Abstract
Although it is well established that there are alterations in type 2A serotonin receptors (5-HT2ARs) in the basolateral nuclear complex of the amygdala (BLC) in several neuropsychiatric disorders, very little is known about the neuronal localization of these receptors in this brain region. Single-labeling and dual-labeling immunohistochemical techniques were utilized in the rat to address this question. Three different 5-HT2AR antibodies were used, each producing distinct but overlapping patterns of immunostaining. Two of three 5-HT2AR antibodies mainly stained pyramidal projection neurons in the BLC. The third antibody only stained pyramidal cells in the dorsolateral subdivision of the lateral amygdalar nucleus. With one of the antibodies, the most intensely stained neurons were a population of large nonpyramidal neurons whose morphology and distribution closely resembled those shown in previous studies to project to the mediodorsal thalamic nucleus (MD). This was confirmed in the present study using a technique that combined 5-HT2AR immunohistochemistry with fluorogold retrograde tract-tracing. Two of three 5-HT2AR antibodies stained large numbers of parvalbumin-containing interneurons in the BLC. One of these two antibodies also stained a subpopulation of somatostatin-containing neurons. None of the 5-HT2AR antibodies stained significant numbers of the other two main interneuronal subpopulations, the large cholecystokinin-positive neurons or the small interneurons that exhibit extensive colocalization of calretinin and cholecystokinin. Since each of the three antibodies was raised against a distinct immunizing antigen, they may recognize different conformations of 5-HT2AR in different neuronal domains. The expression of 5-HT2ARs in pyramidal cells and parvalbumin-positive interneurons in the BLC is consistent with the results of previous electrophysiological studies, and suggests that serotonin may produce excitation of several neuronal populations in the BLC via type 2A serotonin receptors.
Keywords: serotonin, pyramidal cells, interneurons, peptides, calcium-binding proteins, mediodorsal thalamic nucleus
Introduction
In vivo microdialysis studies indicate that there is increased serotonin (5-HT) release in the amygdala during behavioral arousal and stress (Kawahara et al., 1993; Rueter and Jacobs, 1996). Variations in human serotonin transporter and tryptophan hydroxylase genes, which presumably result in altered extracellular serotonin levels, are associated with increased activation of the amygdala by emotional stimuli, as well as the generation of anxiety and depression (Canli et al., 2005; Hariri and Holmes, 2006). There is substantial evidence from experiments in animals and humans that activation of type 2 5-HT receptors (5-HT2Rs), including type 2A receptors (5-HT2ARs), in the basolateral nuclear complex of the amygdala (BLC) produce alterations in anxiety (Zangrossi and Graeff, 1994; Graeff et al., 1996; Maissonnette et al., 2000; Schiller et al., 2003). It is also known that there is an increase in 5-HT2R binding in the amygdala in unmedicated depressives that commit suicide (Hrdina et al., 1993), and that atypical antipsychotic drugs bind to 5-HT2Rs in the human amygdala (Andorn et al., 2003). These data suggest that knowledge of the anatomy, physiology, and pharmacology of 5-HT2ARs in the BLC may contribute to a better understanding of the etiology and treatment of several neuropsychiatric disorders, including anxiety, depression, and schizophrenia.
5-HT2ARs are typical G-protein coupled receptors consisting of an extracellular N-terminal segment, 7 transmembrane segments, and an intracellular C-terminal segment (Roth 1998). Activation of 5-HT2ARs produces a depolarization due to a decrease in potassium conductances. In addition, there is evidence that 5-HT2ARs are positively coupled to phospholipase C (Aghajanian, 1995; Barnes and Sharp, 1999). Receptor binding autoradiography using the preferential 5-HT2AR antagonist ketanserin has shown light to moderate receptor levels in most nuclei of the amygdala, but higher densities of receptor binding in the cortical nucleus and dorsolateral subdivision of the lateral nucleus (Pazos et al., 1985). Likewise, in situ hydridization studies indicate that most nuclei of the amygdala have moderate levels of 5-HT2ARmRNA, but higher levels were seen in the cortical nucleus and dorsolateral subdivision of the lateral nucleus (Wright et al., 1995).
There have been no detailed studies of 5-HT2AR staining in the amygdala using immunohistochemical techniques, and the studies that did provide brief comments on the amygdala were contradictory. Thus, one study reported that 5-HT2AR-positive (5-HT2AR+) neurons in the amygdala were mainly confined to the BLC and cortical nucleus, and all were intensely-stained nonpyramidal interneurons (Morilak et al., 1993). Two other studies, both using the same monoclonal antibody, reported that most amygdalar nuclei had numerous immunostained neurons and processes, suggesting that mainly principal neurons were stained (Cornea-Hebért et al., 1999; Xu and Pandey, 2000). Because of the potential importance of the BLC as a site of action of antipsychotic and antidepressant drugs that target 5-HT2ARs, it is critical to gain a better understanding of the neuronal localization of these receptors in this brain region.
Previous studies have shown that there are two major cell classes in the BLC, pyramidal neurons and nonpyramidal neurons. Although these cells do not exhibit a laminar organization, their morphology, synaptology, electrophysiology, and pharmacology are remarkably similar to their counterparts in the cerebral cortex (Carlsen and Heimer, 1988; McDonald, 1992; Washburn and Moises, 1992; Rainnie et al., 1993; Paré et al., 2003). Thus, pyramidal neurons in the BLC are projection neurons with spiny dendrites that utilize glutamate as an excitatory neurotransmitter, whereas most nonpyramidal neurons are spine-sparse interneurons that utilize GABA as an inhibitory neurotransmitter. Recent dual-labeling immunohistochemical studies suggest that the BLC contains at least four distinct subpopulations of GABAergic interneurons that can be distinguished on the basis of their content of calcium-binding proteins and peptides. These subpopulations are: (1) parvalbumin+/calbindin+ neurons, (2) somatostatin+/calbindin+ neurons, (3) large multipolar cholecystokinin+ neurons that are often calbindin+ and (4) small bipolar and bitufted interneurons that exhibit extensive colocalization of calretinin, cholecystokinin, and vasoactive intestinal peptide (McDonald and Betette, 2001; McDonald and Mascagni, 2001, 2002, Mascagni and McDonald, 2003; Kemppainen and Pitkänen, 2000).
Since different subpopulations of BLC neurons exhibit specific connections (McDonald et al., 2005; Muller et al., 2003, 2005, 2006, 2007), understanding the effects of serotonergic neurotransmission on information processing by the BLC will require knowledge of the expression of 5-HT2AR, and other serotonin receptors, by distinct cell types. In the present study single- and double-labeling immunohistochemical techniques were used to address this question. Because previous studies have shown considerable variability in 5-HT2AR staining with different antibodies, three different 5-HT2AR antibodies were used in the present study.
EXPERIMENTAL PROCEDURES
Tissue Preparation
A total of 28 male Sprague-Dawley rats (250-350g; Harlan, Indianapolis, IN) were used in this study. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use and Care Committee (IACUC) of the University of South Carolina. All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable scientific data.
Rats were anesthetized with chloral hydrate (350 mg/kg) or sodium pentobarbital (50 mg/kg) and perfused intracardially with phosphate buffered saline (PBS; pH 7.4) containing 0.5% sodium nitrite (50 ml) followed by 4.0% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4 (500 ml). Fifteen of these rats received bilateral injections of colchicine (100 μg total; Sigma Chemical Co., St. Louis, MO) into the lateral cerebral ventricles one day before perfusion (see below for details). Following perfusion, brains were removed and postfixed for 3.5 hours in 4.0% paraformaldehyde.
Brains were sectioned on a vibratome at a thickness of 50 μm in the coronal plane. Sections were processed for immunohistochemistry in wells of tissue culture plates. All antibodies were diluted in 0.1M PBS containing Triton X-100 (0.3-0.5%) and 1% normal goat serum.
Immunoperoxidase experiments
Localization of 5-HT2AR was performed in 15 rats using the avidin-biotin immunoperoxidase (ABC) technique. Three primary antibodies to the 5-HT2AR were used: (1) a rabbit polyclonal antibody raised against amino acids 22-41 of the N-terminus of the receptor (1:100; Oncogene/Calbiochem #PC176L) was used in 7 rats (3 colchicine-injected, 4 non-colchicine-injected), (2) a mouse monoclonal antibody raised against a recombinant fusion protein of glutathione S-transferase and a peptide containing amino acids 1-76 of the human 5-HT2AR (i.e., the entire extracellular N-terminal domain) (1:100; BD Pharmingen #556326) wasused in 5 rats (3 colchicine-injected, 2 non-colchicine-injected), and (3) a rabbit polyclonal antibody raised against amino acids 22-41 of the N-terminus of the receptor conjugated to keyhole limpet hemocyanin using glutaraldehyde (1:4000; Ab51, generously donated by Dr.Bryan Roth, University of North Carolina School of Medicine) was used in 3 rats (2 colchicine-injected, 1 non-colchicine-injected).
In experiments using the rabbit polyclonal antibodies, sections were incubated in primary antibody overnight at 4° C and then processed for the avidin-biotin immunoperoxidase technique using a Vectastain rabbit ABC kit (Vector Laboratories, Burlingame, CA). In experiments using the mouse monoclonal antibody, sections were incubated in primary antibody overnight at 4° C and then processed for the avidin-biotin immunoperoxidase technique using a biotinylated goat anti-mouse secondary antibody (1:500; Jackson ImmunoResearch Laboratories, West Grove PA) and a Standard Vectastain ABC kit. Nickel-enhanced DAB (3, 3’-diaminobenzidine-4HCl, Sigma Chemical Co., St. Louis, MO) was used as a chromogen to generate a black reaction product for all antibodies (Hancock, 1986).
Following the immunohistochemical procedures, sections were mounted on gelatinized slides, dried overnight, dehydrated in ethanol, cleared in xylene, and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA). In some animals adjacent sections were counterstained with cresyl violet to identify nuclear borders. Sections were analyzed using an Olympus BHA and Nikon Eclipse E600 microscopes. Digital light micrographs were taken with a QImaging MicroPublisher 5.0 CCD camera (QImaging Corp., Burnaby, British Columbia). Brightness and contrast were adjusted using Photoshop 6.0 software.
Dual-labeling immunofluorescence experiments
Previous double-labeling studies have demonstrated that the rat BLC contains at least four distinct subpopulations of GABAergic interneurons: 1) parvalbumin+/calbindin+ neurons, 2) somatostatin+/calbindin+ neurons, 3) large multipolar CCK+ neurons that are often calbindin+, and 4) small bipolar and bitufted interneurons that exhibit extensive colocalization of vasoactive intestinal peptide, calretinin, and cholecystokinin (McDonald and Betette, 2001; Kemppainen and Pitkänen, 2000; McDonald and Mascagni, 2001, 2002; Mascagni and McDonald, 2003). Dual localization of 5-HT2AR with specific protein/peptide interneuronal markers (parvalbumin [PV], somatostatin [SOM], calretinin [CR], or cholecystokinin [CCK]) was investigated in 10 rats to determine if 5-HT2ARs were expressed by specific interneuronal subpopulations.
Sections were incubated in a cocktail of two primary antibodies (one of the 5-HT2AR antibodies and one of the interneuronal marker antibodies) overnight at 4° C. In each case one of the primary antibodies was a rabbit polyclonal and the other was a mouse or rat monoclonal. The Oncogene/Calbiochem rabbit polyclonal and BD Pharmingen mouse monoclonal 5-HT2AR antibodies were used at a dilution of 1:50, whereas the Ab51 rabbit polyclonal antibody was used at 1:2000. The following interneuronal marker antibodies were used in conjunction with the rabbit 5-HT2AR antibodies: mouse anti-PV (1:2000, Sigma Chemical Co., St. Louis, MO), rat anti-SOM (1:400; Chemicon International, Temecula, CA), mouse anti-CR (1:3000, Chemicon), and mouse anti-CCK (1:500, antibody 9303 generously donated by Dr. John H. Walsh, UCLA). The following interneuronal marker antibodies were used in conjunction with the mouse 5-HT2AR antibody: rabbit anti-PV (1:2000; generously donated by Dr. Kenneth Baimbridge, University of British Columbia), rabbit anti-CR (1:1000; Chemicon), and rabbit anti-SOM (1:4000; Peninsula Laboratories, San Carlos, CA). Four rats were used in the studies using the Oncogene/Calbiochem rabbit polyclonal antibody (all non-colchicine injected). Three rats were used in the studies using the BD Pharmingen mouse monoclonal antibody (all colchicine-injected). Three rats were used in the studies using the Ab51 rabbit polyclonal antibody (2 colchicine-injected, 1 non-colchicine injected). In all cases, 3-4 parallel series of sections at 250 μm intervals were stained for each of the interneuronal markers.
After incubation in the primary antibody cocktail, sections were rinsed in 3 changes of PBS (10 min each), and then incubated in a cocktail of species-appropriate Alexa-568 and Alexa-488 labeled secondary antibodies (1:400; Molecular Probes, Eugene, OR) for 3 hrs at room temperature. All secondary antibodies were highly cross-adsorbed by the manufacturer to ensure specificity for primary antibodies raised in particular species. Sections were then rinsed in 3 changes of PBS (10 min each) and mounted on glass slides using Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
Sections were examined with a Bio-Rad MRC-1024 confocal laser scanning system equipped with an argon-krypton laser attached to a Nikon Eclipse E800M microscope. Fluorescence of Alexa 488 (green) and Alexa 568 (red) dyes was analyzed using filter configurations for sequential excitation/imaging via 488 nm and 568 nm channels. Digital images were adjusted for brightness and contrast using Photoshop 6.0 software. In each of the confocal immunofluorescence cases some control sections were processed with one of the two primary antibodies omitted. In all cases only the color of the corresponding secondary fluorescent antibody was observed, and only on the appropriate channel. These results indicated that the secondary antibodies were specific for rabbit or mouse IgGs and that there was no “crosstalk” between the red and green channels (Wouterlood et al., 1998).
The rat BLC consists of three main nuclei: (1) lateral nucleus, (2) basolateral nucleus, and (3) basomedial nucleus (Paxinos and Watson, 1997). Each nucleus can be divided into two or more subdivisions. We focused our quantitative immunofluorescence studies on two distinct subdivisions in the basolateral and lateral nuclei of the BLC: (1) the anterior subdivision of the basolateral nucleus (BLa) and (2) the ventromedial subdivision of the lateral nucleus (Lvm) (Paxinos and Watson, 1997), to determine if the phenotypic organization of 5-HT receptor-expressing neurons might differ in different regions of the BLC. The BLa was chosen for quantitative analysis since it was the focus of a recent whole cell patch-clamp electrophysiological study that demonstrated 5-HT2R-mediated responses in amygdalar interneurons (Rainnie, 1999), and because it is the focus of our ongoing electron microscopic analysis of the serotoninergic innervation of BLC neurons (Muller et al., 2005). The Lvm was sampled because it is the most prominent of the three subdivisions of the lateral nucleus. Qualitative observations of other nuclear subdivisions within the BLC revealed that the types of receptor/marker colocalization seen in the BLa and Lvm was observed throughout the BLC, but the exact extent of colocalization was not quantitated in these nuclei, and probably varies.
Analysis of dual-labeling immunofluorescence preparations revealed that several subpopulations of nonpyramidal interneurons were labeled with the Oncogene/Calbiochem 5-HT2AR antibody, and that many PV+ interneurons were labeled with the BD Pharmingen 5-HT2AR antibody. To determine the percentage of each interneuronal subpopulation labeled by the Oncogene/Calbiochem antibody, and the percentage of PV+ interneurons labeled by the BD Pharmingen antibody, cell counts of single-labeled and double-labeled neurons in the BLa and Lvm were conducted in these preparations. Bilateral counts were pooled from 2-3 animals for each colocalization combination studied (approximately 5-6 sections for each subdivision in each animal for each marker). At 200X magnification, cell counts were made from the image of a 400 μm X 400 μm field displayed for merged (red/green) channels on the computer screen (double-labeled cells appear yellow). Images of the non-merged red and green channels were also displayed. Depending on the size of the nuclear subdivision at different levels of the amygdala, counts were made from either one such field positioned in the center of the subdivision (and involving about 80-90% of its cross-sectional area), or two adjacent non-overlapping fields. Only somata of nonpyramidal neurons were counted. Although some pyramidal cells contain low levels of CR, they were easily distinguished from the intensely-stained nonpyramidal neurons at the antibody dilutions used in this study (McDonald and Mascagni, 2001). Since dendrites of BLC nonpyramidal neurons are less than 3 μm wide, it was not difficult to distinguish somata from dendrites.
Sequential two–color immunoperoxidase dual-labeling experiment
A sequential two–color immunoperoxidase dual-labeling technique was used to study possible colocalization of BD Pharmingen monoclonal 5-HT2AR immunoreactivity with PV and CR immunoreactivity in one colchicine-injected rat. 5-HT2AR+ structures were stained black using nickel-enhanced DAB as described above. Sections were then blocked using an Avidin/Biotin Blocking kit (Vector Laboratories), incubated in either rabbit anti-PV antibody (1:2000; see above) or rabbit anti-CR antibody (1:1000; see above), and then processed for the avidin-biotin immunoperoxidase technique using a rabbit Vectastain ABC kit (Vector Laboratories). Non-enhanced DAB was used as a chromogen to generate a brown reaction product.
Retrograde tract-tracing experiments
With the BD Pharmingen monoclonal 5-HT2AR antibody, but not the polyclonal antibodies, there was a subpopulation of intensely-stained nonpyramidal neurons whose morphology and distribution closely resembled that of a neuronal subpopulation that projects to the mediodorsal thalamic nucleus (MD; McDonald, 1987). To determine if these 5-HT2AR+ cells were MD-projecting neurons, a technique combining 5-HT2AR immunohistochemistry with fluorogold (FG) retrograde tract tracing was performed in two rats. Rats were anesthetized with sodium pentobarbital (50 mg/kg) and positioned in a stereotaxic head holder (Stoelting Company, Wood Dale, Illinois). The dorsal surface of the skull was exposed and bilateral holes over the intended injection sites were made with a dental drill. Bilateral pressure injections of 2% FG (hydroxystilbamidine, Invitrogen, Grand Island, NY) in saline (0.2 μl) were made into the mediodorsal thalamic nucleus using a 1.0 μl Hamilton microsyringe equipped with a 30 gauge needle, using coordinates obtained from an atlas of the rat brain (AP: -2.8 mm from bregma, L: ±0.5 mm, H: 6.0 mm ventral to bregma; Paxinos and Watson, 1997). The needle was left in place for 10 min after each injection to prevent spread of injectate along the needle track. After a 5 day survival, rats were anesthetized with sodium pentobarbital (50 mg/kg) and received bilateral injections of colchicine (100 μg total; Sigma Chemical Co., St. Louis, MO) into the lateral cerebral ventricles. One day later rats were perfused with 4.0% paraformaldehyde as described above. Following perfusion, brains were removed and postfixed for 3.5 hours in 4.0% paraformaldehyde. Brains were sectioned on a vibratome at a thickness of 50 μm in the coronal plane and processed for immunohistochemistry.
Sections were incubated in a cocktail of the BD Pharmingen mouse monoclonal 5-HT2AR antibody (1:25) and a guinea pig polyclonal antibody to FG (1:3000; kindly donated by Dr. Lothar Jennes, University of Kentucky) overnight at 4° C. After incubation in the primary antibody cocktail, sections were rinsed in 3 changes of PBS (10 min each), and then incubated in a cocktail of goat anti-mouse Alexa-568 and goat-anti-guinea pig Alexa-488 labeled secondary antibodies (1:400; Molecular Probes, Eugene, OR) for 3 hrs at room temperature. Sections were then rinsed in 3 changes of PBS (10 min each) and mounted on glass slides using Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Sections were examined with a Bio-Rad MRC-1024 confocal laser scanning system equipped with an argon-krypton laser attached to a Nikon Eclipse E800M microscope. Fluorescence of Alexa 488 (green) and Alexa 568 (red) dyes was analyzed using filter configurations for sequential excitation/imaging via 488 nm and 568 nm channels. Digital images were adjusted for brightness and contrast using Photoshop 6.0 software.
Antibody Specificity
The monoclonal antibody to 5-HT2AR (clone G186-117; BD Pharmingen # 556326) was raised against a recombinant fusion protein of glutathione S-transferase and a peptide containing amino acids 1-76 of the human 5-HT2AR receptor (Wu et al., 1998), although a recent report indicates that the targeted epitope is comprised of a sequence between amino acids 64-71 (Cornea-Hébert et al., 2002). The specificity of the antibody was determined in Western blot analyses; it labeled a single band of 55 kDa. Staining was completely eliminated by preadsorption of the antibody with the GST-5HT2AR fusion protein (200 μg protein/100μg antibody), but not with GST-5HT2BR or GST-5HT2CR fusion proteins (Wu et al., 1998).
The Oncogene/Calbiochem polyclonal antibody to 5-HT2AR (#PC176L) is an affinity purified antibody raised in rabbit by repeated immunization with a synthetic peptide corresponding to amino acids 22-41 of the receptor. The specificity of the antibody was determined in Western blot analyses conducted by the manufacturer; it labeled a single band of approximately 53 kDa. In studies conducted by the manufacturer, tissue staining in the amygdala, cortex, and hippocampus was completely eliminated by preadsorption of the antiserum with the immunizing peptide at a concentration of 5 μg/ml.
The other rabbit polyclonal antibody (Ab51) was raised against amino acids 22-41 of the receptor conjugated to keyhole limpet hemocyanin using glutaraldehyde (generously donated by Dr. Bryan Roth, University of North Carolina School of Medicine). This antibody has been characterized in previous studies (Roth et al., 1995; Berry et al., 1996). Staining was greatly attenuated by co-incubation of the antibody with 1 μM of the synthetic peptide antigen. In the cortex and striatum, this antibody produces staining that is very similar to that produced by the BD Pharmingen monoclonal antibody, and another antibody raised against the carboxy terminus of 5-HT2AR (Willins et al., 19997; Bubser et al., 2001). The finding that neither the polyclonal nor monoclonal antibodies used in this study stain the choroid plexus (personal observations of AJM) suggests that neither of these antibodies recognizes 5-HT2CRs (Pazos et al., 1985; Wright et al., 1995).
The interneuronal marker antibodies used in this investigation have been characterized and used extensively in previous studies of the cerebral cortex and basolateral amygdala (Conde et al., 1994; Kawaguchi and Kubota, 1996; Sloviter et al., 2001; McDonald and Betette, 2001; McDonald and Mascagni, 2001, 2002, 2004; Mascagni and McDonald, 2003). Each antibody produced the characteristic pattern of labeling for each of the interneuronal subpopulations seen in previous studies.
RESULTS
The pattern of immunostaining obtained in the amygdala with the three 5-HT2AR antibodies was different (Figs. 1-7), and each will be described separately.
Fig. 1.
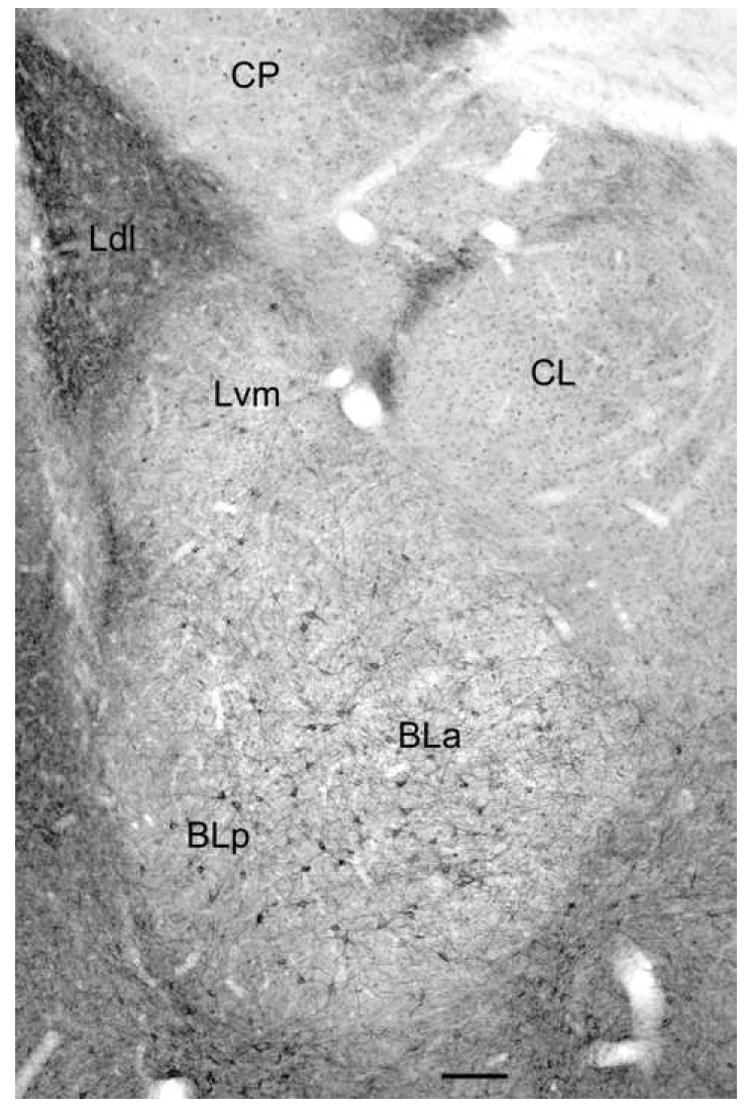
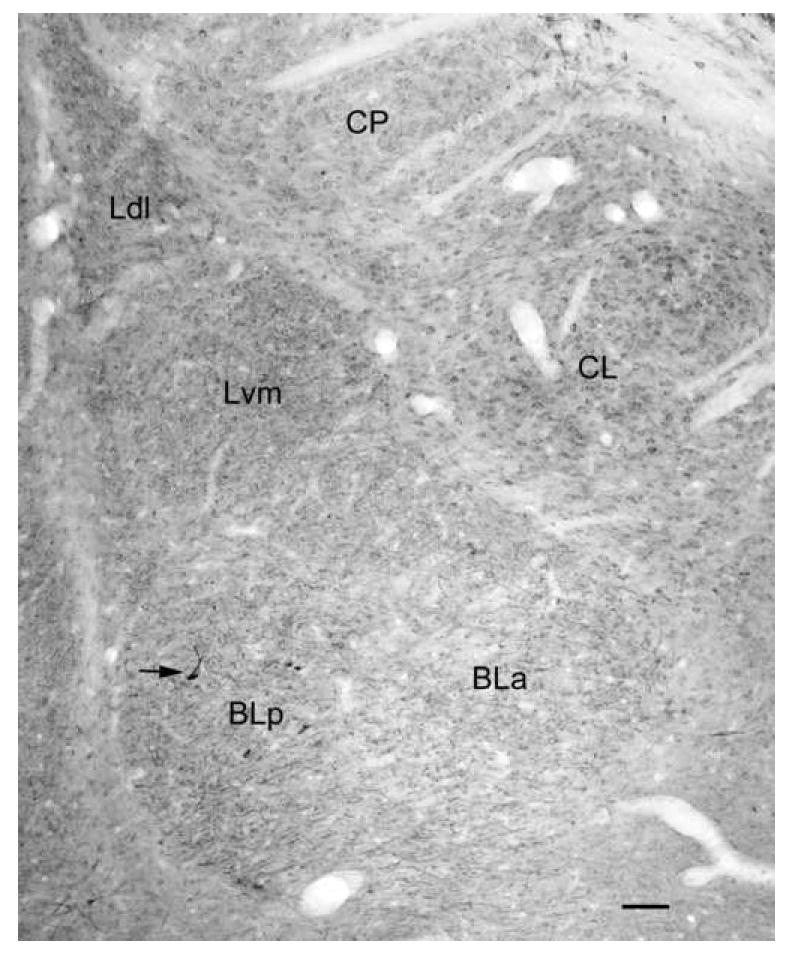
Digital photomicrograph of 5-HT2AR immunoreactivity in the rat amygdala demonstrated with the Oncogene/Calbiochem polyclonal antibody (bregma -2.6 level of Paxinos and Watson, 1997). Note staining of numerous nonpyramidal neurons in the anterior and posterior subdivisions of the basolateral nucleus (BLa and BLp) and the ventromedial subdivision of the lateral nucleus (Lvm). There are similar neurons in the dorsolateral subdivision of the lateral nucleus (Ldl; see Fig. 4), but these are obscured at this low magnification by the dense neuropilar staining. There is also light staining of glial cell nuclei that is mainly evident in the lateral subdivision of the central nucleus (CL) and the caudatoputamen (CP). Scale bar = 100 μm.
Fig. 7.
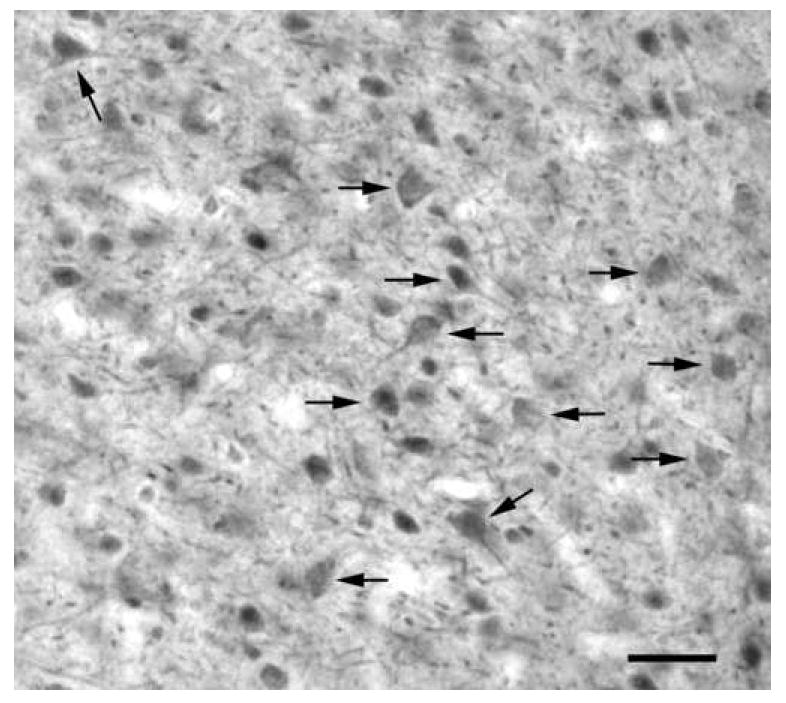
Digital photomicrograph of 5-HT2AR immunoreactivity in the basolateral nucleus using the Ab51 polyclonal antibody. Note staining of many presumptive pyramidal cell somata (arrows) and dendrites. The neuropil contains a very large number of small-caliber structures that appear to be distal dendrites of the immunostained somata. Scale bar = 50 μm.
5-HT2AR Localization: Staining obtained with the Oncogene/Calbiochem polyclonal 5-HT2AR antibody
Immunoperoxidase preparations revealed that there was a moderate density of 5-HT2AR+ neurons in all nuclei of the BLC (Fig. 1). There was a lower density of immunostained neurons in most of the other amygdalar nuclei, especially in the caudal portions of the central nucleus where very few immunostained cells were seen (Fig. 1). All of the 5-HT2AR+ neurons in the BLC appeared to be nonpyramidal interneurons (Figs. 3A, B). The somata of these neurons were 14-20 μm long and 10-12 μm wide in the basolateral nucleus, and tended to be slightly smaller in the lateral nucleus. These cells had 2-4 aspiny dendrites that branched sparingly and could sometimes be followed for over 300 μm from the soma. Although most of the staining was intracellular, the immunolabeling in some dendritic segments was concentrated along the plasma membrane. There was very dense neuropilar staining of the dorsolateral subdivision of the lateral nucleus (Ldl; Figs. 1, 3A, 4), but little or no neuropilar staining in the basolateral nucleus or ventromedial subdivision of the lateral nucleus (Figs. 1, 3). The immunolabeled processes in the neuropil of Ldl appeared to be lightly-stained dendrites, but their structure was difficult to resolve. The pattern of immunostaining in colchicine-injected rats was identical to that seen in non-colchicine-injected rats.
Fig. 3.
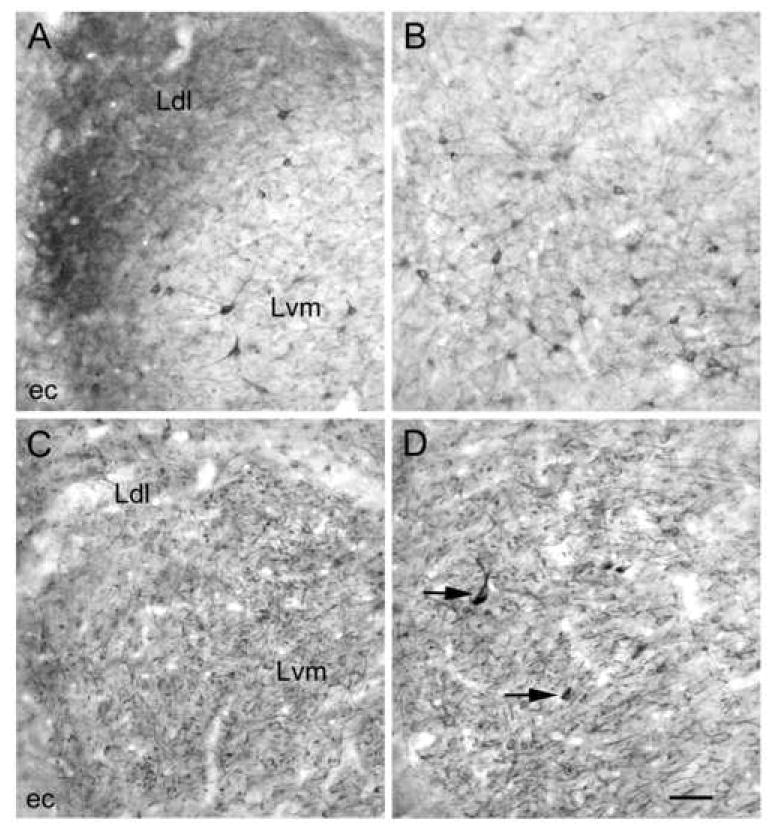
Higher power micrographs of 5-HT2AR staining obtained with the Oncogene/Calbiochem polyclonal antibody (A, B) and the BD Pharmingen monoclonal antibody (C, D). A) Oncogene/Calbiochem polyclonal 5-HT2AR immunoreactivity in the ventromedial (Lvm) and dorsolateral (Ldl) subdivisions of the lateral nucleus. All immunostained neurons appear to be nonpyramidal. These cells are most obvious in the Lvm. ec, external capsule. B) Oncogene/Calbiochem polyclonal 5-HT2AR immunoreactivity in the basolateral nucleus. All immunostained neurons appear to be nonpyramidal. C) BD Pharmingen monoclonal 5-HT2AR immunoreactivity in the ventromedial (Lvm) and dorsolateral (Ldl) subdivisions of the lateral nucleus in a colchicine-injected animal (in the same area of the lateral nucleus depicted in A). All immunostained neurons appear to be pyramidal cells. D) BD Pharmingen monoclonal 5-HT2AR immunoreactivity in the basolateral nucleus in a colchicine-injected animal. All immunostained neurons appear to be pyramidal cells, with the exception of a few intensely-stained nonpyramidal cells (arrows indicate two examples). Scale bar = 50 μm for A-D.
Fig. 4.
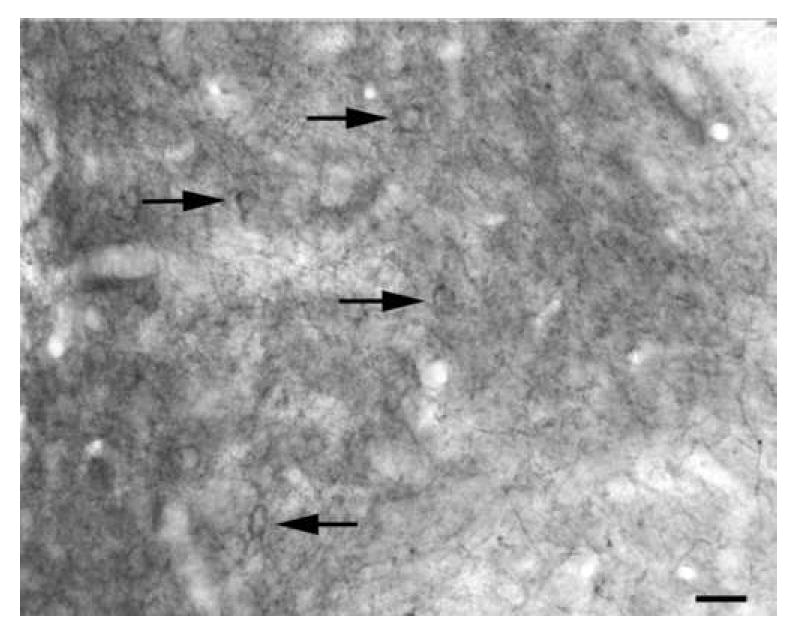
5-HT2AR immunoreactivity in the dorsolateral subdivision of the lateral nucleus (Oncogene/Calbiochem polyclonal antibody). This is the same section as Fig. 1. Arrows indicate immunoreactive perikarya. Scale bar = 20 μm.
Dual-labeling confocal immunofluorescence studies were conducted to determine which interneuronal subpopulations were 5-HT2AR+ (Fig. 5). These investigations revealed that roughly two-thirds to three-quarters (depending on the nucleus) of the 5-HT2AR+ neurons in the BLC were PV+, whereas most of the remaining 5-HT2AR+ neurons were SOM+ (Table 1). These 5-HT2AR+ neurons constituted about two-thirds to three-quarters of the PV+ neurons in the BLC (depending on the nucleus), and about one-third of the SOM+ population. The SOM+ neurons typically exhibited lower levels of 5-HT2AR immunoreactivity than the PV+ neurons. A very small percentage of CR+ neurons exhibited 5-HT2AR immunoreactivity (Figure 5E, Table 1), and no CCK+ neurons were 5-HT2AR+.
Fig. 5.
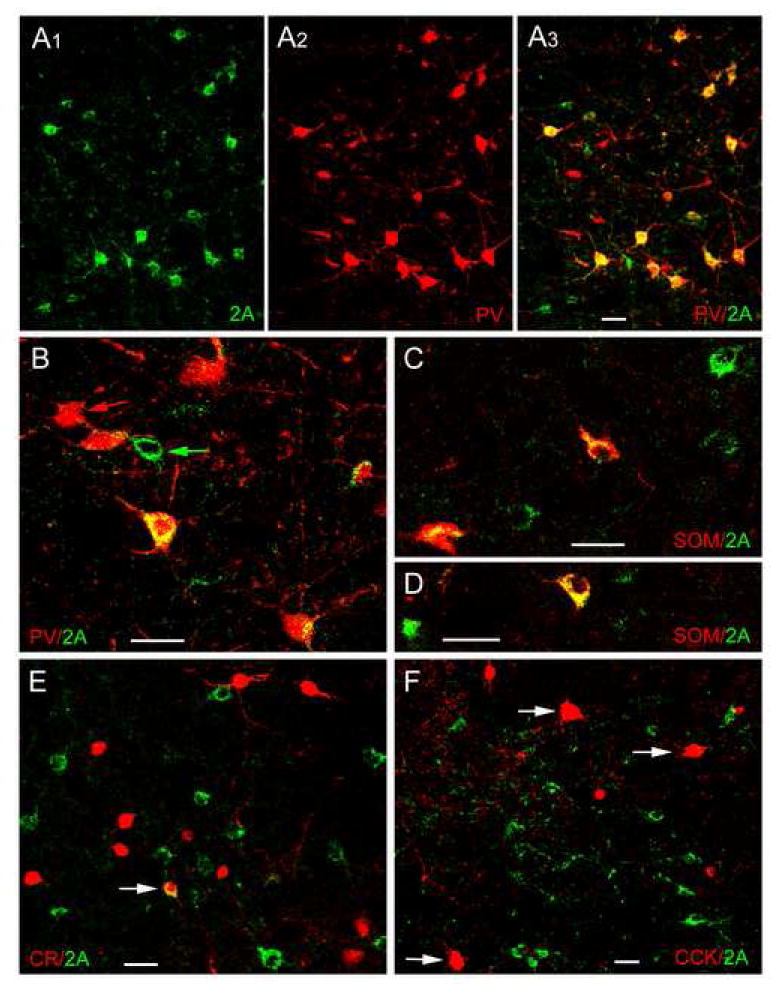
Dual localization of 5-HT2AR immunoreactivity (Oncogene/Calbiochem polyclonal antibody) and interneuronal marker immunoreactivity in the BLa using immunofluorescence confocal laser scanning microscopy. A1-A3) Dual localization of 5-HT2AR and PV in the BLa. A1) 5-HT2AR+ neurons (green). A2) PV+ neurons (red) in the same field. A3) Merging of the red and green channels indicates that there is extensive colocalization of 5-HT2AR and PV immunoreactivity (yellow), but there are also neurons that are single-labeled for 5-HT2AR (green) or PV (red). B) Higher power merged image of 5-HT2AR (green) and PV (red) in the BLa. In addition to five PV+ neurons with variable amounts of 5-HT2AR immunoreactivity (yellow), there is also a single-labeled PV+ neuron (red arrow) and a single-labeled 5-HT2AR + neuron (green arrow). C, D) Dual localization of 5-HT2AR (green) and SOM (red) in the BLa. Double-labeled 5-HT2AR/SOM+ neurons (yellow) and single-labeled 5-HT2AR neurons (green) are observed in these two fields. E) Dual localization of 5-HT2AR (green) and CR (red) in the BLa. Arrow indicates one of two double-labeled neurons found in the BLa (see Table 1). F) Dual localization of 5-HT2AR (green) and CCK (red) in the BLa. No colocalization of 5-HT2AR with CCK was observed in either large Type L CCK neurons (arrows) or small Type S CCK neurons (smaller red cells). All scale bars = 25 μm.
Table 1.
Colocalization of 5-HT2AR and interneuronal markers in the lateral and basolateral nuclei of the amygdala using the Oncogene/Calbiochem polyclonal 5-HT2AR antibody
| Marker | Nuclear Subdivision | 5-HT2AR+ Single-labeled Neurons | Marker+ Single-labeled Neurons | Double-labeled Neurons | Percentage of 5-HT2AR+ neurons Double-labeled | Percentage of Marker+ Neurons Double-labeled |
|---|---|---|---|---|---|---|
| PV | BLa | 36 | 55 | 170 | 82.5% (170/206) | 75.6% (170/225) |
| Lvm | 53 | 78 | 116 | 68.6% (116/169) | 59.8% (116/194) | |
| SOM | BLa | 109 | 116 | 56 | 33.9% (56/165) | 32.6% (56/172) |
| Lvm | 115 | 101 | 50 | 30.3% (50/165) | 33.1% (50/151) | |
| CR | BLa | 121 | 136 | 2 | 1.6% (2/123) | 1.4% (2/138) |
| Lvm | 107 | 170 | 1 | 0.9% (1/108) | 0.6% (1/171) | |
| CCK | BLa | 206 | 100 | 0 | 0.0% (0/206) | 0.0% (0/100) |
| Lvm | 105 | 102 | 0 | 0.0% (0/105) | 0.0% (0/102) |
5-HT2AR Localization: Staining obtained with the BD Pharmingen monoclonal 5-HT2AR antibody
The staining pattern obtained with the BD Pharmingen monoclonal 5-HT2AR antibody in the BLC was quite different from that seen with the Oncogene/Calbiochem polyclonal 5-HT2AR antibody. Initial immunoperoxidase experiments were performed on sections from non-colchicine-injected brains. These preparations were characterized by numerous moderately-stained dendrites and some lightly-stained pyramidal cell somata that appeared to give rise to most of the immunostained dendrites. As in a previous study using this antibody (Luttgen et al., 2004), it was found that injection of colchicine 24 hours prior to sacrifice enhanced staining in these structures; subsequent experiments used only colchicine-injected rats (Figs. 2, 3C, 3D). Immunostained somata were 14-20 μm long and 12-14 μm wide in the basolateral nucleus, and were slightly smaller in the lateral nucleus. In addition, there was a small number of morphologically-heterogeneous nonpyramidal cells in the BLC that were more intensely–stained than the pyramidal cells (Figs. 2, 3D). These intensely–stained nonpyramidal cells had polygonal, irregular or elongated somata (14-28 μm long and 8-12 μm wide) and 2-4 dendrites that branched sparingly. Although some of these nonpyramidal cells were found within the BLa and the posterior subdivision of the basolateral nucleus (BLp), most were located near the external and internuclear borders of the BLC. Thus, these 5-HT2AR+ nonpyramidal cells were mainly located: (1) along the external border of the BLC (especially in the external capsule and intermediate capsule separating the BLC from the central nucleus and caudatoputamen), (2) along the BLa/BLp border, and (3) along the border separating the lateral and basolateral nuclei. The morphology and distribution of these intensely–stained 5-HT2AR+nonpyramidal cells closely resembled that of the BLC neurons that project to the mediodorsal thalamic nucleus (MD; McDonald, 1987), and they were, indeed, selectively labeled by injections of fluorogold into the MD (see below).
Fig. 2.
Digital photomicrograph of 5-HT2AR immunoreactivity in the rat amygdala demonstrated with the BD Pharmingen monoclonal antibody (bregma -3.0 level of Paxinos and Watson, 1997). Note staining of numerous pyramidal neurons in all portions of the BLC (BLa, BLp, Lvm, and Ldl), and a small number of intensely-stained nonpyramidal neurons in the BLp (one of which is indicated by an arrow; see Fig 3D for a higher power view). There is also staining of principal neurons in the lateral subdivision of the central nucleus (CL) and the caudatoputamen (CP). Scale bar = 100 μm.
In both pyramidal cells and the intensely-labeled nonpyramidal cells (and in both colchicine-injected and non-colchicine-injected rats) most of the staining was intracellular, but the immunolabeling in some dendritic segments was concentrated along the plasma membrane. The predominant principal cell staining seen in the BLC was also seen in all of the other amygdalar nuclei (e.g., the central nucleus, Fig. 2), with the notable exception of the intercalated nuclei, where none of the small principal neurons were stained.
Dual-labeling confocal immunofluorescence studies (n = 3 colchicine-injected brains) and two-color immunoperoxidase studies (n = 1 colchicine-injected brain) were conducted to determine if any of the three main interneuronal subpopulations in the BLC (i.e, PV+, CR+ and SOM+ interneurons) were stained with the monoclonal 5-HT2AR antibody. Only an occasional SOM+ or CR+ neuron exhibited 5-HT2AR immunoreactivity. However, in both the two-color immunoperoxidase and dual-labeling immunofluorescence preparations (Fig. 6A, B) there were numerous PV+ neurons with light to moderate 5-HT2AR immunoreactivity. Cell counts pooled from two dual-labeling immunofluorescence preparations revealed that 64.5% (69/107) of PV+ neurons in the BLa, and 34.9% (37/106) of PV+ neurons in the Lvm, were 5-HT2AR+. In contrast, none of the intensely-labeled (i.e., MD-projecting, see below) 5-HT2AR+ nonpyramidal cells were PV+, CR+ or SOM+ (Fig 6A, arrow).
Fig. 6.
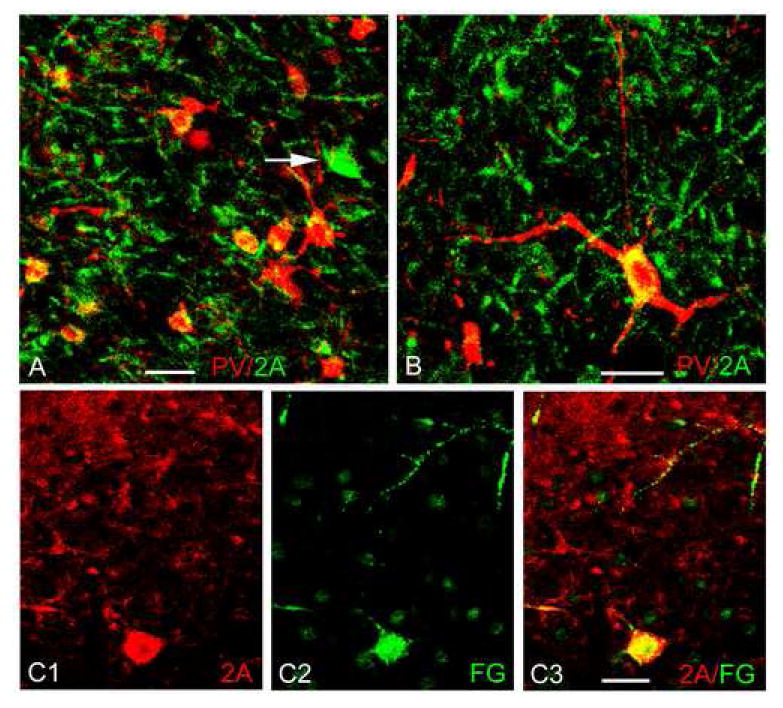
Dual localization of 5-HT2AR immunoreactivity (BD Pharmingen monoclonal antibody) and neuronal markers in the BLa using immunofluorescence confocal laser scanning microscopy. A, B) Dual localization of 5-HT2AR (green) with PV (red). Colocalization is indicated by yellow. Note light to moderate 5-HT2AR immunoreactivity in some of the PV+ neurons. Arrow in A indicates one of the large intensely-stained 5-HT2AR+ nonpyramidal cells that are labeled by injections of fluorogold into the mediodorsal thalamic nucleus (see C1-C3); none of these cells expressed the interneuronal markers PV, SOM or CR. C1-C3) Dual localization of 5-HT2AR (red) and fluorogold (FG; green), with injections of FG into the mediodorsal thalamic nucleus. Note that a large intensely-stained 5-HT2AR+ nonpyramidal cell (lower part of field) is retrogradely labeled by fluorogold. All scale bars = 25 μm.
As in previous studies (McDonald, 1987), fluorogold injections (FG) into the mediodorsal thalamic nucleus (MD) resulted in the retrograde labeling of a population of relatively large nonpyramidal cells located in the anterior and posterior subdivisions of the basolateral nucleus, as well as along the borders of these nuclei. Virtually all of these retrogradely labeled neurons had high levels of 5-HT2AR immunoreactivity (Fig. 6C) and clearly corresponded to the intensely–stained 5-HT2AR+nonpyramidal cells seen in immunoperoxidase preparations.
5-HT2AR Localization: Staining obtained with the Ab51 polyclonal 5-HT2AR antibody
As in the cortex and striatum (Willins et al., 1997; Bubser et al., 2001) the staining obtained with the Ab51 polyclonal 5-HT2AR antibody in the BLC in immunoperoxidase preparations was similar to that obtained with the BD Pharmingen monoclonal antibody. Thus, pyramidal cells and their processes appeared to be the main 5-HT2AR+ structures, although some glial cell nuclei were also stained. The staining of the perikarya of these cells appeared to be slightly enhanced in colchicine-injected animals (Fig. 7). Unlike the monoclonal antibody, however, antibody Ab51 did not produce robust staining of the population of large nonpyramidal cells that project to the mediodorsal thalamic nucleus. Although most of the staining was intracellular, the immunolabeling in some dendritic segments was concentrated along the plasma membrane.
Dual-labeling confocal immunofluorescence studies were conducted in two colchicine-injected brains and one non-colchicine-injected brain to determine whether PV+, CR+, CCK+, or SOM + interneuronal subpopulations were stained with the Ab51 antibody. Only occasional cells in each of these four subpopulations exhibited immunoreactivity; the staining in these neurons was very light and barely above background levels.
DISCUSSION
Although there is evidence that the monoclonal and polyclonal antibodies used in this study are specific for 5-HT2AR (see above), their staining patterns in the BLC were different. The Oncogene/Calbiochem polyclonal antibody (OC antibody) mainly stained nonpyramidal interneurons that expressed PV or SOM, although numerous dendrite-like processes were stained in the Ldl. The density of the latter processes indicates that they probably arise from pyramidal cells, the principal neurons of the BLC. In contrast, the BD Pharmingen monoclonal antibody (BD antibody) appeared to stain all of the pyramidal cells in the BLC. In addition, the BD antibody stained a specific subpopulation of large nonpyramidal cells that project to the mediodorsal thalamus, but that do not express interneuronal marker peptides/proteins. There was also light to moderate staining of PV+ interneurons by the BD antibody. The third antibody, the Ab51 polyclonal antibody, mainly stained pyramidal cells.
Staining differences exhibited by the 5-HT2AR antibodies
All three primary antibodies were raised against sequences of amino acids in the N-terminal portion of 5-HT2AR that are specific to this receptor, and not shared by 5-HT2BR or 5-HT2CR (Julius et al., 1988). However, the immunizing antigen for each antibody was distinct (see Experimental Procedures). Presumably differences in the nature and presentation of the immunizing antigen affect the epitope recognition site of the antibodies, and the subsequent immunohistochemical staining (Boersma et al., 1993). The conjugation of synthetic peptides to protein carriers may create three-dimensional conformations of peptides that mimic those seen in fixed neural tissue, but may also lead to the disappearance of particular antigenic determinants. It is possible, therefore, that these differences in the preparation of the antigen might result in epitopes with distinct molecular configurations, which could produce antibodies that recognize distinct conformations of the 5-HT2A receptor protein located in different neuronal domains, similar to the situation with different 5-HT3R antibodies (Morales et al., 1998; Miquel et al. 2002). It is also possible that these antibodies might recognize different post-translational modifications of the receptor proteins, as has been shown for different 5-HT1AR antibodies (Anthony and Azmitia, 1997). It is of interest is this regard that biochemical studies indicate that the BD antibody may recognize an immaturely glycosylated form of the 5-HT2AR which is not associated with the surface membrane (Nocjar et al., 2002).
The staining of BLC pyramidal cells by both the BD and Ab51 antibodies is consistent with the similar staining pattern of these two antibodies in the cortex and striatum (Willins et al., 1997; Bubser et al., 2001). Since the immunizing antigens were different for each antibody, the fact that they both stain pyramidal cells suggests that BLC pyramidal cells may express 5-HT2ARs. It is not clear why the OC antibody only stains pyramidal cells in the Ldl.
The staining of PV+ interneurons by both the BD and OC antibodies suggests that these interneurons express 5-HT2ARs. It is also of interest that a 5-HT2AR antibody raised against the 22-41 amino acid sequence of the receptor conjugated to keyhole limpet hemocyanin using an N-terminal cysteine only stained interneurons in the forebrain, including the BLC (Garlow et al. 1993; Morilak et al, 1993); the laminar distribution of these interneurons in the hippocampus (i.e., in the pyramidal layer and stratum oriens) suggests that they were PV+ and SOM+ interneurons (Freund and Buzsaki, 1996), similar to the findings with the OC antibody in the BLC in the present study.
The BD antibody and the Ab51 antibody have been used in several studies of the neocortex and produced a very similar staining pattern (Willins et al., 1997; Bubser et al., 2001). Since there have been numerous electrophysiological studies and receptor binding autoradiographic studies of neocortical 5-HT2ARs, it is of interest to correlate the staining of these two antibodies, as well as the less widely used OC antibody, with the results of these studies. Comparison of the staining produced by the BD and OC antibodies in the cortex (Fig. 8) suggests that the staining produced by the latter may be more closely related to receptors that are capable of ligand-binding. Thus, although there is electrophysiological evidence that the apical dendrites, but not the perikarya, of layer V pyramidal cells exhibit postsynaptic 5-HT2AR responses (Aghajanian and Marek, 1997), the BD antibody (Fig. 8A), as well as the Ab51 antibody, stained both of these compartments. In contrast, only the apical dendrites of layer V pyramidal cells were stained with the OC antibody (Fig. 8B). In addition, the pattern of 5-HT2AR receptor binding autoradiographic studies in the BLC and neocortex closely matches that of the staining with the OC antibody, but not the BD antibody. Thus, 5-HT2A receptor binding autoradiography demonstrates a dense band in the neocortex that has been ascribed to layer IV and/or the superficial portion of layer V (Pazos et al., 1985; Blue et al., 1988); this band is seen with the OC antibody (Fig. 8B), and with another 5-HT2AR antibody (Hamada et al., 1998), but not with the BD antibody (Fig. 8A) or the Ab51 antibody. Likewise, the dorsolateral subdivision of the lateral amygdalar nucleus exhibits especially dense 5-HT2A receptor binding (Pazos et al., 1985) that matches the dense immunostaining seen with OC antibody, but not with the BD or Ab51 antibodies.
Fig. 8.

Digital photomicrographs of 5-HT2AR immunoreactivity in the primary somatosensory cortex of the rat obtained with the BD Pharmingen monoclonal (A) and Oncogene/Calbiochem polyclonal antibodies (B). The borders of layer V were determined using an adjacent Nisslstained section. A) Note that the monoclonal antibody stains the somata and basal dendrites of layer V pyramidal cells, as well as the apical dendrites of these cells that extend through the superficial layers of the cortex to the pial surface. B) The polyclonal antibody stains the apical dendrites of these cells in the superficial layers, but not the somata and basal dendrites. This antibody also stains a band of neuropil in the superficial part of layer V (Layer Va). Scale bar = 100 μm.
Colchicine, which inhibits axonal and dendritic transport by disrupting microtubules, enhanced the staining of pyramidal cell somata in preparations stained with the BD and Ab51 antibodies, but had no observable effect on OC antibody staining. However, since the somata and dendrites of the nonpyramidal cells stained with the OC antibody were very intensely stained in non-colchicine-injected brains, increases in staining with colchicine would be less likely to be noticed.
5-HT2AR expression in pyramidal cells of the BLC
The pattern of immunostaining obtained with the BD and Ab51 antibodies suggests that virtually all pyramidal cells in the BLC express 5-HT2ARs that are mainly associated with dendrites. In contrast, staining of presumptive pyramidal cell dendrites was confined to the dorsolateral subdivision of the lateral nucleus (Ldl) with the OC antibody. The latter staining closely correlates with 5-HT2R receptor binding autoradiography (Pazos et al., 1985) and in situ hydridization studies for 5-HT2AR mRNA (Wright et al., 1995) which demonstrate dense labeling in the Ldl, and lighter labeling in other portions of the BLC. Although these results suggest that Ldl pyramidal cell dendrites exhibit 5-HT2ARs, an electrophysiological study which focused on Ldl found no significant increases in pyramidal cell firing by iontophoretically applied 5-HT, only decreases in pyramidal cell firing due to activation of serotonergic receptors on GABAergic interneurons (Stutzmann and LeDoux, 1999). It is possible that the strong inhibition of pyramidal cells by 5-HT-activated interneurons (see below) countered the 5-HT2AR- mediated excitation of pyramidal cell dendrites.
The failure of the OC antiserum to stain pyramidal cells in other BLC nuclei could indicate that the 5-HT2AR epitope targeted by this antibody is masked in those cells. Electrophysiological evidence on this issue is equivocal. Rainnie (1999) found no evidence for 5-HT2R-mediated pyramidal cell depolarization in his whole-cell patch clamp studies of the BLa. In contrast, Stein et al. (2000) noted an increase in presumptive pyramidal cell firing in the BLC using in vivo extracellular unit recording with microiontophoretic application of DOI, a 2A/2C agonist, and Chen et al. (2003)reported that 5-HT2R-activation enhances NMDA receptor-mediated synaptic plasticity in presumptive pyramidal cells in the BLC.
In addition to moderately labeling pyramidal cells, the BD antibody produced robust staining of 5-HT2AR+ nonpyramidal cells in the basolateral nucleus that were retrogradely labeled by fluorogold injections into the mediodorsal thalamic nucleus, the main thalamic nucleus targeting the prefrontal cortex (PFC). The intense staining of these cells suggests that they express very high levels of 5-HT2ARs. In addition to this indirect projection to the PFC, the basolateral nucleus of the amygdala also provides a direct topographically-organized projection to this cortical region via its pyramidal cells (McDonald, 1987). Thus, it appears that the activity of BLC neurons providing both direct and indirect projections to the PFC may be modulated by 5-HT2ARs.
5-HT2AR expression in GABAergic interneurons of the BLC
The OC antibody demonstrated that two of the four main subpopulations of GABAergic interneurons identified in previous studies on the basis of their content of peptides and calcium-binding proteins express 5-HT2ARs. The majority of 5-HT2AR+ interneurons were PV+, and most of the remaining 5-HT2AR+ interneurons were SOM+. There was also significant staining of PV+ interneurons by the BD antibody. This staining of PV+ neurons by both antibodies correlates with the finding that PV+ interneurons are postsynaptic targets of 5-HT+ axon terminals in the BLC (Muller et al., 2005), although the receptors associated with these 5-HT+ terminals have not been determined. None of the antibodies stained significant numbers of large CCK+ interneurons (Type L CCK+ neurons; Mascagni and McDonald, 2003) or the small CR/CCK interneuronal subpopulation. Since both PV+ and SOM+ interneurons innervate BLC pyramidal cells (Muller et al., 2006, 2007), 5-HT2AR-mediated excitation of these GABAergic interneuronal subpopulations would be expected to result in indirect inhibition of pyramidal cells. This is consistent with electrophysiological studies which have demonstrated that the inhibition of BLC pyramidal cells by 5-HT or 5-HT2 agonists is blocked by GABA antagonists (Stutzmann and LeDoux, 1999; Stein et al., 2000), although some of the pyramidal cell inhibition produced by 5-HT is probably mediated in part by excitation of 5-HT3AR+ interneurons (Morales et al., 1998; Stein et al., 2000; Mascagni and McDonald, 2007).
Similarly, using whole-cell patch clamp techniques, Rainnie (1999) demonstrated that 5-HT or 5-HT2 agonists, but not 5-HT1A or 5-HT1B agonists, directly depolarized about 75% of the interneurons recorded from the BLa. Since the remaining 25% were unresponsive, these data suggest that only a subpopulation of BLa interneurons express 5-HT2 receptors, consistent with our results. Also in agreement with our findings that PV+ neurons were the main 5-HT2AR+ interneuronal subpopulation stained with both the OC and BD antibodies, the 5-HT2-responsive interneurons in Rainnie’s study typically exhibited a distinctive burst-firing discharge, which has subsequently been shown to characterize a subpopulation of PV+ interneurons in the BLa (Rainnie et al., 2006). Rainnie (1999) also provided evidence that the 5-HT2R-mediated activation of these interneurons subsequently produces a GABAA-mediated inhibition of BLa pyramidal neurons.
PV+ neurons constitute the largest subpopulation of GABAergic interneurons in the BLC (McDonald and Mascagni, 2001). These PV+ interneurons, which are tightly interconnected by both chemical synapses and gap junctions, appear to constitute an interneuronal network that may generate fast rhythmic oscillations during emotional arousal (Paré and Collins, 2000; Paré et al., 2002; Muller et al., 2005). Collectively, this PV+ interneuronal network provides a massive inhibitory innervation of both the perisomatic and distal dendritic compartments of BLC pyramidal cells (Muller et al., 2006). Anatomical evidence indicates that individual PV+ basket cells may innervate more than 100 surrounding pyramidal cells, and that the axons of approximately 10-20 PV+ basket cells may converge onto each pyramidal cell body (Muller et al. 2006; Rainnie et al., 2006). The extensive divergence and convergence of the axons of PV+ basket cells (and also chandelier cells) innervating the perisomatic domains of neighboring pyramidal cells could entrain the firing of these cells.
Thus, it would be expected that the 5-HT2AR-mediated activation of the PV+ interneuronal network suggested by the present study would not simply inhibit BLC projection neurons, but would modulate synchronized rhythmic oscillations in the BLC. This is consistent with electrophysiological studies which demonstrated that 5-HT2R-mediated excitation of interneurons that exhibited rhythmic burst-firing discharges characteristic of PV+ neurons increased the frequency of rhythmic IPSPs observed in pyramidal neurons (Rainnie, 1999; Rainnie et al., 2006). Since network rhythms are critical for synaptic plasticity associated with the formation of emotional memories (Paré et al, 2002; Pape and Stork, 2003), it is not surprising that selective serotonin reuptake inhibitors alter fear conditioning (Inoue et al., 2004; Burghardt et al., 2004; Izumi et al., 2006).
Acknowledgments
The authors would like to thank the following investigators for their donations of the antibodies used in this study: Dr. Kenneth Baimbridge (University of British Columbia) for the rabbit polyclonal antibody to parvalbumin, Dr. John H. Walsh (CURE/Digestive Diseases Research Center, Antibody/RIA Core, NIH Grant #DK41301, Los Angeles, CA) for the mouse anti-CCK antibody, Dr. Bryan Roth (University of North Carolina School of Medicine) for the Ab51 polyclonal 5-HT2AR antibody, and Dr. Lothar Jennes (University of Kentucky) for the guinea pig polyclonal antibody to fluorogold. We would also like to thank Drs. Donald Rainnie (Emory University School of Medicine) and Jay Muller (University of South Carolina School of Medicine) for comments on an earlier draft of this manuscript. This work was supported by NIH Grant NS38998.
Abbreviations
- 5-HT
serotonin
- 5-HT2R
serotonin type 2 receptor
- 5-HT2AR
serotonin type 2A receptor
- BD antibody
BD Pharmingen 5-HT2AR antibody
- BLa
anterior subdivision of the basolateral amygdalar nucleus
- BLp
posterior subdivision of the basolateral amygdalar nucleus
- BLC
basolateral nuclear complex of the amygdala
- CCK
cholecystokinin
- CL
lateral subdivision of the central nucleus
- CR
calretinin
- ec
external capsule
- GABA
gamma aminobutyric acid
- Ldl
dorsolateral subdivision of the lateral amygdalar nucleus
- Lvm
ventromedial subdivision of the lateral amygdalar nucleus
- MD
mediodorsal thalamic nucleus
- OC antibody
Oncogene/Calbiochem 5-HT2AR antibody
- PV
parvalbumin
- SOM
somatostatin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. Electrophysiology of serotonin receptor subtypes and signal transduction pathways. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. Raven Press; New York: 1995. pp. 451–460. [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Andorn AC, Bennett TL, Gallagher KK, Hogan D. Antipsychotic drug interactions with specific [3H]ketanserin binding sites in membrane fragments derived from human prefrontal cortex and human amygdala. Brain Res. 2003;971:66–72. doi: 10.1016/s0006-8993(03)02356-4. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Azmitia EC. Molecular characterization of antipeptide antibodies against the 5-HT1A receptor: evidence for state-dependent antibody binding. Brain Res Mol Brain Res. 1997;50:277–284. doi: 10.1016/s0169-328x(97)00201-5. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Berry SA, Shah MC, Khan N, Roth BL. Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol Pharmacol. 1996;50:306–313. [PubMed] [Google Scholar]
- Blue ME, Yagaloff KA, Mamounas LA, Hartig PR, Molliver MR. Correspondence between 5HT2 receptors and serotonergic axons in rat neocortex. Brain Res. 1988;453:315–328. doi: 10.1016/0006-8993(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Boersma WJA, Haaijman JJ, Claassen E. Use of synthetic peptide determinants for the production of antibodies. In: Cuello AC, editor. Immunohistochemistry II. Chichester England: John Wiley and Sons; 1993. pp. 1–78. [Google Scholar]
- Bubser M, Backstrom JR, Sanders-Bush E, Roth BL, Deutch AY. Distribution of serotonin 5-HT(2A) receptors in afferents of the rat striatum. Synapse. 2001;39:297–304. doi: 10.1002/1098-2396(20010315)39:4<297::AID-SYN1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Canli T, Congdon E, Gutknecht L, Constable RT, Lesch KP. Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. J Neural Transm. 2005;112:1479–1485. doi: 10.1007/s00702-005-0391-4. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Chen A, Hough CJ, Li H. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-D-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience. 2003;119:53–63. doi: 10.1016/s0306-4522(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cornea-Hébert V, Watkins KC, Roth BL, Kroeze WK, Gaudreau P, Leclerc N, Descarries L. Similar ultrastructural distribution of the 5-HT(2A) serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience. 2002;113:23–35. doi: 10.1016/s0306-4522(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Morilak DA, Dean RR, Roth BL, Ciaranello RD. Production and characterization of a specific 5-HT2 receptor antibody. Brain Res. 1993;615:113–120. doi: 10.1016/0006-8993(93)91121-8. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Hamada S, Senzaki K, Hamaguchi-Hamada K, Tabuchi K, Yamamoto H, Yamamoto T, Yoshikawa S, Okano H, Okado N. Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Brain Res Mol Brain Res. 1998;54:199–211. doi: 10.1016/s0169-328x(97)00322-7. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Two-color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat. 1986;75:343–352. doi: 10.1002/aja.1001750216. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- Inoue T, Li XB, Abekawa T, Kitaichi Y, Izumi T, Nakagawa S, Koyama T. Selective serotonin reuptake inhibitor reduces conditioned fear through its effect in the amygdala. Eur J Pharmacol. 2004;497:311–316. doi: 10.1016/j.ejphar.2004.06.061. [DOI] [PubMed] [Google Scholar]
- Izumi T, Inoue T, Kitaichi Y, Nakagawa S, Koyama T. Target brain sites of the anxiolytic effect of citalopram, a selective serotonin reuptake inhibitor. Eur J Pharmacol. 2006;534:129–132. doi: 10.1016/j.ejphar.2005.12.073. [DOI] [PubMed] [Google Scholar]
- Julius D, Huang KN, Livelli TJ, Axel R, Jessell TM. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci USA. 1990;87:928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett. 1993;162:81–84. doi: 10.1016/0304-3940(93)90565-3. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Luttgen M, Ove Ogren S, Meister B. Chemical identity of 5-HT2A receptor immunoreactive neurons of the rat septal complex and dorsal hippocampus. Brain Res. 2004;1010:156–165. doi: 10.1016/j.brainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Maisonnette S, Villela C, Carotti AP, Landeira-Fernandez J. Microinfusion of nefazodone into the basolateral nucleus of the amygdala enhances defensive behavior induced by NMDA stimulation of the inferior colliculus. Physiol Behav. 2000;70:243–247. doi: 10.1016/s0031-9384(00)00256-0. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. A novel subpopulation of 5-HT3A receptor imunoreactive interneurons in the rat basolateral amygdala. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.10.044. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. J Comp Neurol. 1987;262:46–58. doi: 10.1002/cne.902620105. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala. New York: Wiley-Liss; 1992. pp. 67–96. [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of calbindin-D(28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Mania I, Rainnie DG. Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain Res. 2005;1035:32–40. doi: 10.1016/j.brainres.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, Doucet E, Hamon M, Verge D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur J Neurosci. 2002;15:449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- Morilak DA, Garlow SJ, Ciaranello RD. Immunocytochemical localization and description of neurons expressing serotonin2 receptors in the rat brain. Neuroscience. 1993;54:701–717. doi: 10.1016/0306-4522(93)90241-7. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456:217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Targets of serotonin-immunoreactive terminals in the rat basolateral amygdala. Soc Neurosci Abst. 2005;605(6) [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Roth BL, Pehek EA. Localization of 5-HT(2A) receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience. 2002;111:163–176. doi: 10.1016/s0306-4522(01)00593-0. [DOI] [PubMed] [Google Scholar]
- Pape HC, Stork O. Genes and mechanisms in the amygdala involved in the formation of fear memory. Ann N Y Acad Sci. 2003;985:92–105. doi: 10.1111/j.1749-6632.2003.tb07074.x. [DOI] [PubMed] [Google Scholar]
- Paré D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1361. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol. 2006;498:142–161. doi: 10.1002/cne.21049. [DOI] [PubMed] [Google Scholar]
- Roth BL, Palvimaki EP, Berry S, Khan N, Sachs N, Uluer A, Choudhary MS. 5-Hydroxytryptamine2A (5-HT2A) receptor desensitization can occur without down-regulation. J Pharmacol Exp Ther. 1995;275:1638–1646. [PubMed] [Google Scholar]
- Roth BL, Berry SA, Kroeze WK, Willins DL, Kristiansen K. Serotonin 5-HT2A receptors: molecular biology and mechanisms of regulation. Crit Rev Neurobiol. 1998;12:319–338. doi: 10.1615/critrevneurobiol.v12.i4.30. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Schiller L, Jahkel M, Kretzschmar M, Brust P, Oehler J. Autoradiographic analyses of 5-HT1A and 5-HT2A receptors after social isolation in mice. Brain Res. 2003;980:169–178. doi: 10.1016/s0006-8993(03)02832-4. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Ali-Akbarian L, Elliott RC, Bowery BJ, Bowery NG. Localization of GABA(B) (R1) receptors in the rat hippocampus by immunocytochemistry and high resolution autoradiography, with specific reference to its localization in identified hippocampal interneuron subpopulations. Neuropharmacology. 1999;38:1707–1721. doi: 10.1016/s0028-3908(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré J-F, Paré D. Differential innervation of paravalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. 5-HT(1A) receptor-mediated inhibition and 5-HT(2) as well as 5-HT(3) receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38:328–337. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Van Denderen JC, Blijleven N, Van Minnen J, Hartig W. Two-laser dual-immunofluorescence confocal laser scanning microscopy using Cy2- and Cy5-conjugated secondary antibodies: unequivocal detection of co-localization of neuronal markers. Brain Res Brain Res Protoc. 1998;2:149–159. doi: 10.1016/s1385-299x(97)00038-x. [DOI] [PubMed] [Google Scholar]
- Wu C, Yoder EJ, Shih J, Chen K, Dias P, Shi L, Ji XD, Wei J, Conner JM, Kumar S, Ellisman MH, Singh SK. Development and characterization of monoclonal antibodies specific to the serotonin 5-HT2A receptor. J Histochem Cytochem. 1998;46:811–824. doi: 10.1177/002215549804600704. [DOI] [PubMed] [Google Scholar]
- Xu T, Pandey SC. Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Res Bull. 2000;51:499–505. doi: 10.1016/s0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Jr, Graeff FG. Behavioral effects of intra-amygdala injections of GABA and 5-HT in the elevated plus-maze. Braz J Med Biol Res. 1994;27:2453–2456. [PubMed] [Google Scholar]


