Abstract
Adenoid cystic carcinoma is a frequently occurring malignant salivary gland neoplasm. We studied the induction of protease activity by the laminin-derived peptide, SIKVAV, in cells (CAC2) derived from this neoplasm. Laminin α1 and matrix metalloproteinases (MMPs) 2 and 9 were immunolocalized in adenoid cystic carcinoma cells in vivo and in vitro. CAC2 cells cultured on SIKVAV showed a dose-dependent increase of MMP9 as detected by zymography and colocalization of α3 and α6 integrins. Small interfering RNA (siRNA) knockdown of integrin expression in CAC2 cells resulted in decreased adhesion to the peptide. SIKVAV affinity chromatography and immunoblot analysis showed that α3, α6, and β1 integrins were eluted from the SIKVAV column, which was confirmed by mass spectrometry and a solid-phase binding assay. Small interfering RNA experiments also showed that these integrins, through extracellular signal-regulated kinase (ERK) 1/2 signaling, regulate MMP secretion induced by SIKVAV in CAC2 cells. We propose that SIKVAV increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through α3β1 and α6β1 integrins and the ERK 1/2 signaling pathway.
Adenoid cystic carcinoma is a frequently occurring malignant salivary gland neoplasm.1 It shows insidious and slow growth with high level of recurrence and distant metastasis a long time after treatment.2 This neoplasm is histologically characterized by a sheet or island-like proliferation of round or cuboidal epithelial cells, with scanty cytoplasm and hyperchromatic large oval nuclei.2 Growth patterns are solid, tubular, and pseudocystic, and perineural invasion is a common histological finding.1,2,3 Electron microscopy shows both luminal and myoepithelial cells, and these cells are often separated by extracellular matrix (ECM), such as pools of basal lamina, collagen fibers, elastin, and glycosaminoglycans.2 A conspicuous finding in the cribriform variant of adenoid cystic carcinoma is a thickened band of extensively duplicated basement membrane.2 A prominent feature of adenoid cystic carcinoma is its affinity for basement membrane-rich tissues, such as nerves and blood vessels,2 and many immunohistochemical studies have highlighted the presence of basement membrane proteins in this neoplasm.3,4,5,6
It has been suggested that the ECM plays an important role as a regulatory factor of phenotypic differences among salivary gland neoplasms.7,8,9,10,11,12,13,14 The ECM is a three-dimensional network of macromolecules, including collagens, laminins, fibronectin, entactin/nidogen, and heparan sulfate proteoglycans.15,16,17 However, cells produce matrix metalloproteinases that degrade extracellular matrix macromolecules, which weakens the structural integrity of tissues, stimulates cellular invasion, triggers apoptosis or proliferation, and releases matrix-bound growth factors.18 In addition, several lines of evidence suggest that extracellular components contain cryptic domains that are exposed by proteolysis and elicit biological responses distinct from intact molecules.18,19,20 These domains exposed by proteolysis are named matricryptins, matrikines, or cryptic sites.18,19,20 These cryptic sites are represented by fragments and bioactive peptides that are likely to be present in most extracellular matrix molecules.19,20,21
The laminins express multiple cryptic sites,18,19,20,21 including the well-documented bioactive peptide SIKVAV.18,22,23,24,25,26,27 This peptide, located at the carboxy-terminal portion of the laminin α1 chain is liberated during tumor invasion through basement membranes.18,28 SIKVAV is involved in malignant transformation, cell proliferation, angiogenesis, and protease activity in a number of cell types. Because of its biological significance, this peptide has been proposed as a therapeutic agent.29,30,31,32,33,34 In salivary gland neoplasms, we have demonstrated that SIKVAV regulates the morphology and protease activity of a cell line (CAC2) derived from human adenoid cystic carcinoma.11,12 Despite its biological relevance, the mechanisms regulating the bioactivity of SIKVAV are not fully understood.
Here, we studied the regulatory mechanisms resulting in increased protease activity induced by SIKVAV in CAC2 cells. Cells were cultured on SIKVAV, and the secretion of matrix metalloproteinases was measured by zymography. We show that α3β1 and α6β1 integrins elute from SIKVAV affinity columns. Our experiments suggest that integrin signaling, through the extracellular signal-regulated kinase (ERK) pathway, regulates the increased protease activity in CAC2 cells cultured on SIKVAV.
Materials and Methods
Immunolocalization of Laminin α1 and Matrix Metalloproteinases in Adenoid Cystic Carcinoma in Vivo
Ten cases of adenoid cystic carcinoma were retrieved from the files of the Department of Oral Pathology, UNIMES, and the Department of Oral Pathology, School of Dentistry, University of Pará. Growth patterns were cribriform (six cases), tubular (two cases), and solid (two cases). Formalin-fixed paraffin-embedded tissues were studied by immunohistochemistry. Sections (3 μm) were stained by the EnVision method (Dako Corp., Carpinteria, CA). Sections mounted on 3-aminopropyltriethoxy-silane-coated slides (Sigma Chemical Corp., St. Louis, MO) were dewaxed in xylene and hydrated in graded ethanol. Endogenous peroxidase activity was inhibited with 3% H2O2 in methanol for 20 minutes. Antigen retrieval involved treating sections with 1% pepsin in 10 mmol/L HCl for 1 hour at 37°C. Sections were blocked with 1% bovine serum albumin (BSA; Sigma) in Tris-HCl. A goat polyclonal antibody to laminin α1 chain [C-20 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), kindly provided by Dr. Vilma R. Martins, Ludwig Institute for Cancer Research, São Paulo Branch, Brazil] was diluted 1:50 in Tris-HCl. Anti-matrix metalloproteinase (MMP) 2 monoclonal antibody (mAb; clone 42-5D11) was diluted 1:50, and anti-MMP9 mAb (clone 56-2A4) was diluted 1:25 (both from Calbiochem-Novabiochem Co., La Jolla, CA). Diaminobenzidine (Sigma) was used as the chromogen, and sections were counterstained with Mayer’s hematoxylin (Sigma). A nonspecific serum served as negative control.
Cell Culture
The CAC2 cell line was derived from a human adenoid cystic carcinoma and has been characterized previously.11,12 Cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 10% fetal bovine serum (Cultilab; Campinas, São Paulo, Brazil) and 1% antibiotic-antimycotic solution (Sigma). The cells were maintained in 25-cm2 flasks in a humidified atmosphere of 5% CO2 at 37°C.
Immunofluorescence
Cells were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 10 minutes, blocked with 1% BSA in PBS, and stained with goat antibody against laminin α1 chain (1:50 in PBS; Chemicon, Temecula, CA) and an anti-goat Cy3-conjugated secondary antibody (Zymed-Invitrogen Co., Carlsbad, CA). To stain for MMPs, cells were fixed and permeabilized with 0.5% Triton X-100 (Sigma) in PBS for 15 minutes and incubated with mAbs to MMP2 and MMP9 diluted in PBS 1:50 revealed by an anti-mouse Cy3-conjugated secondary antibody (Zymed-Invitrogen). All incubations were performed for 60 minutes at room temperature, and Pro Long (Invitrogen-Molecular Probes, Eugene, OR) was used as a mounting medium. Nuclei were counterstained with either 4,6-diamidino-2-phenylindole or Sytox Green (Invitrogen-Molecular Probes). A nonspecific serum served as negative control.
For immunofluorescence analysis of α3 and α6 integrins, CAC2 cells were cultured on SIKVAV and IVSKVA for 4 hours and subjected to the immunofluorescence protocol described by Hogervorst et al.35 Cells were fixed in 1% paraformaldehyde in PBS for 10 minutes, rinsed, permeabilized with 0.5% Triton X-100 (Sigma) in PBS for 5 minutes, and blocked with 1% BSA for 30 minutes. Samples were then double-labeled with antibodies against α3 (mAb, clone P1B5; Chemicon) and α6 (rat mAb, clone GoH3; Chemicon) integrins diluted 1:100 in PBS for 1 hour. Secondary antibodies were anti-mouse Cy3 (Zymed-Invitrogen) and anti-rat FITC (Zymed-Invitrogen). Nuclei were counterstained with 4,6-diamidino-2-phenylindole and mounted in Pro Long.
Zymography of SIKVAV-Treated CAC2 Cells
CAC2 cells were cultured in six-multiwell plates coated with 100 μl of either SIKVAV or the scrambled control IVSKVA (50 μg/ml, 200 μg/ml, 500 μg/ml, and 1 mg/ml) diluted in Milli-Q water and allowed to evaporate overnight in a hood. CAC2 cells (1 × 104) were plated and allowed to adhere and spread for at least 8 hours. The adherent cells were washed three times with PBS, and the culture medium was replaced by serum-free medium for 24 hours. The presence of MMPs in the conditioned medium was assessed by zymography. The conditioned medium was collected, concentrated (Microcon 30K; Millipore Co., Bedford, MA), and resuspended in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer (without β-mercaptoethanol). The remaining CAC2 cells were lysed, and the amount of protein was estimated by bicinchoninic acid assay (Pierce Biotechnology Inc., Rockford, IL). In zymograms, the volume of conditioned medium loaded per lane was standardized on the basis of protein content in the cell lysate. Samples were separated on 10% polyacrylamide gels containing 0.2% gelatin (Novex-Invitrogen). After electrophoresis, the gels were washed in 2.5% Triton X-100 for 30 minutes, equilibrated in 10 mmol/L Tris, pH 8.0, and incubated at 37°C in a development buffer containing 50 mmol/L Tris, pH 8.0, 5 mmol/L CaCl2, and 0.02% NaN3 for 16 to 24 hours. The gels were stained with 0.2% Coomassie blue R250 (Amersham Co., Arlington Heights, IL) and destained with acetic acid/methanol.
The MMP activity was also inhibited with either 10 mmol/L ethylenediamine tetraacetic acid (EDTA) (calcium chelator; Sigma) or 5 mmol/L 1,10-phenanthroline (heavy metal chelator; Sigma) in the zymogram development buffer to confirm that the bands were MMPs. We also ran parallel polyacrylamide gels with CAC2 cell lysates and did Western blots for β-actin to confirm equal loading conditions.
CAC2 cells treated with small interfering RNA (siRNA) to integrins and syndecan-1 were cultured in six-multiwell plates on SIKVAV or IVSKVA (scrambled control) diluted in serum-free medium (50 μg/ml). The MMP activity in the conditioned medium was analyzed by zymography as described above.
CAC2 Cell Adhesion Assays
Cell adhesion to SIKVAV was compared with laminin-111 and with IVSKVA. Adhesion assays were performed in 96-well round-bottomed plates. Wells were coated overnight at 4°C with 100 μl of SIKVAV (1 μg/well), laminin-111 (1 μg/well), or IVSKVA (1 μg/well). Wells were blocked with 3% BSA for 30 minutes at 37°C and rinsed in PBS with 0.1% BSA, and cells were incubated for 20 minutes at 37°C. Attached cells were fixed/stained for 10 minutes with 0.2% (w/v) crystal violet in 20% (v/v) methanol. After three washes with H2O, cells were dissolved in 10% (w/v) SDS, and the absorbance at 600 nm was measured. We analyzed the effects of EDTA (calcium chelator) on the adhesion of CAC2 cells to SIKVAV. Cells were preincubated with 1 mmol/L EDTA in PBS for 10 minutes before being added to cell adhesion assays.
CAC2 cells treated with siRNA to α3, α6, β1 integrin subunits and syndecan-1 were used in adhesion assays to SIKVAV, laminin-111, type I collagen, and fibronectin. Adhesion assays were performed in 96-well round-bottomed plates. Wells were coated overnight at 4°C with 100 μl of SIKVAV (1 μg/well), laminin-111 (1 μg/well), type I collagen (1 μg/well), and fibronectin (1 μg/well). Adhesion assays were performed as described above. To assess the specificity of the α3 and α6 integrin knockdown on adhesion, we performed assays to substrates where adhesion is not dependent on these integrins, such as fibronectin and type I collagen. Furthermore, we also silenced a nonintegrin receptor, syndecan-1. CAC2 cells with reduced syndecan expression were submitted to the same adhesion assays described above.
RNA Interference
CAC2 cells were transfected with commercially available siRNA targeting α3 and β1 integrins and syndecan-1 (Santa Cruz Biotechnology) and α6 integrin (Invitrogen) following the manufacturer’s instructions. One day before transfection, subconfluent CAC2 cells were cultured in Dulbecco’s modified Eagle’s medium, supplemented by 10% fetal bovine serum without antibiotic-antimycotic solution. Cells were incubated with a complex formed by the siRNA, transfection reagent (Lipofectamine 2000; Invitrogen), and transfection medium (Opti-MEM I; Invitrogen) for 30 hours at 37°C. A siRNA-scrambled sequence (Invitrogen and Santa Cruz proprietary target sequences) was used as negative control. The following oligonucleotide sequences for siRNA were used: α3 integrin (a pool of three mRNA strands), 5′-UUCACUGUGAUGUUCAUCCTTGGAUGAACAUCACAGUGAATT-3′, 5′-UUCAUGAAGACAUAGAUGGTTCCAUCUAUGUCUUCAUGAATT-3′, and 5′-UGAUAGAUGUACACUUUGCTTGCAAAGUGUACAUCUAUCATT-3′; α6 integrin, 5′-UAACCUGGAGGCAUAUCCCACUAGGTT-3′; β1 integrin (a pool of three mRNA strands), 5′-GAGAUGAGGUUCAAUUUGA-3′, 5′-GAUGAGGUUCAAUUUGAAA-3′, and 5′-GUACAGAUCCGAAGUUUCA-3′; and syndecan-1 (a pool of three mRNA strands), 5′-CGCAAAUUGUGGCUACU-AA-3′, 5′-AGCAGGACUUCACCUUUGA-3′, and 5′-CGUGGGGCUCAUCUUUGCU-3′. Transfection efficiency was confirmed by immunoblot and real-time quantitative polymerase chain reaction (PCR).
Western Blots
Immunoblots for α3, α6, or β1 integrins and syndecan-1 were used to test the efficiency of RNA silencing in CAC2 cells. Samples were lysed in radioimmunoprecipitation assay buffer (150 mmol/L NaCl, 1.0% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, and 50 mmol/L Tris, pH 8.0) with protease inhibitor cocktail (Sigma) and centrifuged (10,000 × g) for 10 minutes at 4°C. The supernatants were recovered, quantified (bicinchoninic acid kit; Pierce), and resuspended in Laemmli sample buffer containing 62.5 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% mercaptoethanol, and 0.001% bromphenol blue. Equal amounts (20 μg) of cell lysates were electrophoresed in 4 to 12% polyacrylamide gradient gels. Proteins were transferred to a Hybond enhanced chemiluminescence nitrocellulose membrane (Amersham Co.) and blocked in Tris-buffered saline with 2.5% nonfat milk overnight at 4°C. After one wash in Tris-buffered saline with 0.05% Tween 20, the membrane was probed with anti-α3 (rabbit polyclonal, 1:1000; Chemicon), anti-α6A (mouse monoclonal, clone 1A10, 1:1000; Chemicon), anti-β1 (rabbit polyclonal, 1:1000; Chemicon) integrin antibodies, and anti-syndecan-1 (mouse mAb, clone B-B4, 1:100; Chemicon). Primary antibodies were detected by horseradish peroxidase-conjugated secondary antibodies (1:10,000) and developed using an enhanced chemiluminescence (ECL) substrate (Amersham Co.).
Real-Time PCR
RNA was collected after siRNA treatment using the TRIzol reagent (Invitrogen). Reverse transcription of total RNA (1 μg) with oligo dT (500 μg/ml), 10 mmol/L of each dNTP, 5× first-strand buffer, 0.1 mol/L dithiothreitol, and 200 U of reverse transcriptase (Moloney murine leukemia virus; Promega, Madison, WI) was performed at 70°C for 10 minutes followed by 42°C for 60 minutes and 10 minutes at 95°C. Quantitative reverse transcriptase-PCR was quantified with the ABI Prism 5700 sequence detector (Applied Biosystems, Foster City, CA). The PCR reactions contained 40 to 160 ng/μl cDNA in 25 μl of SYBR Green PCR master mix (Invitrogen) and 50 to 900 nmol/L primers (forward and reverse). Cycling conditions were as follows: 50°C for 2 minutes and 95°C for 10 minutes, followed by 50 cycles of 15 seconds at 95°C and 60 seconds at 60°C. The primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA), with the following sequences: human α3 integrin,36 5′-TAAGAGGCAGAAGGCGGAGATG-3′ (forward) and 5′-CCTGTGGACTGTCGAGGCATAAC-3′ (reverse); human α6 integrin,37 5′-AGATCCCGGCCTGTGATTAA-3′ (forward) and 5′-CCTGTGGACTGTCGAGGCATAAC-3′ (reverse); human β1 integrin (designed by Primer Express Software, Applied Biosystems), 5′-TGCAGTTTGTGGATCACTGATTG-3′ (forward) and 5′-CCTGTGGACTGTCGAGGCATAAC-3′ (reverse); human syndecan-1,38 5′-GGAGCAGGACTTCACCTTTG-3′ (forward) and 5′-CTCCCAGCACCTCTTTCCT-3′ (reverse); and 18S protein, 5′-GTAACCCGTTGAACCCCATT-3′ (forward) and 5′-CCATCCAATCGGTAGTAGCG-3′ (reverse). Accession numbers for human sequences to α3, α6, and β1 integrins, syndecan-1, and 18S rRNA are M59911, AF166343, X07979, J05392, and M11188, respectively. Results were expressed as the ratio of the mRNA level of each gene of interest normalized to 18S.
Peptide Affinity Chromatography
A carboxyl column (diaminodipropylamine/1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride; Pierce) with spacer arms was used rather than an amino column because the amino group of the lysine residue (K) in SIKVAV could result in improper peptide folding without oriented coupling. Diaminodipropylamine resin affinity columns (1 ml) were prepared (Pierce) according to the manufacturer’s instructions. A SIKVAV affinity column and negative control columns were run in parallel. An IVSKVA (scrambled) column was used as a peptide negative control. The columns were equilibrated in conjugation buffer containing 0.1 mol/L 2-(N-morpholino) ethanesulfonic acid and 0.9% NaCl, pH 4.7. The peptides (5 mg/ml) were diluted in conjugation buffer, and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (Pierce) was added; the peptides were coupled to the columns for 3 hours and then washed with 1 mol/L NaCl.
A crude cell membrane fraction was obtained from CAC2 cells using a Mem-PER Eukaryotic Membrane Protein Extraction Reagent kit (Pierce) according to the manufacturer’s instructions: Cells (5 × 106) were centrifuged at 850 × g for 2 minutes, and the pellet was lysed with a proprietary detergent from the kit. A second proprietary detergent was added to solubilize the membrane proteins. Samples were centrifuged at 10,000 × g for 3 minutes at 4°C. The supernatant was removed, incubated for 10 minutes at 37°C, and centrifuged for 2 minutes at 10,000 × g to separate the hydrophobic proteins (bottom layer) from the hydrophilic proteins (top layer) through phase partitioning. The hydrophobic fraction was dialyzed against two changes of 0.1% sodium deoxycholate overnight at 4°C. A 500-μl aliquot of the crude cell membrane fraction (300 μg of total protein) was incubated with the peptide affinity column for 2 to 4 hours at 4°C. SIKVAV and negative control columns were run in parallel. An IVSKVA (SIKVAV scrambled) column was used as a peptide negative control, and a BSA column was used as a protein negative control. The columns were washed with a buffer containing 1% Triton X-100, 20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 1 mmol/L MgCl2 and sequentially eluted with 1-ml aliquots of wash buffer containing SIKVAV (1 mg/ml), 20 mmol/L EDTA, 20 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1.0 mol/L NaCl, or 0.1 mol/L glycine, pH 3.0.
Material eluted from the peptide affinity columns was ethanol precipitated and separated by 4 to 12% SDS-polyacrylamide gel electrophoresis under reducing conditions. Gels were either silver-stained or transferred to nitrocellulose membranes. The membranes were incubated with anti-α1, -α3, or -β1 integrin rabbit polyclonal antibodies or with anti-α6 integrin monoclonal antibody (Chemicon). Primary antibodies were diluted 1:1000. Antibody-reactive material was detected with horseradish peroxidase-conjugated secondary antibodies (1:10,000) and visualized by enhanced chemiluminescence.
Mass Spectrometry
Material eluted from the affinity columns was separated by 4 to 12% SDS-polyacrylamide gel electrophoresis. Proteins were then detected by staining with Coomassie Blue R250 (Amersham Co.). Sample preparation for mass spectrometry was described elsewhere.39 Bands were excised from the gel, destained, and washed with 75 mmol/L ammonium bicarbonate in 40% ethanol, reduced with 5 mmol/L dithiothreitol in 25 mmol/L ammonium bicarbonate at 60°C for 30 minutes, and then alkylated by 55 mmol/L iodoacetamide in 25 mmol/L ammonium bicarbonate in a dark place for 30 minutes at room temperature. The gel was incubated in 10 μl of a 50 ng/μl modified trypsin solution (Promega) in 50 mmol/L ammonium bicarbonate, pH 8.6, and incubated at 37°C overnight. The resulting peptides were extracted first with a 1:1 solution of 25 mmol/L ammonium bicarbonate and acetonitrile and then twice with a 1:1 solution of 5% formic acid and acetonitrile. The extracted tryptic peptides were vacuum-concentrated (Vacufuge Concentrator 5301; Eppendorf, Westbury, NY) and resuspended with 15 to 20 μl of 5% formic acid for mass spectrometric analysis. Samples were analyzed in the Ettan matrix-assisted laser desorption ionization/time of flight (Amersham Biosciences, Piscataway, NJ). Peptide fingerprints were obtained and compared with a protein database (MascotSearch: http://www.matrix-science.com).
Integrin Immunoprecipitation and Solid-Phase Binding Assay
Integrin complexes with α3, α6, and β1 were isolated using immunoprecipitation on an affinity column (Seize protein A immunoprecipitation kit; Pierce). Columns were first coupled with antibodies to these integrins. Total lysates (1% octylglucoside in PBS with protease inhibitor cocktail) of CAC2 cells were centrifuged and applied to the columns, and the eluted fraction was analyzed by electrophoresis under nonreducing conditions to confirm integrin isolation. Integrin complexes from the eluted fractions (5 μg/ml) were adsorbed overnight in a 96-well plate. The wells were then blocked with BSA to avoid nonspecific binding. SIKVAV was biotinylated (EZ link sulfo-NHS-LC-biotinylation kit; Pierce) and incubated in the wells coated with the integrin complexes. Binding of the peptides to the integrin complexes was detected by a colorimetric method [extra avidin peroxidase followed by staining with 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)]. Intensity of the binding was analyzed in a spectrophotometer at 405 nm. Negative controls using biotinylated-BSA and a biotinylated-IVSKVA (scrambled) were run in parallel.
Analysis of ERK Activity in CAC2 Cells
The ERK pathway was analyzed by treating CAC2 cells (103) with a mitogen-activated protein kinase kinase-1 inhibitor U0126 (Cell Signaling Technology, Beverly, MA) over a range of concentrations (10 to 50 μmol/L). The cells were washed three times with PBS, serum-starved for 24 hours, and preincubated for 2 hours with U0126 before 100 μg/ml SIKVAV was added to the serum-free medium. After 24 hours, the conditioned medium was collected, concentrated, and analyzed by zymography as described above. From the same samples, cell lysates were immunoblotted with antibodies against ERK and phospho-ERK (rabbit polyclonal; Santa Cruz Biotechnology) to analyze the phosphorylation levels of ERK.
CAC2 cells were also cultured in serum-free conditions for 24 hours plated on top of SIKVAV (50 μg) or IVSKVA (50 μg) dried to the plate. In addition, CAC2 cells treated with siRNA to either α3 or α6 integrins or a nonsilencing control were also cultured on SIKVAV. Lysates were prepared, quantified, and immunoblotted for phospho-ERK and ERK as described above.
Statistical Analysis
Student’s t-test was performed to evaluate differences between two groups. Differences between three or more groups were assessed by analysis of variance, followed by Bonferroni’s multiple comparisons test. The software used was GraphPad Prism (GraphPad Software, Inc., San Diego, CA).
Results
Laminin α1 and MMPs Are Present in Adenoid Cystic Carcinoma Cells in Vivo and in Vitro
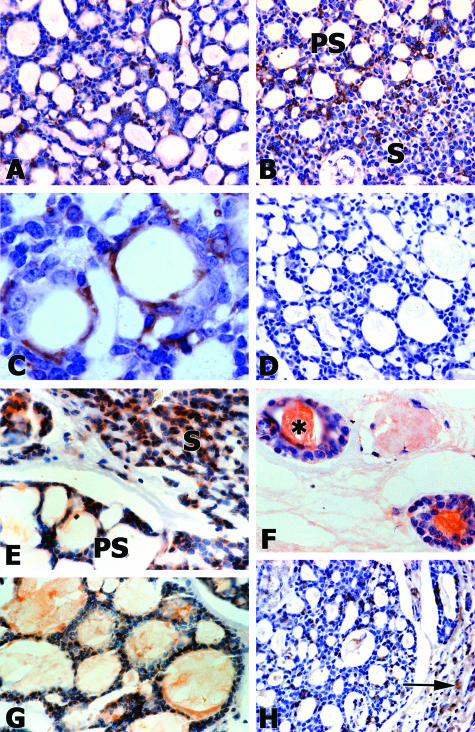
Laminin α1 chain was detected in adenoid cystic carcinoma in vivo (Figure 1, A–C). This protein was observed as a diffuse pattern throughout cells from the cribriform and solid subtypes (Figure 1, A and B). Laminin α1 chain was also visualized as a linear structure in the cribriform subtype (Figure 1C). Negative controls showed no staining in all samples observed (Figure 1D). MMP2 and MMP9 were detected in tubular, cribriform, and solid subtypes of adenoid cystic carcinoma in vivo (Figure 1, E–H). These enzymes were mostly located in the cytoplasm of tumor cells. We also observed MMP2 and MMP9 inside pseudocystic and luminal spaces. A discrete label was found in stromal regions. Negative controls showed no staining in all samples observed (not illustrated).
Figure 1.
Laminin α1, MMP2, and MMP9 are detected in adenoid cystic carcinomas. Laminin α1 appears as diffuse staining throughout the cytoplasm in cribriform and solid subtypes (A and B), with some cell surface staining that appears as a linear structure that is likely in the basement membrane in the cribriform subtype (C). Negative serum controls (D). MMP2 (E and F) and MMP9 (G and H) are observed in pseudocystic (PS), solid (S), and tubular subtypes of the tumor. MMP2 also fills tubular spaces (asterisk in F). MMP9 is present in pseudocystic spaces (G) and in stromal regions (arrow in H). Magnifications: ×200 (A, B, D, and H); ×400 (E and G); and ×600 (C and F).
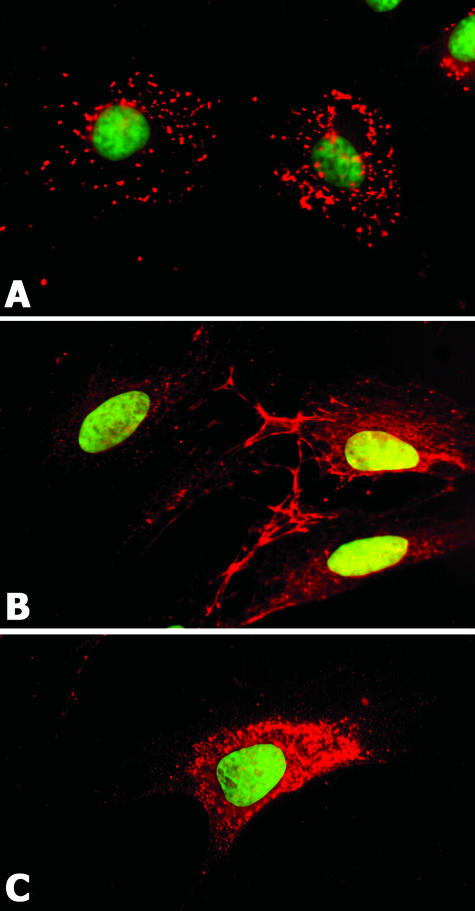
A cell line (CAC2) derived from adenoid cystic carcinoma expressed the laminin α1 chain. This protein was found as dots distributed throughout the cell membrane (Figure 2A). MMP2 and MMP9 were detected in CAC2 cells. MMP2 was present inside the cytoplasm and forming a prominent network at cell edges (Figure 2B). MMP9 showed a cytoplasmic localization in CAC2 cells (Figure 2C).
Figure 2.
CAC2 cells also express laminin α1, MMP2, and MMP9. Laminin α1 appears as punctate staining on the cell membrane (A). MMP2 appears in the cytoplasm and is prominent on the cell periphery (B). MMP9 appears in the cytoplasm (C). Nuclei are counterstained with Sytox green. Magnification, ×630.
SIKVAV Induces MMPs in CAC2 Cells
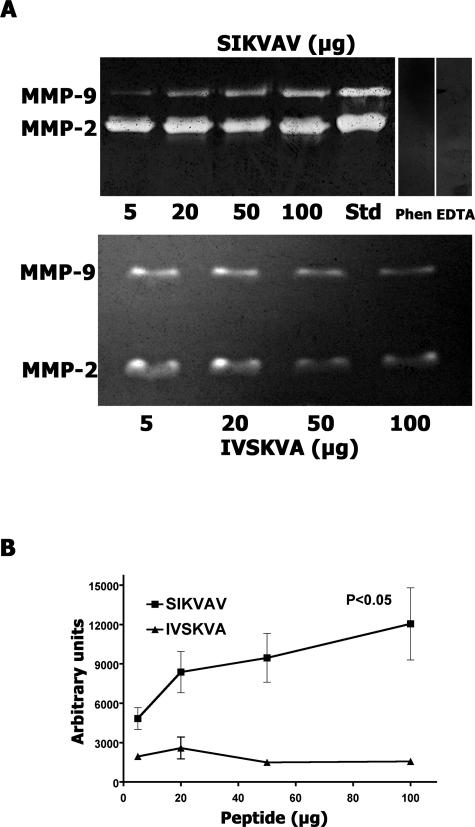
Zymography of the conditioned medium of CAC2 cells grown on different concentrations of SIKVAV showed gelatinolytic bands corresponding to the molecular weights of MMP2 and MMP9 (Figure 3A, top zymogram). MMP2- and MMP9-positive controls were analyzed on the same gel to confirm the result. The zymogram resolved only one MMP9 band, but both the latent and the active forms of MMP2 were observed. Cells grown on different concentrations of IVSKVA (scrambled peptide control) also produced MMP2 and MMP9 (Figure 3A, bottom zymogram). To determine whether these bands were MMP zymograms of conditioned medium from SIKVAV-treated CAC2 cells, cells were incubated in the presence of the calcium chelator EDTA and the heavy metal chelator 1,10-phenanthroline. Both of these treatments resulted in the loss of gelatinase activity, demonstrating that the gelatinolytic bands were MMPs (Figure 3A, top zymogram). Gel densitometry (Image J software; NIH, Bethesda, MD) of gelatinolytic bands showed that SIKVAV induced a significant dose-dependent increase of MMP-9 compared with the scrambled peptide control (Figure 3B). The volume of conditioned medium loaded on the zymogram gel was normalized to the amount of protein in the CAC2 cell lysate. Western blot analysis of β-actin in the CAC2 cells lysate confirmed that equal amounts of lysate were used to estimate the volume of conditioned medium (data not shown). Zymographic experiments were performed at least six times with consistent results.
Figure 3.
SIKVAV induces MMP9 in a dose-dependent manner. The conditioned medium of CAC2 cells cultured on SIKVAV or a scrambled (IVSKVA) control peptide (5, 20, 50, and 100 μg) were analyzed by zymography (A). MMP2- and MMP9-positive controls (Std) are included, and treatments with either 1,10-phenantroline or EDTA (negative controls) demonstrate that the bands are MMPs (A, top). The volume of conditioned medium loaded on the zymogram gel was normalized to the amount of protein in the CAC2 cell lysate. Gel densitometry of the zymograms shows the dose-dependent increase of MMP9 compared with control (B). Results represent a mean ± SEM of six experiments.
Integrins Interact with SIKVAV in CAC2 Cells
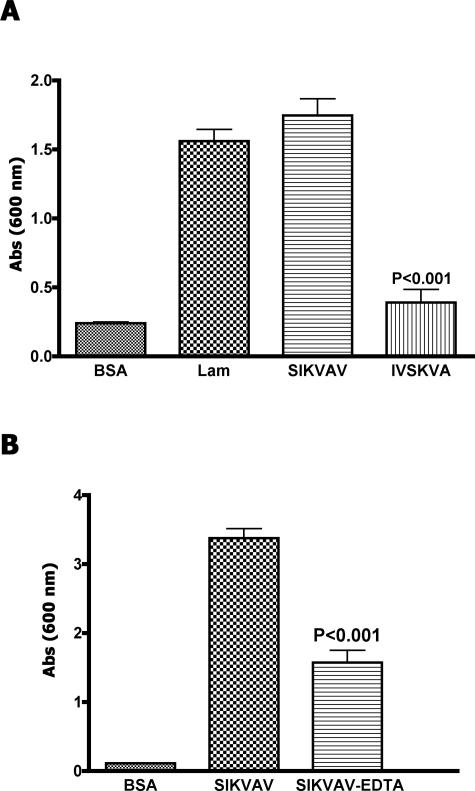
Adhesion assays demonstrated that SIKVAV is an adhesive peptide for CAC2 cells (Figure 4A). The adhesion induced by the peptide is similar to the adhesion induced by laminin-111. Cells grown on the scrambled peptide (IVSKVA) showed negligible adhesion. On the other hand, CAC2 cells treated with a calcium chelator (EDTA) exhibited decreased adhesion to SIKVAV (Figure 4B), suggesting that the adhesion of CAC2 cells to SIKVAV is calcium-dependent.
Figure 4.
CAC2 adhesion to SIKVAV is sensitive to EDTA. CAC2 cells adhere to laminin and SIKVAV but not to BSA and a scrambled peptide (IVSKVA) in a cell adhesion assay (A). EDTA decreases CAC2 adhesion to SIKVAV (B). The results (±SEM) are triplicate experiments performed at least three times.
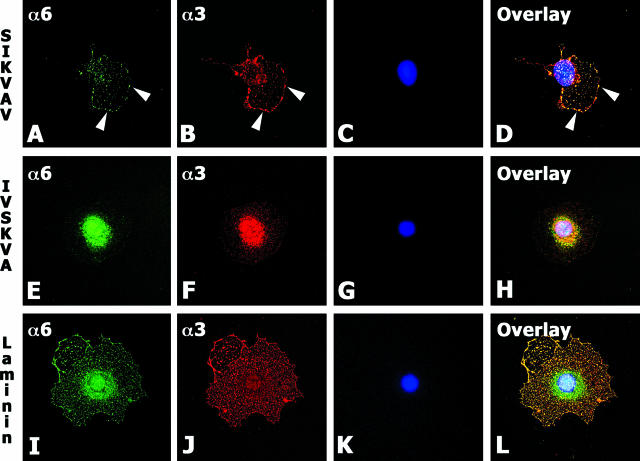
Integrins are divalent ion-dependent receptors; therefore, we investigated whether integrins from CAC2 cells could bind to SIKVAV. CAC2 cells cultured on SIKVAV for 4 hours clustered and colocalized α3 and α6 integrin subunits on the cell surface (Figure 5, A–D). This result was not observed in cells plated on scrambled peptide IVSKVA (Figure 5, E–H). The SIKVAV-induced colocalization of α3 and α6 integrins appeared as punctate staining that seems similar to podosomes (Figure 5, A, B, and D). Podosomes constitute dot-like matrix contacts that differ from focal contacts structurally and functionally.40 Structurally, their most distinguishing feature is their two-part architecture: they have a core of F-actin that is surrounded by a ring structure consisting of plaque proteins such as vinculin, paxillin, and talin.40,41 Functionally, the ability of podosomes to engage in matrix degradation clearly sets them apart from other cell-matrix contacts.40,41 CAC2 cells grown on SIKVAV showed that these podosome-like structures formed by α3 and α6 integrins also expressed vinculin (Supplemental Figure 1 at http://ajp.amjpathol.org).
Figure 5.
Integrins α3 and α6 colocalize on CAC2 cells cultured on SIKVAV and laminin-111. Immunofluorescence staining of CAC cells cultured on SIKVAV, IVSKVA, and laminin-111 shows α6 integrin (green in A, E, and I), α3 integrin (red in B, F, and J), and nuclei (blue in C, G, and K) counterstained with 4,6-diamidino-2-phenylindole. Colocalization appears yellow in the overlay image (D, H, and L). Cells grown on SIKVAV show discrete areas of colocalization (white arrowheads in D), whereas cells on laminin-111 show extensive overlap of α3 and α6 integrins (L). Magnification, ×630.
RNA Interference
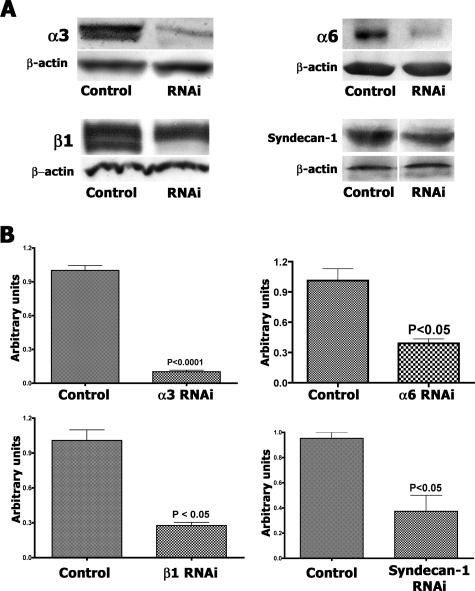
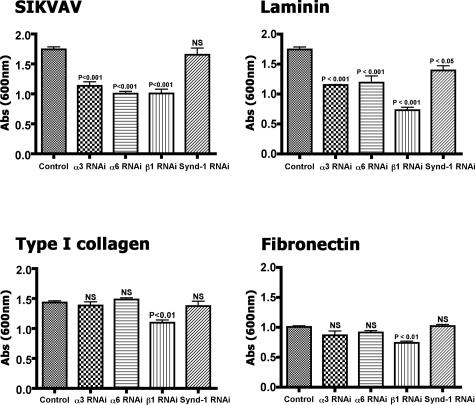
We decreased integrin expression in CAC2 cells with siRNAs to integrin subunits, which provided evidence that SIKVAV interacts with integrins. Immunoblot and quantitative PCR confirmed the efficiency of siRNA transfection and knockdown of both mRNA and protein levels (Figure 6). The integrin subunits α3 and α6 were our primary targets, but we also knocked down β1 integrin, because this molecule forms heterodimers with both α3 and α6 integrin to bind laminin. There was at least a 50% decrease in protein levels for all proteins targeted (Figure 6A) and an even greater decrease in the mRNA levels (Figure 6B). Analysis of knockdown was done after 30 hours of siRNA treatment, which is a relatively short time to evaluate protein knockdown. Although the Western blots for α3 and α6 integrin appear as single bands, the antibody used for the β1 integrin Western blot recognizes a doublet with different glycosylated β1 integrin isoforms: one is faster migrating on a gel and presumably less glycosylated, whereas the other is a fully glycosylated form. The siRNA resulted in a reduction of the less glycosylated isoforms first, which is apparent by 30 hours of treatment in the Western blot. Syndecan-1, a nonintegrin receptor, was silenced to study whether nonintegrin-dependent cell adhesion was involved or whether integrin adhesion to SIKVAV was specific. Cells with decreased receptors levels were submitted to adhesion assays to SIKVAV and to others substrates (Figure 7). CAC2 cells with decreased expression of either α3 or α6 integrins showed reduced adhesion to SIKVAV compared with controls (Figure 7, SIKVAV graph), and decreasing the β1 subunit showed a similar result. The decrease of syndecan-1 showed no effect on the adhesion of CAC2 cells to SIKVAV. This result suggests that the effect of α3, α6, and β1 siRNAs are specific and that other nonintegrin adhesion mechanisms are not affected. A decrease in the adhesion to laminin was observed in all groups of cells with silenced receptors (Figure 7, laminin graph), which is expected because laminin binds integrins and syndecan-1. Adhesion assays to type I collagen and fibronectin provided more information on the specificity of the interaction between SIKVAV and integrins, because α3 and α6 subunits do not bind these substrates. Silencing either α3 or α6 integrins produced no effect in the adhesion of CAC2 cells to type I collagen (Figure 7, type I collagen graph). The same effect was observed for cells grown on fibronectin (Figure 7, fibronectin graph). Taken together, the results observed in the adhesion assays in type I collagen and fibronectin strongly suggest that α3 and α6 integrins cooperate specifically with SIKVAV. Finally, the knockdown of β1 integrin decreased the adhesion of CAC2 cells to all substrates. This was expected, because this integrin binds laminin, type I collagen, and fibronectin. Furthermore, the experiment with CAC2 cells with decreased β1 expression also suggested that the heterodimers α3β1 and α6β1 might interact with SIKVAV. Therefore, we identified the putative receptors for SIKVAV with peptide affinity chromatography, mass spectrometry, and solid-phase binding assays.
Figure 6.
siRNA knockdown of α3, α6, and β1 integrins and syndecan-1 in CAC2 cells results in decreased protein and mRNA expression. CAC2 cells were cultured for 30 hours after siRNA treatment, and protein levels were analyzed by Western blot (A) of the integrin subunit as well as β-actin to show equal protein loading in the gel. There was at least 50% decrease in protein levels for all proteins targeted. After 30 hours of siRNA treatment, the less glycosylated β1 integrin isoform, which is faster migrating on a gel, is not detected whereas some fully glycosylated β1 remains. Quantitative PCR (B) confirms the knockdown of gene expression. The control is transfected with the nonsilencing siRNA. Quantitative PCR results (±SEM) are triplicates repeated at least three times.
Figure 7.
There is decreased CAC2 cell adhesion to SIKVAV after siRNA knockdown of α3, α6, and β1 integrins but not syndecan-1. The adhesion of CAC2 cells with decreased levels of α3, α6, and β1 integrins and syndecan-1 were compared with SIKVAV, laminin-111, type I collagen, and fibronectin. Decreasing either α3 or α6 integrins results in decreased adhesion to SIKVAV and laminin-111, whereas knockdown of β1 integrin reduces cell adhesion to all substrates. Decreasing syndecan-1 has no effect on adhesion to SIKVAV, type I collagen, or fibronectin. The control is transfected with the nonsilencing siRNA. Results (±SEM) are triplicates repeated at least three times.
Peptide Affinity Chromatography, Mass Spectrometry, and Solid-Phase Binding Assays Show That Integrins α3, α6, and β1 Interact with SIKVAV
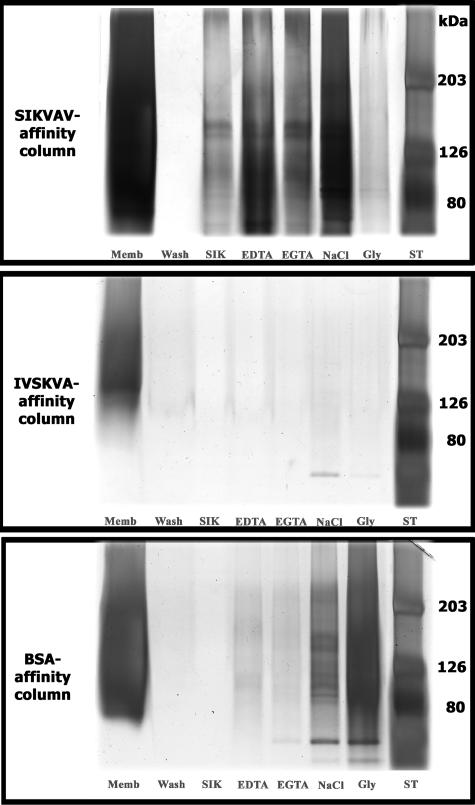
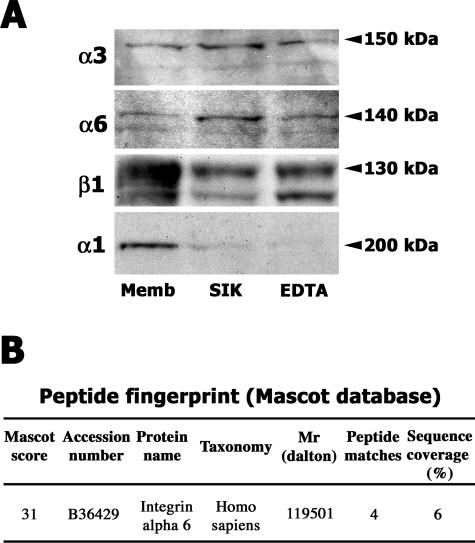
Crude cell membranes from CAC2 cells were prepared and chromatographed on SIKVAV affinity columns. Bound fractions eluted with SIKVAV were visualized by silver staining and appeared as a high-molecular weight smear with discrete bands with a molecular mass of ∼130 kd (Figure 8, top gel, lane SIK). Importantly, columns prepared with either a scrambled peptide, IVSKVA (Figure 8, middle gel), or BSA (Figure 8, bottom gel) identified no putative ligands when eluted with SIKVAV.
Figure 8.
SIKVAV affinity chromatography of CAC2 cell membranes identifies putative SIKVAV ligands. SIKVAV affinity columns were compared with columns with either IVSKVA (scrambled control) or BSA, and fractions eluted from the columns were visualized by silver staining after SDS-polyacrylamide gel electrophoresis. The fraction eluted with SIKVAV appears as a broad high-molecular weight smear with discrete bands of ∼130 kd (top gel, lane SIK). Similar bands are observed in fractions eluted with either EDTA or ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) (top gel). Fractions are also eluted with high salt (NaCl) and glycine (Gly). ST, molecular weight standards.
Crude membrane- and SIKVAV-eluted fractions were immunoblotted with anti-α3, anti-α6, and anti-β1 integrin antibodies. Reactive bands at the expected molecular weights were observed in both crude membrane fraction and in the eluate (Figure 9A). The same bands appeared in EDTA-eluted fractions (Figure 9A). To assess the specificity of these reactive bands, the same membranes were stripped and reprobed with antibodies against the α1 integrin subunit, which also forms heterodimers with β1 integrin. The α1 integrin subunit was more predominant in the membrane fraction, suggesting that it is not a ligand for SIKVAV (Figure 9A), and appeared as a 200-kd band in the crude membrane fraction. Taken together, the affinity chromatography and immunoblot suggested that SIKVAV interacts with integrin heterodimers α3β1 and α6β1, either directly or in an adhesion complex. Mass spectrometry supported the result with affinity chromatography (Figure 9B; Supplemental Figure 2 at http://ajp.amjpathol.org). Coomassie-stained band eluted from SIKVAV affinity column was submitted to in-gel digestion with trypsin (Supplemental Figure 2 at http://ajp.amjpathol.org). Tryptic peptides were searched in Mascot database and matched with α6 integrin sequence (Figure 9B).
Figure 9.
Integrins α3, α6, and β1 are present in the fraction eluted by SIKVAV from the peptide affinity column (A). Western blot analysis identified α3, α6, and β1 integrins in the membrane preparation, in the SIKVAV-eluted fraction, and in EDTA-eluted fraction. The same membranes were reprobed for integrin α1 integrin, which is more predominant in the membrane fraction. Mass spectrometry also identified the α6 integrin in the fraction eluted by SIKVAV from the peptide affinity column (B). Coomassie-stained band eluted from SIKVAV affinity column was submitted to in-gel digestion with trypsin and analyzed by mass spectrometry, the tryptic peptides matched the α6 integrin sequence.
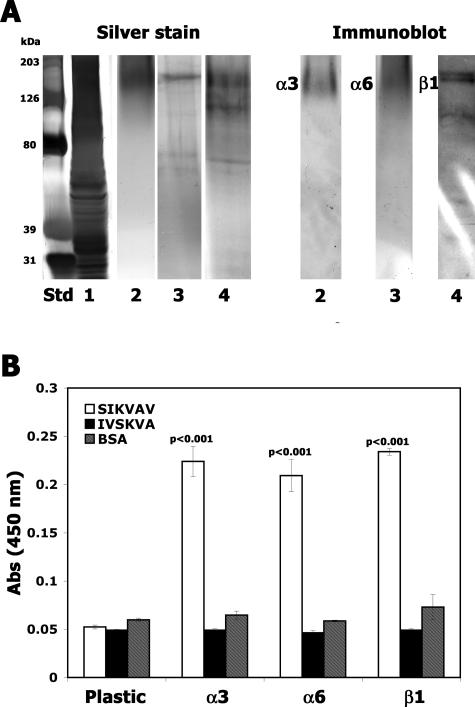
In addition, we immunoprecipitated integrin complexes and used them in solid-phase assays to corroborate the results of the affinity chromatography and mass spectrometry. We observed that biotinylated SIKVAV bound α3, α6 and β1 integrin complexes (Figure 10). Neither the scrambled peptide control (IVSKVA) nor the protein control (BSA) bound to integrin complexes. Taken together, affinity chromatography, mass spectrometry, and solid-phase assays strongly suggested that the integrin complexes containing α3β1 and α6β1 interact with SIKVAV.
Figure 10.
SIKVAV binds to integrin antibody IP complexes in a solid-phase binding assay. Integrin α3, α6, and β1 were immunoprecipitated from CAC2 cell lysates. A: A silver-stained gel and subsequent Western blot of lanes 2, 3, and 4 with anti-integrin antibodies. Lane Std, molecular weight marker; lane 1, whole-cell lysate; lane 2, IP with α3 integrin antibody; lane 3, IP with α6 integrin antibody; lane 4, IP with β1 integrin antibody. B: The IP complexes were adsorbed to plastic wells (5 μg/well), and a solid-phase assay with biotinylated SIKVAV was used to show that SIKVAV binds α3, α6, or β1 integrin antibody IP complexes and does not bind the plastic well. Biotinylated IVSKVA or BSA did not bind to integrin IP complexes. Solid-phase results (±SEM) are triplicates repeated at least three times.
Integrins Signaling Downstream of SIKVAV Increases MMP Production by CAC2 Cells
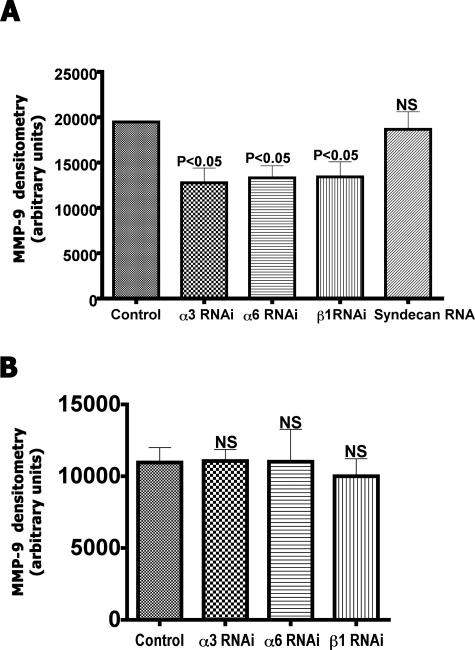
CAC2 cells treated with siRNA for integrin receptors were treated with SIKVAV, and the presence of MMPs in the conditioned medium was assessed by zymography. Silencing of α3, α6, or β1 integrin expression induced a significant decrease in protease activity (Figure 11A). These results suggest that downstream signaling from α3β1 and α6β1 are involved in regulating the SIKVAV-induced MMP2 production in CAC2 cells. Cells treated with siRNAs and the scrambled peptide control (IVSKVA) exhibited no differences in protease activity (Figure 11B).
Figure 11.
There is a significant decrease in SIKVAV-induced MMP9 activity with knockdown of α3, α6, and β1 integrins. CAC2 cells treated with siRNA to integrins were cultured with either SIKVAV (A) or the control peptide IVSKVA (B), and the MMP9 activity was measured by zymography. These results suggest that α3β1 and α6β1 signaling affects protease activity in CAC2 cells. The control is transfected with the nonsilencing siRNA. Densitometric results (±SEM) are six lanes of zymography combined and repeated at least three times. NS, not significant.
The ERK Pathway Is Downstream of SIKVAV and Integrins in CAC2 Cells
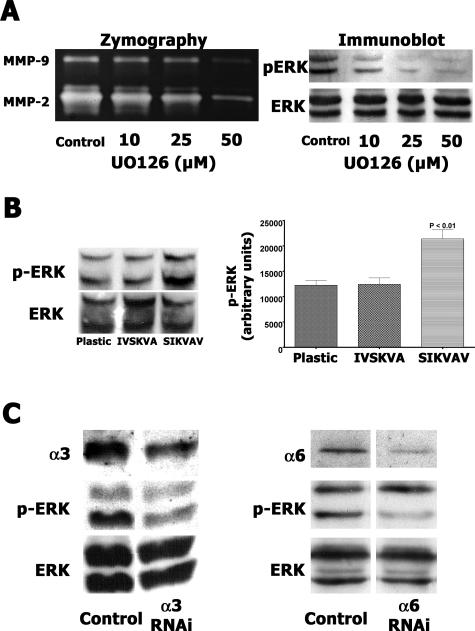
The mitogen-activated protein kinase kinase-specific inhibitor UO126 was used to analyze the role of ERK signaling during SIKVAV-induced protease production in CAC2 cells. A dose-dependent decrease of MMP2 and MMP9 activities was observed in CAC2 cells treated by UO126 (Figure 12A, zymography panel), suggesting that baseline levels of MMP production in CAC2 cells require ERK signaling. Immunoblot analysis of phospho-ERK showed that the inhibitory effect induced by UO126 resulted in decreased ERK phosphorylation (Figure 12A, immunoblot panel). We also measured a twofold increase in ERK 1/2 phosphorylation compared with controls (Figure 12B) in CAC2 cells cultured on SIKVAV. In addition, CAC2 cells with reduced α3 or α6 integrin expression exhibited a decrease in ERK 1/2 phosphorylation when cultured on SIKVAV (Figure 12C). Therefore, we conclude that SIKVAV-induced adhesion complexes on CAC2 cells containing α3 and α6 integrins signal via the ERK pathway to increase MMP9 production.
Figure 12.
SIKVAV-induced MMP secretion in CAC2 cells is dependent on ERK signaling, and decreasing α3 and α6 integrin expression reduces SIKVAV-induced p-ERK. A: CAC2 cells were pretreated with U0126 (10, 25, and 50 μmol/L) for 2 hours before the addition of 100 μg/ml SIKVAV to the medium. A dose-dependent decrease of MMP activity and p-ERK levels are observed by zymography and Western blot analysis with U0126 treatment. A carrier control group (A) was treated with methanol. B: SIKVAV induces p-ERK twofold in CAC2 cells compared with cells cultured on plastic or scrambled peptide IVSKVA. The p-ERK Western blot analysis was quantitated and normalized to total ERK levels. The graph represents triplicates (±SEM) repeated at least three times. C: Decreased levels of α3 and α6 integrins result in reduced p-ERK levels after SIKVAV treatment. Cells transfected with nonsilencing siRNA are the controls.
Discussion
We have previously demonstrated that laminin-111 and SIKVAV regulate the morphology and protease activity of an adenoid cystic carcinoma cell line (CAC2).11,12 Here, we investigate the mechanisms regulating MMP9 production in these cells. Laminin-111 (formerly laminin-1) is composed of α1, β1, and γ1 chains,15 and the α1 chain contains the SIKVAV peptide. Laminin α1 is expressed in vivo in adenoid cystic carcinoma and in CAC2 cells in vitro and is present in both normal and neoplastic salivary glands.6,42 Immunolocalization of the α1 chain in adenoid cystic carcinoma appears in both the basement membrane (described as a linear pattern) and as diffuse staining distributed throughout the tumor. The diffuse nonlinear distribution may suggest a breakdown of the basement membrane present in the neoplasm. Disruption of the basement membrane in the invading area of adenoid cystic carcinoma has been reported.28 We also observed expression of MMP2 and MMP9 at similar locations to laminin α1 in adenoid cystic carcinoma in vivo. Laminins are substrates for MMPs, and adenoid cystic carcinoma cells secrete laminin, which could be processed by MMPs, resulting in the formation of laminin fragments containing bioactive peptides and cryptic sites.18,19,20,21 Many bioactive peptides have been shown to regulate cell behavior,22,23,24,43,44,45,46 and cryptic domains contained in the ECM are exposed by proteolysis and stimulate biological responses.18,19,20,21 The growing number of activities found to be buried in the extracellular matrix structural scaffold suggests that cryptic sites might be part of a general strategy adopted during evolution for controlling cell function.18,19,20,21 Cryptic sites are probably a means for positioning instructional cues to be used by cells during tissue organization, repair, or remodeling processes.20 Such a strategy offers the advantage that extracellular cues can be kept masked until their presence is required. This precludes the need for inhibition or blockage of an activity that is not needed at a particular point in time.20 Understanding the complexity and the cryptic nature of the extracellular matrix will probably uncover information that can be used to address many different physiological and pathological conditions.
Among the cryptic sites of the extracellular matrix, laminin-derived peptides are involved in different biological activities, including cell adhesion, spreading, growth, neurite outgrowth, tumor metastasis, and MMP secretion.22,23,26,43,44,46,47,48,49,50,51,52 Several active sequences on laminin-111 have been identified using proteolytic fragments, recombinant proteins, and systematic peptide screening.53,54,55 The YIGSR sequence located on the β1 chain promotes cell adhesion and migration and inhibits angiogenesis and metastasis.45,48,56 The PDGSR and F-9 (RYVVLPR) sequences located on the β1 chain also promote cell adhesion.44,47 SIKVAV and AG73, from the α1 chain, are involved in cell adhesion, proliferation, neurite outgrowth, acinar formation, and protease activity.11,12,18,22,46,52 Furthermore SIKVAV also induces angiogenesis and experimental metastasis.23,26,49 This peptide is used as soluble ligand in competitive assays for laminin-mediated cell attachment.52,57 Our experiments showed similar results using SIKVAV either as soluble ligand or as immobilized factor. Thus, our rationale for investigating the function of SIKVAV in our assays was to mimic a microenvironment in which CAC2 cells are exposed to a cryptic but bioactive peptide from laminin α1. CAC2 cells cultured on SIKVAV showed a dose-dependent increase of MMP secretion. SIKVAV is involved in different biological functions, such as protease activity11,22,24,58; however, the mechanisms underlying SIKVAV activity remain elusive. This prompted us to identify the putative receptors for SIKVAV and their signaling pathway in CAC2 cells.
Integrins are likely candidates to interact with laminin-derived peptides such as SIKVAV. We have already shown that integrins influence CAC2 cell interactions with laminin. Inhibiting the function of α3β1 integrin, a receptor expressed in adenoid cystic carcinoma in vivo,3 decreased the attachment of CAC2 cells to laminin.11 Furthermore, this experimental approach inhibited laminin- and SIKVAV-induced morphological changes in these cells.11 Thus we decided to study whether integrins from CAC2 cells would cooperate with SIKVAV. Integrins are a large family of adhesion receptors composed of two transmembrane glycoprotein subunits designated α and β.59 They bind laminins and many other ECM ligands, and modulate intracellular signaling pathways in response to this binding.60
CAC2 cells express multiple integrins, and adhesion to SIKVAV induces colocalization of α3 and α6 integrins on the cell surface potentially clustered in an adhesion complex. siRNA knockdown of integrins decreased adhesion to SIKVAV, whereas knockdown of the nonintegrin receptor syndecan-1 showed no effect on the adhesion to SIKVAV but did decrease adhesion to laminin-111, showing that knockdown of different types of cell adhesion receptors had specific effects. This was further supported by use of adhesion of cells to substrates where binding is α3 and α6 integrin-independent, such as type I collagen and fibronectin. Silencing either α3 or α6 integrin did not affect adhesion of CAC2 cells to either type I collagen or fibronectin, whereas decreasing β1 decreased adhesion to all substrates. Decreasing expression of individual integrin subunits did not completely abolish cell adhesion to SIKVAV, suggesting that one integrin isoform may compensate for the other or that there may be nonintegrin components in the adhesion complex that are not affected.
The mass spectrometry analysis detected only the α6 integrin subunit in the SIKVAV eluate. The hydrophobic nature of membrane proteins keep them consistently underrepresented in proteomic analysis of samples from one-dimensional gel electrophoresis.61 Both α3 and α6 integrins could be at the same molecular weight in the gel from the SIKVAV-eluted fraction, as demonstrated by affinity chromatography. However, peptide fingerprint detected only one integrin subunit. Two-dimensional electrophoresis may enhance our results, although this approach has major obstacles. Many hydrophobic proteins are not solubilized in the nondetergent isoeletric focusing sample buffer.61 Furthermore, solubilized proteins are prone to precipitation at their isoeletric point.61 It is important to emphasize that mass spectrometry did confirm one of the results obtained with a variety of other experimental approaches.
Our results suggest that α3 and α6 integrins interact with SIKVAV; however, we cannot exclude the possibility that the interaction is not direct, and these integrins are associated with other molecules, forming an ad-hesion complex that binds the peptide. SIKVAV has been widely studied, and other receptors have been identified in other cell types. Others have identified a 110-kd nonintegrin cell surface laminin-binding protein which recognized the SIKVAV-containing peptide PA22-2 (CSARKQAASIKVAVSADR).62 Sequence analysis from the amino terminus yielded an unexpected identity to nucleolin, a major 110-kd nuclear phosphoprotein.62 Our results suggest that α3, α6, and β1 integrins are eluted from the column whereas other integrins such as α1 integrin do not preferentially bind SIKVAV. However, our data show that EDTA only partially inhibits adhesion of CAC2 cells to this peptide (Figure 4). This result would suggest that integrins and nonintegrin receptors could be involved in the attachment of CAC2 cells to the peptide. We investigated whether other molecules could potentially interact with SIKVAV using competition experiments treating CAC2 cells with either chondroitin sulfate A or heparin followed by adhesion assays to SIKVAV. These treatments significantly decreased the adhesion of CAC2 cells to SIKVAV (Supplemental Figure 3 at http://ajp.amjpathol.org). Thus SIKVAV may associate with heparan sulfate/chondroitin sulfate-containing proteoglycans that complex with integrins. Our column chromatography shows that a high-molecular weight smear elutes with the integrins, and the immunoprecipitation (IP) of integrin complexes for use in the solid-phase assay could also contain other components. Proteoglycans can interact with numerous extracellular ligands, including adhesion molecules, extracellular proteases, protease inhibitors, growth factors, and extracellular matrix proteins.63 Our results with CAC2 cells with silenced syndecan-1 suggested that this receptor does not interact with SIKVAV. However, other syndecan isoforms and proteoglycans need to be evaluated as potential components in the integrin-containing adhesion complex that binds SIKVAV on CAC2 cells.
Integrins modulate cell behavior in response to a range of events largely by generating signals via their cyto-plasmic tails.64 Among the many downstream effects, integrin-mediated signals activate signal transduction pathways terminating in the synthesis of MMPs.64 Matrix metalloproteinases are key players in these processes and are regulated by integrins via ERK1/2. The interaction between integrins and MMPs has been investigated in many models, and a large body of evidence supports the role played by integrins regulating MMP2 and MMP9.65,66 The activation of ERK1/2 is a major signaling pathway by which integrins regulate gene expression.18,58 In addition to extracellular matrix-integrin interactions, ERK1/2 is activated by ligand binding to tyrosine kinase receptors, cytokines, and G-coupled receptors.18,58 Activated ERK1/2 regulates different transcription factors that play an important role in physiological and pathological processes, and include embryogenesis, wound healing, and tumor progression.18,58,64 In CAC2 cells, SIKVAV interactions with α3β1 and α6β1 integrins increase ERK phosphorylation twofold and increase MMP production. Reducing integrin levels with siRNA decreased ERK phosphorylation and SIKVAV-mediated MMP production. Taken together, these results suggest that SIKVAV-induced MMP expression requires signals transduced by integrins and the ERK pathway.
Extracellular proteolytic modifications of laminin play a critical role in determining important domains of this molecule.67 Biochemical assays isolating proteolytic fragments obtained through controlled enzymatic digestion of laminin have been widely used to elucidate the influence of individual domains on cellular behavior. Elastase digestion of laminin has shown that SIKVAV is located in the fragment E8.68 This fragment has been shown to control diverse biological processes such as cell adhesion, migration, and proliferation.67 The E8 fragment binds different integrins and nonintegrin molecules, such as dystroglycan, heparin, and syndecan.46,69 Further experiments are required to determine whether laminin fragments regulate MMP production in CAC2 cells. SIKVAV binding to cellular receptors can initiate signal transduction pathways that alter matrix metalloproteinase expression.67 The enzymes induced may participate in limited proteolytic modification of laminin present in the pericellular matrix, releasing cryptic peptides. Furthermore, proteolytic removal of specific functional domains may have dramatic effects on cellular interactions that control adhesion, motility, and invasion of CAC2 cells.
Spreading of malignant tumors involves a large number of molecules, including proteolytic enzymes, adhesion molecules and other membrane receptors, cytokines, and growth factors. A complex system of signal transduction pathways relays messages from the extracellular matrix via membrane receptors and intracellular molecules to the nucleus, resulting in the activation of transcription factors and synthesis of different genes. It is evident that understanding the interactions between host and cancer cells is crucial for the study of cancer pathogenesis. Based on our experimental findings, we propose that cells from adenoid cystic carcinoma (CAC2) bind SIKVAV and signal through α3β1 and α6β1 integrins via an ERK1/2 pathway, resulting in increased secretion of MMP9. The functional consequences of increased MMP9 production for CAC2 cells remain to be elucidated.
Supplementary Material
Footnotes
Address reprint requests to Ruy G. Jaeger, Universidade de São Paulo, Instituto de Ciências Biomédicas, Departamento de Biologia Celular e do Desenvolvimento, Av. Prof. Lineu Prestes 1524, Ed Biomédicas 1, sala 405, São Paulo SP 05508-900 Brazil. E-mail: rgjaeger@usp.br.
Supported by The State of São Paulo Research Foundation (FAPESP grants 2002/04208-00, 2003/02724-4, and 02/12387-2) and the Brazilian Research Council (CNPq grant 471751/2003-0).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Seifert G, Sobin LH. The World Health Organization’s Histological Classification of Salivary Gland Tumors: a commentary on the second edition. Cancer. 1992;70:379–385. doi: 10.1002/1097-0142(19920715)70:2<379::aid-cncr2820700202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dardick I. Salivary Gland Tumor Pathology. New York: Igaku-Shoin,; 1996 [Google Scholar]
- Loducca SV, Raitz R, Araujo NS, Araujo VC. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: distinct architectural composition revealed by collagen IV, laminin and their integrin ligands (α2β1 and α3β1). Histopathology. 2000;37:118–123. doi: 10.1046/j.1365-2559.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Irie T, Munakata R, Kimura S, Nakamura H, He RG, Lui AR, Saku T. Biosynthesis of basement membrane molecules by salivary adenoid cystic carcinoma cells: an immunofluorescence and confocal microscopic study. Virchows Arch. 1995;426:577–586. doi: 10.1007/BF00192112. [DOI] [PubMed] [Google Scholar]
- Cheng J, Saku T, Okabe H, Furthmayr H. Basement membranes in adenoid cystic carcinoma. An immunohistochemical study. Cancer. 1992;69:2631–2640. doi: 10.1002/1097-0142(19920601)69:11<2631::aid-cncr2820691103>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Raitz R, Martins MD, Araujo VC. A study of the extracellular matrix in salivary gland tumors. J Oral Pathol Med. 2003;32:290–296. doi: 10.1034/j.1600-0714.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- Capuano AC, Jaeger RG. The effect of laminin and its peptide SIKVAV on a human salivary gland myoepithelioma cell line. Oral Oncol. 2004;40:36–42. doi: 10.1016/s1368-8375(03)00130-1. [DOI] [PubMed] [Google Scholar]
- de Oliveira PT, Jaeger MM, Miyagi SP, Jaeger RG. The effect of a reconstituted basement membrane (matrigel) on a human salivary gland myoepithelioma cell line. Virchows Arch. 2001;439:571–578. doi: 10.1007/s004280000380. [DOI] [PubMed] [Google Scholar]
- França CM, Jaeger MM, Jaeger RG, Araujo NS. The role of basement membrane proteins on the expression of neural cell adhesion molecule (N-CAM) in an adenoid cystic carcinoma cell line. Oral Oncol. 2000;36:248–252. doi: 10.1016/s1368-8375(99)00087-1. [DOI] [PubMed] [Google Scholar]
- França CM, Jaeger RG, Freitas VM, Araujo NS, Jaeger MM. Effect of N-CAM on in vitro invasion of human adenoid cystic carcinoma cells. Oral Oncol. 2001;37:638–642. doi: 10.1016/s1368-8375(01)00007-0. [DOI] [PubMed] [Google Scholar]
- Freitas VM, Jaeger RG. The effect of laminin and its peptide SIKVAV on a human salivary gland adenoid cystic carcinoma cell line. Virchows Arch. 2002;441:569–576. doi: 10.1007/s00428-002-0678-x. [DOI] [PubMed] [Google Scholar]
- Freitas VM, Scheremeta B, Hoffman MP, Jaeger RG. Laminin-1 and SIKVAV a laminin-1-derived peptide, regulate the morphology and protease activity of a human salivary gland adenoid cystic carcinoma cell line. Oral Oncol. 2004;40:483–489. doi: 10.1016/j.oraloncology.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Jaeger MM, Araujo VC, Kachar B, Jaeger RG. Effect of spatial arrangement of the basement membrane on cultured pleomorphic adenoma cells: study by immunocytochemistry and electron and confocal microscopy. Virchows Arch. 1997;430:467–477. doi: 10.1007/s004280050057. [DOI] [PubMed] [Google Scholar]
- Jaeger RG, de Oliveira PT, Jaeger MM, de Araujo VC. Expression of smooth-muscle actin in cultured cells from human plasmacytoid myoepithelioma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:663–667. doi: 10.1016/s1079-2104(97)90369-3. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ekblom P. Extracellular matrix and cell adhesion molecules in nephrogenesis. Exp Nephrol. 1996;4:92–96. [PubMed] [Google Scholar]
- Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526–532. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Faisal Khan KM, Laurie GW, McCaffrey TA, Falcone DJ. Exposure of cryptic domains in the alpha 1-chain of laminin-1 by elastase stimulates macrophages urokinase and matrix metalloproteinase-9 expression. J Biol Chem. 2002;277:13778–13786. doi: 10.1074/jbc.M111290200. [DOI] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 2003;13:366–375. doi: 10.1016/s0962-8924(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran ML, Kibbey MC, Kleinman HK, Wahl LM. Laminin SIKVAV peptide induction of monocyte/macrophage prostaglandin E2 and matrix metalloproteinases. J Biol Chem. 1995;270:10365–10368. doi: 10.1074/jbc.270.18.10365. [DOI] [PubMed] [Google Scholar]
- Grant DS, Kinsella JL, Fridman R, Auerbach R, Piasecki BA, Yamada Y, Zain M, Kleinman HK. Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J Cell Physiol. 1992;153:614–625. doi: 10.1002/jcp.1041530324. [DOI] [PubMed] [Google Scholar]
- Khan KM, Falcone DJ. Selective activation of MAPK(erk1/2) by laminin-1 peptide alpha1:Ser: (2091)-Arg(2108) regulates macrophage degradative phenotype. J Biol Chem. 2000;275:4492–4498. doi: 10.1074/jbc.275.6.4492. [DOI] [PubMed] [Google Scholar]
- Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992;84:1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- Royce LS, Martin GR, Kleinman HK. Induction of an invasive phenotype in benign tumor cells with a laminin A-chain synthetic peptide. Invasion Metastasis. 1992;12:149–155. [PubMed] [Google Scholar]
- Sephel GC, Tashiro KI, Sasaki M, Greatorex D, Martin GR, Yamada Y, Kleinman HK. Laminin A chain synthetic peptide which supports neurite outgrowth. Biochem Biophys Res Commun. 1989;162:821–829. doi: 10.1016/0006-291x(89)92384-x. [DOI] [PubMed] [Google Scholar]
- Shintani S, Alcalde RE, Matsumura T, Terakado N. Extracellular matrices expression in invasion area of adenoid cystic carcinoma of salivary glands. Cancer Lett. 1997;116:9–14. doi: 10.1016/s0304-3835(97)04730-7. [DOI] [PubMed] [Google Scholar]
- Kasai S, Ohga Y, Mochizuki M, Nishi N, Kadoya Y, Nomizu M. Multifunctional peptide fibrils for biomedical materials. Biopolymers. 2004;76:27–33. doi: 10.1002/bip.10565. [DOI] [PubMed] [Google Scholar]
- Almiñana N, Grau-Oliete MR, Reig F, Rivera-Fillat MP. In vitro effects of SIKVAV retro and retro-enantio analogues on tumor metastatic events. Peptides. 2004;25:251–259. doi: 10.1016/j.peptides.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Almiñana N, Alsina MA, Espina M, Reig F. Synthesis and physicochemical study of the laminin active sequence: SIKVAV. J Colloid Interface Sci. 2003;263:432–440. doi: 10.1016/s0021-9797(03)00344-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Suzuki Y, Tanihara M, Kakimaru Y, Suzuki K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials. 2004;25:1407–1414. doi: 10.1016/j.biomaterials.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tong YW, Shoichet MS. Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials. 2001;22:1029–1034. doi: 10.1016/s0142-9612(00)00338-0. [DOI] [PubMed] [Google Scholar]
- Grant DS, Zukowska Z. Revascularization of ischemic tissues with SIKVAV and neuropeptide Y (NPY). Adv Exp Med Biol. 2000;476:139–154. doi: 10.1007/978-1-4615-4221-6_12. [DOI] [PubMed] [Google Scholar]
- Hogervorst F, Admiraal LG, Niessen C, Kuikman I, Janssen H, Daams H, Sonnenberg A. Biochemical characterization and tissue distribution of the A and B variants of the integrin α6 subunit. J Cell Biol. 1993;121:179–191. doi: 10.1083/jcb.121.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M, Fujita H, Ida H, Hoshina H, Inoue T, Seki Y, Ohnishi M, Ohyama T, Shingaki S, Kaji M, Saku T, Takagi R. Identification of potential biomarkers of lymph node metastasis in oral squamous cell carcinoma by cDNA microarray analysis. Int J Cancer. 2003;106:683–689. doi: 10.1002/ijc.11283. [DOI] [PubMed] [Google Scholar]
- Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004;145:3473–3486. doi: 10.1210/en.2003-1682. [DOI] [PubMed] [Google Scholar]
- Sun H, Berquin IM, Edwards IJ. Omega-3 polyunsaturated fatty acids regulate syndecan-1 expression in human breast cancer cells. Cancer Res. 2005;65:4442–4447. doi: 10.1158/0008-5472.CAN-04-4200. [DOI] [PubMed] [Google Scholar]
- Lim H, Eng J, Yates JR, 3rd, Tollaksen SL, Giometti CS, Holden JF, Adams MW, Reich CI, Olsen GJ, Hays LG. Identification of 2D-gel proteins: a comparison of MALDI/TOF peptide mass mapping to μ LC-ESI tandem mass spectrometry. J Am Soc Mass Spectrom. 2003;14:957–970. doi: 10.1016/s1044-0305(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Linder S, Kopp P. Podosomes at a glance. J Cell Sci. 2005;118:2079–2082. doi: 10.1242/jcs.02390. [DOI] [PubMed] [Google Scholar]
- Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Yamashina S. Salivary gland morphogenesis and basement membranes. Anat Sci Int. 2005;80:71–79. doi: 10.1111/j.1447-073x.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- Kuratomi Y, Nomizu M, Tanaka K, Ponce ML, Komiyama S, Kleinman HK, Yamada Y. Laminin γ1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16–F10 mouse melanoma cells. Br J Cancer. 2002;86:1169–1173. doi: 10.1038/sj.bjc.6600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz AP, McCarthy JB, Zhao Q, Yi XY, Furcht LT. Definition of a sequence, RYVVLPR, within laminin peptide F-9 that mediates metastatic fibrosarcoma cell adhesion and spreading. Cancer Res. 1990;50:7612–7622. [PubMed] [Google Scholar]
- Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238:1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Hoffman MP, Nomizu M, Roque E, Lee S, Jung DW, Yamada Y, Kleinman HK. Laminin-1 and laminin-2 G-domain synthetic peptides bind syndecan-1 and are involved in acinar formation of a human submandibular gland cell line. J Biol Chem. 1998;273:28633–28641. doi: 10.1074/jbc.273.44.28633. [DOI] [PubMed] [Google Scholar]
- Charonis AS, Skubitz AP, Koliakos GG, Reger LA, Dege J, Vogel AM, Wohlhueter R, Furcht LT. A novel synthetic peptide from the B1 chain of laminin with heparin-binding and cell adhesion-promoting activities. J Cell Biol. 1988;107:1253–1260. doi: 10.1083/jcb.107.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, Ogle RC, Robey FA, Sasaki M, Martin GR, Yamada Y, Kleinman HK. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor. Biochemistry. 1987;26:6896–6900. doi: 10.1021/bi00396a004. [DOI] [PubMed] [Google Scholar]
- Kanemoto T, Reich R, Royce L, Greatorex D, Adler SH, Shiraishi N, Martin GR, Yamada Y, Kleinman HK. Identification of an amino acid sequence from the laminin A chain that stimulates metastasis and collagenase IV production. Proc Natl Acad Sci USA. 1990;87:2279–2283. doi: 10.1073/pnas.87.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Graf J, Iwamoto Y, Sasaki M, Schasteen CS, Yamada Y, Martin GR, Robey FA. Identification of a second active site in laminin for promotion of cell adhesion and migration and inhibition of in vivo melanoma lung colonization. Arch Biochem Biophys. 1989;272:39–45. doi: 10.1016/0003-9861(89)90192-6. [DOI] [PubMed] [Google Scholar]
- Kuratomi Y, Nomizu M, Nielsen PK, Tanaka K, Song SY, Kleinman HK, Yamada Y. Identification of metastasis-promoting sequences in the mouse laminin α1 chain. Exp Cell Res. 1999;249:386–395. doi: 10.1006/excr.1999.4497. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, Kleinman HK, Yamada Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264:16174–16182. [PubMed] [Google Scholar]
- Nomizu M, Kuratomi Y, Malinda KM, Song SY, Miyoshi K, Otaka A, Powell SK, Hoffman MP, Kleinman HK, Yamada Y. Cell binding sequences in mouse laminin α1 chain. J Biol Chem. 1998;273:32491–32499. doi: 10.1074/jbc.273.49.32491. [DOI] [PubMed] [Google Scholar]
- Nomizu M, Kuratomi Y, Ponce ML, Song SY, Miyoshi K, Otaka A, Powell SK, Hoffman MP, Kleinman HK, Yamada Y. Cell adhesive sequences in mouse laminin β1 chain. Arch Biochem Biophys. 2000;378:311–320. doi: 10.1006/abbi.2000.1828. [DOI] [PubMed] [Google Scholar]
- Nomizu M, Kuratomi Y, Song SY, Ponce ML, Hoffman MP, Powell SK, Miyoshi K, Otaka A, Kleinman HK, Yamada Y. Identification of cell binding sequences in mouse laminin gamma1 chain by systematic peptide screening. J Biol Chem. 1997;272:32198–32205. doi: 10.1074/jbc.272.51.32198. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Graf J, Sasaki M, Kleinman HK, Greatorex DR, Martin GR, Robey FA, Yamada Y. Synthetic pentapeptide from the B1 chain of laminin promotes B16F10 melanoma cell migration. J Cell Physiol. 1988;134:287–291. doi: 10.1002/jcp.1041340216. [DOI] [PubMed] [Google Scholar]
- Nomizu M, Utani A, Shiraishi N, Kibbey MC, Yamada Y, Roller PP. The all-d-configuration segment containing the IKVAV sequence of laminin A chain has similar activities to the all-l-peptide in vitro and in vivo. J Biol Chem. 1992;267:14118–14121. [PubMed] [Google Scholar]
- Khan KM, Falcone DJ. Role of laminin in matrix induction of macrophage urokinase-type plasminogen activator and 92-kDa metalloproteinase expression. J Biol Chem. 1997;272:8270–8275. doi: 10.1074/jbc.272.13.8270. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- Wu CC, Mac Coss MJ, Howell KE, Yates JR., III A method for the comprehensive proteomic analysis of membrane proteins. Nat Biotechnol. 2003;21:532–538. doi: 10.1038/nbt819. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Weeks BS, Cannon FB, Sweeney TM, Sephel GC, Clement B, Zain M, Olson MO, Jucker M, Burrous BA. Identification of a 110-kDa nonintegrin cell surface laminin-binding protein which recognizes an A chain neurite-promoting peptide. Arch Biochem Biophys. 1991;290:320–325. doi: 10.1016/0003-9861(91)90547-v. [DOI] [PubMed] [Google Scholar]
- Engbring JA, Hoffman MP, Karmand AJ, Kleinman HK. The B16F10 cell receptor for a metastasis-promoting site on laminin-1 is a heparan sulfate/chondroitin sulfate-containing proteoglycan. Cancer Res. 2002;62:3549–3554. [PubMed] [Google Scholar]
- Morgan MR, Thomas GJ, Russell A, Hart IR, Marshall JF. The integrin cytoplasmic-tail motif EKQKVDLSTDC is sufficient to promote tumor cell invasion mediated by matrix metalloproteinase (MMP)-2 or MMP-9. J Biol Chem. 2004;279:26533–26539. doi: 10.1074/jbc.M401736200. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Risberg B, Ben-Baruch G, Reich R. Coordinated expression of integrin subunits, matrix metalloproteinases (MMP), angiogenic genes and Ets transcription factors in advanced-stage ovarian carcinoma: a possible activation pathway? Cancer Metastasis Rev. 2003;22:103–115. doi: 10.1023/a:1022272204045. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Bergamini C, Fransvea E, Marinosci F, Quaranta V, Antonaci S. Human hepatocellular carcinoma (HCC) cells require both alpha3beta1 integrin and matrix metalloproteinases activity for migration and invasion. Lab Invest. 2001;81:613–627. doi: 10.1038/labinvest.3780270. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Stack MS. Proteolytic modification of laminins: functional consequences. Microsc Res Tech. 2000;51:238–246. doi: 10.1002/1097-0029(20001101)51:3<238::AID-JEMT4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Paulsson M, Deutzmann R, Timpl R, Dalzoppo D, Odermatt E, Engel J. Evidence for coiled-coil α-helical regions in the long arm of laminin. EMBO J. 1985;4:309–316. doi: 10.1002/j.1460-2075.1985.tb03630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.