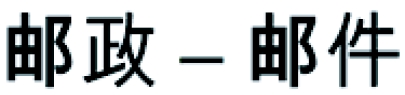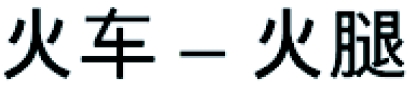Abstract
Whether the native language of bilingual individuals is active during second-language comprehension is the subject of lively debate. Studies of bilingualism have often used a mix of first- and second-language words, thereby creating an artificial “dual-language” context. Here, using event-related brain potentials, we demonstrate implicit access to the first language when bilinguals read words exclusively in their second language. Chinese–English bilinguals were required to decide whether English words presented in pairs were related in meaning or not; they were unaware of the fact that half of the words concealed a character repetition when translated into Chinese. Whereas the hidden factor failed to affect behavioral performance, it significantly modulated brain potentials in the expected direction, establishing that English words were automatically and unconsciously translated into Chinese. Critically, the same modulation was found in Chinese monolinguals reading the same words in Chinese, i.e., when Chinese character repetition was evident. Finally, we replicated this pattern of results in the auditory modality by using a listening comprehension task. These findings demonstrate that native-language activation is an unconscious correlate of second-language comprehension.
Keywords: bilingualism, event-related potentials, language access, semantic priming, unconscious priming
Some studies in cognitive neuroscience have suggested that fluent bilinguals can effectively inhibit their first language when accessing word meaning in their second language based on the word form (1). However, this finding conflicts with functional neuroimaging data showing overlapping cortical representation of the two languages (2, 3). A number of psycholinguistic experiments have also suggested that the two languages mastered by one individual are constantly coactivated and interactive (4–7), whereas others have provided evidence for language independence (8, 9). It therefore remains an open question whether or not bilingual individuals can effectively suppress all interference from their first language when processing their second language (10).
Previous studies have made extensive use of cross-language priming (6, 9, 11) or overt translation tasks (12, 13) to compare native- and second-language activation in bilinguals. For example, reaction time is reduced in French–English bilinguals when the English word money is presented after the French word coin “corner” relative to when it is presented after feuille “leaf.” However, mixing stimuli from two languages creates an artificial context that necessarily biases the output of behavioral tests toward a bilingual or “dual-language” activation pattern (14). For that matter, translation tasks are even more biased because they require conscious access to both languages. In fact, any experiment mixing stimuli from two languages or using interlingual homographs is likely to activate both languages, even if native-language activation is not automatic during everyday second-language comprehension. By contrast, studies using the masked priming paradigm, in which participants are generally unaware of the prime, can be considered functionally monolingual (15–17). However, in such studies, the magnitude of the priming effect is strongly influenced by the level of masking, especially when the prime is presented very briefly (e.g., 50 ms) in the second language (18, 19). Here, we avoided artificial dual-language activation and attenuations relating to masking by testing implicit native-language access in conditions where both the prime and the target are fully visible in a second-language context free of any explicit reference to the first language.
Event-related potentials (ERPs) provide a continuous account of brain activity time-locked to an external stimulus. The fine temporal resolution of ERPs makes them an ideal tool for investigating neural stages of language comprehension. One well established ERP correlate of language processing is the N400, which has been shown to index semantic integration processes (20, 21) and unconscious priming (ref. 22; for a review, see ref. 23). ERP studies have revealed aspects of second-language processing that cannot be detected on the basis of behavioral measurements alone (refs. 24–26; for a review, see ref. 27).
We collected behavioral and electrophysiological data in 15 Chinese–English bilinguals who acquired English after the age of 12 (late fluent bilinguals) and 15 monolingual controls performing a semantic relatedness task exclusively on English word pairs. In each trial the prime and target words were either related in meaning (e.g., post–mail) or not (e.g., train–ham; Table 1). Unknown to the participants, half of the word pairs were chosen such that they shared a character when translated into Chinese. The words train and ham, for instance, are not related in meaning but their Chinese translations Huo Che and Huo Tui have a Chinese character in common. This made the design a fully balanced 2 × 2 factorial design with one overt factor (semantic relatedness) and one hidden factor (character repetition in Chinese). Mandarin Chinese is an “ideographic language,” radically different from languages written in the Roman alphabet (e.g., English or French). Therefore, any significant effect of the concealed Chinese character repetition in bilinguals reading English words would demonstrate spontaneous activation of the native language. We also tested 15 Chinese monolingual controls on Chinese translations of the English material. Finally, the full experimental design was tested again in the auditory modality in 45 other participants (15 in each of the three experimental groups).
Table 1.
Experimental design and stimulus examples
Each cell contains one example of an English word pair, its Mandarin Chinese translation, the corresponding Chinese Pin Yin (alphabetic transposition of the phonological form), and the mean semantic relatedness of the words in English (SRE) and Chinese (SRC). Standard deviation of the mean relatedness is given in parentheses. SRE of word pairs was rated on a scale from 1 to 5 by a group of 25 native English speakers, and the Chinese translations (SRC) were rated by a group of 21 native Chinese speakers. None of the evaluators were involved in the ERP experiments. Difference in semantic relatedness was highly significant between S+ and S− pairs (P < 0.0001 for all pairwise comparisons), but there was no difference in semantic relatedness induced by Chinese character repetition, whether it was hidden (English) or visible (Chinese; P > 0.1 for all pairwise comparisons).
Results
The main results reported here are those of the reading experiment and the listening experiment is regarded as a replication study.
Behavioral (Surface) Effects.
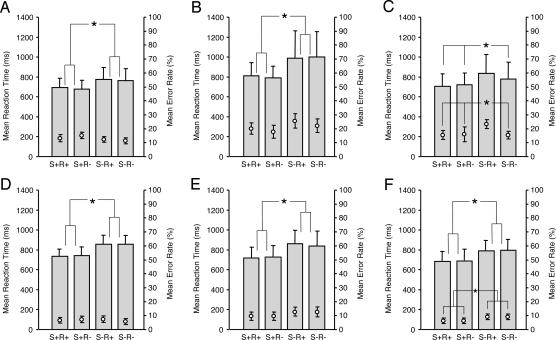
In the reading experiment, as expected, English participants responded faster to semantically related than to unrelated word pairs (F1,14 = 32.2, P < 0.001; Fig. 1A) and showed no effect of concealed Chinese character repetition (F1,14 = 1.9, P > 0.1). Error rates were unaffected by semantic relatedness (F1,14 = 1.7, P > 0.1) or Chinese character repetition (F1,14 = 0.7, P > 0.1).
Fig. 1.
Reaction times (bars, left axis) and error rates (bullets, right axis) for the Chinese–English bilinguals (B and E) and the two monolingual control groups [English (A and D) and Chinese (C and F)]. (A–C) Reading experiment. (D–F) Listening experiment. Conditions in which the target was semantically related/unrelated are labeled S+/S−, respectively. Conditions in which one Chinese character was repeated/not repeated are labeled R+/R−, respectively. Stars indicate significant differences (P < 0.05). Error bars depict standard deviation in all cases.
The same overall pattern of performance was found in the Chinese–English bilingual participants (Fig. 1B). Semantically related word pairs were responded to faster than semantically unrelated word pairs (F1,14 = 28.4, P < 0.001) and no effect of Chinese character repetition was found (F1,14 = 0.2, P > 0.1). Similarly, error rates were not significantly affected by either factor (semantic relatedness, F1,14 = 2.2, P > 0.1; Chinese character repetition, F1,14 = 3.6, P = 0.08).
In the Chinese monolingual participants reading Chinese translations of the English words, semantically related word pairs were responded to faster than semantically unrelated word pairs (F1,14 = 10.4, P < 0.001) but we found an interaction between semantic relatedness and Chinese character repetition for both reaction times (F1,14 = 20.6, P < 0.001) and error rates (F1,14 = 11.6, P < 0.01; Fig. 1C). Post hoc pairwise comparisons showed that semantically unrelated words sharing a Chinese character yielded significantly longer reaction time and higher error rates than all other conditions (all P < 0.01).
In the listening experiment, the same overall pattern of behavioral performance was found in the English monolinguals and the Chinese–English bilinguals (all P < 0.001; Fig. 1 D and E). In Chinese monolinguals, however, there was a main effect of semantic relatedness on error rates (F1, 14 = 4.88, P < 0.05) and reaction times (F1, 14 = 35.1, P < 0.001), such that semantic relatedness increased error rates and decreased reaction times (Fig. 1F).
ERP (Implicit) Effects.
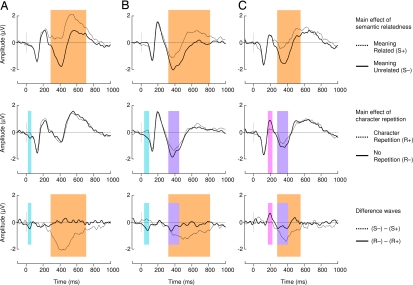
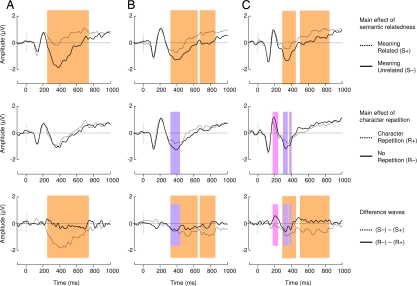
In the reading experiment, we found early differences between the repeated character and unrepeated character conditions between ≈30 and ≈90 ms in English monolinguals and Chinese–English bilinguals but not Chinese monolinguals (Fig. 2, blue boxes). In the interest of clarity, these differences, attributed to low-level perceptual processing induced by inexorable word length differences (see Discussion and Experimental Procedures), are addressed separately.
Fig. 2.
ERP results in the reading experiment for English monolinguals (A), Chinese–English bilinguals (B), and Chinese monolinguals (C). All waveforms depict brain potential variations in the linear derivation of a group of nine electrodes centered on Cz where the N400 component is typically maximal (FC1, FC2, FCz, C1, C2, Cz, CP1, CP2, CPz). Color boxes indicate significant differences elicited by semantic relatedness in the N400 range (orange) and significant differences elicited by form repetition in the P2 range (pink) and the N400 range (purple). Early perceptual variations attributed to differences in word length are highlighted in blue. Note that the latter do not perseverate into the N1/P2 window.
In English monolinguals, semantic relatedness reduced ERP mean amplitude significantly between 350 and 500 ms (F1,14 = 89, P < 0.0001), which is the N400 component typical window (20, 21). Hidden Chinese character repetition had no effect in the N400 range in this group (F1,14 = 1.89, P > 0.1), and no other amplitude modulation was found on other main ERP components (Fig. 2A).
In Chinese–English bilinguals, there was a main effect of semantic relatedness (F1,14 = 12.2, P < 0.004), which was significantly smaller in magnitude than that found in English monolinguals (F1,29 = 14.79, P < 0.001). Critically, we also found a hidden Chinese character repetition main effect (F1,14 = 8.3, P < 0.01), which did not interact with the semantic effect (F1,14 = 0.18, P > 0.1). Mean N400 amplitude was reduced for semantically related targets as compared with unrelated targets and for targets that shared a Chinese character with the prime through translation as compared with targets with no character repetition. Moreover, the N400 modulation elicited by semantic relatedness was of greater magnitude and lasted longer than that induced by character repetition. No other ERP peak was modulated in amplitude or latency by the experimental factors (Fig. 2B).
In Chinese monolinguals who read Chinese translations of the English stimuli, the same pattern of priming was found as was seen in bilinguals (Fig. 2C). There was a main effect of semantic relatedness (F1,14 = 23.5, P < 0.0001) and overt Chinese character repetition (F1,14 = 5.13, P < 0.04), but no interaction (F1,14 = 0.53, P > 0.1). Interestingly, the N400 modulation induced by semantic relatedness was greater and more durable than that elicited by character repetition, reproducing the pattern of variations found in Chinese–English bilinguals. In addition, in this group we found a main effect of overt Chinese character repetition on the amplitude of the P2 component (F1,14 = 8.1, P < 0.02), between 150 and 200 ms. The P2 was reduced by character repetition priming but was insensitive to semantic priming (F1,14 = 0.02, P > 0.1) and there was no interaction (F1,14 = 0.09, P > 0.1).
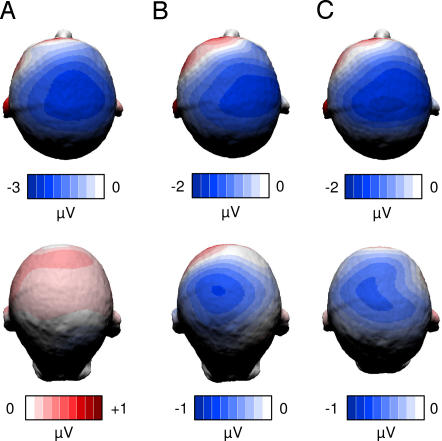
ERP scalp topographies were not significantly different either between the three groups with regard to the semantic relatedness main effect or between the Chinese–English bilinguals and Chinese monolinguals with regard to the Chinese character repetition main effect (Fig. 3).
Fig. 3.
Scalp topographies of ERP differences elicited by the two experimental factors [semantic relatedness (Upper) and character repetition (Lower)] for English monolinguals (A), Chinese–English bilinguals (B), and Chinese monolinguals (C).
Replication of ERP Effects in the Auditory Modality.
In the listening experiment, ERP effects overall replicated those found in the reading experiment. (i) In English monolinguals, semantic relatedness reduced ERP mean amplitude significantly between 350 and 500 ms (F1,14 = 24.3, P < 0.0001) but Chinese character repetition had no effect (F1,14 = 0.33, P > 0.1) and there was no interaction (F1,14 = 0, P > 0.1; Fig. 4A). (ii) In Chinese–English bilinguals, there was a main effect of semantic relatedness (F1,14 = 19.3, P < 0.001) and the Chinese character repetition also reduced N400 amplitude significantly (F1,14 = 5.2, P < 0.05), in the absence of an interaction between the two factors (F1,14 = 0.3, P > 0.1; Fig. 4B). (iii) In Chinese monolinguals who listened to Chinese translations, there was a main effect of semantic relatedness (F1,14 = 20.5, P < 0.0001) and overt Chinese character repetition (F1,14 = 4.9, P < 0.05) and no interaction (F1,14 = 0.05, P > 0.1; Fig. 4C). As in the reading experiment, the N400 modulation induced by semantic relatedness was greater and more durable than that elicited by character repetition in both the Chinese–English bilinguals and the Chinese monolingual controls. In the latter group, moreover, the P2 was reduced by character repetition priming (F1,14 = 7.5, P < 0.02) but was insensitive to semantic priming (F1,14 = 1.5, P > 0.1) and there was no interaction (F1,14 = 0.1, P > 0.1).
Fig. 4.
ERP results in the listening experiment for English monolinguals (A), Chinese–English bilinguals (B), and Chinese monolinguals (C). All waveforms depict brain potential variations in the linear derivation of a group of nine electrodes centered on Cz where the N400 component is typically maximal (FC1, FC2, FCz, C1, C2, Cz, CP1, CP2, CPz). Color boxes indicate significant differences elicited by semantic relatedness in the N400 range (orange) and significant differences elicited by form repetition in the P2 range (pink) and the N400 range (purple).
The only results that differed in the listening experiment from those in the reading experiment were: (i) the absence of differences between 30 and 90 ms in all groups and contrasts, and (ii) the more extended time course of the N400 modulation by semantic relatedness (slightly earlier onset and longer duration).
Discussion
Using an implicit priming paradigm, we tested whether Chinese–English bilinguals spontaneously access Chinese translations when reading or listening to English words. Despite the absence of any measurable effect of concealed Chinese character repetition on the behavioral performance of bilingual participants, this hidden repetition modulated ERPs, just as it did in monolingual Chinese controls overtly exposed to character repetition in Chinese.
The character repetition priming was indexed by an amplitude reduction of the N400 component, which is known to be sensitive to overt (20, 21) and unconscious (22) semantic priming and to repetition priming (28, 29). ERP modulations elicited by the two factors appeared in the same temporal window and can only be explained by activation of Chinese translations in bilinguals, because semantic relatedness and character repetition were built in as independent factors (Table 1). Furthermore, the activation of translation equivalents was unconscious because, at debriefing, none of the bilingual participants reported being aware of the hidden factor when questioned about the English words presented.
All participants showed the well established N400 modulation by semantic priming (20, 21), whether words were presented in their first or their second language and whether they were presented visually or auditorily. It is noteworthy, however, that the magnitude of the N400 modulation was larger in English monolinguals than in Chinese–English bilinguals, even though the two groups of participants read the same words. Such observations have been made previously (30–32) and can be related to the relative efficiency of semantic access in first and second languages, respectively.§
The fact that English monolinguals only showed an effect of semantic relatedness in the ERPs confirmed that the N400 modulation by Chinese character repetition seen in the bilinguals was not caused by spurious, confounding semantic effects but was genuinely induced by implicit character repetition priming. Furthermore, the pattern of semantic relatedness and character repetition priming seen in bilinguals was remarkably similar to that found in Chinese monolinguals reading Chinese translations. In particular, both groups of Chinese participants displayed large N400 modulations by semantic priming and smaller, less durable N400 modulations by character repetition, whether the latter was implicit (Chinese–English bilinguals) or overtly perceived (Chinese monolinguals). This pattern is consistent with previous reports of weaker variations in the N400 range elicited by orthographic and/or phonological overlap between words as compared with semantic relationships (34, 35). Critically, the character repetition effect was of similar amplitude in Chinese–English bilinguals and Chinese bilinguals, which is fully consistent with spontaneous activation of translation equivalents in the bilinguals. Furthermore, this character repetition effect was found in both a reading and a listening task, which demonstrates that it is modality-independent. Note, however, that this effect need not be symmetrical, i.e., effects of second-language knowledge on first-language processing are likely to be weaker (36, 37).
Our previous attempt to identify spontaneous translation effects failed to show Chinese activation in the absence of interference with semantic processing in English (10). We see two reasons the independence of the two factors described here was never shown before to our knowledge. (i) In ref. 10, word concreteness (see ref. 38 for a definition) was not controlled and post hoc comparisons of available concreteness ratings (39) for the stimuli used at the time revealed significant differences between conditions. (ii) The Chinese translations of the previous stimulus set were one to three Chinese characters in length, and the repeated character was not systematically positioned at the same place in the translations. The first issue might have affected the route by which bilingual participants accessed the meaning of English words in the different conditions (40–42). Moreover, word concreteness is known to affect the amplitude of the N400, such that concrete words tend to elicit greater N400 amplitudes than abstract words (43, 44). In sum, uncontrolled concreteness effects probably introduced noise into the response pattern of monolingual English controls and not necessarily with the same effect and to the same extent as in Chinese–English bilinguals. The second issue is likely to reduce repetition priming because no systematic unconscious template can be formed in which to expect character repetition to occur. In addition, the degree to which repetition priming is reduced need not be the same for semantically related and unrelated conditions.
Here, (i) we matched words for lexical frequency and concreteness between conditions, (ii) translations systematically involved two Chinese characters, (iii) character repetition consistently appeared at the same position within Chinese translations of each word pair (see Experimental Procedures), and, critically, (iv) we also tested a control group of 15 Chinese monolinguals presented with the Chinese translations of the English material. The parallel results obtained for Chinese–English bilinguals and Chinese monolingual controls strongly support the conclusion that the mechanisms operating explicitly in monolinguals and implicitly in the bilinguals are analogous. This conclusion is further supported by the replication in the auditory modality.
Because Chinese monolingual participants actually saw or heard the repeated Chinese characters, we expected to see some early orthographic and/or phonological priming effect of Chinese character repetition in these groups. Indeed, the P2 component sensitive to perceptual priming (29, 45) was significantly reduced when a Chinese character was repeated but was unaffected by semantic relatedness (Figs. 2C and 4C). This P2 modulation, which preceded the N400 effect by at least 100 ms, was seen in neither Chinese–English bilinguals nor English monolinguals. The absence of a priming effect before the N400 window in bilinguals also suggests that translation took place at a late, possibly postlexical processing stage, i.e., during and after word meaning retrieval.
The only measurable effect of Chinese character repetition in the behavioral data were found in the reading experiment in Chinese monolingual participants, who were explicitly aware of the repetition. Reaction time and error rate were both significantly greater when the second word of a pair shared a Chinese character but was unrelated in meaning to the first (S−R+). Here, the conflict may have arisen in semantically unrelated pairs that share a Chinese character because the repetition implicitly hinted at a semantic link that was not actually present. The absence of such a behavioral effect in the bilingual participants further supports the view that first-language activation was implicit and unconscious. In the listening experiment, however, the S−R+ condition did not yield longer reaction times or greater error rates than the S+R+ condition. There are two possible explanations for this result. When words were presented auditorily, (i) the repeated characters were temporally further apart than when words were presented visually, and (ii) characters were perceived phonologically whereas their visual form was likely to activate both orthographic and phonological representations.
One peculiarity of the reading experiment data was the finding of significant differences between ≈30 and ≈90 ms between the R+ and R− conditions in the English monolinguals and the Chinese–English bilinguals (Fig. 2 D and E). We interpret this difference as a consequence of word length differences between conditions (see Experimental Procedures) because such differences (i) have been found to elicit ERP modulations within 100 ms of stimulus onset (46–48), (ii) were significant in both Chinese–English bilinguals and English monolinguals who were exposed to the same stimuli, (iii) were not found in the Chinese monolinguals who read Chinese translations of equal length in all conditions, (iv) were not found in comparisons between S+ and S− conditions, which did not differ with respect to average stimulus word length, and (v) did not persist beyond 100 ms in either the Chinese–English bilinguals or the English monolinguals. Critically, these early differences did not affect the N1/P2 complex and therefore cannot account for significant main effects of character repetition later seen in the N400 time window. Finally, it is noteworthy that such early differences were not seen at all in any of the groups in the listening experiment, and yet a clear N400 effect was also seen for character repetition in that experiment.
In sum, our electrophysiological results reveal an automatic translation process in late fluent bilinguals that could not be detected with traditional behavioral measures. This finding provides an account for parallel, language-nonselective activation models of bilingual word recognition (49, 50). In fact, although we found no evidence of prelexical access to native translations when bilinguals read or listen to words in their second language, the postlexical translation mechanism revealed by the N400 reduction appears to be totally automatic and unconscious (22). Access to word meaning in a second language may thus well be direct but it nevertheless spontaneously activates the native language lexicon.
Conclusion
Neuroimaging studies have shown common or partially overlapped cortical areas associated with the two languages of bilinguals in a variety of tasks (3, 51–56). These tasks systematically involve spoken or written words from the two languages or require switching mentally between languages. The present study makes a direct observation of spontaneous lexical activation of the native language during an experiment involving only second-language stimuli. This result suggests that native-language activation operates in everyday second-language use, in the absence of awareness on the part of the bilingual speaker. Future studies will determine how proficiency in a second language affects implicit native-language activation and the extent to which interactions between first and second languages are asymmetrical.
Experimental Procedures
Participants.
We tested 90 participants in total: 30 native English speakers, 30 Chinese monolinguals, and 30 Chinese–English bilinguals. All had normal or corrected-to-normal vision and self-reported normal hearing. They gave written consent to take part in the experiments that were approved by the ethics committee of the University of Wales. Participants were controlled for age (19–25 years), level of education, and handedness (right) across groups. The bilingual participants were first exposed to English at the age of 12; by the time of testing, they were studying at a British university and had lived in the United Kingdom for a mean of 18.3 months (±4.78). All bilinguals used English in their everyday life and had an English proficiency score of 6 or 6.5 as measured by the International English Language Testing System (www.ielts.org/candidates/findoutmore/article255.aspx).
Stimuli.
The 200 word pairs used were matched across experimental conditions for lexical frequency and word concreteness (39). The repeated Chinese characters were both logographically and phonologically identical and always appeared in the same position in the two words of a pair (Table 1). In the reading experiment, no English word had >11 letters and all Chinese translations featured two Chinese characters. Conditions were not balanced for average word length in English, however, because of the need to control the other experimental factors. As a consequence, average visual word length was significantly longer in the repeated character conditions (R+) as compared with the unrepeated conditions (R−; P < 0.001). There were no other differences in visual word length between conditions. Participants viewed two blocks of 100 word pairs presented in a pseudorandomized order. After a prestimulus interval of 200 ms, the first word was flashed for 500 ms at fixation followed by the second word after a variable interstimulus interval of 500, 600, or 700 ms. In the listening experiment, the average length of English words was 4.9 ± 2 phonemes. There were no significant differences in the number of phonemes in pairwise comparisons between conditions (all P > 0.1). Participants heard digitized words pronounced by a native female speaker of English or Chinese. Prime words were presented within a 1,000-ms time window followed by a target word after a variable interval of 500, 600, or 700 ms. No word was repeated in either of the studies. Participants were instructed to indicate whether the second word was related in meaning to the first by pressing keys. Response sides were fully counterbalanced between blocks and participants.
ERP Recording.
Electrophysiological data were recorded in reference to Cz at a rate of 1 kHz from 64 Ag/AgCl electrodes placed according to the extended 10–20 convention. Impedances were kept <5 kΩ. Electroencephalogram activity was filtered on-line band pass between 0.1 and 200 Hz and refiltered off-line with a 25-Hz, low-pass, zero-phase shift digital filter. Eye blinks were mathematically corrected, and remaining artefacts were manually dismissed. There was a minimum of 30 valid epochs per condition in every subject. Epochs ranged from −100 to 1,000 ms after the onset of the second word. Baseline correction was performed in reference to prestimulus activity, and individual averages were digitally re-referenced to the global average reference. ERP data were collected simultaneously to behavioral data.
ERP Data Analysis.
Peak detection was carried out automatically, time-locked to the latency of the peak at the electrode of maximal amplitude on the grand-average ERP. Temporal windows for peak detection were determined based on variations of the Global Field Power measured across the scalp (57). Peak amplitudes were subjected to a repeated measures ANOVA with semantic relatedness (related/unrelated), character repetition (repeated/unrepeated), and electrode (63 levels) as factors using a Greenhouse-Geisser correction where applicable. Pairwise differences between conditions were considered significant when differences were above threshold (P < 0.01) for >30 ms over a minimum of three clustered electrodes. Topographical analyses were based on mean amplitudes measured over 63 electrodes distributed over the entire scalp. Between-group comparisons involved calculating main-effect contrasts (semantically unrelated–semantically related and no character repetition–character repetition) and differences in mean amplitudes were entered into a between-subject repeated measures ANOVA with 63 levels of electrodes. Interactions involving the electrode factor were controlled by using within condition vector normalization (58).
Acknowledgments
We thank Jean-François Démonet, Tim Fosker, Steve Luck, Clara Martin, Marilyn Vihman, and two reviewers for useful discussions and Mark Roberts for technical assistance. This work was supported by Economic and Social Research Council Grant ES/E024556/1 and Biotechnology and Biological Science Research Council U.K. Grant S18007. Y.J.W. is supported by the Overseas Research Scheme.
Abbreviation
- ERP
event-related potential.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
We note that the waveform structure in the semantically related condition differed between English monolinguals and Chinese–English bilinguals. This difference may be accounted for by partial overlap with P300-type activity peaking ≈600 ms in the case of lexical–semantic tasks and associated with target detection in English monolinguals (33).
References
- 1.Rodriguez-Fornells A, Rotte M, Heinze HJ, Nosselt T, Munte TF. Nature. 2002;415:1026–1029. doi: 10.1038/4151026a. [DOI] [PubMed] [Google Scholar]
- 2.Kim KH, Relkin NR, Lee KM, Hirsch J. Nature. 1997;388:171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- 3.Chee MW, Tan EW, Thiel T. J Neurosci. 1999;19:3050–3056. doi: 10.1523/JNEUROSCI.19-08-03050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroll JF, De Groot AMB. Handbook of Bilingualism: Psycholinguistic Approaches. New York: Oxford Univ Press; 2005. [Google Scholar]
- 5.Kroll JF, Stewart E. J Mem Lang. 1994;33:149–174. [Google Scholar]
- 6.Dijkstra T, Timmermans M, Schriefers H. J Mem Lang. 2000;42:455–464. [Google Scholar]
- 7.Dijkstra T, Van Heuven WJB. Biling Lang Cogn. 2002;5:175–197. [Google Scholar]
- 8.Scarborough DL, Gerard L, Cortese C. J Verb Learn Verb Behav. 1984;23:84–99. [Google Scholar]
- 9.Gerard LD, Scarborough DL. J Exp Psychol Learn Mem Cogn. 1989;15:305–313. [Google Scholar]
- 10.Thierry G, Wu YJ. NeuroReport. 2004;15:1555–1558. doi: 10.1097/01.wnr.0000134214.57469.c2. [DOI] [PubMed] [Google Scholar]
- 11.Beauvillain C, Grainger J. J Mem Lang. 1987;26:658–672. [Google Scholar]
- 12.De Groot AMB, Hoeks JCJ. Lang Learn. 1995;45:683–724. [Google Scholar]
- 13.Dufour R, Kroll JF. Mem Cogn. 1995;23:166–180. doi: 10.3758/bf03197219. [DOI] [PubMed] [Google Scholar]
- 14.Grosjean F. Biling Lang Cogn. 1998;1:131–149. [Google Scholar]
- 15.De Groot AMB, Nas GLJ. J Mem Lang. 1991;30:90–123. [Google Scholar]
- 16.Gollan TH, Forster KI, Frost R. J Exp Psychol Learn Mem Cogn. 1997;23:1122–1139. doi: 10.1037//0278-7393.23.5.1122. [DOI] [PubMed] [Google Scholar]
- 17.Brysbaert M, Van Dyck G, Van de Poel M. J Exp Psychol Hum Percept Perform. 1999;25:137–148. doi: 10.1037//0096-1523.25.1.137. [DOI] [PubMed] [Google Scholar]
- 18.Grainger J, Frenck-Mestre C. Lang Cogn Proc. 1998;13:601–623. [Google Scholar]
- 19.Jiang N. Biling Lang Cogn. 1999;2:59–75. [Google Scholar]
- 20.Kutas M, Hillyard SA. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- 21.Kutas M, Hillyard SA. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- 22.Luck SJ, Vogel EK, Shapiro KL. Nature. 1996;383:616–618. doi: 10.1038/383616a0. [DOI] [PubMed] [Google Scholar]
- 23.Kutas M, Federmeier K. Trends Cogn Sci. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- 24.Kotz SA, Elston-Guttler KE. J Neurolingu. 2004;17:215–235. [Google Scholar]
- 25.McLaughlin J, Osterhout L, Kim A. Nat Neurosci. 2004;7:703–704. doi: 10.1038/nn1264. [DOI] [PubMed] [Google Scholar]
- 26.Tokowicz N, MacWhinney B. Stud Second Lang Acquisition. 2005;27:173–204. [Google Scholar]
- 27.Mueller JL. Second Lang Res. 2005;21:152–174. [Google Scholar]
- 28.Osterhout L, Holcomb P. In: Electrophysiological Studies of Human Cognitive Function. Coles MRM, editor. Oxford: Oxford Univ Press; 1995. pp. 171–215. [Google Scholar]
- 29.Liu Y, Perfetti CA, Hart L. J Exp Psychol Learn Mem Cogn. 2003;29:1231–1247. doi: 10.1037/0278-7393.29.6.1231. [DOI] [PubMed] [Google Scholar]
- 30.Ardal S, Donald MW, Meuter R, Muldrew S, Luce M. Brain Lang. 1990;39:187–205. doi: 10.1016/0093-934x(90)90011-5. [DOI] [PubMed] [Google Scholar]
- 31.Kutas M, Kluender R. In: Cognitive Electrophysiology: Basic and Clinical Applications. Heinze HJ, Munte TF, Mangun GR, editors. Boston: Birkhauser; 1991. pp. 183–210. [Google Scholar]
- 32.Hahne A. J Psycholinguist Res. 2001;30:251–266. doi: 10.1023/a:1010490917575. [DOI] [PubMed] [Google Scholar]
- 33.Polich J, Donchin E. Electroencephalogr Clin Neurophysiol. 1988;70:33–45. doi: 10.1016/0013-4694(88)90192-7. [DOI] [PubMed] [Google Scholar]
- 34.Rugg MD, Barrett SE. Brain Lang. 1987;32:336–361. doi: 10.1016/0093-934x(87)90132-5. [DOI] [PubMed] [Google Scholar]
- 35.Perrin F, Garcia-Larrea L. Brain Res Cogn Brain Res. 2003;17:36–47. doi: 10.1016/s0926-6410(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 36.Van Wijnendaele I, Brysbaert M. J Exp Psychol Hum Percept Perform. 2002;28:616–627. [PubMed] [Google Scholar]
- 37.Van Hell JG, Dijkstra T. Psychon Bull Rev. 2002;9:780–789. doi: 10.3758/bf03196335. [DOI] [PubMed] [Google Scholar]
- 38.Fliessbach K, Weis S, Klaver P, Elger CE, Weber B. NeuroImage. 2006;32:1413–1421. doi: 10.1016/j.neuroimage.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Coltheart M. Q J Exp Psychol. 1981;33:497–505. [Google Scholar]
- 40.Paivio A, Clark JM, Lambert WE. J Exp Psychol Learn Mem Cogn. 1988;14:163–172. [Google Scholar]
- 41.Paivio A, Desrochers A. Can J Psychol. 1980;34:388–399. [Google Scholar]
- 42.De Groot AMB. J Exp Psychol Learn Mem Cogn. 1992;18:1001–1018. [Google Scholar]
- 43.Kounios J, Holcomb PJ. J Exp Psychol Learn Mem Cogn. 1994;20:804–823. doi: 10.1037//0278-7393.20.4.804. [DOI] [PubMed] [Google Scholar]
- 44.West WC, Holcomb PJ. J Cogn Neurosci. 2000;12:1024–1037. doi: 10.1162/08989290051137558. [DOI] [PubMed] [Google Scholar]
- 45.van Schie HT, Wijers AA, Kellenbach ML, Stowe LA. Brain Lang. 2003;86:300–325. doi: 10.1016/s0093-934x(02)00546-1. [DOI] [PubMed] [Google Scholar]
- 46.Assadollahi R, Pulvermuller F. NeuroReport. 2003;14:1183–1187. doi: 10.1097/00001756-200306110-00016. [DOI] [PubMed] [Google Scholar]
- 47.Hauk O, Pulvermuller F. Clin Neurophysiol. 2004;115:1090–1103. doi: 10.1016/j.clinph.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 48.Hauk O, Davis MH, Ford M, Pulvermuller F, Marslen-Wilson WD. NeuroImage. 2006;30:1383–1400. doi: 10.1016/j.neuroimage.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 49.Dijkstra T, Van Heuven WJB. In: Localist Connectionist Approaches to Human Cognition. Grainger J, Jacobs AM, editors. Hillsdale, NJ: Lawrence Erlbaum; 1998. pp. 189–225. [Google Scholar]
- 50.Lemhofer K, Dijkstra T. Mem Cogn. 2004;35:533–550. doi: 10.3758/bf03195845. [DOI] [PubMed] [Google Scholar]
- 51.Chee MW, Soon CS, Lee HL. J Cogn Neurosci. 2003;15:85–97. doi: 10.1162/089892903321107846. [DOI] [PubMed] [Google Scholar]
- 52.Illes J, Francis WS, Desmond JE, Gabrieli JD, Glover GH, Poldrack R, Lee CJ, Wagner AD. Brain Lang. 1999;70:347–363. doi: 10.1006/brln.1999.2186. [DOI] [PubMed] [Google Scholar]
- 53.Frenck-Mestre C, Anton JL, Roth M, Vaid J, Viallet F. NeuroReport. 2005;16:761–765. doi: 10.1097/00001756-200505120-00021. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Fornells A, van der Lugt A, Rotte M, Britti B, Heinze HJ, Munte TF. J Cogn Neurosci. 2005;17:422–433. doi: 10.1162/0898929053279559. [DOI] [PubMed] [Google Scholar]
- 55.Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, Fox PT, Gao JH. Hum Brain Mapp. 2003;18:158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crinion J, Turner R, Grogan A, Hanakawa T, Noppeney U, Devlin JT, Aso T, Urayama S, Fukuyama H, Stockton K, et al. Science. 2006;312:1537–1540. doi: 10.1126/science.1127761. [DOI] [PubMed] [Google Scholar]
- 57.Picton TW, van Roon P, Armilio ML, Berg P, Ille N, Scherg M. Clin Neurophysiol. 2000;111:53–65. doi: 10.1016/s1388-2457(99)00227-8. [DOI] [PubMed] [Google Scholar]
- 58.McCarthy G, Wood CC. Electroencephalogr Clin Neurophysiol. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]