Abstract
We examined the effect of inoculum dose on SHIV transmission and infection. We found that repeated low-dose intravaginal exposure with either R5-SHIVSF162P3 or X4-SHIVSF33A results in infections that are blunted and rapidly controlled. Interestingly, although the transmission rate after all repeated exposures is comparable for the two viruses, the probability of low-dose vaginal transmission is greater for the X4 than R5 virus. Furthermore, X4-SHIVSF33A replication predominates in low-dose dually-exposed macaques, suggesting that it is better at establishing a systemic infection following transmission. However, X4-SHIVSF33A advantage in transmission and infection is not observed in macaques inoculated intravenously with low-dose mixed inoculum. The finding that although matched in tissue culture infectious dose, the X4 inoculum is more complex leads us to hypothesize that the greater genetic heterogeneity of the X4 virus population may have rendered it less susceptible to the severe bottleneck effects imposed by IVAG inoculation with small doses, allowing for greater probability of transmission and establishment of a generalized infection. These data have implications for HIV-1 transmission and infection in humans.
Keywords: SHIV, vaginal transmission, dose effect
Introduction
It is generally believed that a successful HIV-1 infection, on a per exposure basis, is a low-probability event (Gray et al., 2001), but increases in persons who have a history of multiple exposures or other sexually transmitted diseases(DeGruttola et al., 1989; Galvin and Cohen, 2004; Jewell and Shiboski, 1990). Furthermore, a correlation between plasma viral load and transmission probability has been reported(Garcia et al., 1999; Gray et al., 2001; Quinn et al., 2000), with recent studies showing that the risk of HIV-1 transmission is increased in the setting of primary infection when viral burden is highest(Pilcher et al., 2004; Wawer et al., 2005). The relationship between dose and HIV-1 transmission is noteworthy, suggesting that intervention or vaccine strategies that lower the viral load might have a significant impact in reducing transmission and/or ameliorating disease course(Gray et al., 2003; Mellors et al., 1996).
Because of the similarities existing between humans and non-human primates with regard to the populations of target cells, the physiology and immunology of the female genital tract(Hendrickx and Cukierski, 1987; Miller and Lu, 2003), simian immunodeficiency virus (SIV) or chimeric simian/human immunodeficiency virus (SHIV) infection of rhesus macaques has been commonly used to study HIV-1 transmission in a controlled manner (Miller, 1994)and to screen intervention strategies aimed at reducing HIV-1 transmission or infection(Shattock and Moore, 2003). To achieve high infection rates and limit group sizes for these animal studies, virus doses ranging from 103 to 105 50% tissue culture infectious doses (TCID50) are routinely used. These doses are far greater than that required to infect humans and may underestimate beneficial effects of strategies that could protect against infections in real life settings. Animal models that more closely mimic HIV-1 transmission by repeated low-dose exposure, therefore, are needed for studies of the biology of HIV-1 transmission and for identification of effective vaccine and microbicide candidates.
In this study, rhesus macaques (RM) were exposed intravaginally (IVAG) to single high or multiple low dose of the pathogenic X4 SHIVSF33A, R5 SHIVSF162P3, and mixtures containing varying proportions of the two viruses. Intravaginal (IVAG) exposures were chosen because male to female transmission causes the majority of HIV-1 infections worldwide2005. The probability of transmission and the biology of infection in macaques infected as a result of exposures to small but repeated inocula doses were compared to that seen in macaques infected with high dose inocula to determine if there is an inoculum effect. We report that infection outcome differs in macaques infected with high- and low-dose SHIV inocula. Both R5 SHIVSF162P3 and X4 SHIVSF33A can be transmitted efficiently, even at low doses, but the probability of transmission and infection with low-dose X4 SHIVSF33A is greater than with R5 SHIVSF162P3. Because the heterogeneous genetic nature of HIV-1, which is drastically reduced in the presence of transmission bottleneck, is thought to play a central role in the establishment of viral infection and persistence by allowing for rapid evolution and adaptation (Coffin, 1995), we hypothesize that the greater efficiency of low-dose vaginal transmission of the X4 over the R5 SHIV virus is linked to the greater genetic complexity of the X4 viral quasispecies.
Results
Pathogenic infection in macaques exposed IVAG to single high dose of R5 SHIVSF162P3 or X4 SHIVSF33A
Four RM each were inoculated with a single high inoculum dose of R5 SHIVSF162P3 or X4 SHIVSF33A. All four macaques inoculated with 5000 TCID50 of R5 SHIVSF162P3 were infected, with varying peak (105 – 108 RNA copies/ml plasma) and chronic (102 – 104) viremia (Fig. 1A). Progressive loss of peripheral CD4+ T cells was seen in R183 and BC81, the two infected macaques with sustained viremia. R183 was a rapid progressor and was euthanized at 5.5 wpi. At the time of death, R183 had high viral loads, low CD4 T cell count, a body weight loss greater than 20%, chronic diarrhea nonresponsive to therapy, moderate enterocolitis, severe thymic dysinvolution and generalized lymphoid dysplasia. No definitive evidence of an opportunistic infection was found but the animal did have a multifocal pneumonia consistent with a viral infection. BC81 also progressed to disease at 81 wpi, but the other two infected macaques controlled their infection at 10–15 wpi and have been clinically healthy for over 2 years. Thus, consistent with previous findings(Harouse et al., 2001), IVAG infection with R5 SHIVSF162P3 in RM mimics HIV infection of humans, with natural variation in the level of viremia and disease course(Mellors et al., 1995).
Figure 1.
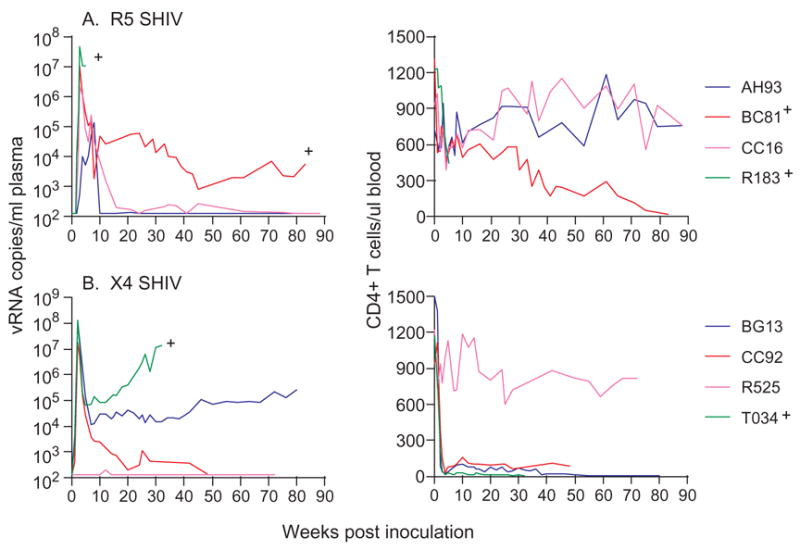
Viremia (viral RNA copies/ml plasma) and CD4+ T cell counts (per μl blood) in macaques infected IVAG with a single high dose of (A) R5 SHIVSF162P3 and (B) X4 SHIVSF33A. +Indicates progression to simian AIDS (SAIDS).
Three of four macaques inoculated once with 3000 TCID50 of X4 SHIVSF33A were also systemically infected, reaching peak viremia of 107 – 108 RNA copies/ml plasma (Fig. 1B). arying degree of virus replication was seen in these infected animals as well, with T034, the macaque with the highest viral burden progressing to SAIDS at 38 wpi. A rapid and precipitous drop in peripheral CD4+ T cell numbers, characteristic of pathogenic X4 SHIV infection, accompanied the rise in viremia. Table 1 provides a summary, based on results from contemporaneous inoculations of eight macaques as well as historic studies using different virus stocks, infection characteristics with high dose IVAG challenge of the two viruses, showing a trend towards greater probability of high dose R5 SHIVSF162P3 (0.85 per exposure) than X4 SHIVSF33A (0.56 per exposure) transmission (p=0.13), but one-log higher peak and chronic virus loads in animals infected with the latter.
Table 1.
Culmulative transmission, replication and pathogenesis data from smacaques exposed IVAG to high dose (>3000 TCID50) of different R5 SHIVSF162P3 and X4 SHIVSF33A virus stocks.
| Virus | Infected/exposed | Probability of transmission | Peak Viremia | Viral setpoint | SAIDS/infected | Time to SAIDS |
|---|---|---|---|---|---|---|
| R5-SHIVSF162P3 | 11/13 | 0.85 | 105 – 108 | 102 – 106 | 6/11 | 5.5 – 104 weeks |
| X4-SHIVSF33A | 9/16 | 0.56 | 106 – 109 | 102 – 107 | 7/9 | 30 – 156 weeks |
Results shown are tabulated from contemporaneous study of eight macaques and historic studies in which nine and twelve macaques were exposed IVAG to R5 and X4 SHIV respectively. The historic studies were conducted using different virus stocks and with challenge doses that are equal or greater for the X4 SHIV.
Transient and attenuated infections in macaques repeatedly exposed IVAG to low dose R5 or X4 SHIV
Six RM each were exposed to weekly inoculum dose containing 50 TCID50 of the same viruses used for high dose challenge. A variable number of exposures, ranging from 1–13, were required to establish systemic infection in all five R5 SHIVSF162P3 exposed macaques (one macaque died of unrelated causes 3 weeks into the study), with a probability of transmission of 0.161 (per exposure) (Fig. 2A). The resulting systemic infection by multiple low-dose exposures differs from that of classic systemic R5 SHIVSF162P3 infection which follows high dose inoculations with the same virus stock (Fig. 1A). Peak viremia of 103 – 105 RNA copies/ml was 2–3 logs lower and none of the infected animals in the low-dose group sustained viremia above the detection limit of 125 copies/ml. Furthermore, there was no evidence of peripheral CD4+ T cell loss. Of note, viral “blips” preceded the onset of a systemic infection in 2 of 5 infected macaques (V558 and V785)(Fig. 2C). Additionally, in monkey N172 for example, multiple peaks in viremia that progressively decreased in magnitude were seen. Since this animal received a total of 6 inoculations, the last one at 5 weeks post-inoculation, it is unlikely that these viral peaks occurring at weeks 12 and 16 post-inoculation are due to re-infection. Collectively, the data show that the infection is attenuated and the pattern of virus replication more variable in the low-dose than high-dose R5 SHIVSF162P3 IVAG infected animals.
Figure 2.
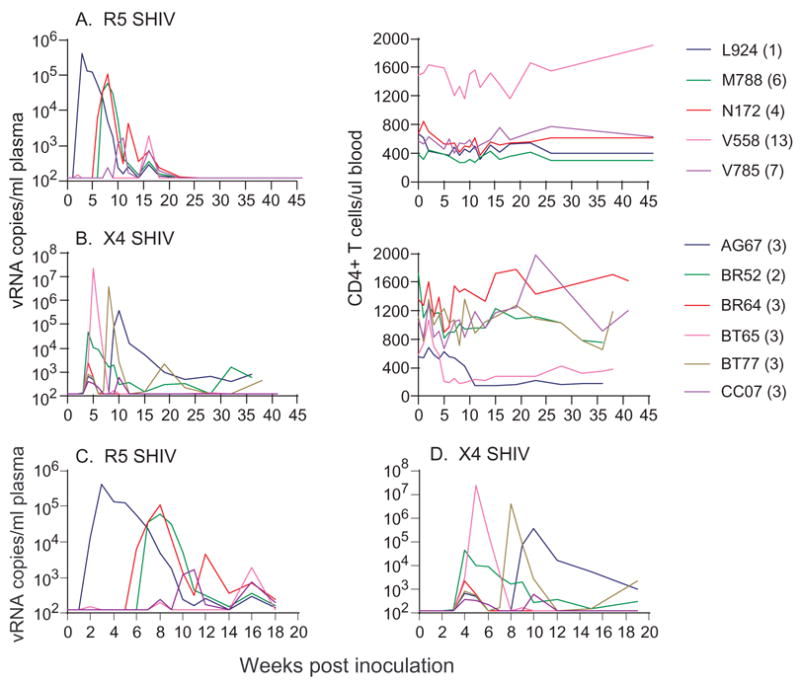
Viremia (viral RNA copies/ml plasma) and CD4+ T cell counts (per μl blood) in macaques exposed weekly to 50 TCID50 of (A) R5 SHIVSF162P3 and (B) X4 SHIVSF33A. Numbers in brackets indicate the number of exposures required to establish systemic infection in each of the macaque. Panels C and D highlight the variability in viremia of low dose R5 SHIVSF162P3 and X4 SHIVSF33A infected macaques respectively.
Interestingly, only 2–3 exposures were required to establish systemic infection in all six macaques that were inoculated weekly with 50 TCID50 of X4 SHIVSF33A (Fig. 2B), with a probability of transmission of 0.353. Thus, although a 100% transmission rate is seen with 50 TCID50 inoculum dose of the R5 (5/5 animals) and X4 (6/6 animals) SHIV after all repeated exposures, the probability of low-dose vaginal transmission is significantly higher for the X4 than R5 virus (p=0.048). The resulting infection in low-dose X4 SHIVSF33A infected macaques also differs from the classic systemic infection that follows a single high-dose exposure (Fig. 1B). With the exception of macaque BT65 where peak viremia of 2 × 107 RNA copies/ml was reached, values in the low-dose infected animals were substantially lower, ranging from 4 × 102 – 4 × 104 RNA copies/ml. Consequently, only BT65 showed peripheral CD4+ T cell loss, but the extent of this loss (~50%) was less than that seen in the high dose infected animals (>95%). Viremia was rapidly controlled in all but BR52, where low level virus replication persisted. However, a second rise in viremia at wk 8 and 9 post-inoculation respectively was seen in macaques BT77 and AG67 (Fig. 2D). Since all the animals received a total of 8 inoculations, the last one at week 7, the increase in virus replication could be the result of re-infection. Interestingly, the rise in peak viremia to values of 105 – 106 RNA copies/ml plasma was accompanied by peripheral CD4+ T cell loss in AG67 but not BT77. Taken together, the data show that infection with low-dose inoculum of X4 SHIVSF33A is also attenuated. However, although a dilution effect was seen for both viruses, the finding that the difference in the probability of R5 high- (n=4) vs repeated R5 low-dose (n=5) IVAG transmission (p=0.024) is of greater significance than that seen for X4 high- (n=4) vs repeated X4 low-dose (p=0.045) suggests that R5 SHIVSF162P3 transmission is more susceptible to the inoculum effect. Furthermore, since strong correlation between peak viral load and the extent of CD4+ T cell depletion in acute infection has been reported for X4 SHIV89.6P (Davenport et al., 2006), the lack of CD4+ T cell depletion in BT77 raises the possibility of transmission of less pathogenic viral variants.
Transmission of variants in macaques repeatedly exposed IVAG to low dose R5 or X4 SHIV
To determine whether the attenuated replication phenotype seen in both X4 and R5 SHIV low dose infected macaques is due to the transmission of virus variants with lower pathogenic potential, heteroduplex mobility assay (HMA) was employed to examine the composition of the transmitted virus. Results showed transmission of the major variant in all four of the R5 high-dose infected macaques as well as four of the five macaques infected by low-dose exposures (Fig. 3A). The exception was V785, in which HMA pattern revealed a slight difference in band migration.
Figure 3.
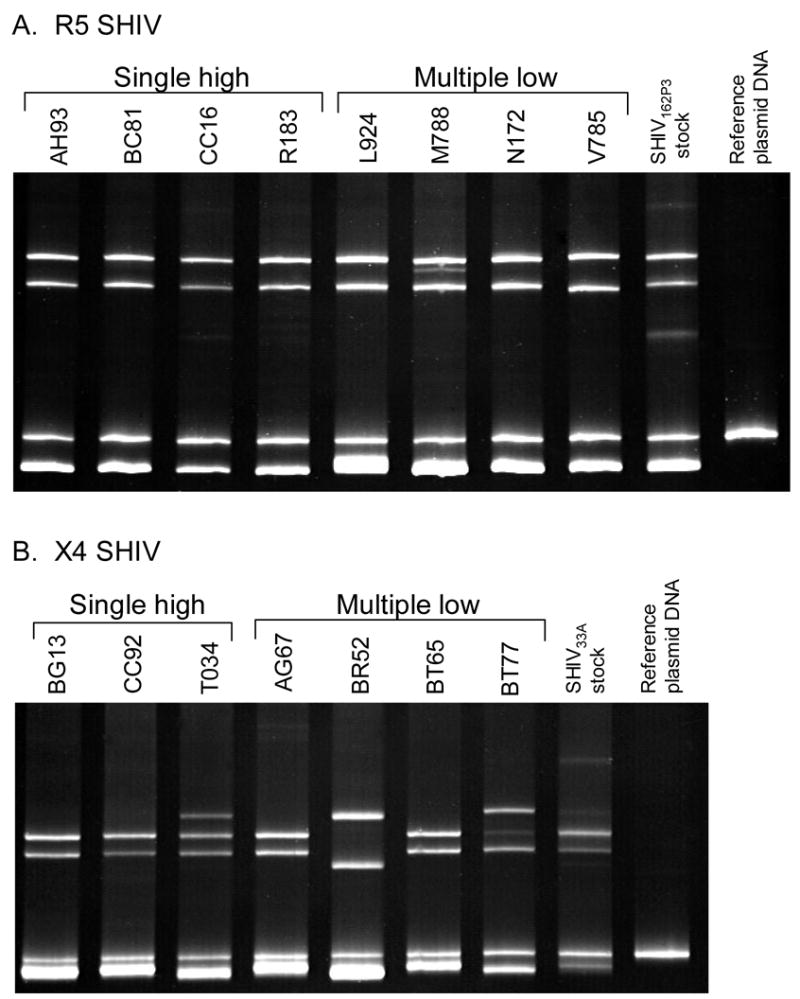
Envelope variants in high and low dose (A) R5 SHIVSF162P3 and (B) X4 SHIVSF33A infected macaques. Variants were analyzed in plasma collected at 2–3 weeks post-infection and HMA profiles are shown.
Analysis of the X4 infected macaques also indicated transmission of the most common variant in the inoculum in all three high dose infected macaques. Although viral loads in two of the low-dose infected macaques were too low for successful amplification, analysis of transmitted viruses in the other four animals (Fig. 3B) showed transmission of minor variants in BT77 and BR52. Interestingly, these are the two macaques that had unusual viral replication patterns (Figs. 2B & D). High viremia peak but little CD4 depletion was seen in macaque BT77 while virus replication in BR52 persisted, albeit at low levels. Thus, there was a tendency for transmission of minor variants with apparent differential phenotypic and replicative properties following low-dose X4 SHIVSF33A IVAG infection. Nevertheless, the finding that similar to high-dose challenge macaques, infection in most multiple low-dose challenge monkeys is initiated with the most common variant indicates that the attenuated infections seen in these animals cannot be attributed solely to the transmission of variants that are less fit.
Selective advantage of X4 SHIVSF33A transmission and replication in macaques repeatedly exposed IVAG to low dose mixed inocula
We previously reported that while both X4 SHIVSF33A and R5 SHIVSF162P3 were simultaneously transmitted as high-dose mixed inocula, replication of R5 SHIV predominated following primary infection(Harouse et al., 2003). The unexpected finding of greater probability of transmission with low-dose X4 SHIVSF33A than R5 SHIVSF162P3 prompted us to examine whether the selective advantage of the X4 virus is manifested in the setting of repeated low-dose exposures with mixed inoculum. Six female RM therefore were exposed to weekly inocula containing 25 TCID50 of each virus. Results showed that a varying number of exposures, ranging from 1–12 were required to achieve systemic infection in five of the six animals (probability of transmission of 0.128), with macaque AP06 remaining unsuccessfully challenged after 13 exposures (Fig. 4A). Peak viremia of 104 to 106 RNA copies/ml was reached, with one animal (R024) sustaining high viral load and progression to SAIDS at 38 wpi. Precipitous drop in peripheral CD4+ T cells, indicative of the presence of X4 SHIVSF33A, was seen in macaque R024. CD4+ T cell loss, although less sustained, was also present in three other infected animals (BV09, CC70, CL49) but was not noticeable in macaque CD03.
Figure 4.
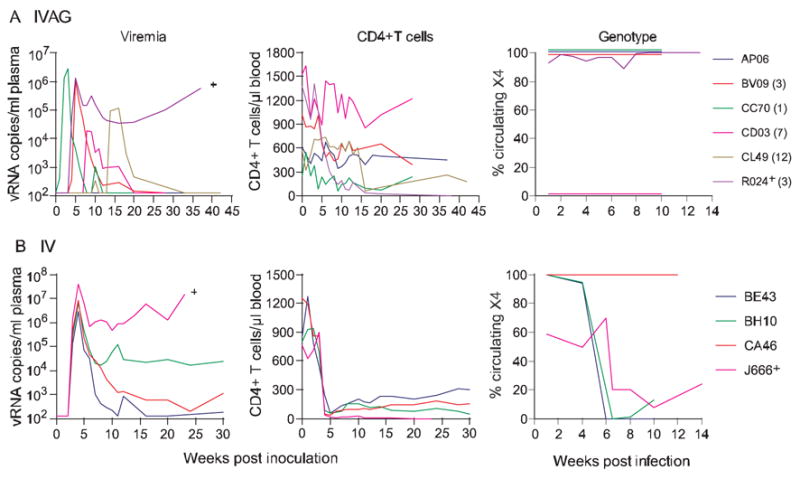
Viremia, CD4+ T cell counts and genotype of replicating virus in macaques exposed weekly intravaginally (A) or intravenously (B) to mixed incoula consisting of 25 TCID50 each of R5 SHIVSF162P3 and X4 SHIVSF33A. Genotype of replicating virus in IVAG and IV infected macaques was determined by real-time PCR. Percentage of circulating X4 virus is shown. + Indicates progression to simian AIDS (SAIDS).
Genotyping results showed the presence of both X4 and R5 SHIVs in macaque R024, but only X4 SHIVSF33A in BV09, CC70 and CL49, and R5 SHIV162P3 in CD03. In the dually-infected macaque R024, the X4 virus dominated. The finding of either X4 or R5 circulating virus in four of the five low-dose dually-exposed macaques is consistent with the stochastic nature of IVAG infection(Greenier et al., 2001), which is accentuated with the use of small inoculum dose(Wick and Self, 2000). However, the trend for selective transmission of X4 SHIVSF33A in low- dose dually-exposed macaques and its dominance in the dually-infected macaque contrast with findings we previously reported using high dose challenges (Harouse et al., 2003).
The selective advantage of low-dose IVAG X4, compared to R5 SHIV transmission, is not linked to differences in infectious inoculum dose of the two viruses and is abrogated with intravenous inoculation
The greater probability of transmission and growth of X4 SHIVSF33A under low-dose IVAG co-infection setting could be due to an inherent replicative property of the X4 virus. Indeed, in vivo replication had been suggested to predict efficiency of vaginal transmission of SIV and SHIV in RM (Miller et al., 1998). Alternatively, the inoculum dose, which is based on TCID50, could be underestimated for the R5 virus as a result of in vitro titration conditions. Although we deem this latter possibility less likely in light of the similar transmission rates seen after all repeated vaginal exposures to 50 TCID50 of the two viruses (Figs. 1 and 2), we inoculated four RM intravenously (IV) with the same dose of mixed inoculum (25 TCID50 of X4 and R5 SHIVs) as the one used for IVAG co-challenge and monitor the genotype of the replicating virus. As there is no bottleneck, all variants present in the inoculum should be transmitted with IV inoculation. As expected, all four animals inoculated IV were infected, reaching peak titers of 106–107 RNA copies/ml with one (J666) progressing to SAIDS at w24 (Fig. 4B). Precipitous peripheral CD4+ T cell loss, indicative of the presence of X4 SHIVSF33A, was detected in all four infected macaques. Genotyping readily detected both viruses in three of the four IV dually-exposed macaques. The exception being CA46 where <2% of circulating virus was of the R5 genotype. Importantly, in those macaques where both viruses could be readily detected, the R5 virus dominated. The finding of similar transmission rates of the X4 and R5 virus with 25 TCID50 each in IV co-challenge further illustrates that the R5 SHIV162P3 inoculum dose is not underestimated, and predominance of the R5 SHIV in three of four low-dose IV dually-infected macaques indicates that the X4 virus is not intrinsically better fit for replication if there is no transmission bottleneck.
Increased representation of the R5 virus in the mixed inoculum favors its transmission by the mucosal route
Genetic bottlenecks present during IVAG transmission results in a restriction of the quasispecies and may influence the ability of the virus to establish infection within the host. To determine whether we can bias towards R5 SHIVSF162P3 vaginal transmission and favor its replication by increasing its relative frequency in the mixed inocula, six RM were exposed weekly to 100 TCID50 mix incoula containing 25 TCID50 of X4 SHIVSF33A and 75 TCID50 of R5 SHIVSF162P3, a three-fold increase in R5 representation. Six of six exposed macaques were shown to be systemically infected after 13 exposures, with a probability of transmission of 0.167. Peak viremia varied ~4 log among the infected animals. BG15 and CE75, the two macaques with the highest peak viremia (>106 RNA copies/ml) sustained viral load of > 104 RNA copies/ml plasma, and progressed to SAIDS at 47 and 42 wpi respectively (Fig. 5). Consistent with the precipitous drop in peripheral CD4+ T cells seen in these two macaques, genotyping revealed the presence of both viruses, but only the R5 virus was detected in BG34, BH71, CB16 and CT25. Thus, it is indeed possible to increase the risk of R5 virus transmission vaginally by increasing its representation in the mixed inoculum. The increased probability of transmission of the R5 SHIV in the 3:1 (0.167) compared to 1:1 (0.03) mixed inoculum is significant (p=0.034).
Fig. 5.
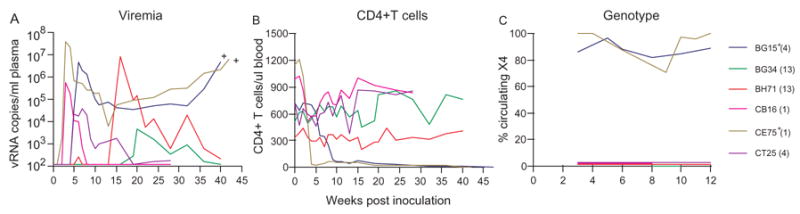
Viremia (A), CD4+ T cell counts (B) and genotype (C) of the replicating virus in macaques exposed weekly to mixed inocula consisting of 75 TCID50 of R5 SHIVSF162P3 and 25 TCID50 of X4 SHIVSF33A. For genotyping, the percentage of circulating X4 virus is shown. + Indicates progression to simian AIDS (SAIDS).
It is worthy of note that when X4 virus is transmitted with R5 virus under low-dose mixed vaginal challenge settings, the X4 virus has an advantage in outgrowing the R5 virus (BG15 and CE75; and R024 in Fig. 4A). Collectively, these data suggest that in the presence of transmission bottleneck, variants of HIV-1 are transmitted in a frequency proportional to their representation in the inoculum. However, in low-dose exposed macaques where both viruses are transmitted simultaneously, X4 virus replication predominates. Significantly, this X4 dominance is not seen after IV low-dose mixed challenges (Fig. 4B) or, as previously reported, in high-dose co-infected macaques (Harouse et al., 2003). This then raises the possibility that there are properties of the X4 inoculum that allow it to establish a systemic infection more efficiently following low-dose vaginal transmission, but which are not necessary or play a lesser role in intravenous infections and high-dose vaginal challenges.
Discussion
In this study, we determine whether transmission and infection outcome differ in RM exposed IVAG to repeated small doses or to a single large inoculum dose, and whether there are differences in susceptibility of X4 and R5 viruses to the “inoculum effects”. Exposure to repeated low doses of pathogenic R5 SHIVSF162P3 or X4 SHIVSF33A results in infections that are blunted and are rapidly controlled. Surprisingly, we find that transmission of the R5 virus is more susceptible than X4 to the inoculum effect. The probability of low-dose IVAG transmission is greater for the X4 SHIV than R5 SHIV, and in contrast to the observations previously made with high-dose mixed inoculum, the X4 virus predominates in low-dose IVAG dually-infected macaques. Thus, vaginal mucosal transmission and infection with X4 SHIVSF33A can be achieved quite efficiently at low doses. Microbicides and vaccination intervention strategies, therefore, should not target only R5, but both X4 and R5 viruses.
Contrary to what is observed with single high-dose IVAG inoculations, a number of exposures are needed to establish systemic infection with low-dose SHIV challenges. Furthermore, significantly lower values of the geometric mean peak viremia are seen in the low- compared to high-dose X4 or R5 SHIV infected macaques (p≤0.02)(Fig. 6). Considerable bottlenecks are operational in vaginal transmission, resulting in fitness loss and random transmission of variants (Greenier et al., 2001; Muller, 1964; Neildez et al., 1998; Zhu et al., 1993), as well as spontaneous resolution of infection (Wick and Self, 2000). In this regard, the attenuated infection and the transmission of minor viral variants seen in X4 and R5 low-dose infected macaques (Figs. 2 and 3), and the presence of either X4 or R5 virus in a majority of macaques exposed to multiple low doses of mixed inocula (Figs. 4 and 5) are consistent with the fitness loss and stochastic nature of IVAG infection, and which are accentuated with the use of small inoculum dose.
Fig. 6.
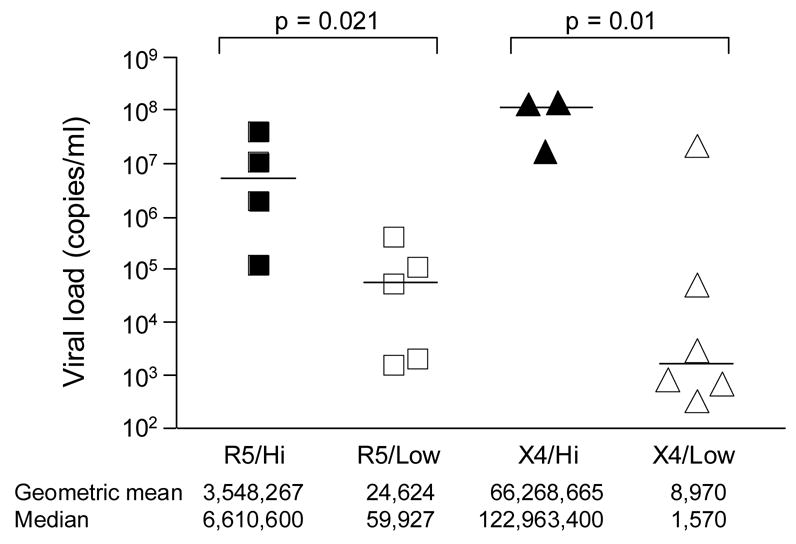
Comparison of peak viremia in macaques infected with high and repeated low dose R5 or X4 SHIVs. The bar indicates median value for the various groups. Viral load data was transformed to logarithmic values for statistical comparison. p values, two-tailed t tests.
The dose effect seen here for both X4 and R5 SHIV IVAG infection differs from reports for the pathogenic SIVmac251 isolate and its molecular clone derivative SIVmac239. Although a period of transient viremia precedes the onset of systemic infection with low-dose SIVmac251 or SIVmac239 mucosal exposures, once established, viral RNA levels in the low-dose infected macaques were similar to that seen in classic systemic infection that follows high-dose challenge (Ma et al., 2004; McDermott et al., 2004; Neildez et al., 1998). Although different routes of inoculation and challenge dose were employed in the various studies, it is conceivable that optimally adapted and highly virulent strains, especially very pathogenic molecular clones, are less susceptible to a dose effect. Indeed, SIVmac251 was derived through several passages in RM over a period of 10 years following the inadvertent transmission of SIVsm from sooty mangabeys to macaques (Mansfield et al., 1995). In contrast, X4 SHIVSF33A was recovered from a macaque infected with the molecular clone SHIVSF33 two years after infection (Luciw et al., 1999), and R5 SHIVSF162P3 was generated through three successive, rapid serial passages of clone SHIVSF162 in RM (Harouse et al., 2001), with a total passage time in RM of ~26 weeks. These SHIVs, in particular the R5 SHIV, are likely to be less well adapted to RM compared to SIV. The finding that R5 SHIVSF162P3 is more susceptible to an inoculum effect than X4 SHIVSF33A is consistent with it being less well adapted. When choosing an animal model to use for testing intervention strategies, these differences in characteristics of repetitive mucosal SIV and SHIV challenges should be taken into consideration.
Intriguingly, we observe that the probability of transmission with repeated low-dose X4 exposure is greater than with the R5 virus (p=0.048). The selective advantage of low- dose IVAG X4 SHIVSF33A transmission was also seen in the context of equal mixed infection experiments, and contrary to what we previously reported in high-dose dually-infected macaques (Harouse et al., 2003), the X4 virus dominated in low-dose dually-infected animals (Figs. 4A & 5). Our inocula are based on tissue culture and not animal infectious dose of the two viruses and it could be argued that due to in vitro titration conditions, the R5 inoculum dose used was in fact lower. However, we find similar transmission rates after repeated vaginal exposures to 50 TCID50 or IV inoculations with 25 TCID50 of the two viruses. And, once infected, the geometric mean or median peak viremia in macaques infected IVAG with 50 TCID50 of the R5 SHIVSF162P3 are not lower than those infected with equal TCID50 of X4 SHIVSF33A (R5/low vs X4/low, Fig. 6), which would have been expected if the infectious dose of the R5 virus is indeed underestimated. Furthermore, the selective advantage of low-dose X4 SHIV transmission and its dominance is route dependent (Fig. 4), seen with IVAG and not IV co-challenge, suggesting that the X4 inoculum is not more infectious if there is no genetic bottleneck.
What then, could be the underlying basis for the selective advantage of X4 SHIVSF33A transmission and efficiency in establishing a generalized infection following low- dose intravaginal challenges if the inoculum dose is indeed matched. It is conceivable that differences in target cell availability or susceptibility of X4 and R5 SHIVs to innate immunity in the vaginal mucosa are playing a role. However, the finding that the probability of low-dose transmission of the R5 virus can be increased by tripling its representation in the inocula makes these possibilities less plausible. Instead, we favor the interpretation that because the X4 SHIVSF33A virus population has been replicating and subjected to selection in the macaque host for a longer time period than R5 SHIVSF162P3, it is better adapted and is more heterogeneous in terms of genetic diversity and complexity. These in turn, render the X4 virus less susceptible to the genetic bottlenecks imposed by IVAG inoculation, allowing for better establishment of a generalized infection. This interpretation is supported by several observations. (i) The X4 inoculum is more complex, containing approximately four times more RNA copies per tissue culture infectious dose than R5. Since variants of HIV-1 are transmitted in a frequency dependent manner (Frater et al., 2006) (Fig. 5), more X4 variants are expected to be transmitted compared to R5 even when the infectious doses are matched. And, although the vast majority of variants within the virus population appear to be defective given the high RNA copy number to tissue culture infectious dose ratios, these genomes can nevertheless contribute to increase host cell tropism and virulence through complementation and/or recombination (Charpentier et al., 2006; Lori et al., 1992). (ii) As each round of replication generates mutations, the X4 inoculum which contains four times greater RNA copy numbers is expected to be more diverse as well. While env and gag sequencing did not reveal significant differences in the average genetic distance between major variants within the X4 and R5 virus populations (Fig. 7), heterogeneity among minor variants, or heterogeneity in other regions of the genome that contribute to viral diversity, cannot be excluded. The difference in passage history of the two viruses, as well as a greater tendency for transmission of minor variants in the X4 inoculum (Fig. 3) is also consistent with it being more heterogeneous than the R5 SHIV. (iii) Since the severity of the bottleneck effects is dependent on inoculum dose, this would explain for our findings of X4 dominance under low- (Fig. 4) but not high-dose vaginal co-infection as reported previously(Harouse et al., 2003). Further studies are needed to fully elucidate the underlying basis for efficient low-dose vaginal transmission of X4 SHIVSF33A.
Figure 7.
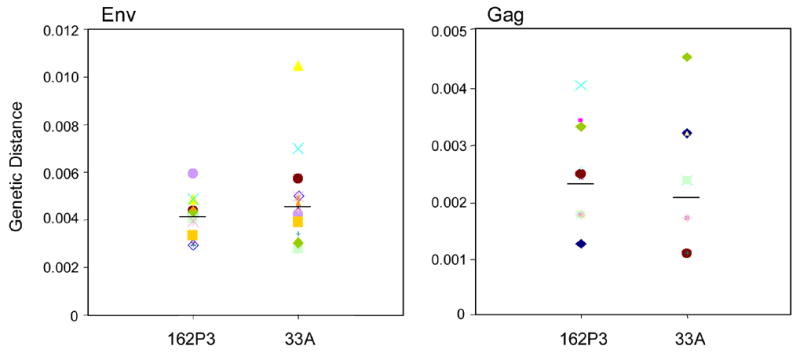
Viral population diversity of X4 SHIVSF33A and R5 SHIVSF162P3 inocula. Genetic distances between all possible pairs of Env and Gag nucleotide sequences for viral RNA in the inocula are shown. Sequences from at least 20 clones each were analysed and bars indicate median values.
Efficient X4 SHIVSF33A transmission in RM vaginally challenged with small inoculum dose, and even from inocula containing both X4 and R5 SHIVs raises the question of why natural sexual transmission of HIV-1 X4 viruses is relatively rare in humans (Moore et al., 2004). We suggest that in addition to or in spite of tropism, the predominance of HIV-1 R5 early in infection in humans is the consequence of greater diversity and quantitative representation of R5 genomes in the viral inocula. R5 viruses persist during all stages of infection while X4 variants appear in about 50% of infected individuals at end stage disease (Koot et al., 1993). The genome copy number of R5 virus in inocula from individuals transmitting during the early stages of their infection is therefore in vast excess compared to those of the X4 virus. At late stage infection and with the appearance of X4 variants, the R5 virus may still have an advantage since its quasispecies is expected to be more diverse, having been replicating in the transmitters for longer periods of time. Furthermore, it is likely that patients at end stage disease are less likely to participate actively in sexual activities.
In summary, our study describes characteristics of repeated low dose vaginal transmission and infection with viruses that resemble HIV-1. Multiple low-dose exposure models that use the number of challenges required to establish infection rather than reduction in viral load as end points should aid in the development and pre-clinical evaluation of vaccine and microbicide strategies using experimental models that have a high degree of variability in infection outcome but nevertheless, may be more relevant to natural infection. Importantly, the unexpected finding of efficient low dose X4 SHIVSF33A IVAG transmission leads to the hypothesis of a link between greater genetic heterogeneity of an inoculum and its enhanced vaginal transmission capacity. We propose that this is among the reasons for selective sexual transmission of CCR5-utilizing HIV-1 in human.
Materials and methods
Virus and animal inoculations
Cell-free stocks of X4 SHIVSF33A and R5 SHIVSF162P3 were propagated and titrated in a CEMX174.CCR5 cell line (5.25.eGFP.Luc.M7) (Brandt et al., 2002). The in vitro titer of R5 SHIVSF162P3 was 5.13 × 103 TCID50 and 9 × 108 RNA copies/ml, while that of X4 SHIVSF33A was 1.58 × 103 TCID50 and 1.1 × 109 RNA copies/ml. For IVAG inoculations, 1–2 ml of virus, either undiluted or diluted in media to produce an inoculum containing 50–100 TCID50 of X4, R5 or varying proportions of the two was deposited atraumatically onto the cervicovaginal mucosa. The animals were kept with their pelvis elevated for 20 minutes after virus exposures. All exposures were carried out with female Indian RM (Macaca mulatta) individually housed at the Tulane National Primate Research Center (TNPRC) in compliance with the Guide for the Care and Use of Laboratory Animals, and the studies were reviewed and approved by the Institutional Animal Care and Use Committee at TNPRC. Animals were not experimentally cycled and were confirmed to be serologically negative for simian type D retrovirus, SIV and simian T-cell lymphotropic virus before use. A single high dose inoculation was performed whereas inoculation with low dose virus was repeated weekly for a maximum of 13 exposures or until systemic SHIV infection could be documented. Systemic infection is defined as at least two consecutive plasma viral RNA (vRNA) positive time points above the limit of detection (125 RNA copies/ml), regardless of viral load. Whole blood was collected at weekly intervals and plasma virus was quantified by an SIV-specific branched DNA (bDNA) signal amplification assay (Bayer Diagnostics, Emeryville, CA). T cell subsets (CD3+, CD4+, and CD8+ T lymphocytes) were determined by Trucount flow cytometry analysis (Becton Dickinson, San Jose, CA). Animals with clinical signs of simian AIDS (SAIDS) or at the end of the study were euthanized by intravenous administration of ketamine-HCL followed by an overdose of sodium pentobarbital. For this study, any two of the following clinical signs [opportunistic infections (MAI or CMV), >20% weight loss, muscle wasting, diarrhea] coupled with virological and immunological indicators and histological findings were used as criteria for defining simian AIDS (SAIDS).
Virus genotype
The relative proportion of replicating X4 and R5 virus in the plasma of dually-exposed macaques was determined by real-time PCR that distinguishes the two viruses(Boadi et al., 2005). Briefly, viral RNA was extracted from plasma, reversed transcribed and the V1–V5 region of envelope gp120 amplified with the conserved ED5/ED12 primers(Delwart et al., 1993). Ten-fold dilutions of the first round PCR products were then subjected to separate X4- and R5-specific real-time PCR reactions. Standard curves were generated in parallel with serial 10-fold dilutions of a known quantity of the X4 or R5 reference strain plasmid. Copy numbers of X4 SHVSF33A and R5 SHIVSF162P3 sequences were calculated from the standard curves and expressed as relative percentages.
Heteroduplex Mobility Assay (HMA)
A 648-bp fragment encompassing the V3–V5 region of gp120 was amplified from cDNA using the ED5/ED12 and the ED7/ED8 primers in a nested PCR reaction. A corresponding fragment was also prepared from the reference HIV-1SF128A plasmid DNA. An excess amount of the HIV-1SF128A amplified fragment was then mixed with the PCR products to promote heteroduplex formation as previously described (Delwart et al., 1993). Samples were run on non-denaturing 5% polyacrylamide gels and stained with ethidium. To avoid genetic variations introduced in response to immune pressure, d14–d21 post-infection plasma samples obtained before full seroconversion were analyzed.
DNA sequencing and analysis
Nested PCR was used to amplify a 900-bp SIV gag fragment from cDNA as described (Chen et al., 1996). The gag product, together with a 1200-bp V1–V5 Env PCR product amplified using the ED5/ED12 primers were subjected to TOPO TA cloning (Invitrogen, Carlsbad, CA), and the ligates were used for transformation. Plasmid DNA of at least 20 clones from each transformation was purified using the QIAprep Miniprep Kit (Qiagen, Valencia, CA), and sequencing was performed by SeqWright (Fisher Scientific, Houston, Tx). Nucleotide sequences were aligned using the CLUSTALX 1.81 program, and further adjusted manually (Jeanmougin et al., 1998). Pairwise genetic distances were calculated using Kimura’s 2-parameter model of molecular evolution (Kimura, 1980).
Statistics
Fisher’s exact or unpaired tests were used to compare characteristics of R5 and X4 SHIV infections, and logrank Mantel-Cox test for the Kaplan–Meier analysis was performed to determine the significance of the difference in probability of transmission with repeated low dose R5 and X4 SHIV.
Acknowledgments
This work was supported by grants from a subproject (MSA-03-362) provided by CONRAD, Eastern Virginia Medical School under a Cooperative Agreement (HRN-A-00-98-00020-00) with the United States Agency for International Development (USAID), and the NIH (R01AI46980, R37AI41945). We thank Dr. Allen Mayer for critical comments, Dr. Zhiwei Chen for advice on sequence analyses, and Wendy Chen for help with graphics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 2005. UNAIDS/WHO AIDS Epidemic Update: December 2005. Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO).
- Boadi T, Schneider E, Chung S, Tsai L, Gettie A, Ratterree M, Blanchard J, Neurath AR, Cheng-Mayer C. Cellulose acetate 1,2-benzenedicarboxylate protects against challenge with pathogenic X4 and R5 simian/human immunodeficiency virus. Aids. 2005;19(15):1587–94. doi: 10.1097/01.aids.0000186020.24426.62. [DOI] [PubMed] [Google Scholar]
- Brandt SM, Mariani R, Holland AU, Hope TJ, Landau NR. Association of chemokine-mediated block to HIV entry with coreceptor internalization. J Biol Chem. 2002;277(19):17291–9. doi: 10.1074/jbc.M108232200. [DOI] [PubMed] [Google Scholar]
- Charpentier C, Nora T, Tenaillon O, Clavel F, Hance AJ. Extensive recombination among human immunodeficiency virus type 1 quasispecies makes an important contribution to viral diversity in individual patients. J Virol. 2006;80(5):2472–82. doi: 10.1128/JVI.80.5.2472-2482.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Telfier P, Gettie A, Reed P, Zhang L, Ho DD, Marx PA. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70(6):3617–27. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267(5197):483–9. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- Davenport MP, Zhang L, Shiver JW, Casmiro DR, Ribeiro RM, Perelson AS. Influence of peak viral load on the extent of CD4+ T-cell depletion in simian HIV infection. J Acquir Immune Defic Syndr. 2006;41(3):259–65. doi: 10.1097/01.qai.0000199232.31340.d3. [DOI] [PubMed] [Google Scholar]
- DeGruttola V, Seage GR, 3rd, Mayer KH, Horsburgh CR., Jr Infectiousness of HIV between male homosexual partners. J Clin Epidemiol. 1989;42(9):849–56. doi: 10.1016/0895-4356(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Delwart EL, Shpaer EG, Louwagie J, McCutchan FE, Grez M, Rubsamen-Waigmann H, Mullins JI. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993;262(5137):1257–61. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- Frater AJ, Edwards CT, McCarthy N, Fox J, Brown H, Milicic A, Mackie N, Pillay T, Drijfhout JW, Dustan S, Clarke JR, Holmes EC, Zhang HT, Pfafferott K, Goulder PJ, McClure MO, Weber J, Phillips RE, Fidler S. Passive sexual transmission of human immunodeficiency virus type 1 variants and adaptation in new hosts. J Virol. 2006;80(14):7226–34. doi: 10.1128/JVI.02014-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2(1):33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew JF. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341(6):394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- Gray RH, Li X, Wawer MJ, Gange SJ, Serwadda D, Sewankambo NK, Moore R, Wabwire-Mangen F, Lutalo T, Quinn TC. Stochastic simulation of the impact of antiretroviral therapy and HIV vaccines on HIV transmission; Rakai, Uganda. Aids. 2003;17(13):1941–51. doi: 10.1097/00002030-200309050-00013. [DOI] [PubMed] [Google Scholar]
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- Greenier JL, Miller CJ, Lu D, Dailey PJ, Lu FX, Kunstman KJ, Wolinsky SM, Marthas ML. Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J Virol. 2001;75(8):3753–65. doi: 10.1128/JVI.75.8.3753-3765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harouse JM, Buckner C, Gettie A, Fuller R, Bohm R, Blanchard J, Cheng-Mayer C. CD8+ T cell-mediated CXC chemokine receptor 4-simian/human immunodeficiency virus suppression in dually infected rhesus macaques. Proc Natl Acad Sci U S A. 2003;100(19):10977–82. doi: 10.1073/pnas.1933268100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harouse JM, Gettie A, Eshetu T, Tan RC, Bohm R, Blanchard J, Baskin G, Cheng-Mayer C. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75(4):1990–5. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx AG, Cukierski MA. Reproductive and developmental toxicology in nonhuman primates. Prog Clin Biol Res. 1987;235:73–88. [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23(10):403–5. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Jewell NP, Shiboski SC. Statistical analysis of HIV infectivity based on partner studies. Biometrics. 1990;46(4):1133–50. [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–20. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Koot M, Keet IP, Vos AH, de Goede RE, Roos MT, Coutinho RA, Miedema F, Schellekens PT, Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118(9):681–8. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- Lori F, Hall L, Lusso P, Popovic M, Markham P, Franchini G, Reitz MS., Jr Effect of reciprocal complementation of two defective human immunodeficiency virus type 1 (HIV-1) molecular clones on HIV-1 cell tropism and virulence. J Virol. 1992;66(9):5553–60. doi: 10.1128/jvi.66.9.5553-5560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw PA, Mandell CP, Himathongkham S, Li J, Low TA, Schmidt KA, Shaw KE, Cheng-Mayer C. Fatal immunopathogenesis by SIV/HIV-1 (SHIV) containing a variant form of the HIV-1SF33 env gene in juvenile and newborn rhesus macaques. Virology. 1999;263(1):112–27. doi: 10.1006/viro.1999.9908. [DOI] [PubMed] [Google Scholar]
- Ma ZM, Abel K, Rourke T, Wang Y, Miller CJ. A period of transient viremia and occult infection precedes persistent viremia and antiviral immune responses during multiple low-dose intravaginal simian immunodeficiency virus inoculations. J Virol. 2004;78(24):14048–52. doi: 10.1128/JVI.78.24.14048-14052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KG, Lerch NW, Gardner MB, Lackner AA. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. Med Primatol. 1995;24:116–22. doi: 10.1111/j.1600-0684.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- McDermott AB, Mitchen J, Piaskowski S, De Souza I, Yant LJ, Stephany J, Furlott J, Watkins DI. Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: a model for the evaluation of vaccine efficacy in nonhuman primates. J Virol. 2004;78(6):3140–4. doi: 10.1128/JVI.78.6.3140-3144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Kingsley LA, Rinaldo CR, Jr, Todd JA, Hoo BS, Kokka RP, Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122(8):573–9. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272(5265):1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Miller CJ. Mucosal transmission of simian immunodeficiency virus. Curr Top Microbiol Immunol. 1994;188:107–22. doi: 10.1007/978-3-642-78536-8_6. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Lu FX. Anti-HIV and -SIV immunity in the vagina. Int Rev Immunol. 2003;22(1):65–76. doi: 10.1080/08830180305230. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Marthas M, Greenier J, Lu D, Dailey PJ, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72(4):3248–58. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors--central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20(1):111–26. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- Muller HJ. The Relation of Recombination to Mutational Advance. Mutat Res. 1964;106:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Neildez O, Le Grand R, Caufour P, Vaslin B, Cheret A, Matheux F, Theodoro F, Roques P, Dormont D. Selective quasispecies transmission after systemic or mucosal exposure of macaques to simian immunodeficiency virus. Virology. 1998;243(1):12–20. doi: 10.1006/viro.1997.9026. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, Goh LE, Cohen MS. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–92. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1(1):25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- Wick D, Self SG. Early HIV infection in vivo: branching-process model for studying timing of immune responses and drug therapy. Math Biosci. 2000;165(2):115–34. doi: 10.1016/s0025-5564(00)00013-4. [DOI] [PubMed] [Google Scholar]
- Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261(5125):1179–81. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]


