Abstract
TAR DNA-binding protein 43 (TDP-43) is a major pathological protein of sporadic and familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions (FTLD-U) with or without motor neuron disease (MND). Thus, TDP-43 defines a novel class of neurodegenerative diseases called TDP-43 proteinopathies. We performed ubiquitin and TDP-43 immunohistochemistry on 193 cases of familial and sporadic FTLD with or without MND. On selected cases, immunoelectron microscopy and biochemistry were performed. Clinically defined frontotemporal dementias (FTDs) included four groups: 1) familial FTD with mutations in progranulin (n = 36), valosin-containing protein (n = 5), charged multivesicular body protein 2B (n = 4), and linked to chromosome 9p (n = 7); 2) familial cases of FTD with unknown gene association (n = 29); 3) sporadic FTD (n = 72); and 4) familial and sporadic FTD with MND (n = 40). Our studies confirm that the spectrum of TDP-43 proteinopathies includes most cases of sporadic and familial FTLD-U with and without MND and expand this disease spectrum to include reported families with FTD linked to chromosome 9p but not FTD with charged multivesicular body protein 2B mutations. Thus, despite significant clinical, genetic, and neuropathological heterogeneity of FTLD-U, TDP-43 is a common pathological substrate underlying a large subset of these disorders, thereby implicating TDP-43 in novel and unifying mechanisms of FTLD pathogenesis.
The frontotemporal dementias (FTDs) are a clinically, genetically, and neuropathologically heterogeneous group of diseases accounting for up to 20% of presenile dementia cases. FTD is characterized by behavioral and/or language dysfunction and may co-occur with motor neuron disease (MND).1,2 Frontotemporal lobar degeneration (FTLD) with ubiquitin-positive, tau-negative inclusions (FTLD-U) is the most common underlying pathology in FTD with and without MND.3 TAR DNA-binding protein 43 (TDP-43), a nuclear protein implicated in exon skipping and transcription regulation,4,5,6 was recently identified as a major protein component of the ubiquitin-immunoreactive inclusions characteristic of sporadic and familial FTLD-U, with and without MND, as well as in sporadic amyotrophic lateral sclerosis (ALS)7,8 and has been rapidly confirmed by others.9,10,11 TDP-43 in these disorders is abnormally phosphorylated, ubiquitinated, and cleaved to generate C-terminal fragments and is recovered only from areas with ubiquitin-immunoreactive inclusions, including hippocampus, neocortex, and spinal cord.8 Therefore, the presence of abnormal aggregates of phosphorylated and ubiquitinated TDP-43 defines a novel class of neurodegenerative diseases that we propose to call “TDP-43 proteinopathies” that includes FTLD-U, FTLD-MND, and ALS. The neuropathology of these conditions is characterized by ubiquitin- and TDP-43-positive neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions (NIIs), dystrophic neurites (DNs), and glial cytoplasmic inclusions8,10,11,12 that are negative for tau, α-synuclein, β-amyloid, neuronal intermediate filaments, and expanded polyglutamines. The variability in the morphological types of neuronal inclusions, their distribution, density, and immunohistochemical profile has led to the development of the classification of FTLD-U into four pathological subtypes.8,10,13,14 Recently, the molecular genetic basis of non-tau familial FTD linked to chromosome 17 was discovered as being mutations in the progranulin gene (PGRN).15,16,17 The neuropathology in these cases is FTLD-U with ubiquitin-positive neurites, NCI and, most characteristically, NII.17,18,19 As demonstrated by immunohistochemical and biochemical investigation, the ubiquitinated pathological protein in these cases is not progranulin but TDP-43.8,9 Pathological TDP-43 is detected biochemically in both affected gray and white matter, suggesting that both glial and neuronal pathology may contribute to the pathogenesis of FTLD-U caused by PGRN mutations.8,11
Inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia is a rare autosomal dominant disorder caused by mutations in the valosin-containing protein gene (VCP).10,13,20 VCP, a member of the AAA-ATPase gene super family (ATPase associated with diverse cellular activities), has multiple cellular functions, including acting as a molecular chaperone in endoplasmic reticulum-associated protein degradation, stress response, programmed cell death, and interactions with the ubiquitin-proteasome system.21 The neuropathology in inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia is a unique subtype of FTLD-U characterized by numerous NIIs and relatively few NCIs and DNs.10,13 Once again, the ubiquitinated pathology is not primarily composed of the mutated protein (VCP) but rather TDP-43.10 Phosphorylated TDP-43 is detected only in the insoluble brain extracts from affected regions, indicating that the VCP gene mutations cause a dominant-negative loss of function or alteration of VCP function, leading to impaired metabolism of TDP-43.10
Mutations in the charged multivesicular body protein 2B gene (CHMP2B) were recently identified as the cause of FTD linked to chromosome 3 in a large Danish pedigree.22 Human CHMP2B is a protein of 213 amino acids with a predicted coil-coil domain and is a component of the endosomal secretory complex III required for transport. Neuropathology was originally described as “dementia lacking distinctive histopathology,” but more recent studies have revealed ubiquitin-positive granular NCIs in frontal neocortex and hippocampus.23 TDP-43 immunohistochemistry, electron microscopy, and biochemistry have not previously been undertaken in these cases.
Recently, a new genetic locus on chromosome 9p for familial FTD-MND has been described.24,25 In one family, candidate gene sequencing revealed the presence of a putative disease segregating stop codon mutation (Q342X) in the intraflagellar transport protein 74 gene (IFT74).26 IFT74 is a 600-amino acid protein with a coiled-coil domain-containing protein that localizes to the intracellular vesicle compartment and is a component of the intraflagellar transport system responsible for vesicular transport of material synthesized within the cell body into and along dendrites and axons. Neuropathology in a single case with the IFT74 gene mutation was reported as showing all of the signs of FTLD-U (ubiquitinated NCI, DN, and NII).26 TDP-43 immunohistochemistry and biochemistry have not previously been reported in this or other chromosome 9-linked FTD families.
Therefore, previous studies indicate that TDP-43-immunoreactive inclusions constitute a common pathological finding linking many cases of sporadic FTLD-U, familial FTLD-U with PGRN and VCP mutations, and FTLD-MND. However, each of the aforementioned studies included relatively small numbers of cases in each disease category. The aims of the present study were as follows: 1) to define the frequency of TDP-43 proteinopathy in a much larger collection of familial and sporadic cases of FTLD-U and FTLD-MND, collected at multiple sites in North America and Europe; 2) to determine whether FTLD-U in reported families linked to chromosome 9p and FTLD-U linked to chromosome 3 are TDP-43 proteinopathies; and 3) to examine the presence of pathological TDP-43 in a wider range of FTLDs and other neurodegenerative conditions.
Materials and Methods
Tissue Collection and Processing
Brain tissues from clinically and neuropathologically characterized cases of sporadic and familial FTLD-U, with or without MND, other FTLDs, and other neurodegenerative diseases were obtained from Canada, Denmark, Germany, The Netherlands, and the United States (Table 1; Supplemental Table 1, see http://ajp.amjpathol.org). Cases of FTLD-U showed characteristic pathology3,17,18,19,27 and had a clinical diagnosis of one of the FTD subtypes (including frontal variant FTD, primary progressive aphasia, or corticobasal syndrome), MND with dementia, or simply “dementia.”1,2,27,28 Those with the primary clinical diagnosis of either ALS or MND,29 in the absence of clinical dementia, were excluded from this study. The FTLD-U group included 1) familial cases with PGRN,17,18,19,30 VCP,11,13 and CHMP2B22 mutations and cases linked to chromosome 9p,24,25 including one case with IFT74 gene mutation26; 2) other familial cases of FTLD-U in which the genetic defect was not known; 3) cases with sporadic FTLD-U; and 4) familial and sporadic cases of MND with dementia.1,2,27 FTLDs with tauopathy included Pick disease,27 corticobasal degeneration,27,28 progressive supranuclear palsy,27 FTLD with MAPT mutations, also called frontotemporal dementia with parkinsonism linked to chromosome 17,27 tangle-only dementia,27 and argyrophilic grain disease.31 Other cases fulfilling clinical and/or neuropathological diagnostic criteria for FTLD included neuronal intermediate filament inclusion disease,32 hereditary diffuse leukoencephalopathy with spheroids,33 and basophilic inclusion body disease.34 Synucleinopathies included dementia with Lewy bodies,35 Parkinson’s disease,35,36,37 and multiple system atrophy.38,39 Other neurological controls included Alzheimer’s disease (AD),40 polyglutamine expansion diseases (Huntington’s disease and spinocerebellar ataxia),41,42 dementia lacking distinctive histopathology,43 and hippocampal sclerosis (HS).44 In addition, normal aged controls were studied. Clinical, genetic, and neuropathological data and tissue samples were obtained from the following collaborating centers: Alzheimer’s Disease Research Center, Washington University School of Medicine (St. Louis, MO); Northwestern University Cognitive Neurology and Alzheimer Disease Center (Chicago, IL); Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine (Philadelphia, PA); Center for Neuropathology and Prion Research, Ludwig-Maximilians University (Munich, Germany); Department of Pathology and Laboratory Medicine, Vancouver General Hospital (Vancouver, BC, Canada); Department of Pathology, Aalborg Hospital (Aalborg, Denmark); Department of Neuropathology, Academic Medical Centre (Amsterdam, The Netherlands); University of Texas Southwestern School of Medicine (Dallas, TX); and Rush Alzheimer’s Disease Center, Rush University Medical School (Chicago, IL). After death, the consent of the next-of-kin was obtained for brain removal, following approved Local Ethics Committee procedures in accordance with state law. Brain tissue was preserved in either buffered 10% formal saline for up to 3 weeks or 4% paraformaldehyde for 30 to 72 hours followed by cryopreservation in sucrose or glycerol. When available, tissue was frozen at −70°C for biochemistry.
Table 1.
Demographic, Clinical, Genetic, and Neuropathologic Data of Cases
| Familial and sporadic FTDs | Demographic data
|
Diagnosis
|
||||
|---|---|---|---|---|---|---|
| Onset (years) [mean (range)] | Duration (years) [mean (range)] | Gender | Clinical diagnosis (n, %) | Pathological diagnosis (n, %) | Pathological diagnosis-other (n, %) | |
| Familial FTD with PGRN mutation (n = 36) | ||||||
| 59.8 (50 to 74) | 7.06 (3 to 15) | 20 F/16 M | FTD (27, 75) | FTLD-U (36, 100) | AD (8, 22) | |
| PPA (2, 6) | AD, HS (2, 6) | |||||
| FTD + CBD (2, 6) | AD, MS (1, 3) | |||||
| DAT/dementia (5, 14) | CVD (4, 11) | |||||
| HS (1, 3) | ||||||
| DLB, PD (1, 3) | ||||||
| Familial FTD with VCP mutation (n = 5) | ||||||
| 51.4 (38 to 62) | 7.2 (5 to 9) | 4 F/1 M | FTD (5, 100) | FTLD-U (5, 100) | ||
| Familial FTD with CHMP2Bmutation (n= 4) | ||||||
| 53.3 (50 to 48) | 12.8 (9 to 21) | 3 F/1 M | FTD (4, 100) | FTLD-U (4, 100) | ||
| Familial FTD with/without MND linked to chromosome 9 (n= 7) | ||||||
| 53.8 (39 to 59) | 3.7 (2 to 11) | 4 F/3 M | FTD (1, 14) | FTLD-U (2, 29) | ||
| FTD + MND (4, 57) | FTLD-MND (5, 71) | |||||
| MND (2, 28) | ||||||
| Other familial FTD cases (n = 29) | ||||||
| 57.1 (33 to 69) | 7.4 (2 to 19) | 15 F/14 M | FTD (21, 72) | FTLD-U (27, 93) | AD (2, 7) | |
| PPA (2, 7) | FTLD-MND (2, 7) | AGD (3, 10) | ||||
| DAT/dementia (6, 21) | HS (1, 3) | |||||
| AD, DLB (1, 3) | ||||||
| Sporadic FTD cases (n = 72) | ||||||
| 60.5 (33 to 89) | 7.5 (2 to 18) | 28 F/44 M | FTD (49, 68) | FTLD-U (61, 85) | AD (9, 13) | |
| PPA (6, 8) | FTLD-MND (11, 15) | HS (3, 4) | ||||
| CBD (3, 4) | AGD (2, 3) | |||||
| PPA + CBD (1, 1) | CBD (1, 1) | |||||
| DAT/dementia (13, 18) | ||||||
| Familial FTD and MND (n = 17) | ||||||
| 51.0 (44 to 63) | 6.6 (1 to 6) | 5 F/12 M | PPA + MND (1, 6) | FTLD-MND (17, 100) | AD (2, 12) | |
| FTD + MND (16, 94) | CVD (1, 6) | |||||
| Sporadic FTD and MND (n = 23) | ||||||
| 54.7 (35 to 72) | 4.0 (1 to 11) | 6 F/19 M | FTD + MND (21, 92) | FTLD-U (1, 4) | ||
| PPA + MND (2, 8) | FTLD-MND (22, 96) | |||||
| Other familial and sporadic FTD cases (non-FTLD-U) FTLD (n = 2) | ||||||
| 75.5 (62 to 89) | 6 (3 to 9) | 2 F/0 M | FTD (1, 50) | FTLD (2, 100) | Infarcts (1, 50) | |
| DAT (1, 50) | ||||||
| 65 (NA) | 8 (NA) | 5 F/6 M | FTD (11, 84) | PICK (13, 100) | AD (3, 23) | |
| DAT (1, 8) | ||||||
| CJD (1, 8) | ||||||
| Corticobasal degeneration (n = 19) | ||||||
| 70.0 (57 to 78) | 7.6 (5 to 12) | 8 F/6 M | FTD (10, 53) | CBD (19, 100) | AD, HS (1, 5) | |
| CBD (9, 47) | ||||||
| Progressive supranuclear palsy (n = 4) | ||||||
| 73 (NA) | 3 (NA) | NA | FTD + PD (4, 100) | PSP (4, 100) | AD (1, 25) | |
| FTD with MAPTmutation (n = 5) | ||||||
| 60 (57 to 63) | 14 (10 to 18) | 0 F/2 M | FTD (4, 80) | FTD-MAPT (5, 100) | ||
| Dementia (1, 20) | ||||||
| Neuronal intermediate filament inclusion disease (n = 6) | ||||||
| 37 (25 to 48) | 3.7 (3 to 4) | 2 F/2 M | FTD (5, 83) | NIFID (6, 100) | ||
| PLS (1, 17) | ||||||
| Basophilic inclusion body disease (n = 2) | ||||||
| 29 (NA) | 10 (NA) | 0 F/2 M | FTD (1, 50) | BIBD (2, 100) | ||
| CBD (1, 50) | ||||||
| Hereditary diffuse leukoencephalopathy with spheroids (n = 2) | ||||||
| 37.5 (36 to 39) | 8.5 (7 to 10) | 2 F/0 M | FTD (2, 100) | HDLS (2, 100) | ||
| Other diseases | ||||||
| Alzheimer’s disease (n = 19) | ||||||
| 73 (59 to 99) | 8 (4 to 13) | 7 F/9 M | DAT (18, 95) | AD (19, 100) | Lewy bodies (3, 16) | |
| FTD (1, 5) | Infarct (1, 5) | |||||
| HS (1, 5) | ||||||
| Argyrophilic grain disease (n = 2) | ||||||
| 70.5 (54 to 87) | 8.5 (7 to 10) | 2 F/0 M | FTD (1, 50) | AGD (2, 100) | ||
| Dementia (1, 50) | ||||||
| Tangle-only dementia (n = 2) | ||||||
| 74 (NA) | 4 (NA) | 1 F/1 M | DAT/dementia (2, 100) | TOD (2, 100) | ||
| Parkinson’s disease (n = 3) | ||||||
| 51 (35 to 67) | 16 (10 to 20) | 1 F/2 M | PD (3, 100) | PD (3, 100) | DLB (2, 67) | |
| HS (1, 33) | ||||||
| Dementia with Lewy bodies (n= 8) | ||||||
| 65.5 (62 to 69) | 13 (5 to 13) | 4 F/4 M | DLB (3, 38) | DLB (8, 100) | AD (7, 87) | |
| DAT + PD (2, 25) | PD (1, 13) | |||||
| DAT/dementia (3, 38) | ||||||
| Multiple system atrophy (n = 3) | ||||||
| NA | NA | NA | MSA (3, 100) | MSA (3, 100) | ||
| Trinucleotide repeat disease (n = 3) | ||||||
| 38 (NA) | 6 (NA) | 1 F/2 M | HD (1, 33) | HD (1, 33) | Tauopathy | |
| HD + FTD (1, 33) | SCA (2, 67) | |||||
| FTD (1, 33) | ||||||
| Hippocampal sclerosis (n = 2) | ||||||
| 43 (NA) | 6 | 2 F/0 M | DAT (1, 50) | HS (2, 100) | AD (1, 50) | |
| PD + DAT (1, 50) | ||||||
| Normal adult brain (n = 19) | ||||||
| 83.8** (73 to 98) | NA | 8 F/11 M | NL (15, 79) | NL (19, 100) | Gliosis (1, 5) | |
| Schizophrenia (4, 21) | ||||||
AGD, argyrophilic grain disease; BIBD, basophilic inclusion body disease; CVD, cerebrovascular disease; PGRN, progranulin; VCP, valosin-containing protein; CHMP2B, charged multivesicular body protein 2B; CJD, Creutzfeldt-Jakob disease; DAT, dementia of the Alzheimer’s type; DLB, dementia with Lewy bodies; CBD, corticobasal degeneration; FTD, frontotemporal dementia; FTD-MAPT, FTD with tau mutation; FTLD, frontotemporal lobar degeneration; FTLD-MND, FTLD with ubiquitin-positive, tau-negative inclusions and MND; FTLD-U, FTLD with ubiquitin-positive, tau-negative inclusions but without MND; HD, Huntington’s disease; HDLS, hereditary diffuse leukoencephalopathy with neuroaxonal spheroids; HS, hippocampal sclerosis; MS, multiple sclerosis; MND, motor neuron disease; MSA, multiple system atrophy; NIFID, neuronal intermediate filament inclusion disease; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; SCA, spinocerebellar ataxia; PICK, Pick disease; PPA, primary progressive aphasia; TOD, tangle-only dementia; NA, not available; NL, normal adult brain with no neurologic or psychiatric disease; F, female; M, male.
Age at death.
Histology and Immunohistochemistry
Paraffin sections from all cases of FTLD-U included the frontal lobe (middle frontal gyrus and Brodmann areas 8, 9, and 46), temporal lobe (middle/superior temporal gyrus and Brodmann areas 21 and 22), parahippocampal gyrus, and hippocampus and, when available, striatum, precentral gyrus, medulla oblongata, and spinal cord. TDP-43 immunohistochemistry (IHC) was also performed on sections with disease-representative pathology from cases with other neurodegenerative conditions and normal aged control cases.
Antigen retrieval was performed by microwaving tissue sections in a solution of 0.1 mol/L citrate buffer, pH 6.0, at 100°C for 10 minutes. IHC was undertaken on 4- to 10-μm-thick sections prepared from formalin-fixed, paraffin-embedded tissue blocks using the avidin-biotin complex detection system (Vector Laboratories, Burlingame, CA) and the chromogen 3,3′-diaminobenzidine, and sections were counterstained with hematoxylin as previously described,8,14 or sections were immunostained with an automated staining procedure.45 Antibodies used included those that recognized epitopes of ubiquitin (rabbit polyclonal, 1:1000; Dako, Glostrup, Denmark) (mouse monoclonal, 1510; Chemicon, Temecula, CA) and TDP-43 (rabbit polyclonal, 1:1000; ProteinTech Inc., Chicago, IL). In addition, in selected cases of FTLD-U, novel monoclonal antibodies 137 and 182 were used, as previously described.8,10,14
The pattern of FTLD-U pathology was subclassified, based on the system proposed by Sampathu et al.14 Type 1 cases were characterized by an abundance of long DNs predominantly in superficial cortical laminae, with few or no NCIs or NIIs. Type 2 was characterized by numerous NCIs in both superficial and deep cortical laminae as well as infrequent DNs and sparse or no NIIs. Type 3 was characterized by pathology predominantly in the superficial cortical layers with numerous NCIs, DNs, and variable numbers of NIIs. An additional class was added (type 4) that was distinguished by numerous NIIs and infrequent NCIs and DNs in neocortical areas with relative sparing of the hippocampus, consistent with the pathology previously described in cases with VCP mutations.10,13 Each center performed its own severity rating of pathology and classification of cases using ubiquitin and TDP-43 IHC using established FTLD-U subtype criteria. The severity of ubiquitin- or TDP-43-positive inclusions was rated semiquantitatively where 0 = no inclusions; 1 = rare to mild; 2 = moderate; and 3 = severe. To determine intercenter reliability of FTLD-U subclassification, three or more cases (where available) classified in each subtype group, from Washington University School of Medicine and from University of British Columbia, were blindly reviewed by Dr. Neumann (Ludwig-Maximilians University, Munich, Germany). Agreement between the raters was good with κ = 0.75 (95% CI, 0.52 to 0.98).
Electron Microscopy
Samples of hippocampus and temporal cortex collected at autopsy were fixed overnight in 0.1% glutaraldehyde and 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline, sliced on a vibratome, and stored in cryoprotection solution at −20°C. Tissue for routine electron microscopy was washed and fixed for an additional 20 hours at 4°C in 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.4, postfixed for 1 hour on ice in 1% osmium tetroxide and 1.5% potassium ferrocyanide in cacodylate buffer, dehydrated in ethanol, and embedded in Epon. Vibratome sections of hippocampus were fixed for an additional 15 minutes in 2.5% glutaraldehyde cacodylate buffer before immunostaining. Electron microscopy immunostaining was performed according to the method of Llewellyn-Smith and Minson.46 The primary antibodies used were a rabbit polyclonal antibody to TDP-43 and normal rabbit IgG. The 3,3′-diaminobenzidine reaction was completed by incubating for 15 minutes in 0.05% 3,3′-diaminobenzidine with 10 mmol/L imidazole in 0.1 mol/L Tris buffer, pH 7.4, without H2O2 and for 5 to 10 minutes with 0.1% H2O2 added. Sections were postfixed for 2 hours in 1% osmium tetroxide and 1.5% potassium ferrocyanide in 0.1 mol/L cacodylate buffer, dehydrated in ethanol, and embedded in Araldite. Selected semithin sections were re-embedded for thin sectioning.
Biochemistry
Postmortem brain tissue was dissected, weighed, and sequentially extracted with buffers of increasing strength as previously described.8,10,11 In brief, gray and white matter was extracted at 5 ml/g (v/w) with low-salt buffer (10 mmol/L Tris, pH 7.5, 5 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L dithiothreitol, 10% sucrose, and a cocktail of protease inhibitors), high-salt Triton X buffer (low-salt buffer, 1% Triton X-100, and 0.5 mol/L NaCl), myelin floatation buffer (Triton X buffer containing 30% sucrose), and sarkosyl buffer (low-salt buffer, 1% N-lauroyl-sarcosine, and 0.5 mol/L NaCl). The detergent-insoluble materials were extracted in 0.25 ml/g urea buffer (7 mol/L urea, 2 mol/L thiourea, 4% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, and 30 mmol/L Tris, pH 8.5). For Western blot analysis, protein extracts were resolved in Tris-glycine 5 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA), and probed with antibodies to TDP-43. Primary antibodies were detected with alkaline phosphatase-conjugated anti-mouse or anti-rabbit IgG (Dako) and visualized by incubation with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche Molecular Biochemicals, Mannheim, Germany). Where indicated, TDP-43 was dephosphorylated by dialysis (50 mmol/L Tris and 0.2 mmol/L ethylenediamine tetraacetic acid, pH 8.0) and treated with Escherichia coli alkaline phosphatase (Sigma-Aldrich, St. Louis, MO) for 2 hours at 56°C.
Results
TDP-43 Immunohistochemistry Indicates Neuropathological Heterogeneity in FTLD-U
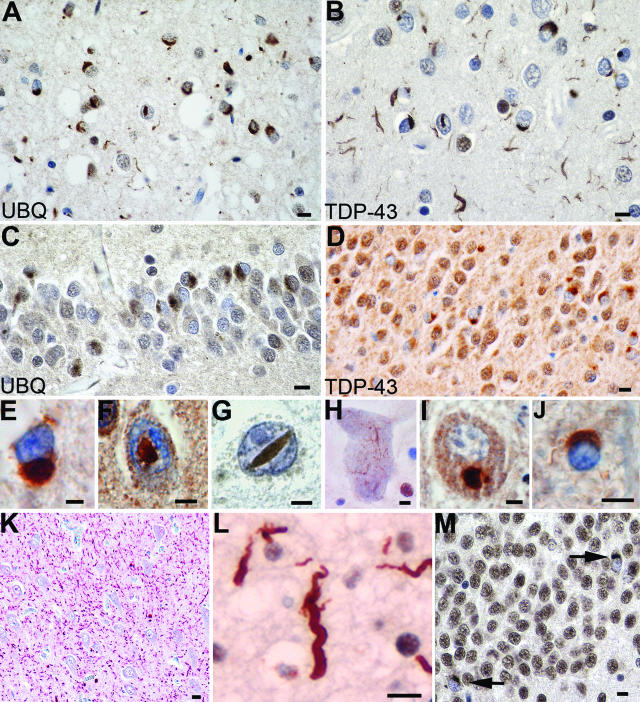
Immunohistochemistry for ubiquitin and TDP-43 revealed similar findings, which included a spectrum of neuronal (NCIs, DNs, and NIIs) and glial inclusions (Figure 1). Most NCIs, DNs, and NIIs that were ubiquitin immunoreactive were also TDP-43-positive. In contrast, glial cytoplasmic inclusions, which were readily seen by TDP-43 IHC, were only variably labeled with ubiquitin, possibly indicating an early stage in pathogenesis. When present, DNs were most numerous in neocortical areas of the frontal and temporal lobes; rarely, numerous DNs were seen in the CA1 subfield of the hippocampus (Figure 1K). In agreement with our previous studies, four distinct patterns of FTLD-U pathology were identified, and a number of correlations with clinical and genetic subgroups were apparent (Table 1; Figure 2; Supplemental Table 1 at http://ajp.amjpathol.org).
Figure 1.
Spectrum of TDP-43 pathology in FTLD-U. Adjacent sections of superficial frontal neocortex showing NCIs, DNs, and isolated NIIs, stained for both ubiquitin (A) and TDP-43 (B). NCIs in the dentate granule cells stained for ubiquitin (C) and TDP-43 (D). Neuronal and glial inclusions include NCIs (E), round and lentiform NIIs (F and G); skein-like (H) and compact round (I) NCIs in lower motor neurons; and a glial cytoplasmic inclusion (J). Low-power micrograph showing numerous DNs in the hippocampus CA1 subfield (K). High-power micrograph showing a tortuous DN in a case of FTLD-U, subtype 1 (L). NCIs in the dentate fascia of a case of hippocampal sclerosis (M). A and C: Ubiquitin immunohistochemistry. B, D, E–M: TDP-43 immunohistochemistry. Bars = 10 μm (A–D and K–M); 5 μm (E–J).
Figure 2.
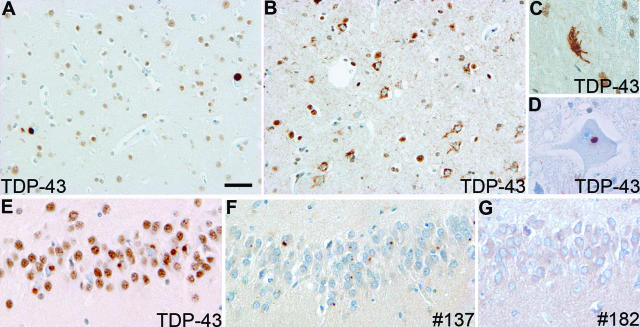
FTLD-U subtypes 1 to 4. A: Type 1 is characterized by long and tortuous DNs in laminae II/III with relatively few NCIs and no NII. B: Type 2 has numerous NCIs, relatively few DNs, and no NII. C: Type 3 has numerous NCIs and DNs and an occasional NII in lamina II. D: Type-4 pathology is characterized by numerous NIIs and DNs but few NCIs. TDP-43 immunohistochemistry. Bar = 10 μm.
We found that the majority (97.4%) of cases of FTLD-U with or without MND (Table 2) could be classified according to the previously published schemes.10,13,14 Most cases in this large, multicenter series showed type-3 pathology (49.2%), whereas type-2 and -1 pathologies represented 28.5 and 17.1%, respectively (Table 2). The pathological subtypes distinguished familial cases with different known gene defects. Cases with PGRN mutations were exclusively type 3 (100%), those with VCP mutations all had type-4 pathology, and cases linked to chromosome 9 were all type 2 (see below). Familial FTLD-U and FTLD-MND in which the genetic association was unknown were mostly type 3 (55.2%), whereas type-2 cases represented 34.5%, and 10.3% were type 1 (Table 2). Sporadic FTD with FTLD-U and/or FTLD-MND were almost evenly split between type-1 (36.1%), type-2 (30.6%), and type-3 (33.1%) pathology. Most cases of sporadic MND with dementia had either type-2 (39.1%) or type-3 (52.2%) pathology, and a comparable distribution was found in cases of familial MND-dementia (41.2% were type 2, and 41.2% were type 3). One case of familial MND-dementia had too little cortical pathology to allow classification in this system.
Table 2.
TDP-43 and FTLD-U Subtypes
| Familial and sporadic FTDs | Neuropathologic diagnosis | FTLD-U subtype
|
|
|---|---|---|---|
| Ubiquitin type (n, %) | TDP-43 type (n, %) | ||
| Familial FTD with PGRN mutation (n = 36) | FTLD-U | 3 (36, 100) | 3 (36, 100) |
| Familial FTD with VCP mutation (n= 5) | FTLD-U | 4 (5, 100) | 4 (5, 100) |
| Familial FTD with CHMP2B mutation (n = 4) | FTLD-U | a (4, 100) | 0 (4, 100) |
| Familial FTD with/without MND linked to chromosome 9 (n = 7) | FTLD-U | 2 (2, 29) | 2 (2, 29) |
| FTLD-MND | 2 (5, 71) | 2 (5, 71) | |
| Other familial FTD cases (n = 29) | FTLD-U | 1 (3, 10) | 1 (3, 10) |
| FTLD-U | 2 (8, 28) | 2 (8, 28) | |
| FTLD-U | 3 (16, 55) | 3 (16, 55) | |
| FTLD-MND | 2 (2, 7) | 2 (2, 7) | |
| Sporadic FTD cases with ubiquitin- or TDP-43-positive inclusions (n = 72) | FTLD-U | 1 (26, 36) | 1 (26, 36) |
| FTLD-U | 2 (15, 21) | 2 (15, 21) | |
| FTLD-U | 2 (1, 1) | 0 (1, 1) | |
| FTLD-U | 3 (18, 25) | 3 (18, 25) | |
| FTLD-U | 3 (1, 1) | 0 (1, 1) | |
| FTLD-MND | 2 (6, 8) | 2 (6, 8) | |
| FTLD-MND | 3 (5, 7) | 3 (5, 7) | |
| Familial FTD and MND (n = 17) | FTLD-MND | 1 (2, 12) | 1 (2, 12) |
| FTLD-MND | 2 (7, 41) | 2 (7, 41) | |
| FTLD-MND | 3 (7, 41) | 3 (7, 41) | |
| FTLD-MND | b (1, 6) | b (1, 6) | |
| Sporadic FTD and MND (n = 23) | FTLD-U | 3 (1, 4) | 3 (1, 4) |
| FTLD-MND | 1 (2, 9) | 1 (2, 9) | |
| FTLD-MND | 2 (9, 39) | 2 (9, 39) | |
| FTLD-MND | 3 (11, 48) | 3 (11, 48) | |
| Total of all familial and sporadic FTD cases with/without MND (n = 193) | 1 to 4 (188, 97.4) | 1 to 4 (186, 96.4) | |
| Other familial and sporadic FTD cases (non-FTLD-U) | |||
| FTLD (n = 2) | FTLD | 0 (2, 100) | 0 (2, 100) |
| Pick disease (n = 13) | PICK | 0 (12, 100) | 0 (12, 100) |
| Corticobasal degeneration (n = 20) | CBD | 0 (20, 100) | 0 (20, 100) |
| Progressive supranuclear palsy (n = 4) | PSP | 0 (4, 100) | 0 (4, 100) |
| FTD with MAPT mutation (n = 5) | FTD-MAPT | 0 (5, 100) | 0 (5, 100) |
| Neuronal intermediate filament inclusion disease (n = 6) | NIFID | c (6, 100) | 0 (6, 100) |
| Basophilic inclusion body disease (n = 2) | BIBD | d (2, 100) | 0 (2, 100) |
| Hereditary diffuse leukoencephalopathy with spheroids (n = 2) | HDLS | 0 (2, 100) | 0 (2, 100) |
| Other diseases | |||
| Alzheimer’s disease (n = 19) | AD | 0 (19, 100) | 0 (19, 100) |
| Argyrophilic grain disease (n = 2) | AGD | 0 (2, 100) | 0 (2, 100) |
| Tangle-only dementia (n = 2) | TOD | 0 (2, 100) | 0 (2, 100) |
| Parkinson’s disease (n = 3) | PD | 0 (3, 100) | 0 (3, 100) |
| Dementia with Lewy bodies (n = 8) | DLB | 0 (8, 100) | 0 (8, 100) |
| Multiple system atrophy (n = 3) | MSA | 0 (3, 100) | 0 (3, 100) |
| Trinucleotide repeat disease (n = 3) | HD | 0 (1, 33) | 0 (1, 33) |
| SCA | 0 (2, 67) | 0 (2, 67) | |
| Hippocampal sclerosis (n = 2) | HS | e (2, 100) | e (2, 100) |
| Normal adult brain (n = 18) | NL | 0 (18, 100) | 0 (18, 100) |
Cases that are inconsistent with FTLD-U subtypes 1 to 4: a, FTLD with CHMP2B mutation cases with ubiquitin-positive and TDP-43-negative inclusions; b, FTLD-MND case with ubiquitin- and TDP-positive inclusions but insufficient cortical pathology for FTLD-U subtyping; c, NIFID cases with NII or NCI that are ubiquitin-positive and TDP-43-negative; d, BIBD cases with NCI that are ubiquitin-positive and TDP-43-negative; and e, HS cases with NCI in dentate gyrus that are ubiquitin- and TDP-43-positive.
AD, Alzheimer’s disease; AGD, argyrophilic grain disease; BIBD, basophilic inclusion body disease; DLB, dementia with Lewy bodies; CBD, corticobasal degeneration; FTD-MAPT, FTD with τ mutation; HD, Huntington disease; HDLS, hereditary diffuse leukoencephalopathy with neuroaxonal spheroids; HS, hippocampal sclerosis; MSA, multiple system atrophy; NIFID, neuronal intermediate filament inclusion disease; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; SCA, spinocerebellar ataxia; PICK, Pick disease; TOD, tangle-only dementia.
Chromosome 9p-Linked FTD with or without MND Is a TDP-43 Proteinopathy
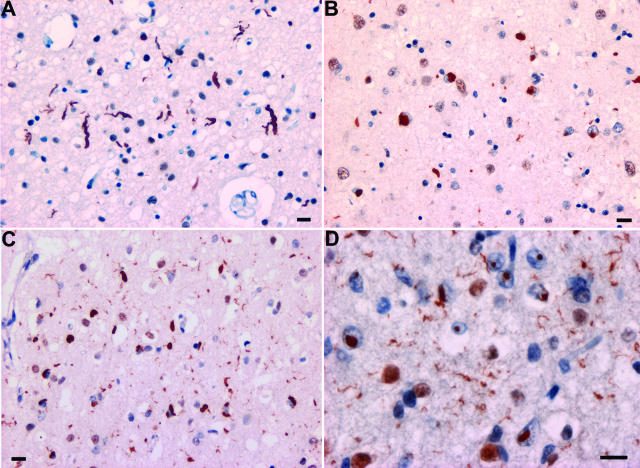
Cases of familial FTD with genetic linkage to chromosome 9p showed moderate numbers of TDP-43-positive NCIs, relatively few DNs and, in one case, rare NIIs (Figure 3). In addition to well-formed NCIs in the frontal and temporal neocortices and hippocampus, TDP-43 IHC revealed additional granular “pre-inclusions” (Figure 3B) that were not stained with ubiquitin. Both the NCIs and DNs were most numerous in the upper cortical laminae but were also present in neurons throughout the entire cortical thickness. Consistent with findings in other subtypes of TDP-43 proteinopathy, neurons with inclusions showed loss of the normal physiological TDP-43 staining of nuclei (Figure 3E). The majority of chromosome 9p-linked cases also had evidence of ubiquitin- and TDP-positive inclusions in upper and lower motor neurons, identical to those encountered in sporadic ALS/MND (Figure 3, C and D) but were not seen in the one case with FTD only. Furthermore, the inclusions were immunostained with monoclonal antibody to FTLD-U type 2 (#137) but not to type 1 (#182) (Figure 3, F and G). These data indicate that the pathology of FTD linked to chromosome 9p is a specific subtype of FTLD-U (type 2) and that TDP-43 is the disease-associated protein.
Figure 3.
Neuropathology of FTLD-U linked to chromosome 9p. TDP-43 immunohistochemistry showing compact TDP-43-positive cytoplasmic inclusions in neurons in the lower cortical layers (A); numerous neurons with diffuse granular cytoplasmic TDP-43 immunoreactivity in superficial frontal cortex (B); skein-like (C) and round (D) inclusion in motor neurons; and cytoplasmic inclusions in dentate granule cells (E). Note the absence of physiological, nuclear TDP-43 immunoreactivity in neurons with NCI. Inclusions in the dentate gyrus stained with monoclonal antibody 137 (F) but were negative with MAB 182 (G). Bars = 50 μm (A and B); 25 μm (C–G).
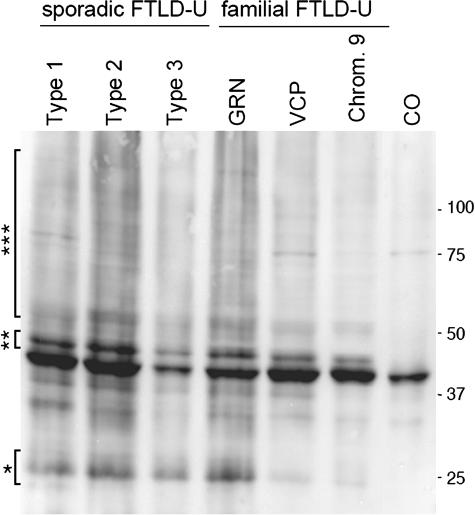
To characterize TDP-43 biochemically, Western blot analysis was performed on samples of cortical gray matter from patients with sporadic FTLD-U and FTLD-U linked to chromosome 9p that were sequentially extracted with buffers of increasing strength. Full-length TDP-43 protein of ∼43 kd was detected in all soluble and insoluble fractions of affected and unaffected brain regions from FTLD-U linked to chromosome 9p cases, similar to sporadic FTLD-U and control brains (data not shown). Additional protein bands of ∼25 and 45 kd, as well as a high molecular smear, were detected in detergent-insoluble, urea fractions from affected regions of cases of FTLD-U linked to chromosome 9p and FTLD-U but not controls (Figure 4). The quantity of these modified TDP-43 species was variable but correlated with the amount of pathology detected by immunohistochemistry. Furthermore, the 45-kd species was collapsed into the 43-kd band on dephosphorylation with alkaline phosphatase, indicating that TDP-43 is abnormally phosphorylated (data not shown). Thus, these data suggest that, despite the distinct genetic alterations and pattern of ubiquitin pathology in FTLD-U linked to chromosome 9p, the molecular signature of the TDP-43 disease protein is similar to that seen in both sporadic and familial FTLD-U, including those individuals with PGRN and VCP gene mutations.
Figure 4.
Biochemical analysis of TDP-43. Immunoblot analysis probed with anti-TDP-43 antibody demonstrated a distinct pathological profile of TDP-43 with variable presence of pathological ∼25-kd bands (*), 45-kd bands (**), and high molecular smear (***) in all sporadic and familial FTLD-U brains (FTLD-U subtypes 1 to 3), including those with progranulin (GRN) and VCP mutations and those linked to chromosome 9p (Chrom. 9). Pathological TDP-43 bands were not detected in control brains (CO).
Chromosome 3-Linked FTD Is Not a TDP-43 Proteinopathy
Ubiquitin IHC revealed NCIs in the dentate fascia and sparse inclusions in the frontal neocortex of cases with familial FTD with CHMP2B mutation. However, the absence of DN and the presence of granular, ubiquitin-positive structures within the neocortex distinguished this FTLD-U subtype from types 1 to 4 described above. In addition, TDP-43 IHC failed to label any of the ubiquitinated inclusions. Thus, familial FTD with CHMP2B mutation is a unique pathological subtype of FTLD-U and is not a TDP-43 proteinopathy on the basis of IHC.
TDP-43 Is a Component of the Ubiquitinated Inclusions of Hippocampal Sclerosis
HS was found to be a coexisting pathology in seven cases of FTLD-U with or without MND. In an additional two cases classified as pathologically pure HS, small numbers of ubiquitin-positive and TDP-43-positive inclusions were found exclusively in the dentate granule cells (Figure 1M). In one case of corticobasal degeneration with HS, the NCIs were ubiquitin-positive but TDP-43- negative. The dense network of fine TDP-43-positive neurites seen occasionally in CA1 in FTLD-U (Figure 1K) was not observed in any case of HS with or without FTLD-U. These data indicate that some cases of HS are TDP-43 proteinopathies, but further studies on a larger sample of “pure HS” and biochemical studies are required to determine the nosological status of HS.
TDP-43 Is Not a Component of Inclusions of Other Neurological Diseases
To determine the specificity of TDP-43 proteinopathy among the spectrum of FTLDs and other neurological diseases, we performed ubiquitin and TDP-43 IHC on sections with disease-representative pathology. In six cases of neuronal intermediate filament inclusion disease (Table 1), the ubiquitin-immunoreactive NCI and NII were not labeled with antibodies to TDP-43. In none of the FTLDs with tauopathy was TDP-43 a component of the inclusions as demonstrated by IHC. TDP-43 was also absent from other FTLDs, including basophilic inclusion body disease (n = 2) and hereditary diffuse leukoencephalopathy with spheroids (n = 2), other neurological diseases including AD (n = 19), argyrophilic grain disease (n = 2), tangle-only dementia (n = 2), synucleinopathies [dementia with Lewy bodies (n = 8), Parkinson’s disease (n = 3), and multiple system atrophy (n = 3)], trinucleotide repeat diseases (n = 3), and normal aged brain (n = 18) (Table 1; Supplemental Table 1 at http://ajp.amjpathol.org).
Fine Structure of Inclusions of FTLD-U
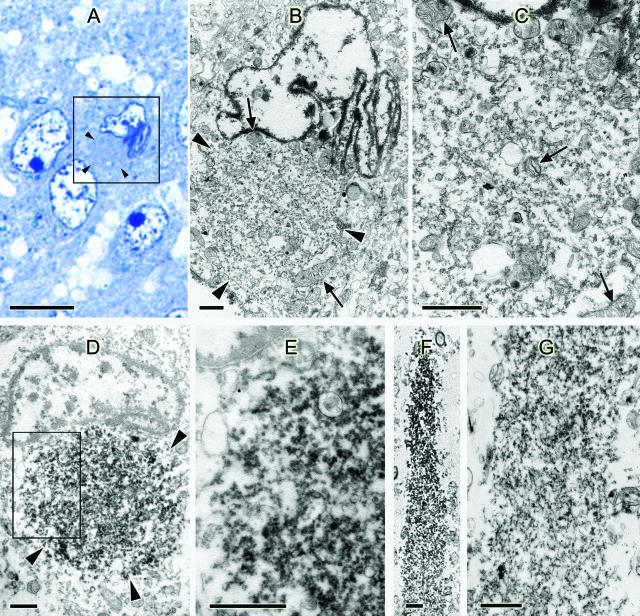
Light microscopic examination of semithin sections from the hippocampus of a case of FTLD-U revealed round cytoplasmic bodies identical to the NCIs immunolabeled with ubiquitin and TDP-43 antibodies in paraffin sections. Cells containing the NCIs often had a variably indented nucleus, sometimes clefted or corrugated (Figure 5A, inset). Electron microscopy of the same cells showed an accumulation of granular and membranous material with a few mitochondria. Mitochondria and lipofuscin surrounded the NCI (Figure 5, B and C). An occasional filament or microtubule was seen in the inclusions, but these were a minor component. Immunostained sections showed similar NCIs that were TDP-43-positive (Figure 5, D and E). Sections of the temporal lobe showed TDP-43-positive DNs that contained somewhat more filamentous material, but no dense or compact accumulations of filaments were seen (Figure 5, F and G).
Figure 5.
Fine structure of inclusions of FTLD-U. A: Light microscopy semithin section from the dentate gyrus of the hippocampus. Toluidine blue stain. Inset: Neuron with a round body (arrowheads) is adjacent to the nucleus. The nucleus is corrugated and indented compared with the other three normal neuronal nuclei. B and C: Re-embedded ultrathin section of the boxed cell in A and counterstained with uranyl acetate and bismuth subnitrate. B: Low-magnification electron micrograph showing a round body (arrowheads) of an NCI surrounded by mitochondria. C: Higher magnification of B shows an accumulation of granular and membranous material with a few mitochondria (arrows, mitochondria). D: Ultrathin section of a neuron from the dentate gyrus of the hippocampus, immunostained for TDP-43. An NCI (outlined by arrowheads) indenting the nucleus is labeled (no counterstain). E: Higher magnification of boxed area of D. F: Dystrophic neurite from the temporal lobe immunostained for TDP-43 (no counterstain). G: Same neurite as in F in an adjacent section counterstained with uranyl acetate and bismuth subnitrate. More filamentous material is seen than in the cell body of C, but no dense accumulations of filaments are present. Bars = 10 μm (A); 1 μm (B–G).
Discussion
TDP-43 is a recently identified pathological protein of the signature lesions of a spectrum of diseases, including FTLD-U, FTLD-MND, and sporadic MND.7,8,10,11 The purpose of this study was to determine the frequency of TDP-43 proteinopathy in a broad spectrum of clinically and neuropathologically characterized cases of FTLDs obtained from several dementia research centers in Europe and North America. This study demonstrates that TDP proteinopathy is the most frequently found pathology in both familial and sporadic cases of FTLD-U with or without MND.
In our previous studies, we identified three subtypes of FTLD-U, which were distinguished by the morphology, immunohistochemical profile, and cellular localization of the inclusions (NCI, NII, or DN).8,14 Recently, we have used ubiquitin and TDP-43 immunohistochemistry to characterize the inclusions of FTLD-U with VCP mutations.10,13 Unlike FTLD-U subtypes 1 to 3, which have predominantly NCIs and/or DNs, FTLD-U subtype 4 is distinguished by predominantly NIIs.10,13 Taken together, these studies show that four pathological subtypes of FTLD-U may be identified based on the morphology and distribution of ubiquitin-positive pathology.8,10,14 In this study, the order of frequency was: type 3 > type 2 > type 1 > type 4. This differs from our previous study, in which we found that type 1 was the most frequent FTLD-U subtype.14 However, in the present multicenter study, the relatively low proportion of type 1 cases and high proportion of type 3 cases likely represents an acquisition bias and reflects the research interests in familial dementias at centers in this study. For instance, the present series included 37 cases (36 familial cases and one sporadic case) with PGRN mutations, which is characterized by type-3 pathology. Additional studies from other centers and population-based studies are required to determine the true prevalence of these subtypes.
A family history of dementia is present in up to 40% of FTD patients, implicating a strong genetic influence. To date, mutations in three genes have been linked to familial FTD with FTLD-U pathology: VCP, CHMP2B, and PGRN. We have previously shown that pathological TDP-43 is a component of the inclusions of FTLD-U with PGRN and VCP mutations.8,10,11 Here, for the first time, we show that familial FTLD-U and FTLD-MND linked to chromosome 9p are also TDP-43 proteinopathies. In one case, a putative mutation in the IFT74 gene has been linked to this clinicopathological entity,26 but this requires confirmation.
The seven cases with genetic linkage to chromosome 9p had a pattern of pathology consistent with FTLD-U type 2 but with some additional, unique features. First, many neurons in neocortical and archicortical regions had granular TDP-43 immunoreactivity in the neuronal cytoplasm that was not seen with antibodies to ubiquitin. We speculate that this may represent an early stage in aggregate formation, analogous to pre-tangles commonly encountered in AD. Second, the majority of chromosome 9-linked cases also had evidence of ubiquitin- and TDP-positive inclusions in upper and lower motor neurons, identical to those encountered in sporadic ALS/MND.
A disease-specific biochemical signature of pathologically altered TDP-43 has previously been reported in sporadic and familial FTLD-U.8,10,11 In affected regions of cases of FTLD-U linked to chromosome 9p, additional protein bands of ∼25 and 45 kd and a high molecular smear identical to the pattern found in familial FTLD-U with PGRN and VCP mutations were detected in sporadic FTLD-U and MND but not in controls. Thus, these data suggest that despite the distinct genetic alterations and pattern of ubiquitin pathology in FTLD-U linked to chromosome 9p, the molecular signature of the pathological TDP-43 protein is similar to that found in sporadic and other genetic causes of familial FTLD-U.
In familial FTLD-U, the morphology and distribution of ubiquitinated and TDP-43-positive inclusions correlated with genotype. Cases with PGRN mutations were exclusively FTLD-U type 3, cases with VCP mutations were FTLD-U type 4, FTLD-U cases linked to chromosome 9 were FTLD-U type 2, and cases with mutations in CHMPB2 had a unique pattern of ubiquitin pathology that was negative for TDP-43. In contrast, sporadic cases with a similar clinical phenotype were much more heterogeneous according to FTLD-U subtype, possibly reflecting variability in genotype and environmental factors.
TDP-43 was not a component of the inclusions of other FTLDs (neuronal intermediate filament inclusion disease, hereditary diffuse leukoencephalopathy with spheroids, and basophilic inclusion body disease), AD, the tauopathies (Pick disease, corticobasal degeneration, progressive supranuclear palsy, argyrophilic grain disease, tangle-only dementia, and FTLD with MAPT mutation), synucleinopathies (Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy), and trinucleotide repeat diseases (Huntington’s disease and spinocerebellar ataxia). The one exception was HS in which we found TDP-43-positive NCIs in the dentate gyrus. Although only one case of HS without coexisting neurodegenerative disease was available in this study, the presence of TDP-43 epitopes within the NCI indicates that a proportion of pure HS cases may be considered part of the spectrum of FTLD-U.44 The biochemistry of this disorder remains to be evaluated.
The mechanisms leading to inclusion formation in FTLD-U remain unknown. However, we have shown for the first time by electron microscopy that the majority of inclusions are composed of granular material that contains epitopes of TDP-43. The fine structure of the inclusions of FTLD-U may be contrasted with the inclusions of other protein-folding diseases, including tauopathies and synucleinopathies, in which monomeric species aggregate to form fibrils that make up the inclusion. These fibrillary proteins are typically amyloidogenic, in that they stain with amyloid-binding dyes, including Congo red and thioflavin S. In contrast, the inclusions of FTLD-U are not labeled by amyloid dyes, and this corresponds with the sparsity of fibrillary structure as visualized by electron microscopy. This biophysical property distinguishes the TDP-43 proteinopathies from other neurodegenerative diseases.
How defects in different genes cause TDP-43 protein aggregation remains to be elucidated. Furthermore, the common mechanisms leading to TDP-43 phosphorylation, ubiquitination, and aggregation in the nucleus, cytoplasm and processes or neurons are unknown. The ubiquitin pathology of FTLD-U and FTLD-MND may result from a primary defect of the ubiquitin-proteasome system.47 Alternatively, there may be an abnormal metabolism of TDP-43, resulting in pathological species that then become ubiquitinated, a mechanism similar to that proposed for other neurodegenerative diseases, including the tauopathies, synucleinopathies, and trinucleotide repeat diseases.
TDP-43 is a ubiquitously expressed and highly conserved protein found mainly in the nucleus.48 It may act as an activator of exon skipping,5,6 a transcription repressor, and/or as a scaffold for nuclear bodies through interactions with survival motor neuron protein.49 In unaffected neurons, TDP-43 IHC showed the normal diffuse nuclear staining pattern but did not reveal significant levels in the neuronal cytoplasm, axons, or dendrites. However, in cells with inclusions, there was a marked reduction of TDP-43 immunoreactivity within the nucleus.8 The relocation of the nuclear protein TDP-43 resembles the relocation of peptidyl-prolyl cis-trans isomerase (Pin1) from the nucleus to the cytoplasm of inclusion-containing neurons in AD, FTLDs with tauopathy and neuronal intermediate filament inclusion disease.50 This relocation of a nuclear protein may lead to loss of TDP-43 nuclear function or cause neurodegeneration by apoptotic mechanisms as with Pin1 translocation. The loss of nuclear TDP-43 immunoreactivity may result from reduced protein expression, relocation from the nucleus to the cytoplasm, and sequestration within ubiquitinated inclusions. However, because some TDP-43-positive neuronal and glial inclusions were detected that were not labeled by ubiquitin antibodies, abnormal TDP-43 phosphorylation and/or aggregation may precede ubiquitination in the pathogenesis of inclusions of FTLD-U. The exact mechanism and pathological significance of the relocation of TDP-43 from the nucleus requires further study.
In summary, this study shows that TDP-43 is a major component of the pathological inclusions in four clinical groups characterized by FTLD-U pathology: 1) familial FTD with PGRN and VCP mutations and cases linked to chromosome 9p; 2) familial FTD with unknown genetic association; 3) sporadic FTD; and 4) MND with dementia. Familial FTD with CHMP2B mutation is an exception, being a familial FTLD-U without pathological TDP-43. Although pathological TDP-43 was not found to be a component of the inclusions of any other FTD, other neurodegenerative disease, or normal aging, it was observed in most cases of HS, indicating that some of these cases have both clinical and pathological similarities with other TDP-43 proteinopathies. There was a remarkable correlation between genotype and FTLD-U subtype for familial cases with known gene defects. In contrast, there was much more heterogeneity in the pathological subtype of sporadic cases of FTD. The identification of granular TDP-43-positive pre-inclusions, particularly in FTD linked to chromosome 9p cases, may indicate that TDP-43 proteinopathy is an early event in pathogenesis and precedes ubiquitination.11 In conclusion, the identification of TDP-43 proteinopathy in a diverse group of FTLDs illuminates a new target for investigation and therapeutic intervention.
Supplementary Material
Acknowledgments
We thank the clinical, genetic, pathology, and technical staff of the collaborating centers for making information and tissue samples available for this study, and we thank the families of patients whose generosity made this research possible.
Footnotes
Address reprint requests to Nigel J. Cairns, Ph.D., MRCPath, Department of Pathology and Immunology, Washington University School of Medicine, Campus Box 8118, 660 South Euclid Ave., St. Louis, MO 63110. E-mail: cairns@wustl.edu.
Supported by grants from the National Institute on Aging of the National Institutes of Health (AG03991, AG05681, AG13854, AG10124, AG17586, AG10124, AG17586, AG16976, and AG12300), the Buchanan Fund, the Winspear Family Center for Research on the Neuropathology of Alzheimer Disease, the McCune Foundation, and the Canadian Institutes of Health Research (74580).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Neary D, Snowden JS, Mann DM. Classification and description of frontotemporal dementias. Ann NY Acad Sci. 2000;920:46–51. doi: 10.1111/j.1749-6632.2000.tb06904.x. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Mann DM, Northen B, Goulding PJ, Macdermott N. Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry. 1990;53:23–32. doi: 10.1136/jnnp.53.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton AM, White CL, III, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol (Berl) 2004;108:379–385. doi: 10.1007/s00401-004-0900-9. [DOI] [PubMed] [Google Scholar]
- Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti E, Brindisi A, Pagani F, Baralle FE. Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet. 2004;74:1322–1325. doi: 10.1086/420978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado PA, Ayala YM, Romano M, Buratti E, Baralle FE. Depletion of TDP 43 overrides the need for exonic and intronic splicing enhancers in the human apoA-II gene. Nucleic Acids Res. 2005;33:6000–6010. doi: 10.1093/nar/gki897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DM. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol (Berl) 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor J Paul, Kretzschmar HA, Kimonis V, Forman MS. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP mutations. J Neuropathol Exp Neurol. 2007;66:152–157. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]
- Neumann M, Kwong LK, Truax AC, Vanmassenhove B, Kretzschmar HA, Van Deerlin VM, Clark CM, Grossman M, Miller BL, Trojanowski JQ, Lee VMY. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66:177–183. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- Woulfe J, Kertesz A, Munoz DG. Frontotemporal dementia with ubiquitinated cytoplasmic and intranuclear inclusions. Acta Neuropathol (Berl) 2001;102:94–102. doi: 10.1007/s004010000346. [DOI] [PubMed] [Google Scholar]
- Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, Jhaveri BS, Karlawish JH, Pestronk A, Smith TW, Tu PH, Watts GD, Markesbery WR, Smith CD, Kimonis VE. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol. 2006;65:571–581. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]
- Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der ZJ, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, van den BM, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Mukherjee O, Pastor P, Cairns NJ, Chakraverty S, Kauwe JS, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, Levitch D, Taylor-Reinwald L, Gitcho M, Tu PH, Tenenholz GL, Liscic RM, Armendariz J, Morris JC, Goate AM. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol. 2006;60:314–322. doi: 10.1002/ana.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Baker M, Pickering-Brown S, Hsiung GY, Lindholm C, Dwosh E, Gass J, Cannon A, Rademakers R, Hutton M, Feldman HH. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain. 2006;129:3081–3090. doi: 10.1093/brain/awl271. [DOI] [PubMed] [Google Scholar]
- Behrens MI, Mukherjee O, Tu PH, Liscic RM, Grinberg LT, Carter D, Paulsmeyer K, Taylor-Reinwald L, Gitcho M, Norton JB, Chakraverty S, Goate AM, Morris JC, Cairns NJ. Neuropathologic heterogeneity in HDDD1: a familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alzheimer Dis Assoc Disord. 2007;21:1–7. doi: 10.1097/WAD.0b013e31803083f2. [DOI] [PubMed] [Google Scholar]
- Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- Wang Q, Song C, Li CC. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol. 2004;146:44–57. doi: 10.1016/j.jsb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sorensen SA, Gydesen S, Fisher EM, Collinge J. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- Holm IE. Ubiquitin-positive inclusions in frontotemporal dementia linked to chromosome 3 (FTD-3). Brain Pathol. 2006;16:S43. [Google Scholar]
- Morita M, Al Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, Mitchell JE, Habgood JJ, de Belleroche J, Xi J, Jongjaroenprasert W, Horvitz HR, Gunnarsson LG, Brown RH., Jr A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- Vance C, Al Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, Siddique T, Schelhaas HJ, Kusters B, Troost D, Baas F, de Jong V, Shaw CE. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain. 2006;129:868–876. doi: 10.1093/brain/awl030. [DOI] [PubMed] [Google Scholar]
- Momeni P, Schymick J, Jain S, Cookson MR, Cairns NJ, Greggio E, Greenway MJ, Berger S, Pickering-Brown S, Chio A, Fung HC, Holtzman DM, Huey ED, Wassermann EM, Adamson J, Hutton ML, Rogaeva E, St George-Hyslop P, Rothstein JD, Hardiman O, Grafman J, Singleton A, Hardy J, Traynor BJ. Analysis of IFT74 as a candidate gene for chromosome 9p-linked ALS-FTD. BMC Neurol. 2006;6:44. doi: 10.1186/1471-2377-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis: Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, Pickering-Brown SM, Graff-Radford N, Uitti R, Dickson D, Wszolek Z, Gonzalez J, Beach TG, Bigio E, Johnson N, Weintraub S, Mesulam M, White CL, III, Woodruff B, Caselli R, Hsiung GY, Feldman H, Knopman D, Hutton M, Rademakers R. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, de Silva R, Lees A, Dickson DW. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol. 2002;61:547–556. doi: 10.1093/jnen/61.6.547. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Grossman M, Arnold SE, Burn DJ, Jaros E, Perry RH, Duyckaerts C, Stankoff B, Pillon B, Skullerud K, Cruz-Sanchez FF, Bigio EH, Mackenzie IR, Gearing M, Juncos JL, Glass JD, Yokoo H, Nakazato Y, Mosaheb S, Thorpe JR, Uryu K, Lee VM, Trojanowski JQ. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology. 2004;63:1376–1384. doi: 10.1212/01.wnl.0000139809.16817.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson R, Roytta M, Sourander P, Akesson HO, Andersen O. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl. 1984;314:1–65. [PubMed] [Google Scholar]
- Munoz DG. The pathology of pick complex. Kertesz A, Munoz DG, editors. New York: Wiley-Liss,; Pick’s Disease and Pick Complex. 1998:pp 211–241. [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- Gilman S, Low PA, Quinn N, Albanese A, Ben Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- The National Institute on Aging, Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Robitaille Y, Lopes-Cendes I, Becher M, Rouleau G, Clark AW. The neuropathology of CAG repeat diseases: review and update of genetic and molecular features. Brain Pathol. 1997;7:901–926. doi: 10.1111/j.1750-3639.1997.tb00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuereb JH, MacMillan JC, Snell R, Davies P, Harper PS. Neuropathological diagnosis and CAG repeat expansion in Huntington’s disease. J Neurol Neurosurg Psychiatry. 1996;60:78–81. doi: 10.1136/jnnp.60.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS. Overview of dementia lacking distinctive histology: pathological designation of a progressive dementia. Dementia. 1993;4:132–136. doi: 10.1159/000107354. [DOI] [PubMed] [Google Scholar]
- Hatanpaa KJ, Blass DM, Pletnikova O, Crain BJ, Bigio EH, Hedreen JC, White CL, III, Troncoso JC. Most cases of dementia with hippocampal sclerosis may represent frontotemporal dementia. Neurology. 2004;63:538–542. doi: 10.1212/01.wnl.0000129543.46734.c0. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Shi J, Shaw CL, Duplessis D, Neary D, Snowden JS, Mann DM. Dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol (Berl) 2006;112:551–559. doi: 10.1007/s00401-006-0123-3. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Minson JB. Complete penetration of antibodies into vibratome sections after glutaraldehyde fixation and ethanol treatment: light and electron microscopy for neuropeptides. J Histochem Cytochem. 1992;40:1741–1749. doi: 10.1177/40.11.1431060. [DOI] [PubMed] [Google Scholar]
- Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- Ayala YM, Pantano S, D’Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila, and C. elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348:575–588. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Wang IF, Reddy NM, Shen CK. Higher order arrangement of the eukaryotic nuclear bodies. Proc Natl Acad Sci USA. 2002;99:13583–13588. doi: 10.1073/pnas.212483099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe JR, Mosaheb S, Hashemzadeh-Bonehi L, Cairns NJ, Kay JE, Morley SJ, Rulten SL. Shortfalls in the peptidyl-prolyl cis-trans isomerase protein Pin1 in neurons are associated with frontotemporal dementias. Neurobiol Dis. 2004;17:237–249. doi: 10.1016/j.nbd.2004.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.