Abstract
Cells undergo apoptosis in development, tissue homeostasis, and disease and are subsequently cleared by professional and nonprofessional phagocytes. There is now overwhelming evidence that phagocyte function is profoundly altered following apoptotic cell uptake, with consequences for the ensuing innate and adaptive immune response. Pathogens and tumors exploit the changes in macrophage function following apoptotic cell uptake. Here, we will outline the consequences of apoptotic cell phagocytosis and illustrate how apoptotic cells could be used to manipulate the immune response for therapeutic gain.
Apoptosis Happens All of the Time in All Places
Apoptosis is programed cell death usually associated with retention of plasma membrane integrity, condensation and cleavage of nuclear and cytoplasmic proteins, and cell shrinkage or the formation of apoptotic bodies. Apoptotic cells are rapidly engulfed by phagocytes in a process akin to macropinocytosis, coined efferocytosis (taken from the Latin effero, meaning to take to the grave).1 The effective clearance of apoptotic cells followed by replenishment of cells and tissues is essential for development, homeostasis, and response to injury.
During development organogenesis requires repeated remodeling, and cell turnover occurs at a staggering rate. Even tissues with low turnover rates in adults show extensive turnover during organogenesis, exemplified by the developing mammalian brain where up to 50% of cells are deleted.2 Deletion of unwanted cells is also critical for the development and maintenance of the innate and adaptive immune system. Greater than 1 × 1011 circulating neutrophils are eliminated each day, mostly from the blood by liver and spleen but also by in situ phagocytosis of apoptotic neutrophils that have migrated into tissues and been replaced in a process that leaves no obvious trace.3 Only 5% of developing thymocytes are exported as mature T cells as the vast majority undergo apoptosis in a process known as negative selection, which allows for removal of self-reactive and potentially autoimmune lymphocytes.4 Clearance defects in the negative selection process, as illustrated in dexamethasone-induced thymocyte apoptosis in mer(kd) mice, can lead to autoantibody production and autoimmunity.5
Effectively, almost every cell in our bodies is replaced during our lifetime and some many times over. This includes the deletion of red blood cells, or eryptosis, a special form of programed cell death that displays all features of apoptosis (except of course nuclear condensation) and occurs at a rate of 3000 cells per second. Another example is the shedding of intact cell fragments illustrated by work from Finnemann’s group,6 showing that photoreceptor rods continuously renew their light-sensitive outer segments with the onset of light. Rod shedding precedes a synchronized burst of retinal pigment epithelia phagocytosis, which rapidly clears shed photoreceptor outer segment fragments from the retina. Retinal pigment epithelial cells phagocytose more material over a lifetime than any other cell in the body. Clearance failure causes accumulation of undigested photoreceptor components associated with storage bodies containing lipofuscin associated with retinal disease including the development of age-related macular degeneration, which is the leading cause of blindness among the elderly.
Physiological changes associated with growth, age, or pregnancy can generate additional large numbers of apoptotic cells, and one striking example is the involuting mammary gland where mammary epithelial cells clear dying cells and restore the organ to pre-pregnancy conditions7 (further illustrated in Figure 1). Finally, tissue injury and ensuing inflammation are invariably associated with cell death and apoptosis of tissue cells or infiltrating cells of the immune system and have been described in numerous experimental models and human diseases.8,9 Given the incredible number of apoptotic cells generated and cleared in health and disease, it is not surprising that the process was initially thought to be immunologically inert.
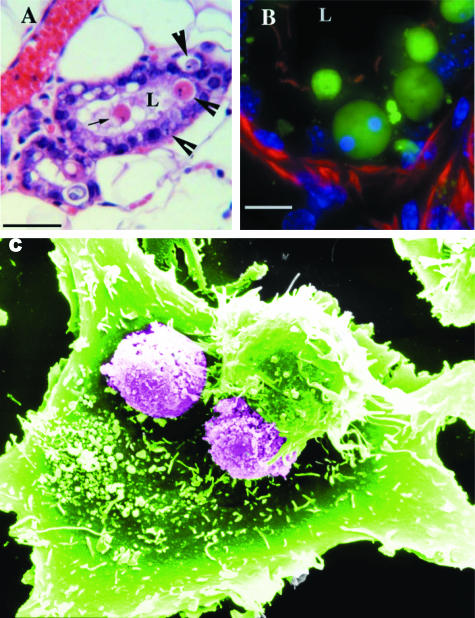
Figure 1.
Phagocytosis of apoptotic cells. A and B: Engulfment of apoptotic cells by mammary epithelial cells in vivo. A: H&E staining of paraffin-embedded tissue taken 3 days after weaning. The arrow shows an apoptotic cell shed into the lumen (L). Arrowheads point to apoptotic bodies, which appear to be contained within viable neighbors. Bar = 50 μm. B: Frozen section (8 μm thick) of mammary gland tissue harvested 3 days after weaning stained with Cytodeath, an antibody recognizing caspase-cleaved keratin-18, and counterstained with rhodamine-phalloidin and 4,6-diamidino-2-phenylindole. Optical slices through the tissue are shown as the maximum intensity projection of the data. Intact and fragmented apoptotic bodies within phagosomes in viable epithelial cells are shown. Bar = 10 μm. Kindly provided by Jen Monks, National Jewish Medical and Research Center, Denver, CO. C: Scanning electron micrograph obtained of phagocytosis of apoptotic eosinophils by a small airway bronchial epithelial cell. Two partially phagocytosed eosinophils are clearly visible by their globular surface features, whereas another is almost completely engulfed by an encroaching smooth small airway bronchial epithelial cell membrane. The membrane advances further to cover an adjacent eosinophil and projections of small airway bronchial epithelial cell membrane clearly extend around the apoptotic eosinophil. Original magnification, ×3200. Kindly provided by Drs. Garry Walsh and Darren Sexton, University of Aberdeen.
Inflammatory Mediator Production following Apoptotic Cell Uptake
Early studies supported the notion that apoptotic cell uptake is immunologically neutral, because of the lack of proinflammatory mediator production following uptake of apoptotic eosinophils and neutrophils, whereas postapoptotic eosinophils or opsonized neutrophils and zymosan particles induce production of granulocyte-macrophage colony-stimulating factor, N-acetyl-β-d-glucosaminidase, and thromboxane.10,11 However, there is now compelling evidence that binding or uptake of apoptotic cells to phagocytes induces production of transforming growth factor β (TGF-β) and in some model systems interleukin-10 in vitro.12,13 These anti-inflammatory cytokines have direct autocrine and paracrine effects on proinflammatory cytokine production as illustrated by inhibition of lipopolysaccharide-induced tumor necrosis factor α production.12 More recently, the importance of TGF-β signaling in the consequences of apoptotic cell uptake were examined in more detail: arachidonic acid release, cyclooxygenase 2, and prostaglandin synthase expression were shown to be dependent on TGF-β production as well as inhibition of thromboxane synthase, sulfidopeptide leukotrienes, nitric-oxide synthase, and nitric oxide.14
Interestingly, apoptotic cell uptake stimulates lipid mediators such as 15-lipoxygenase and 15-hydroxyeicosatetraenoic acid, acting through peroxisome proliferator-activated receptor-γ and inducing lipoxin A4 production, which enhances uptake of apoptotic cells by phagocytes15 and, together with resolvins and protectins, dominates the resolution phase of the inflammatory response.16 In addition to autocrine and paracrine effects mediated through cytokines and lipid mediators, Ucker and colleagues17 have described direct effects of apoptotic cells on the proinflammatory transcriptional machinery of macrophages. They show that apoptotic cell binding causes immediate-early inhibition of proinflammatory cytokine gene transcription independent of subsequent engulfment and soluble factors.
Nonprofessional phagocytes such as endothelial or epithelial cells that phagocytose neighboring apoptotic cells subsequently produce survival and growth factors such as vascular endothelial growth factor and hepatocyte growth factor,18,19 which probably contribute to tissue replenishment and restoration of endothelial and epithelial boundaries. Interestingly, recent work in the model organism Drosophila suggests that the secretory factors decapentaplegic (a TGF-β homolog) and wingless are directly produced by cells undergoing apoptosis and induce signaling cascades for compensatory proliferation of neighboring cells20 (illustrated in Figure 2). In this context, it is important to underline the role of nonprofessional phagocytes such as airway epithelial cells in the lung or mesangial cells in the kidney, which play an important role in the clearance process. Chronic lung disease, including cystic fibrosis and chronic obstructive pulmonary disease, are characterized by increased numbers of apoptotic cells, and this is not just a consequence of increased induction of apoptosis but also because of impaired clearance by airway epithelial cells.8
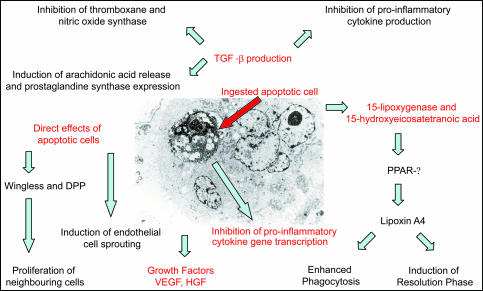
Figure 2.
Inflammatory mediator release in the context of apoptotic cell phagocytosis. Electron micrograph of a apoptotic endothelial cell ingested by a viable neighboring endothelial cell and diagram of inflammatory mediators produced in the context of this process. Kindly provided by Mike Greaves and Isobel Ford, University of Aberdeen.
In contrast to the countless reports detailing the anti-inflammatory consequences of apoptotic cell uptake, a small number of studies that cannot be disregarded show that very early apoptotic cells can be cleared silently without release of either pro- or anti-inflammatory mediators21 or describe proinflammatory consequences, including the release of interleukin-8 with subsequent neutrophil chemotaxis22 and release of Fas ligand. The recognition mechanism involved in uptake may be critically important for the immunological consequences as suggested by studies showing that phosphatidylserine-dependent ingestion of necrotic cells is immunologically neutral23 and data suggesting that the dual function of bridge molecules such as surfactant protein (SP) A (SP-A) and surfactant protein D (SP-D) is to enhance proinflammatory mediator production when binding to calreticulin/CD91 and to inhibit inflammation when binding to signal regulatory protein-α.24
Apoptotic cell uptake predominantly initiates mechanisms that contribute to resolution of injury and repair, but this must be seen in the context of other signals that impinge on the surface receptors of phagocytes. Generally, not all phagocytes within a given population take up apoptotic cells, and those that do frequently take up more than one apoptotic target, suggesting that the activation state and differential receptor expression markedly influences not only phagocytic capacity but also subsequent responses. Necrotic cells and pathogens share many of the ligands of apoptotic cells but usually induce different responses at least partially because they also engage pattern recognition receptors and signaling pathways not activated by apoptotic corpses. On the single cell level, apoptotic cell uptake activates Rho GTPases,25 which in turn markedly inhibit phagocytosis.26 This may eventually lead to clearance failure or uptake by phagocytes that initially were not primed for uptake, and so far, we can only speculate whether this alters the phagocyte and subsequent immune response in an inflamed focus with ongoing cell death. Macrophage function within complex environments (ie, inflamed tissue) is notoriously difficult to study, but existing data suggest that macrophage function is not an amalgamate of all of the signals received but rather a programed response induced by the first dominant stimulus to which the cells are exposed.27 It is therefore conceivable that apoptotic cell uptake does not immediately switch individual phagocyte function but only does so after a critical number of cells have contributed to an overall change in the microenvironment.
Importantly, a series of in vivo experiments show the anti-inflammatory effects of apoptotic cell phagocytosis. Deliberate instillation of apoptotic cells into sites of local inflammation in the lungs and peritonea increased production of TGF-β as well as enhanced resolution of injury.28 Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia,29 and defective clearance of apoptotic cells in CD44 knockout mice leads to unremitting inflammation following noninfectious lung injury.30
Thus, the innate response to apoptotic cell phagocytosis is dominated by anti-inflammatory signals originating from the professional and nonprofessional phagocytes. Considerably less is known about the direct effects of apoptotic cells, but recent observations suggest that changes in surface composition and surface charge may directly influence restoration of endothelial layers and angiogenesis.31
T-Cell Activation following Apoptotic Cell Uptake
Dendritic cells (DCs) are the primary antigen-presenting cells for initiating primary immune responses, but macrophages are abundant in inflammatory sites and can act as antigen-presenting cells and perpetuate or terminate immune responses depending on their state of activation.9,27 After uptake of necrotic neutrophils, macrophages up-regulate the costimulatory molecule CD40 and stimulate significantly higher T-cell proliferation than macrophages that have ingested apoptotic neutrophils.32 The production of the proinflammatory cytokine interleukin-12 by macrophages is transcriptionally suppressed following apoptotic cell uptake or treatment with phosphatidylserine.33 Immature DCs are capable of extensive phagocytosis, and DC maturation can be inhibited by the engulfment of apoptotic cells with suppressed expression of the costimulatory molecule CD86 and similarly to macrophages decreased interleukin-12 production.34 Interestingly, studies conducted in the 1970s show improved allograft survival following repeated blood transfusions, and this may be due to the presence of apoptotic granulocytes and lymphocytes in blood stored for clinical transfusion.35 In this context, it is intriguing that the infusion of donor apoptotic lymphocytes in a rat heart transplantation model induced allograft tolerance and was shown to be dependent on intact efferocytosis.36
Despite this compelling evidence that ingestion of apoptotic cells can inhibit antigen presentation and dendritic cell maturation, earlier studies indicate that antigen-derived from ingested apoptotic cells could access the cytoplasm of the ingesting cell and be cross-presented on major histocompatibility class I molecules.37 More recent work by Nussenzweig and colleagues38 established that CD8+ CD205+ DCs, which seem to be specialized for uptake of dying cells, are much better than CD8− 33D1+ DCs for cross-presentation on major histocompatibility class I.
This is concerning in that the uptake of apoptotic cells and associated protein cleavage, which could generate neo-autoantigens, might provoke autoimmune responses. However, apoptotic cell phagosomes within dendritic cells mature at a significantly slower rate than in macrophages,39 and DCs have been shown to be lysosomal protease-poor, resulting in a limited capacity for lysosomal degradation.40 In this context, it is not surprising that apoptotic cells contained in DC phagosomes can be observed in the afferent lymphatics of the gut41 en route to lymph nodes. The slow degradation of ingested material by dendritic cells may allow an extended period of time to sample the microenvironment for danger signals, which in turn instruct the dendritic cells to initiate an immune response or assist in the maintenance of self-tolerance. Danger signals include not only signals received through pattern recognition receptors such as Toll-like receptors but also necrotic cells.42 Necrotic but not apoptotic cell death has been shown to release heat shock proteins, which in turn deliver a partial maturation signal to dendritic cells and activate their nuclear factor κB pathway.43 Furthermore, protein fragments chaperoned by heat shock proteins and not intact proteins have recently been shown to be crucially important for the cross-presentation of antigens from cancer or infected cells for priming of naïve CD8+ T cells.44
Therefore, the microenvironment in which apoptotic cell phagocytosis takes place seems to be critically important for the subsequent adaptive immune response. It is likely that apoptotic cells ingested by DCs in the absence of danger signals or concomitant necrotic cell death contribute to tolerance but otherwise might provoke autoimmune responses. It is important to keep in mind that the microenvironment is profoundly influenced by the molecules produced by the corpse-eating phagocytes and that the balance of appropriately disposed apoptotic cells versus primary necrotic or secondary necrotic (ie, in clearance failure) cells may be critically important.
Failed Clearance and Autoimmunity
The prototypic autoimmune disease systemic lupus erythematosus is characterized by development of specific antibodies to intracellular antigens that are clustered on the surface of apoptotic cells.45 The notion that apoptotic cell debris represents the autoantigen led to a series of studies that showed that immunization with apoptotic cells can drive the immune response and result in the production of autoantibodies.46 This suggests that apoptotic cell clearance defects contribute to the development of autoimmunity. Indeed, patients with C1q deficiency and other defects of the complement pathway develop systemic lupus erythematosus,47 and C1q knockout mice develop spontaneous systemic autoimmunity with a marked excess of noningested apoptotic cells in the kidney.48 Experiments using knockout mice of other components of the complement pathway including C3 and C4, also associated with susceptibility to systemic lupus erythematosus, show delayed clearance of apoptotic bodies by resident peritoneal macrophages.49 Mice deficient in other receptors and bridge molecules implicated in clearance of apoptotic cells such as MFG-E8,7 Mer,5 and CD3150 also exhibit autoimmune disease. It is important to keep in mind, however, that genes from the nonautoimmune strains 129 and C57BL/6(B6) commonly used for generating knockout mice can induce a lupus-like disease and that a 129-derived interval on distal chromosome 1 is strongly linked to autoantibody production in the absence of any disrupted gene.51
Mannose-binding lectin, a member of the collectin family, is a serum protein with functional and structural similarities to C1q that can activate complement through the lectin pathway. Similar to the lung collectins SP-D and SP-A, mannose-binding lectin can bind cells undergoing apoptosis in vitro.52 SP-D and mannose-binding lectin knockout mice show defective clearance of apoptotic cells.53 Despite this, mannose-binding lectin knockout mice do not develop autoimmunity, even when they are aged on a lupus-prone background for 18 months,54 showing that clearance failure does not inescapably lead to autoimmunity. Intriguingly, mice deficient in CD14 also show a clearance defect leading to persistence of apoptotic cells in multiple organs and do not develop autoantibodies or autoimmune disease.55 This is of particular interest because this receptor serves to present lipopolysaccharide to pattern recognition receptors and is known to induce proinflammatory signals, but CD14-dependent uptake of apoptotic cells is not accompanied by proinflammatory responses.
Taken together, these data suggest that failure of apoptotic cell clearance in itself is not sufficient to initiate autoimmunity, raising questions regarding other mechanisms that instruct the immune response in the context of clearance failure (further illustrated in Figure 3). It is important to keep in mind that despite clearance failure, strong immunosuppressive signals such as the generation of TGF-β, are induced by those cells that indeed get ingested or even just by binding to the phagocyte.
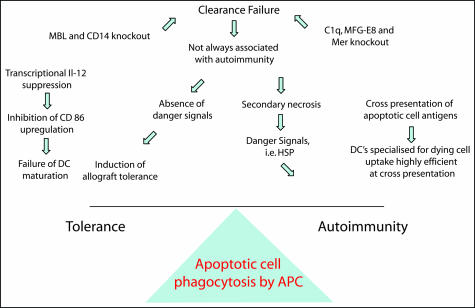
Figure 3.
Consequences for the adaptive immune response. Diagram illustrating the balance of signals that determine the subsequent adaptive immune response following apoptotic cell ingestion.
Exploitation of Apoptotic Cells by Tumors and Pathogens
In some ways, the most convincing evidence for the anti-inflammatory consequences of apoptotic cell phagocytosis is the exploitation of these immune inhibitory signals by pathogens and tumors to aid their survival. Plasmodium falciparum-infected erythrocytes inhibit the maturation of DCs by binding to CD36, a known recognition receptor for apoptotic cells. Infected DCs still secrete tumor necrosis factor α but fail to activate T cells and secrete interleukin-10.56 This response can be mimicked by antibodies to CD36 or apoptotic cells and suggests that the pathogen and apoptotic cells engage the same pathway regulating DC function. It seems that plasmodium almost inadvertently profits from using the same entry mechanism as apoptotic cells, whereas other pathogens not only exploit recognition mechanism but also profit from the microenvironment created by apoptotic cell phagocytosis. Intense lymphocyte apoptosis occurs in Chagas disease, a debilitating cardiac illness caused by the protozoan Trypanosoma cruzi. In a mouse model of the disease, interaction of apoptotic but not necrotic T lymphocytes with macrophages infected with T. cruzi fuels parasite growth in a manner dependent on prostaglandins, TGF-β, and polyamine biosynthesis.57 Work by Freire-de-Lima et al57 further show that the vitronectin receptor is critical in both apoptotic-cell binding to phagocytes and the induction of prostaglandin E2/TGF-β release and ornithine decarboxylase activity in macrophages. These results suggest that continual lymphocyte apoptosis and phagocytosis of apoptotic cells by macrophages have a role in parasite persistence in the host.
A blunted immune response to rapidly growing tumors is frequently observed and thought to be at least partly mediated by the immune inhibitory effects of apoptotic cell phagocytosis. Reiter et al58 showed that exposure of bone marrow-derived macrophages to apoptotic tumor cells (but not necrotic) tumor cell inhibits their cytotoxicity and nitric oxide production in response to interferon γ and lipopolysaccharide. Furthermore, unstimulated bone marrow-derived macrophages exposed to apoptotic tumor cells enhanced growth of live tumor cells by 40%. Therefore, treatment of cancers with chemotherapy or radiation, which leads to massive tumor cell apoptosis, is likely to inhibit macrophage-mediated antitumor responses.
These examples clearly illustrate the profound effects of apoptotic cell recognition on the outcome of the immune response to pathogens and tumors. It shows that pathogens and tumors use endogenous anti-inflammatory pathways to aid their survival, suggesting possibilities for developing similar avenues to treat inflammatory disease. A recent article by Rossi et al59 establishes that we are already in position to apply this principle to treat experimental lung and joint inflammation. They show that human neutrophils contain functionally active cyclin-dependent kinases (CDKs) and that structurally diverse CDK inhibitors induce caspase-dependent apoptosis and override powerful anti-apoptosis signals from survival factors such as granulocyte-macrophage colony-stimulating factor. Furthermore, the CDK inhibitor R-roscovitine markedly enhances resolution of established neutrophil-dependent inflammation in carrageenan-elicited acute pleurisy, bleomycin-induced lung injury, and passively induced arthritis in mice. In the pleurisy model, the caspase inhibitor zVAD-fmk prevents R-roscovitine-enhanced resolution of inflammation, indicating that this CDK inhibitor augments inflammatory cell apoptosis. Thus, they show that CDK inhibitors enhance the resolution of established inflammation by promoting apoptosis of inflammatory cells.
Conclusions
Resolution of inflammation is not a passive process but rather an active response to terminate the immune response.60 We show here that the effective recognition and clearance of apoptotic cells is critically important in this process and that this important endogenous mechanism of controlling the immune response is exploited by pathogens and tumors. The challenge for the future is to manipulate effectively and coordinately the clearance of dying cells to develop new therapies for inflammatory and autoimmune disease and prevent inappropriate immune inhibition in the context of pathogens and cancer.
Note Added in Proof
This Biological Perspectives review aims to provide a conceptual outline of the consequences of apoptotic cell phagocytosis rather than providing a complete review of the existing literature, and we apologize to those whose important relevant contributions are not cited. Detailed differences between necrotic and apoptotic cells in this context and the ligands that apoptotic and necrotic cells and pathogens share have just recently been reviewed elsewhere61,62 and are beyond the scope of the manuscript.
Footnotes
Address reprint requests to Lars-Peter Erwig, Department of Medicine and Therapeutics, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen AB25 2ZD, UK. E-mail: mmd288@abdn.ac.uk.
References
- deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave (review). Essays Biochem. 2003;39:105–117. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory (review). Trends Neurosci. 2000;23:454–458. doi: 10.1016/s0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Palmer E. Negative selection-clearing out the bad apples from the T-cell repertoire (review). Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Vandivier RW, Henson PM, Douglas IS. The impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- Minto AW, Erwig LP, Rees AJ. Heterogeneity of macrophage activation in anti-Thy-1.1 nephritis. Am J Pathol. 2003;163:2033–2041. doi: 10.1016/S0002-9440(10)63561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J Leukoc Biol. 1992;52:269–273. [PubMed] [Google Scholar]
- Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by αvβ3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol. 1996;149:911–921. [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apototic cells, through transforming growth factor-β, co-ordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Cvetanovic M, Ucker DS. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol. 2004;172:880–889. doi: 10.4049/jimmunol.172.2.880. [DOI] [PubMed] [Google Scholar]
- Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004;18:1716–1718. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutorphils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol. 2001;24:608–615. doi: 10.1165/ajrcmb.24.5.4292. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the NJK and the wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Kurosaka K, Takahashi M, Watanabe N, Kobayashi Y. Silent cleanup of very early apoptotic cells by macrophages. J Immunol. 2003;171:4672–4679. doi: 10.4049/jimmunol.171.9.4672. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 2001;20:7085–7095. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- Brouckaert G, Kalai M, Krysko DV, Saelens X, Vercammen D, Ndlovu ’M, Haegeman G, D’Herde K, Vandenabeele P. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell. 2004;15:1089–1110. doi: 10.1091/mbc.E03-09-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Tosello-Trampont AC, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J Biol Chem. 2003;278:49911–49919. doi: 10.1074/jbc.M306079200. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Kluth DC, Walsh GM, Rees AJ. Previous uptake of apoptotic neutrophils or ligation of integrin receptors downmodulates the ability of macrophages to ingest apoptotic neutrophils. Blood. 1999;93:1406–1412. [PubMed] [Google Scholar]
- Erwig LP, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–1988. [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott HM, Hellewell PG, Cross SS, Ince PG, Whyte MKB, Dockrell DH. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in murine model of pneumococcal pneumonia. J Immunol. 2006;177:6480–6488. doi: 10.4049/jimmunol.177.9.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teder R, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Tsan R, Schroit AJ, Fidler IJ. Apoptotic cells initiate endothelial cell sprouting via electrostatic signaling. Cancer Res. 2005;65:11529–11535. doi: 10.1158/0008-5472.CAN-05-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RN, Erwig LP, Hill KSK, Devine A, Pearce WP, Rees AJ. Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clin Exp Immunol. 2002;127:220–225. doi: 10.1046/j.1365-2249.2002.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- Opelz G, Vanrenterghem Y, Kirste G, Gray DW, Horsburgh T, Lachance JG, Largiader F, Lange H, Vujaklija-Stipanovic K, Alvarez-Grande J, Schott W, Hoyer J, Schnuelle P, Descoeudres C, Ruder H, Wujciak T, Schwarz V. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63:964–967. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- Sun E, Gao Y, Chen J, Roberts A, Wang X, Chen Z, Shi Y. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 2004;11:1258–1264. doi: 10.1038/sj.cdd.4401500. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci USA. 2006;103:12825–12830. doi: 10.1073/pnas.0605331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–599. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Botto M. Complement deficiencies in humans and animals: links to autoimmunity. Autoimmunity. 2006;39:367–378. doi: 10.1080/08916930600739233. [DOI] [PubMed] [Google Scholar]
- Walport MJ, Davies KA, Morley BJ, Botto M. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R, Lyons AB, Roberts D, Wong MX, Bartley PA, Jackson DE. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation, and autoimmune disease. Blood. 2002;100:184–193. doi: 10.1182/blood-2002-01-0027. [DOI] [PubMed] [Google Scholar]
- Heidari Y, Bygrave AE, Rigby RJ, Rose KL, Walport MJ, Cook HT, Vyse TJ, Botto M. Identification of chromosome intervals from 129 and C57BL/6 mouse strains linked to the development of systemic lupus erythematosus. Genes Immun. 2006;7:592–599. doi: 10.1038/sj.gene.6364335. [DOI] [PubMed] [Google Scholar]
- Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- Devitt A, Parker KG, Ogden CA, Oldreive C, Clay MF, Melville LA, Bellamy CO, Lacy-Hulbert A, Gangloff SC, Goyert SM, Gregory CD. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14−/− mice. J Cell Biol. 2004;167:1161–1170. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci USA. 2001;98:8750–8755. doi: 10.1073/pnas.151028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- Reiter I, Krammer B, Schwanberger G. Cutting edge: differential effect of apoptotic versus necrotic tumour cells on macrophage antitumour activities. J Immunol. 1999;163:1730–1732. [PubMed] [Google Scholar]
- Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, Gray M, Crescenzi E, Martin MC, Brady HJ, Savill JS, Dransfield I, Haslett C. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckly CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D’Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]