Abstract
Human and rodent erythrocytes are known to be highly permeable to glycerol. Aquaglyceroporin aquaporin (AQP)3 is the major glycerol channel in human and rat erythrocytes. However, AQP3 expression has not been observed in mouse erythrocytes. Here we report the presence of an aquaglyceroporin, AQP9, in mouse erythrocytes. AQP9 levels rise as reticulocytes mature into erythrocytes and as neonatal pups develop into adult mice. Mice bearing targeted disruption of both alleles encoding AQP9 have erythrocytes that appear morphologically normal. Compared with WT cells, erythrocytes from AQP9-null mice are defective in rapid glycerol transport across the cell membrane when measured by osmotic lysis, [14C]glycerol uptake, or stopped-flow light scattering. In contrast, the water and urea permeabilities are intact. Although the physiological role of glycerol in the normal function of erythrocytes is not clear, plasma glycerol is an important substrate for lipid biosynthesis of intraerythrocytic malarial parasites. AQP9-null mice at the age of 4 months infected with Plasmodium berghei survive longer during the initial phase of infection compared with WT mice. We conclude that AQP9 is the major glycerol channel in mouse erythrocytes and suggest that this transport pathway may contribute to the virulence of intraerythrocytic stages of malarial infection.
Keywords: Plasmodium berghei, aquaglyceroporin
Aquaglyceroporins are transmembrane proteins belonging to the aquaporin (AQP) water channel family (1). Aquaglyceroporins are the only known glycerol channels in mammalian cells. Among 13 identified mammalian aquaporins, AQP3, AQP7, AQP9, and AQP10 represent the aquaglyceroporin subfamily on the basis of their amino acid sequences and solute permeabilities (1). Aquaglyceroporins are also permeable to urea and water when expressed in Xenopus laevis oocytes (1).
The first defined water channel, AQP1, was discovered in human erythrocytes (2). The high expression and water permeability of AQP1 in erythrocytes led to the hypothesis that AQP1 is important in adaptation to dramatic osmolarity changes in the circulation (2). In addition to high water permeability, human and rodent erythrocytes are known to be highly permeable to urea and glycerol (3). AQP3 has been identified as the main channel for glycerol transport in human and rat erythrocytes (4). AQP3 has not been detected in mouse erythrocytes, and the glycerol permeability of mouse erythrocytes from AQP3 knockout mice has been shown to not be affected by gene deletion (5). We revisited this question and detected AQP9 expression in mouse erythrocytes. By using AQP9-null mice, we provide evidence that AQP9 is the major pathway for glycerol transport in mouse erythrocytes.
The physiological role of aquaglyceroporins in erythrocytes is still not clear. However, recent studies indicate that aquaglyceroporins AQP7 and AQP9 participate in metabolism. Under fasting conditions, up-regulated AQP7 expression in adipocytes facilitates the release of glycerol, which can then be transported through the blood to the liver, where it is taken up by hepatocytes for gluconeogenesis via AQP9, which is also up-regulated by fasting (6, 7). AQP7-null mice have been shown to exhibit reduced glycerol release from adipocytes into the bloodstream during prolonged fasting (8), and these mice develop adult-onset obesity (9, 10). As expected, the serum glycerol concentration is higher in AQP9-null mice compared with WT mice (11).
Malaria is a major threat to the health of poverty-stricken populations in tropical countries. The intracellular parasite Plasmodium proliferates rapidly in erythrocytes, having the capacity to increase 32-fold within 24 h. A high rate of lipid synthesis is required for cell membrane biogenesis, and malarial parasites are known to incorporate glycerol from host serum efficiently into glycerolipids (12). This requires glycerol to cross three membranes: the erythrocyte plasma membrane, the parasitophorous vacuole membrane, and the parasite plasma membrane. A single aquaglyceroporin, PbAQP, from the mouse malarial parasite Plasmodium berghei, has been shown to facilitate glycerol uptake from host erythrocytes through the parasite plasma membrane (13). To study the effect of the mouse erythrocyte glycerol channel on P. berghei pathogenesis, we infected AQP9-null mice with P. berghei. A lack of AQP9 in erythrocytes correlates with delayed mortality in P. berghei-infected mice.
Results
Expression of AQP9 in Mouse Erythrocytes.
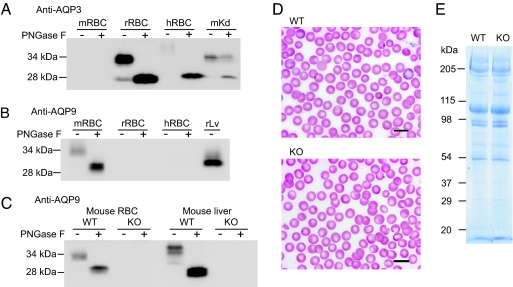
Membranes from human, rat, and mouse erythrocytes were prepared from freshly drawn blood. Protein samples were analyzed by SDS/PAGE with and without peptide-N-glycosidase F (PNGase F) treatment. Immunoblotting with an antibody raised to rat AQP3 revealed that AQP3 was abundantly expressed in human and rat erythrocytes (Fig. 1A). In human erythrocytes AQP3 was highly glycosylated, masking AQP3 from detection with the antibody unless treated with the glycanase PNGase F. AQP3 was not detected in samples prepared from mouse erythrocytes, even though the same antibody recognized AQP3 expression in mouse kidney (Fig. 1A). AQP9 is an aquaglyceroporin closely related to AQP3 that has been detected in lymphocytes (14). To test the possibility that AQP9, instead of AQP3, is expressed in mouse erythrocytes, we probed the same samples with an antibody to rat AQP9. The rat AQP9 antibody recognized a band at ≈34 kDa, which was reduced to 29 kDa after PNGase F treatment, indicating the expression of glycosylated AQP9 in mouse erythrocytes (Fig. 1B). Probing with the rat AQP9 antibody revealed bands of the same size in erythrocyte and liver samples. AQP9 was not detected in erythrocyte or liver samples prepared from AQP9-null mice (Fig. 1C).
Fig. 1.
Expression of AQP9 in mouse erythrocytes. Membrane protein samples (20 μg per lane) from mouse, rat, and human erythrocytes with (+) and without (−) PNGase F digestion analyzed by immunoblotting. (A) Mouse (mRBC), rat (rRBC), and human (hRBC) erythrocyte membrane proteins analyzed by immunoblot using anti-AQP3. Mouse kidney medulla membrane proteins (mKd) were analyzed as positive controls. (B) Mouse, rat, and human erythrocyte membrane proteins analyzed by immunoblot by using anti-AQP9. Rat liver membrane proteins (rLv) were analyzed as positive controls. (C) Erythrocyte and liver membrane proteins from WT and AQP9-null (KO) mice analyzed by immunoblot by using anti-AQP9. (D) The morphology of Wright–Giemsa-stained WT and AQP9-null erythrocytes. (E) Erythrocyte membranes from WT and AQP9-null mice analyzed by SDS/PAGE stained with Coomassie blue. (Scale bars: 10 μm.)
Hematological indices of WT and AQP9-null mice were compared. No apparent differences in erythrocyte morphology (Fig. 1D), erythrocyte number, or reticulocyte count were found between the WT and AQP9-null mice (data not shown). Coomassie-stained SDS/PAGE revealed that deficiency of AQP9 did not affect the abundance or mobility of spectrin, band 3, band 4.1, band 4.2, actin, or other major erythrocyte membrane proteins (Fig. 1E). It should be noted that AQP9 does not stain well with Coomassie blue and was not visible in either sample.
AQP9 Expression During the Development of Mouse Erythrocytes.
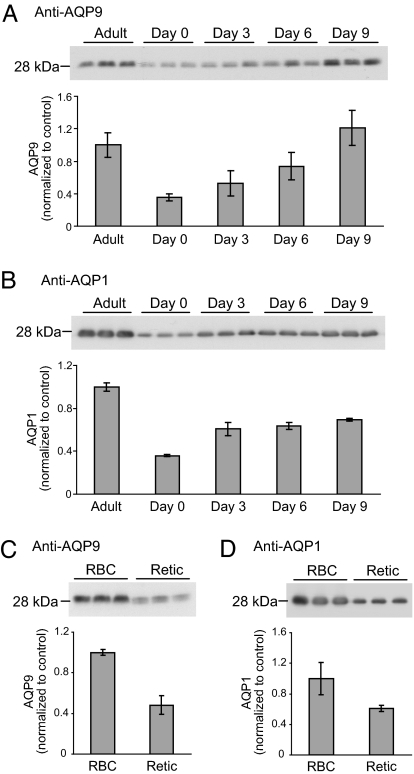
To investigate the physiological role of aquaglyceroporin AQP9 in mouse erythrocytes, the expression levels of AQP9 at different developmental stages were compared. In erythrocytes from neonatal pups, AQP9 was detectable; however, as the mice matured, AQP9 protein expression increased until reaching adult levels at approximately day 9 (Fig. 2A). In a separate experiment, reticulocytes isolated from anemic mice expressed lower levels of AQP9 than normal erythrocytes (Fig. 2C). AQP1 is known to be present in mouse erythrocytes, and AQP1 expression was also higher in mature erythrocytes compared with reticulocytes (Fig. 2 B and D).
Fig. 2.
Expression of AQP9 and AQP1 in various developmental stages of mouse erythrocytes. (Upper) Membrane protein samples from mouse erythrocytes after PNGase F digestion were analyzed by immunoblotting. (Lower) Quantitation of the density of the bands on the immunoblots are shown. Three mice were studied for each age group. Erythrocyte membrane proteins from mice of various ages analyzed by immunoblot using anti-AQP9 (20 μg per lane) (A) or anti-AQP1 (0.1 μg per lane) (B); mature erythrocyte (RBC) and reticulocyte (Retic) membrane proteins analyzed by immunoblot using anti-AQP9 (20 μg per lane) (C) or anti-AQP1 (0.1 μg per lane) (D).
Characterization of Solute Permeabilities of AQP9-Null Erythrocytes.
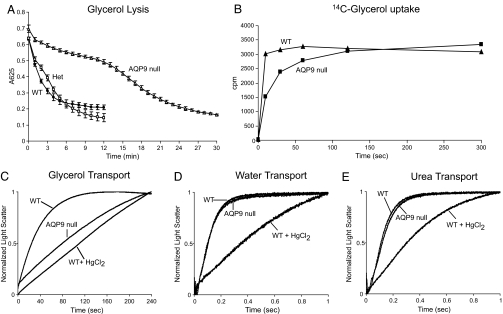
When expressed in Xenopus laevis oocytes, AQP9 was reported to transport glycerol, urea, water, and a wide range of other solutes, including polyols, purines, and pyrimidines (15). WT and AQP9-null erythrocytes were evaluated by osmotic fragility in glycerol. In 0.3 M glycerol, erythrocytes take up glycerol, thereby raising intracellular tonicity, which is diluted by water absorption. This causes WT erythrocytes to lyse rapidly, with a half-time of ≈2.5 min. Lacking glycerol uptake, AQP9-null erythrocytes were much more resistant to glycerol-induced lysis, with a half-time of ≈14 min (Fig. 3A). A similar difference in glycerol transport was seen when comparing [14C]glycerol uptake in erythrocytes from WT and AQP9-null mice (Fig. 3B).
Fig. 3.
Solute permeability of mouse erythrocytes. (A) The glycerol permeability of WT (filled circles; n = 3), AQP9 heterozygous (Het) (open squares; n = 3), and AQP9-null (open triangles; n = 3) erythrocytes assayed by osmotic lysis in a 0.3 M glycerol solution. (B) The glycerol permeability of WT (triangles) and AQP9-null (squares) erythrocytes assayed by [14C]glycerol uptake. Representative data of five independent experiments are shown. (C–E) The solute permeabilities of erythrocytes from WT and AQP9-null mice measured by monitoring light scattering with a stopped-flow spectrophotometer after rapid mixing of solute-loaded erythrocytes with an equal volume of sucrose solution. Inhibition of solute permeability was achieved by incubating erythrocytes with 0.1 mM HgCl2 for 5 min before measurement. The kinetics of 10 measurements were averaged and fitted to a first-order exponential equation. (C) Glycerol permeability. (D) Water permeability. (E) Urea permeability.
Freshly collected mouse erythrocytes were preequilibrated with glycerol and mixed with an isoosmotic buffer containing sucrose as a nonpermeant osmolyte. The decrease in the cell volume caused by efflux of solute was observed as an increase in light scattering by using a stopped-flow spectrophotometer. WT mouse erythrocytes were permeable to glycerol (Kglycerol = 2750E-05 ± 7.70E-05·s−1). However, the glycerol permeability of AQP9-null erythrocytes (Kglycerol = 417E-05 ± 2.33E-05·s−1) was substantially decreased and was similar to the permeability of WT cells pretreated with the aquaporin inhibitor mercuric chloride (Kglycerol = 115E-05 ± 1.76E-05·s−1) (Fig. 3C).
Absence of AQP9 from mouse erythrocytes does not affect the protein expression of AQP1 or urea transporter B, the main water and urea channels in erythrocytes (data not shown). As a consequence, water and urea permeabilities are similar in WT and AQP9-null erythrocytes (Fig. 3 D and E).
Infection of AQP9-Null Mice with P. berghei.
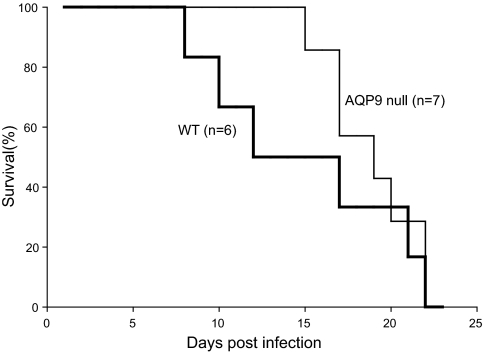
To study the effect of AQP9 on malarial parasite growth and virulence, WT female mice and AQP9-null female mice at the age of 4 months were infected with equal numbers of P. berghei, the mouse malarial parasite. The parasitemia in AQP9-null mice was similar to that of WT mice (data not shown). Nevertheless, AQP9-null mice were more resistant to P. berghei infection in the early stages of infection than were WT mice. All AQP9-null mice were still alive at day 12, whereas only 50% of WT mice survived (Fig. 4). Despite a difference in early survival, none of the mice in either group survived past day 22. In experiments with male mice at the age of 5 months, a smaller difference was observed between WT and AQP9-null mice (data not shown).
Fig. 4.
Effect of AQP9 on malarial virulence. Both WT (bold line; n = 6) and AQP9-null (thin line; n = 7) female mice were infected with equal numbers of P. berghei (105) on day 0. The survival of the mice was examined daily for 23 days.
Discussion
Erythrocytes are known to be highly permeable to solutes, such as water, urea, and glycerol (16, 17). The discovery of aquaporin water channels provided an explanation for the fast water flux across the erythrocyte plasma membrane (2). Urea transporter B, a urea transporter expressed in erythrocytes and testes, has been shown to be the main route for urea to pass through erythrocyte membranes (18). Glycerol permeability of erythrocytes has long been recognized and can be pharmacologically inhibited by phloretin and mercury (16, 19). Nevertheless, the existence of glycerol channels in mouse erythrocytes has been controversial. By using immunoblotting, immunofluorescence, RT-PCR, and other techniques, Roudier et al. (4, 20, 21) have shown that AQP3 is expressed in human and rat erythrocytes. Humans with mutations in the gene encoding AQP3 fail to express the Gil blood group (20), and erythrocytes from Gil-null patients are defective in glycerol transport (20). These studies provide evidence that AQP3 is expressed and functions as the main glycerol channel in human erythrocytes. A study by Yang et al. (5) did not detect AQP3 in normal mouse erythrocytes, and erythrocytes from AQP3-null mice displayed normal glycerol transport activity across the cell membrane, which was blocked by phloretin.
In this paper, we demonstrate the expression of AQP9, but not AQP3, in mouse erythrocytes. The glycerol permeability of mouse erythrocytes was examined by light scattering with a stopped-flow spectrophotometer and the osmotic lysis assay. By using AQP9-null mice, we demonstrated that AQP9 is the main glycerol channel in mouse erythrocytes, whereas a lack of AQP9 in erythrocytes has no detectable effect on water or urea permeability. Interestingly, erythrocyte urea permeability can also be inhibited by 0.1 mM mercuric chloride, but not as effectively as with 0.5 mM phloretin (data not shown). We conclude that AQP9 as a glycerol channel in mouse erythrocytes functions similarly to AQP3 in human and rat erythrocytes.
The reason that different rodent species express different aquaglyceroporins is not obvious; however, the existence of a yet-unidentified physiological need for an aquaglyceroporin in erythrocytes seems likely. The higher expression of AQP9 in mature mouse erythrocytes suggests that the AQP9 protein is very stable and plays an important physiological role in erythrocytes circulating in adult mice. However, the absence of AQP9 protein in erythrocytes does not affect the cell maturation or life span (data not shown).
Glycerol is a small metabolite, which is important for gluconeogenesis. After prolonged fasting, up to 21% of circulating glucose can be derived from blood glycerol in humans (22), and this fraction is even higher in rodents (23). Mouse erythrocytes do not contain glycerol kinase activity and thus glycerol is not used by mature erythrocytes (data not shown). In vitro incubation of erythrocytes did not increase glycerol in the media, indicating that mature erythrocytes do not produce glycerol in culture (data not shown). Because glycerol does not have an obvious physiological role in erythrocytes, it remains possible that the aquaglyceroporins may transport a different solute.
Glycerol is important during malarial infection. The malarial parasites incorporate host plasma glycerol into lipid and membrane during the asexual intraerythrocytic stages of infection (12). A single aquaglyceroporin identified in Plasmodium has been shown to be the major pathway for glycerol uptake from the erythrocyte cytoplasm into the parasite (13, 24). The mouse malarial parasite, P. berghei, lacking the aquaglyceroporin, PbAQP, grows more slowly and is less virulent, indicating that glycerol may be important for the proliferation of the parasite (13).
To enter the parasite, glycerol must first cross the erythrocyte plasma membrane. As shown in this paper, AQP9 is expressed on the mouse erythrocyte surface and is the major route for glycerol penetration. A lack of AQP9 in mouse erythrocytes does not affect P. berghei parasite invasion of erythrocytes because the parasitemia is similar between WT and AQP9-null mice. It must be noted that the AQP9-null mice used in these studies are not bred onto a homogeneous background, potentially confounding definition of the precise role of AQP9 in malarial infection. Nevertheless, AQP9-null mice (4 months of age) showed improved survival during the early stages of infection when compared with WT mice, consistent with a requirement for glycerol transport through the erythrocyte membrane to support P. berghei metabolism and virulence. A possible explanation may be that P. berghei can invade erythrocytes of AQP9-null mice but that the parasite load is lower in AQP9-null mice and thus are somewhat less virulent. This survival advantage is temporary because AQP9-null mice eventually succumb to the infection, and by 22 days, neither group has survivors. Thus, erythrocyte AQP9 is not absolutely necessary for the ultimate virulence of P. berghei. In addition, AQP9-null mice have been shown to have slightly increased glycerol levels in serum that may reduce the effect of the lack of AQP9. Further investigation, including the study of infection of AQP9-null mice with PbAQP-null parasites, will allow us to examine the relationship between Plasmodium and the glycerol permeability of their erythrocyte hosts.
Malaria continues to be an enormous clinical problem worldwide. In addition to defining AQP9 as the major route for glycerol transport in mouse erythrocytes, these studies reinforce the requirement for glycerol in support of malarial virulence and point to disruption of glycerol transport as a potential target for adjunctive therapy in patients with malaria.
Materials and Methods
Animal Studies.
AQP9-knockout mice were generated by targeted gene disruption as described by Rojek et al. (11). Under physiological conditions, these mice have normal embryonic survival, growth, fertility, appearance, behavior, and plasma parameters, except for a significant increase in the level of glycerol and triglycerides. Mouse genotyping was performed by PCR as described in ref. 11.
To enrich for reticulocytes, adult C57BL/6 mice were subjected to tail vein injection of 40 μg/g body weight of phenylhydrazine (Sigma–Aldrich, St. Louis, MO) consecutively for 2 days. Mice were killed with CO2 at day 3 after injection, and blood samples were harvested from the right ventricle of the heart. Reticulocytes were separated from mature erythrocytes and buffy coat with Lymphoprep by spinning at 800 × g for 10 min (Axis-Shield, Oslo, Norway).
Animal protocols were approved by the Institutional Animal Care and Use Committees at The Johns Hopkins University and Duke University and conformed to National Institutes of Health guidelines.
Erythrocyte Membrane Preparation and Immunoblotting.
Sprague–Dawley rats (Taconic, Hudson, NY) were decapitated, and blood was collected into acid citrate dextrose solution. Mice were anesthetized, and blood samples were harvested from the right ventricle of the heart.
Freshly collected blood samples were loaded into Lymphoprep to remove the buffy coat. Erythrocytes were washed with three volumes of PBS (7.5 mM sodium phosphate, pH 7.4/150 mM NaCl) and lysed hypotonically with chilled lysis buffer containing 7.5 mM sodium phosphate, pH 7.4, 1.0 mM Na2EDTA, and a protease inhibitor mixture tablet (Roche Applied Science, Indianapolis, IN). The erythrocyte membranes were isolated by spinning at 39,000 × g as described in ref. 25.
Immunoblotting was performed as described in ref. 26. An appropriate amount of membrane protein sample was subjected to digestion with PNGase F (New England Biolabs, Ipswich, MA) and analyzed with 12% acrylamide gels by using SDS/PAGE; immunoblots were reacted with 3.5 μg/ml rabbit anti-rat AQP9 (Alpha Diagnostic, San Antonio, TX), 1:1,000 rabbit anti-rat AQP1 (Alpha Diagnostic), or 1:1,000 rabbit anti-rat AQP3 (27). After incubation with 1:10,000 HRP-labeled goat anti-rabbit IgG (Amersham, Arlington Heights, IL) for 1 h at room temperature, the targeted proteins were visualized by chemiluminescence (ECL; Amersham).
Hematological Analyses.
The morphology of the erythrocytes was recorded with a light microscope after staining the blood smear with Wright–Giemsa stain (Sigma–Aldrich). The erythrocyte count was determined by counting cells under a microscope with a hemocytometer after diluting the blood in PBS (1:25,000). The reticulocyte count was determined by counting the number of reticulocytes in every 1,000 cells after staining with reticulocyte stain (Sigma–Aldrich).
Erythrocyte Membrane-Transport Studies.
Solute permeabilities were determined by measuring light scattering by using a stopped-flow apparatus (SF-2002; Kin-Tek Instruments, University Park, PA) with a dead time of <1 ms. Mouse blood was collected from the tail (≈50 μl per bleed) and then washed three times with chilled PBS to remove the buffy coat. The erythrocytes were resuspended at a hematocrit of ≈1% in chilled measuring solution. Water, glycerol, and urea permeabilities were measured similarly to the description in Roudier et al. (4). Briefly, the cellular osmotic water permeability was determined by measuring the light scattering after mixing 40 μl of erythrocytes suspended in PBS with an equal volume of PBS solution containing 200 mM sucrose as a nonpermeable osmolyte. For glycerol and urea permeabilities, erythrocytes were preequilibrated in PBS supplemented with 200 mM glycerol or 200 mM urea for more than 15 min on ice. The erythrocytes were then mixed in the stopped flow with an equal volume of 200 mM sucrose in PBS solution. Light scattering was recorded (λ = 600 nm) and first-order rate constants, which represent protein-mediated transport, were calculated. In the inhibition experiments, 0.1 mM mercuric chloride was added to the erythrocyte suspensions 5 min before loading into the stopped-flow apparatus.
The osmotic lysis test was conducted by the method of Zanella et al. (28). Briefly, freshly prepared mouse erythrocytes were resuspended in PBS to a hematocrit of 0.4% and equilibrated at 37°C for 30 min. The erythrocyte suspension (0.4 ml) was mixed in a cuvette with 0.8 ml of lysis solution containing 0.3 M glycerol and 0.3× PBS. The rapid penetration of glycerol into the erythrocytes caused cell lysis, which was recorded as a decrease in light absorption at 625 nm.
[14C]glycerol uptake assay was performed as described in ref. 13, with slight modification. Freshly collected erythrocytes were suspended to a hematocrit of 5% in RPMI medium 1640 containing 1 mM cold glycerol and 0.5 μCi/ml (1 Ci = 37 GBq) [14C]glycerol (Amersham) and were incubated at 37°C. After the reaction was stopped by spinning the cells through a dibutyl phthalate layer, cell pellets were lysed and counted directly without washing.
Phenotype Analysis of Mice Infected with P. berghei.
To study the effect of AQP9 on parasite growth during blood-stage development, 4-month-old WT (n = 6) and AQP9-null (n = 7) female mice were infected i.p. with 105 P. berghei parasites. Parasitemia, the percentage of infected erythrocytes, was examined every 2 days after staining a blood smear with Giemsa stain (Sigma–Aldrich). Survival of the mice was monitored daily.
Acknowledgments
We thank Dr. Eric Beitz and Dr. Joel Anne Chasis for critical reading of the manuscript, Dr. Tim Oliver for assistance with light microscopy, and Linhua Song for help with the animal studies. This work was supported in part by National Institutes of Health Grant HL48268 and The Johns Hopkins Malaria Research Institute Pilot Grant.
Abbreviations
- AQP
aquaporin
- PNGase F
peptide-N-glycosidase F.
Footnotes
The authors declare no conflict of interest.
References
- 1.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preston GM, Carroll TP, Guggino WB, Agre P. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 3.Wessels JM, Veerkamp JH. Biochim Biophys Acta. 1973;291:190–196. doi: 10.1016/0005-2736(73)90411-2. [DOI] [PubMed] [Google Scholar]
- 4.Roudier N, Verbavatz JM, Maurel C, Ripoche P, Tacnet F. J Biol Chem. 1998;273:8407–8412. doi: 10.1074/jbc.273.14.8407. [DOI] [PubMed] [Google Scholar]
- 5.Yang B, Ma T, Verkman AS. J Biol Chem. 2001;276:624–628. doi: 10.1074/jbc.M008664200. [DOI] [PubMed] [Google Scholar]
- 6.Kuriyama H, Shimomura I, Kishida K, Kondo H, Furuyama N, Nishizawa H, Maeda N, Matsuda M, Nagaretani H, Kihara S, et al. Diabetes. 2002;51:2915–2921. doi: 10.2337/diabetes.51.10.2915. [DOI] [PubMed] [Google Scholar]
- 7.Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. Proc Natl Acad Sci USA. 2003;100:2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda N, Funahashi T, Hibuse T, Nagasawa A, Kishida K, Kuriyama H, Nakamura T, Kihara S, Shimomura I, Matsuzawa Y. Proc Natl Acad Sci USA. 2004;101:17801–17806. doi: 10.1073/pnas.0406230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, Mizunoya W, Kishida K, Inoue K, Kuriyama H, Nakamura T, et al. Proc Natl Acad Sci USA. 2005;102:10993–10998. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S, Uchida S, Verkman AS. J Biol Chem. 2005;280:15493–15496. doi: 10.1074/jbc.C500028200. [DOI] [PubMed] [Google Scholar]
- 11.Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, Frokiaer J, Nielsen S. Proc Natl Acad Sci USA. 2007;104:3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vial HJ, Ancelin ML. Subcell Biochem. 1992;18:259–306. [PubMed] [Google Scholar]
- 13.Promeneur D, Liu Y, Maciel J, Agre P, King LS, Kumar N. Proc Natl Acad Sci USA. 2007;104:2211–2216. doi: 10.1073/pnas.0610843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Am J Physiol. 1999;277:F685–F696. doi: 10.1152/ajprenal.1999.277.5.F685. [DOI] [PubMed] [Google Scholar]
- 15.Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, van Hoek AN, Hediger MA. J Biol Chem. 1998;273:24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- 16.Macey RI, Farmer RE. Biochim Biophys Acta. 1970;211:104–106. doi: 10.1016/0005-2736(70)90130-6. [DOI] [PubMed] [Google Scholar]
- 17.Bowyer F. Nature. 1954;174:355–356. doi: 10.1038/174355b0. [DOI] [PubMed] [Google Scholar]
- 18.Olives B, Neau P, Bailly P, Hediger MA, Rousselet G, Cartron JP, Ripoche P. J Biol Chem. 1994;269:31649–31652. [PubMed] [Google Scholar]
- 19.Carlsen A, Wieth JO. Acta Physiol Scand. 1976;97:501–513. doi: 10.1111/j.1748-1716.1976.tb10290.x. [DOI] [PubMed] [Google Scholar]
- 20.Roudier N, Ripoche P, Gane P, Le Pennec PY, Daniels G, Cartron JP, Bailly P. J Biol Chem. 2002;277:45854–45859. doi: 10.1074/jbc.M208999200. [DOI] [PubMed] [Google Scholar]
- 21.Roudier N, Bailly P, Gane P, Lucien N, Gobin R, Cartron JP, Ripoche P. J Biol Chem. 2002;277:7664–7669. doi: 10.1074/jbc.M105411200. [DOI] [PubMed] [Google Scholar]
- 22.Baba H, Zhang XJ, Wolfe RR. Nutrition. 1995;11:149–153. [PubMed] [Google Scholar]
- 23.Peroni O, Large V, Odeon M, Beylot M. Metabolism. 1996;45:897–901. doi: 10.1016/s0026-0495(96)90166-3. [DOI] [PubMed] [Google Scholar]
- 24.Hansen M, Kun JF, Schultz JE, Beitz E. J Biol Chem. 2002;277:4874–4882. doi: 10.1074/jbc.M110683200. [DOI] [PubMed] [Google Scholar]
- 25.Mathai JC, Mori S, Smith BL, Preston GM, Mohandas N, Collins M, van Zijl PC, Zeidel ML, Agre P. J Biol Chem. 1996;271:1309–1313. doi: 10.1074/jbc.271.3.1309. [DOI] [PubMed] [Google Scholar]
- 26.Smith BL, Preston GM, Spring FA, Anstee DJ, Agre P. J Clin Invest. 1994;94:1043–1049. doi: 10.1172/JCI117418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 28.Zanella A, Izzo C, Rebulla P, Zanuso F, Perroni L, Sirchia G. Br J Haematol. 1980;45:481–486. doi: 10.1111/j.1365-2141.1980.tb07167.x. [DOI] [PubMed] [Google Scholar]