient to impair memory tested at 48 h (P < 0.05 vs. vehicle controls). The NE treatment was as effective as anisomycin in producing amnesia (NE vs. anisomycin, P > 0.8). n = 23, vehicle with or without propranolol or clenbuterol; n = 25, anisomycin amnesia controls; n = 8, propranolol plus anisomycin; n = 9, anisomycin plus clenbuterol; n = 7, NE.
The behavioral results are shown in Fig. 5 Lower. Note that both propranolol and clenbuterol, injected at times to block or to compensate for the changes in release of NE, significantly attenuated the amnesia produced by anisomycin (both P values < 0.05 vs. anisomycin-vehicle controls). Separate control groups for the various combinations of vehicle × time of injection with and without anisomycin were combined. Twenty-two of 23 learning rats that did not receive anisomycin had retention scores at the 180-sec cutoff latency. Twenty-two of 25 rats that received anisomycin plus vehicle, i.e., baseline controls for anisomycin-induced amnesia, had latencies below 90 sec.
Thus, anisomycin successfully produced anterograde amnesia for 2 h; a time course for anterograde amnesia after anisomycin injection is shown in supporting information (SI) Fig. 7.
Thus, it appears that both the peak and trough changes in release of NE at the site of intraamygdala anisomycin injections may contribute to the resultant impairments in memory. It should be noted, however, that propranolol also has effects on both 5-HT and DA functions (29, 30). The relative contributions of the monoamines to anisomycin-induced amnesia remain to be elucidated. Also, the attenuation of amnesia was not complete. The partial rather than full reversal of amnesia may reflect the effects of anisomycin on multiple neurotransmitter systems besides the biogenic amines. The relative importance of the increases and decreases in release of the monoamines will require additional testing.
Intraamygdala Injections of NE Are Sufficient to Produce Amnesia.
To mimic the effects of anisomycin on NE release, and to test whether NE itself would impair memory, a separate group of rats received intraamygdala injections of a relatively high dose of NE (31) 20 min before training and were tested for memory 48 h later. As shown in Fig. 5 Lower, this group of rats had amnesia (P < 0.05 vs. vehicle controls), with memory scores comparable to those of the anisomycin amnesia group.
Adrenergic Drugs Attenuate Anisomycin-Induced Amnesia Without Concomitant Attenuation of Protein Synthesis Inhibition.
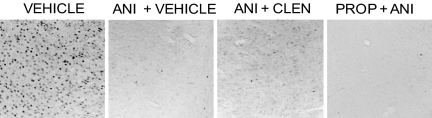
c-Fos immunoreactivity was used as a marker with which to monitor the efficacy of anisomycin-induced inhibition of protein synthesis (27, 28). Fig. 6 shows examples of inhibition of shock-induced c-Fos immunoreactivity in the basolateral amygdala, in sections taken just beyond the cannulae tracks. Brains were removed at 150 min after intraamygdala injections of vehicle, anisomycin, or anisomycin administered with propranolol or clenbuterol at the times used in the behavioral studies. The timing of the samples represented 2 h after injection of anisomycin plus 30 min after training. c-Fos immunoreactivity in the amygdala was essentially abolished by intraamygdala anisomycin injections. Note that the inhibition remained clearly evident in rats that received anisomycin plus propranolol or clenbuterol.
Fig. 6.
Inhibition of c-Fos immunoreactivity in the basolateral amygdala after anisomycin (ANI) and coadministration of propranolol (PROP) or clenbuterol (CLEN). The sections shown were taken one to two sections posterior to the cannula tract, i.e., within 100 μm of the injection. The additional infusion of propranolol or clenbuterol into the amygdala did not substantially affect c-Fos inhibition caused by intraamygdala anisomycin treatment.
Discussion
The findings of these experiments show that the insult of anisomycin injections into the amygdala leads to massive release of biogenic amines near the site of injection at a magnitude well beyond typical physiological values. For comparison to increases of 1,000–15,000% above baseline, behavioral manipulations result in 100–300% increases above baseline in release of these neuromodulators, with recovery to baseline levels typically occurring within 30 min (32–38). The release of neurotransmitters may be a neural response to the onset of protein synthesis inhibition at the site of injection or a secondary effect of anisomycin on monoamine release. Anisomycin injections into the amygdala additionally result in lingering decreases in release, probably resulting from depletion of neurotransmitter stores after the initial surges in release. The sources of the monoamines measured here are from terminals derived from neurons that reside relatively far from the site of injection. It therefore seems unlikely that the neurochemical consequences of anisomycin are based on direct effects of the inhibitor on the monoamine cell bodies of origin. However, anisomycin-induced disruption of the integrity of terminals, followed by compensatory responses to restore release to baseline levels within 48 h, remains possible.
Within the amygdala there is especially good evidence that NE is important for modulating memory (24). The dose–response relationship for the effects on memory of intraamygdala injections of drugs that target NE follow an inverted-U dose–response curve, as do many treatments that enhance memory (39, 40). For example, injections of NE enhance memory at intermediate doses but, as observed in the present studies and others (31, 41), impair memory at high doses; depletion of NE also impairs long-term memory (42). Therefore, either or both the increase and decrease in release of NE might produce the amnesia caused by anisomycin.
There are prior examples of studies showing that adrenergic antagonists block amnesia produced by protein synthesis inhibitors (43). In most studies of this type the attenuating treatments have been drugs that themselves can enhance memory (9, 10, 44). The clenbuterol effects described here fit this category. One interpretation of such studies is that the rescuing treatments might enhance a low level of memory that survives inhibition of protein synthesis and may therefore not directly conflict with the interpretation that protein synthesis inhibition is the primary cause of the amnesia (9, 44). This position offers an alternative explanation for the results obtained with clenbuterol, a treatment that itself enhances memory when injected into the amygdala (24). However, based on the neurochemical results obtained here, we also selected the β-adrenergic receptor antagonist propranolol to attenuate anisomycin-induced amnesia. Propranolol does not have independent memory-enhancing properties and, instead, if the timing of the injection is closer to training, will itself impair memory when injected into the amygdala and related brain areas (e.g., refs. 45–48). Thus, if administered with appropriate timing relative to training and to the neurotransmitter responses to anisomycin, a drug that itself impairs memory when injected into the amygdala under other conditions can attenuate amnesia produced by the protein synthesis inhibitor. It is important to note that both previous (20–23) and present findings indicate that the treatments attenuate amnesia after administration of protein synthesis inhibitors without blocking the level of inhibition of protein synthesis.
The present findings raise several questions regarding the generality of these findings as well as the mechanisms underlying the effects of anisomycin on memory and on neurotransmitter release. Although NE participates significantly in modulation of memory processes, this is a role shared by DA and 5-HT (24–26). In addition, the neurochemical results reported here may extend to neurotransmitters beyond the biogenic amines, a possibility that must also be tested directly in the future. The effects of anisomycin on release of multiple neurotransmitters may explain the partial, rather than full, reversal of amnesia attained with the β-adrenergic receptor agents used in the present experiment. Additional assessments of the generality of these findings to other inhibitors of protein synthesis are also needed. Such experiments would help to determine whether anisomycin-induced effects on neurotransmitter release are a consequence of protein synthesis inhibition generally or are a secondary effect restricted to anisomycin. Moreover, it is unclear whether the neurochemical responses shown here will be evident in brain areas other than the amygdala.
In the present case, the findings support the view that amnesia induced by anisomycin injections into the amygdala may reflect local modulation of memory by NE, DA, and 5-HT (24) rather than identification of de novo protein synthesis necessary for the formation of long-lasting memory (2). A prediction from a theory that memory is established in the amygdala by de novo protein synthesis is that memory tested within hours of training should be intact but memory tested later should be impaired. In contrast, a prediction from a theory that neurotransmitter mechanisms within the amygdala modulate memory formation not only within but also outside of the amygdala is that changes in release of the monoamines might modulate memory at both short and long intervals after training. In one experiment (2), the onset of amnesia followed a nonmonotonic function, with memory impairment evident at 1 and 48 h but not 4 h after training. In another experiment, pretraining intraamygdala injections of anisomycin impaired memory for inhibitory avoidance training assessed at 30 min, 4 h, and 48 h after training (49). These temporal profiles for the development of amnesia are difficult to reconcile with a view that a protein synthesis-dependent memory process follows a protein synthesis-independent memory process. The findings fit more readily into a view that anisomycin results in severe alterations of processes important for modulation of memory, with effects evident at both short and long intervals after training.
Together with the considerable evidence that NE, DA, and 5-HT modulate memory formation, the neurochemical findings presented here support the possibility that the aberrant increases and decreases in release of the biogenic amines may be the mechanism by which intraamygdala injections of anisomycin induce amnesia. The results therefore support the view that anisomycin, as either a direct or indirect consequence of protein synthesis inhibition, imposes abnormal neurochemical changes that disrupt local neuronal function, thereby resulting in interference with memory formation (12).
Thus, at doses that both block protein synthesis and produce amnesia, our results reveal that injections of anisomycin into the amygdala produce substantial release and then depletion of biogenic amines. One of these neurotransmitters in particular, NE, is a well established modulator of memory processes. The memory impairments observed after intraamygdala injections of a high dose of NE provide direct evidence demonstrating that increases in local NE are sufficient to impair memory. Also, timed delivery of noradrenergic receptor antagonists and agonists to mitigate the effects on memory of the release and depletion of NE rescue the memory impairment produced by anisomycin. Thus, our results can explain past findings that the amnestic effects of protein synthesis inhibitors can be reversed by many drugs: The inhibitors produce their effects on memory by altering release of neurotransmitters that modulate memory storage.
The findings described here therefore offer evidence for a substantially different mechanism for the amnesias produced by inhibition of protein synthesis than one of a necessity for de novo protein synthesis for the formation of new long-lasting memories, a conclusion often used as the basis for pursuing changes in gene expression related to memory formation. Importantly, although the present results directly challenge the major interpretation of findings obtained with studies that test effects of protein synthesis inhibitors on memory, the findings offer no direct information regarding the importance, or lack thereof, of de novo protein synthesis in memory formation. Rather, the present findings suggest that tests of such a hypothesis will require a set of approaches other than the use of protein synthesis inhibitors.
Methods
Subjects.
Male Sprague–Dawley rats (90–120 days old; Harlan–Sprague–Dawley, Madison, WI) were housed individually in translucent cages, with food and water available ad libitum. The rats were maintained on a 12:12-h light–dark cycle (lights on at 0700 hours) throughout the experiment.
Surgery.
Rats were anesthetized with isoflurane and then placed in a stereotaxic apparatus with skulls in a horizontal orientation (47). For the microdialysis experiments, a 23-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) was implanted 2 mm above the left amygdala and a microdialysis guide cannula was lowered to 2 mm above the right amygdala [coordinates: −2.9 mm from bregma, ± 4.8 mm lateral; −5.0 mm below dura (50)]. The microdialysis probe was a combination injection–microdialysis probe (MAB 6 injections, 2 mm; SciPro, Sanborn, NY) in which an injection port passes through the microdialysis membrane to the tip of the probe, allowing microinjections during microdialysis. For the adrenergic drug behavioral experiments, 23-gauge guide cannulae (Plastics One) were implanted bilaterally 2 mm above the amygdala. Both the microdialysis probe and microinjection needles extended 2 mm beyond the tip of the guide cannulae. Skull screws were inserted, and the assemblage was anchored in place with dental cement. Stylets flush with the guide cannulae tips were secured in the cannulae. Beginning 1 week after surgery, rats were handled for 5 days before microdialysis, injection, and behavioral procedures.
Microdialysis and Anisomycin Injection Procedures.
In the first microdialysis experiment, rats received bilateral injections of anisomycin 20 min before training, and unilateral (right amygdala) microdialysis was conducted before, during, and after inhibitory avoidance training. Rats were placed in a holding chamber (30 cm long, 30 cm wide, 41 cm deep) with fresh bedding, food, and water during microdialysis. Dialysis probes were inserted into the microdialysis guide cannulae, and brains were perfused continuously at a rate of 2.0 μl/min with artificial cerebrospinal fluid (128 mM NaCl/2.5 mM KCl/1.3 mM CaCl2/2.1 mM MgCl2/0.9 mM NaH2PO4/2.0 mM Na2HPO4/1.0 mM dextrose, adjusted to pH 7.4). To allow equilibration with brain extracellular fluid and to avoid temporary changes in extracellular neurotransmitter levels caused by acute tissue damage (51), the first hour of dialysate was discarded. The time resolution for each microdialysis sample in this experiment was 15 min. Thus, each sample contained 30 μl of dialysate, which was collected into a vial containing 20 μl of 0.2 M acetic acid. After three baseline samples had been collected, injections of anisomycin (Sigma, St. Louis, MO) or vehicle (PBS: 1 mM KH2PO4/155 mM NaCl/3 mM Na2HPO4) were administered bilaterally into the amygdala. Anisomycin was dissolved in 1 M HCl and brought to pH 7.2 with 1 M NaOH and to a concentration of 62.5 μg per 0.5 μl with PBS. The injections were given after the third baseline sample was collected and 20 min before inhibitory avoidance training. Anisomycin injections were administered bilaterally over 2 min (0.25 μl/min) via a CMA/100 microinjection pump (Carnegie Medicin, Stockholm, Sweden). Unilateral microdialysis sampling continued during the injection procedures. After injections, the cannulae were left in place for an additional 1 min before the rats were returned to the holding chamber. The 15-min samples collected from each rat included three baseline samples, one injection sample (in which the microinjection procedure was included), one training sample (in which inhibitory avoidance training was included), and six posttraining samples. Samples were stored in a −20°C freezer until the assay.
The second microdialysis experiment was similar except that the flow rate was slowed to 0.6 μl/min, and microdialysis continued for >8 h after anisomycin or vehicle injections. Samples were collected every 45 min in this experiment, leading to three baseline samples and 11 samples collected during and after the injection. No training was included in this experiment.
Intraamygdala Injections of Anisomycin, Anisomycin Plus Adrenergic Drugs, and NE.
In the adrenergic drug experiments, injection volumes were 0.5 μl per side delivered over 4 min (0.125 μl/min) via a CMA/100 microinjection pump. Rats received bilateral intraamygdala injections of anisomycin (62.5 μg per side), propranolol (propranolol HCl; 1.25 μg per side), clenbuterol (clenbuterol HCl; 100 ng per side), NE (DL-NE HCl; 10 μg per side), or vehicle (PBS or 0.9% saline). Anisomycin was administered 2 h before training. Propranolol was administered 10 min before anisomycin injections. Clenbuterol was administered 10 min before training (110 min after anisomycin injections). Other rats received intraamygdala injections of NE 20 min before training. Vehicle controls were included for each of the drug groups. The experimental design is shown in Fig. 5 Upper.
Behavioral Procedures.
Rats were trained on a one-trial inhibitory avoidance task. The apparatus was a trough-shaped alleyway (91 cm long, 22.9 cm wide at the top, 7.6 cm wide at the bottom, and 15.2 cm deep) divided into lit (31 cm) and dark (60 cm) compartments by a sliding door that could be lowered through the floor. Rats were placed in the lit chamber. Upon entering the dark chamber, the door was closed and the rats received a footshock (0.7 mA, 1.5 sec for microdialysis experiments; 0.5 mA, 1.5 sec for the amnesia attenuation experiment). In the first microdialysis experiment, the rats were returned to the holding cage for continued microdialysis after the shock. In the behavioral experiments, rats were returned to their home cages until memory (latency to enter the dark compartment) was tested 48 h later.
Monoamine Assay Procedures.
Samples were assayed for NE, DA, and 5-HT concentrations using HPLC plus electrochemical detection. Samples were separated by an ODS C18 reverse phase analytical column (HR-80, 3 μm, 100 × 3.2 mm; ESA, Bedford, MA). The mobile phase contained 75 mM NaH2PO4, 1.3 mM SDS, 20 μM EDTA, 10% acetonitrile (vol/vol), 8% methanol (vol/vol), and 0.01% triethylamine (vol/vol) (pH 5.6) and was driven by a solvent delivery system (ESA 580 pump) at a rate of 0.6 ml/min. Samples were automatically injected by a Waters 717plus autoinjector. Electrochemical detection was carried out by ESA Coulochem III detector with Model 5014B analysis cell. The working potentials were set at −175 mV for electrode I, +200 mV for electrode II, and +300 mV for the guard cell. The detection limit of this system was ≈1 pg for each amine.
Histology.
Rats were deeply anesthetized with sodium pentobarbital (125 mg/kg) and were perfused intracardially with 0.9% saline followed by 10% formalin. Brains were removed and placed in a 20% glycerol 0.1 M PB solution for a minimum of 2 days. Frozen sections (50 μm) were collected with a Leica 1800 cryostat. Sections containing the guide cannulae tracts were mounted on slides, dried, and stained with cresyl violet. Behavioral and chemical data were discarded for those rats with one or both injection sites outside the amygdala.
Immunocytochemistry.
In the first experiment, rats received inhibitory avoidance training (0.5 mA/1.5 sec) 20 min after anisomycin or vehicle injections and 60 min before preparation for immunocytochemical assessment of c-Fos immunoreactivity in sections through the infusion sites. In the second experiment, rats received footshock training 30 min before preparation of sections. The groups (n = 2) in Experiment 2 matched those used for inhibitory avoidance training in treatment and timing of treatment: (i) vehicle; (ii) anisomycin plus vehicle; (iii) anisomycin plus clenbuterol; (iv) propranolol plus anisomycin. After footshock training, rats were anesthetized with sodium pentobarbital and were perfused with 0.9% saline followed by freshly prepared 4% paraformaldehyde. Brains were removed and postfixed overnight at 4°C in 4% paraformaldehyde, then cryoprotected in 20% glycerol in 0.1 M PB before sectioning (50 μm) at −30°C. The sections were washed in PBS, followed by incubation in normal goat serum/Triton X-100/H2O2 in PBS for 10 min, then normal goat serum/Triton X-100 in PBS for 15–18 min. The sections were incubated in primary antibody (1:7,500, c-Fos antibody, rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA) for 48 h at 4°C then washed in PBS followed by a 60-min incubation in secondary antibody (1:400 goat anti-rabbit; Santa Cruz Biotechnology). Sections were immunostained by using the ABC Vectastain Elite kit (Vector Laboratories, Burlingame, CA) and diaminobenzidine.
Statistical Analyses.
Inhibitory avoidance scores were analyzed with Mann–Whitney U tests (52). Neurochemical data were analyzed with repeated-measures ANOVAs and post hoc t tests using Statview software. Because the means and standard deviations in treated and untreated groups were extremely different, the data were analyzed by using log10 transforms of the values. For the peak increases and for the later decrease in DA and NE there were no overlaps between groups. Scheffé's post hoc t tests were used to compare anisomycin vs. vehicle results in individual samples.
Supplementary Material
Acknowledgments
We thank Renee Haag and Andrew Flesner for valuable assistance with surgeries and microdialysis. The research was supported by U.S. Public Health Service grants from the National Institute on Aging (Grant AG07648) and the National Institute on Drug Abuse (Grant DA16951).
Abbreviations
- NE
norepinephrine
- DA
dopamine
- 5-HT
serotonin.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705195104/DC1.
References
- 1.Nader K. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 2.Schafe GE, LeDoux JE. J Neurosci. 2000;20(RC96):1–5. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel ER. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 4.Dudai Y. Curr Opin Neurobiol. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 5.Sweatt JD. Curr Biol. 2001;11:R391–R394. doi: 10.1016/s0960-9822(01)00216-0. [DOI] [PubMed] [Google Scholar]
- 6.Routtenberg A, Rekart JL. Trends Neurosci. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Frey U, Morris RG. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 8.Martin KC, Barad M, Kandel ER. Curr Opin Neurobiol. 2000;10:587–592. doi: 10.1016/s0959-4388(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 9.Davis HP, Squire LR. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 10.Martinez JL, Jr, Jensen RA, McGaugh JL. Progr Neurobiol. 1981;16:155–186. doi: 10.1016/0301-0082(81)90011-3. [DOI] [PubMed] [Google Scholar]
- 11.Rudy JW, Biedenkapp JC, Moineau J, Bolding K. Learn Mem. 2006;13:1–3. doi: 10.1101/lm.157806. [DOI] [PubMed] [Google Scholar]
- 12.Gold PE. Learn Mem. 2006;13:506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- 13.Agnihotri NT, Hawkins RD, Kandel ER, Kentros C. Proc Natl Acad Sci USA. 2004;101:3656–3661. doi: 10.1073/pnas.0400385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manahon-Vaughan D, Kulla A, Frey JU. J Neurosci. 2000;20:8572–8576. doi: 10.1523/JNEUROSCI.20-22-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley AE, Andrzejewski ME, Baldwin AE, Hernandez PJ, Pratt WE. Ann NY Acad Sci. 2003;1003:159–168. doi: 10.1196/annals.1300.061. [DOI] [PubMed] [Google Scholar]
- 16.Milekic MH, Alberini CM. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 17.Merlin LR, Bergold PJ, Wong RK. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- 18.Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- 19.Gold PE, McGaugh JL. In: Short Term Memory. Deutsch D, Deutsch JA, editors. New York: Academic; 1975. pp. 355–390. [Google Scholar]
- 20.Flood JF, Jarvik ME, Bennett EL, Orme AE. Behav Biol. 1977;20:168–183. doi: 10.1016/s0091-6773(77)90734-9. [DOI] [PubMed] [Google Scholar]
- 21.Hall ME, Schlesinger K, Stamm E. Pharmacol Biochem Behav. 1976;4:353–355. doi: 10.1016/0091-3057(76)90256-2. [DOI] [PubMed] [Google Scholar]
- 22.Serota RG, Roberts RB, Flexner LB. Proc Natl Acad Sci USA. 1982;69:340–342. doi: 10.1073/pnas.69.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barondes SH, Cohen HD. Proc Natl Acad Sci USA. 1968;61:923–929. doi: 10.1073/pnas.61.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGaugh JL. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 25.LaLumiere RT, Nguyen LT, McGaugh JL. Eur J Neurosci. 2004;20:2804–2810. doi: 10.1111/j.1460-9568.2004.03744.x. [DOI] [PubMed] [Google Scholar]
- 26.Silva RC, Gargaro AC, Brandao ML. Behav Brain Res. 2004;151:93–101. doi: 10.1016/j.bbr.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Inda MC, Delgado-García JM, Carrión ÁM. J Neurosci. 2005;25:2070–2080. doi: 10.1523/JNEUROSCI.4163-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamou CB, Gamache K, Nader K. Nat Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava M, Kapoor NK. J Biosci. 1983;5:261–266. [Google Scholar]
- 30.Alexander BS, Wood MD. J Pharm Pharmacol. 1987;39:664–666. doi: 10.1111/j.2042-7158.1987.tb03452.x. [DOI] [PubMed] [Google Scholar]
- 31.Liang KC, McGaugh JL, Yao HY. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre CK, Hatfield T, McGaugh JL. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 33.Ma S, Morilak DA. J Neuroendocrinol. 2005;17:22–28. doi: 10.1111/j.1365-2826.2005.01279.x. [DOI] [PubMed] [Google Scholar]
- 34.Shearman E, Rossi S, Sershen H, Hashim A, Lajtha A. Neurochem Res. 2005;30:1055–1066. doi: 10.1007/s11064-005-7132-9. [DOI] [PubMed] [Google Scholar]
- 35.Hassert DL, Miyashita T, Williams CL. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- 36.Clayton EC, Williams CL. Behav Brain Res. 2000;112:151–158. doi: 10.1016/s0166-4328(00)00178-9. [DOI] [PubMed] [Google Scholar]
- 37.Miyashita T, Williams CL. Behav Neurosci. 2002;116:13–21. doi: 10.1037//0735-7044.116.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Williams CL, Men D, Clayton EC, Gold PE. Behav Neurosci. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- 39.Koob GF. In: Perspectives on Cognitive Neuroscience. Lister RG, Weingartner HJ, editors. London: Oxford Univ Press; 1991. pp. 300–313. [Google Scholar]
- 40.Gold PE. In: Brain and Memory: Modulation and Mediation of Neural Plasticity. McGaugh JL, Weinberger NM, Lynch GS, editors. New York: Oxford Univ Press; 1995. pp. 41–74. [Google Scholar]
- 41.Decker MW, McGaugh JL. Brain Res. 1989;477:29–37. doi: 10.1016/0006-8993(89)91391-7. [DOI] [PubMed] [Google Scholar]
- 42.Liang KC. Chin J Physiol. 1998;41:223–233. [PubMed] [Google Scholar]
- 43.Gold PE, Sternberg DB. Science. 1978;201:367–369. doi: 10.1126/science.208153. [DOI] [PubMed] [Google Scholar]
- 44.Squire LR. Learn Mem. 2006;13:522–529. doi: 10.1101/lm.310306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lennartz RC, Hellems KL, Mook ER, Gold PE. Behav Neurosci. 1996;110:1–7. doi: 10.1037//0735-7044.110.5.1033. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Proc Natl Acad Sci USA. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaLumiere RT, McGaugh JL. Learn Mem. 2005;12:527–532. doi: 10.1101/lm.97405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Debiec J, LeDoux JE. Ann NY Acad Sci. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- 49.Canal CE, Gold PE. Behav Neurosci. 2007 doi: 10.1037/0735-7044.121.4.732. in press. [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. North Ryde, Australia: Academic; 1986. [Google Scholar]
- 51.Westerink BHC, Timmerman W. Anal Chim Acta. 1999;379:263–274. [Google Scholar]
- 52.Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw–Hill; 1956. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.