Abstract
Endogenous molecules released from disrupted cells and extracellular matrix degradation products activate Toll-like receptors (TLRs) and, thus, might contribute to immune activation after tissue injury. Here, we show that aseptic, cold-induced cortical injury triggered an acute immune response that involves increased production of multiple cytokines/chemokines accompanied by neutrophil recruitment to the lesion site. We observed selective reductions in injury-induced cytokine/chemokine expression as well as in neutrophil accumulation in mice lacking the common TLR signaling adaptor MyD88 compared with wild-type mice. Notably, attenuation of the immune response was paralleled by a reduction in lesion size. Neutrophil depletion of wild-type mice and transplantation of MyD88-deficient bone marrow into lethally irradiated wild-type recipients had no substantial impact on injury-induced expression of cytokines/chemokines and on lesion development. In contrast to MyD88 deficiency, double deficiency of TLR2 and TLR4—despite the two receptors being activated by specific endogenous molecules associated to danger and signal through MyD88—altered neither immune response nor extent of tissue lesion size on injury. Our data indicate modulation of the neuroinflammatory response and lesion development after aseptic cortical injury through MyD88-dependent but TLR2/4-independent signaling by central nervous system resident nonmyeloid cells.
The innate immune system provides first-line defense to protect complex organisms from pathogen invasion. Therefore, it discriminates between infectious nonself and noninfectious self.1 Hosts recognize specific pathogen-associated molecular patterns representing infectious nonself through pattern recognition receptors.2 Among pattern recognition receptors, Toll-like receptors (TLRs) mediate both invading pathogen recognition through direct interaction and signal transduction.1,3 Aside from infection, disruption of the regular homeostasis such as through tissue injury in the absence of challenge with exogenous compounds leads to activation of the innate immune system. For instance, aseptic physical injury to the brain has been shown to elicit an acute immune response that involves activation of resident microglial cells and increased production of pro- and anti-inflammatory cytokines, chemokines, and adhesion molecules followed by the infiltration of blood-borne leukocytes.4,5,6 Causes of noninfectious inflammatory reactions are specific products released by dying cells that alert the immune system.7,8 In vitro experiments implicate necrotic cells as the source of endogenous factors that induce activation of dendritic cells and macrophages,9,10,11 of which heat shock proteins (HSPs) are major danger signals.12,13 In addition to HSPs, several other substances such as uric acid, high mobility group box-1 protein (HMGB-1), and endogenous nucleic acids that are localized within cells normally have been implicated as endogenous immunostimulants.14,15,16,17,18 An additional feature accompanying necrotic cell death is disruption of tissue architecture through break-down of extracellular matrix, which yields a set of distinct signals such as fibrinogen, heparan sulfate, or hyaluronan fragments that may activate the immune system19,20 and trigger dendritic cell maturation.21 Recent reports have implicated TLR2 and TLR4 in sensing endogenous danger signals such as specific HSPs, HMGB-1, uric acid, and hyaluronan fragments.22,23,24,25,26,27 Moreover, fibrinogen and heparan sulfate fragments have been shown to activate antigen-presenting cells through TLR4.20,21
After acute brain injury, an up-regulation and/or release of endogenous TLR ligands including HSP70, HMGB-1, monosodium urate, fibrinogen, and heparan sulfate fragments has been observed in both humans and animal models.28,29,30,31,32 Therefore, we have speculated that endogenous danger signals released from dying cell and/or extracellular matrix on acute brain injury might be recognized through TLR2 and TLR4 to initiate inflammatory responses. TLRs and most important signaling molecules interacting directly with them in the cytoplasm share a Toll-interleukin 1 (IL-1) receptor domain (TIR). Because MyD88 is the prominent molecule of the latter group of molecules and interacts with all TLRs except for TLR3,33 we reasoned that it might be crucial to aseptic brain injury responses. The death domain carried by MyD88 aside of its TIR recruits members of the IL-1 receptor-associated kinases (IRAKs) on which downstream signals lead to activation of transcription factors such as activator protein-1, IRF5, and nuclear factor-κβ.34
Thus, we analyzed the universal TLR cytoplasmic signal transducer MyD88 as well as TLR2 and TLR4 for their roles in inflammation and lesion development after aseptic, cold-induced cortical injury. We demonstrated for the first time substantial impairment of injury-induced immune response in the brains of MyD88−/− mice compared with those in wild-type mice. The attenuated immune response was paralleled by reduced tissue damage on the cortical injury applied. Neutrophil depletion experiments, as well as transplantation of MyD88-deficient bone marrow (BM) into lethally irradiated wild-type recipients implicated nonmyeloid cells as MyD88-dependent modulators of inflammation and lesion development after cold-induced cortical injury. Surprisingly, TLR2/4 double-deficient mice displayed neither altered immune responses nor tissue lesion sizes compared with those in wild-type mice. Our results imply MyD88- and central nervous system (CNS) resident cell dependency but TLR2/4 independency of brain tissue injury-induced immune responses.
Materials and Methods
Mouse Model of Cold-Induced Cortical Injury
To induce aseptic brain tissue damage, a well-established mouse model of cold-induced cortical injury35 was used with minor modifications. In brief, adult male mice (strain C57BL/6) weighing 25 to 30 g were anesthetized by an intraperitoneal injection of avertin (0.5 mg/g) and were placed in a stereotactic frame with the rectal temperature maintained between 37 and 37.5°C using a heating pad. The scalp was incised on the midline, the subcutaneous tissue was retracted from the bone, and the skull was exposed. Then, a circular area overlying the right parietal cortex (3 mm in diameter with center at position 2 mm posterior to bregma and 2 mm right lateral to the sagittal suture) was thinned to translucency using a dental drill. A copper probe (3 mm in diameter), which was precooled in liquid nitrogen, was applied to the thinned skull by force of 100 g for 30 seconds. Thereafter, the skin incision was sutured, and mice were allowed to wake up with free access to water and food. After specific time periods (1 hour, 4 hours, 24 hours, or 72 hours) after injury, mice were deeply anesthetized by intraperitoneal application of ketamine (0.5 mg/g) and xylazine (0.01 mg/g), and perfused transcardially with ice-cold phosphate-buffered saline. Brains were subsequently rapidly removed and frozen to −80°C.
Evaluation of Neurological Clinical Status
Neurological clinical status was evaluated before injury, as well as 4, 24, and, in one experimental group (see below), also 72 hours after injury using a combination of standard tasks (with modifications) including a postural reflex test,36 a beam walk test,37 an inclined plane balancing test,38 and a spontaneous motor activity test.39
For postural reflex test, mice were lifted on fixation of the tail and symmetry in the movement of the four limbs was examined. A score of 0 indicates all four limbs extended symmetrically; 1, limbs on left side extended to a lesser degree or more slowly than those on the right; 2, minimal movement of left side limbs; and 3, lack of movement of left side limbs. The beam walk test assessed fine motor coordination. Therefore, mice were analyzed for their capacity to traverse wooden beams of 30 cm in length and with consecutively decreasing diameters of 13, 9, and 5 mm in diameter by walking. Failure of walking along the thickest beam whose diameter was 13 mm was assigned to a score of 3. Motor ability was further tested using an inclined plane test. In this test, mice were placed on planes with a decreasing inclination angle. If a mouse dropped off from the plane inclined at 75°, 60°, or 45° within 30 seconds, the score was 1, 2, or 3, respectively. For evaluation of spontaneous motor activity, mice were seeded in the center of a circle of 20 cm in diameter. One additional point was assigned to mice that failed to exit the circle within 120 seconds. In addition, one score point was given to mice that had seizures at the time of clinical examination. The maximum neurological score was 11 and indicated severe neurological dysfunction, whereas a score of 0 was associated with healthy uninjured mice.
Assessment of Brain Injury
Coronal sections, 10 μm in thickness, were cut on a cryostat at 0.5-mm intervals from 1 mm anterior to 3.5 mm posterior to the bregma and stained with Cresyl violet. The images of the stained specimen were captured by a digital video camera (JVC TK-C1360B; JVC Germany, Friedberg, Germany) and analyzed by UTHSCSA Image Tool Version 3 for morphometric measurements (developed in the Department of Dental Diagnostic Science, University of Texas Health Science Center, San Antonio, TX). Lesion volumes were determined by multiplying the total lesion area with the section interval thickness.
In addition, three cryosections per mouse were collected from the region spanning parts of the hippocampus (from 1.5 to 2.5. mm posterior to the bregma, at 0.5-mm intervals). A commercial in situ histochemical assay (Klenow-FragEL DNA fragmentation detection kit; Calbiochem, Darmstadt, Germany) was used to detect DNA fragmentation characteristic of apoptosis. In this assay, Klenow fragment binds to exposed ends of DNA fragments generated in response to apoptotic signals and catalyzes the template-dependent addition of biotin-labeled deoxynucleotides. Biotinylated nucleotides are detected with a streptavidin-horseradish peroxidase conjugate. Diaminobenzidine reacts with the labeled sample to generate an insoluble colored substrate at the site of DNA fragmentation. The diaminobenzidine-stained brain sections were imaged at a magnification of 35 using a digital video camera connected to a PC. The number of FragEL-positive (colored) cells in the injured cortex, the hippocampus, and the thalamus was determined by using a UTHSCSA Image Tool Version 3 Macro.
Immunohistochemical Detection of Neutrophils
Ten-μm-thick coronal brain sections cut at bregma levels −1.5, −2, and −2.5 mm were stained with a rat anti-mouse GR-1 monoclonal antibody (RB6-8C5; BD Biosciences, Erembodegem, Belgium) for evaluation of brain neutrophil infiltration. After quenching endogenous peroxidase activity with 0.3% methanolic hydrogen peroxide and blockage of nonspecific binding by 10% normal rabbit serum, brain sections were incubated with the anti-GR-1 antibody diluted 1:10 overnight at 4°C. Labeled cells were visualized using biotinylated rabbit anti-rat IgG at a 1:200 dilution, followed by horseradish peroxidase-conjugated streptavidin and then 3,3-diaminobenzidine as a chromogen (all from Vector Laboratories, Burlingame, CA). After counterstaining with Mayer’s hematoxylin solution, tissue sections were imaged at a magnification of 35 using a digital video camera connected to a PC. The number of positive cells was counted in a blinded manner in the injured and contralateral cerebral cortex. The counts were then normalized to the area of tissue and expressed in mm2.
Protein Array Analysis
Mice brains were screened for containment of 62 cytokines/chemokines using a mouse-specific cytokine antibody array (Array 3.1.; Ray Biotech Inc., Atlanta, GA), performed according to the manufacturer’s instructions. In brief, 88 50-μm-thick cryosections (cut from 0.5 mm anterior to 4.5 mm posterior to the bregma, containing the lesion area) were homogenized in lysis buffer (10 mmol/L HEPES at pH 7.9, 10 mmol/L KC/CXCL1l, 1.5 mmol/L MgCl2, and a mixture of protease inhibitors including phenylmethyl sulfonyl fluoride, aprotinin, leupeptin, and pepstatin A) and then centrifuged at 12,000 × g for 15 minutes at 4°C. Protein concentrations were determined in supernatants using the Nanoquant assay (Carl Roth GmbH, Karlsruhe, Germany), and equal protein amounts of eight separate brain extracts per group were pooled. A total of 1500 μg of pooled protein was applied for incubation with array membranes and handled according to the manufacturer’s instructions. Cytokine-antibody complexes on membranes were detected by enhanced chemiluminescence and recorded with X-ray films. The images were scanned and analyzed by TINA 2.08e software (Raytest, Straubenhardt, Germany). For normalization, optical densities of each spot were expressed as percentage of the average optical densities of the six positive controls contained on each membrane. Expression levels <5% as related to positive controls were considered as originating from unspecific staining and ignored. Greater than twofold changes of protein expression levels as revealed by comparative analysis of different experimental groups were considered as significant.
Immunoassays for IL-1β, IL-6, CXCL16, and CCL-9
Immunoreactive IL-1β, IL-6, CXCL16, and CCL-9 were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Quantikine assay kits; R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany). Briefly, frozen brain sections of a total thickness of 1.8 mm were homogenized in lysis buffer and thereafter centrifuged at 12,000 rpm for 15 minutes at 4°C, and 50 μl of the supernatant was used for ELISA. For normalization, supernatant protein concentration was measured using the Nanoquant assay (Carl Roth GmbH). Concentrations of immunoreactive IL-1β, IL-6, CXCL16, and CCL-9 were expressed as pg/mg brain protein.
Experimental Groups in the Mouse Model
For analyses of cytokine pattern alterations in response to cold-induced cortical injury, 36 C57/BL6 wild-type mice were euthanized immediately before cold injury (n = 6), 1 hour after cold injury (n = 6), 4 hours after cold injury (n = 6), 24 hours after cold injury (n = 10), or 72 hours after cold injury (n = 9). Analysis of the role of MyD88 in the immune response to cold injury was conducted in Myd88-deficient mice and wild-type mice (genetic background: C57/BL6; n = 11 per group) subjected to cold-induced cortical injury and euthanized 24 hours thereafter. MyD88-deficient mice backcrossed eightfold to the C57BL/6 background were kindly provided by Prof. S. Akira (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan). Wild-type C57BL/6 mice were purchased from Charles River Germany (Sulzfeld, Germany). To explore the role of TLR2 and TLR4 in the immune response to cold injury, we also subjected TLR2/4 double-deficient mice backcrossed for five times to the C57/BL6 background: (n = 11) to cold injury. Wild-type mice used for control of TLR2/4 double-deficient mice analyses (n = 10) were outcrossed during TLR2−/− and TLR4−/− mice crossing. Sham-operated wild-type mice (n = 5) served as negative controls. Sham-operated mice and cold-injured mice underwent identical surgery, except that the metal probe was not precooled before being placed on the skull in sham-operated mice. To analyze the contribution of blood-borne leukocytes to injury-induced inflammation, additional wild-type mice were either rendered neutropenic or lethally irradiated and transplanted with MyD88-deficient BM. Mice (n = 5) assigned to the neutropenia group were injected intraperitoneally with 250 μg of rat anti-mouse GR-1 monoclonal antibody (BD Biosciences) 24 hours before cold injury. The control group of mice (n = 5) followed the same dosage schedule, receiving intraperitoneal injection of a purified rat IgG2b isotype control antibody (BD Biosciences). To verify neutrophil depletion, blood was obtained by cardiac puncture before injury and at the time of sacrifice. The total leukocyte count was determined using blood samples diluted in Turk’s solution counted in a Neubauer chamber and differential leukocyte counts were performed on thin blood smears stained by the May-Gruenwald-Giemsa method. Anti-GR-1 treatment resulted in a significant reduction in mean neutrophil counts compared with the isotype controls (eg, at 24 hours after injury: 196 ± 116 neutrophils/μl in anti-GR-1-treated mice versus 1954 ± 388 neutrophils/μl; P < 0.001). BM chimeric mice were generated according to the method described recently by Prinz and colleagues.40 In brief, 8-week-old wild-type mice were exposed to a 137CsCl beam source to receive a radiation dose of 900 cGy (Amersham, Brunswick, Germany) 24 hours before supplementation with BM of untreated mice. BM cells (5 × 106 cells) derived from tibiae and femurs either from wild-type or MyD88-deficient mice were injected into tail veins of recipients (n = 6 in each group). Eight weeks after grafting, reconstitution was assessed using a whole blood bioassay. Therefore, blood samples were obtained by cardiac puncture using heparinized syringes. Then, 100 μl of whole blood was added to 100 μl of RPMI 1640 culture medium with or without 1 μg/ml lipopolysaccharide (LPS) of Escherichia coli 0111:B4 (Sigma Chemicals, Deisenhofen, Germany) in flat-bottom wells of a microtiter plate. After incubation for 24 hours, blood solutions were harvested and centrifuged at 2000 rpm for 10 minutes. The resulting supernatants were assayed for IL-6 containment by a Quantikine ELISA kit (R&D Systems GmbH). Stimulation with LPS led to significantly increased IL-6 release from whole blood from both normal wild-type mice (218.4 ± 95.0 pg/ml) and wild-type mice reconstituted with wild-type BM (205.4 ± 90.2 pg/ml), compared with their nonstimulated controls (12.1 ± 10.1 and 10.7 ± 4.6 pg/ml). In contrast, whole blood cells drawn from wild-type mice reconstituted with MyD88-deficient BM did not respond with increased IL-6 production on exposure to LPS (10.8 ± 2.7 pg/ml). Experiments were approved by the government of Upper Bavaria.
Statistical Analysis
The principal statistical test used for comparison of sham-operated wild-type mice, cold-injured wild-type mice, cold-injured MyD88-deficient mice, and cold-injured TLR2/4 double-deficient mice was one-way analysis of variance and Scheffé’s test. Statistical differences of cytokine expression, lesion size, or clinical score between anti-GR-1- and isotype control antibody-treated cold-injured wild-type mice as well as cold-injured wild-type and MyD88−/− BM chimeras were evaluated using two-tailed unpaired Student’s t-test. To test statistical significance of changes of lesion size, number of FragEL-positive cells, as well as expression of CCL-9 and CXCL16 throughout time, we used unpaired Student’s t-test and Bonferroni corrections for multiple comparisons. Differences were considered significant at P < 0.05. Data are expressed as mean ± SD.
Results
Characteristics of Cold-Induced Cortical Injury
Cold trauma resulted in sharply demarcated cortex lesions, as revealed by Nissl stains. The lesion volume increased by ∼50% between 1 and 24 hours after cold injury; no further rise in lesion size was found between 24 and 72 hours after cold injury (Figures 1 and 2). Cells carrying DNA breaks were detected by Klenow fragment of DNA polymerase I-mediated biotin-dATP nick-end labeling (FragEL) in the injured cortex as early as 1 hour after cold injury; their number peaked at 24 hours after injury and then declined (Figure 2). In lesioned mice, FragEL-positive cells were furthermore detected in the contralateral cerebral cortex, the ipsi- and contralateral hippocampus (Figures 1 and 2), and the ipsi-and contralateral thalamus (not shown), starting at 4 to 24 hours, but being present to a more pronounced degree at the 72-hour time point after cold injury.
Figure 1.
Neuropathology in mice after cold-induced cortical injury. Cold trauma resulted in sharply demarcated cortex lesions, as revealed by Nissl stains (indicated by diamonds; top and middle left images). At 24 hours after cold injury (middle left image), the lesion size was significantly greater than that observed 1 hour after injury (top left image). In addition, DNA fragmentation characteristic for apoptosis was detected in injured brains using a commercial in situ histochemical assay (Klenow-FragEL DNA fragmentation detection kit). In the injured cortex, cells containing DNA breaks (stained brown) were detected as early as 1 hour and were maximal at 24 hours after injury (bottom left image). In the contralateral hippocampus, apoptotic cells were not detected either by the Klenow kit or by Cresyl violet staining until 4 hours (top middle image) and were maximal at 72 hours after injury (middle and bottom middle images). The presence of neutrophils in the injured brain was indicated by immunohistochemistry using an anti GR-1 antibody. GR-1-positive cells (stained brown) were found in the lesioned cortex at 24 hours (middle right image) after cold injury but were not detected or very rare in the contralateral cortex (top right image) or the contralateral hippocampus (bottom right image). Scale bars = 100 μm.
Figure 2.
Lesion development in mice after cold-induced cortical injury. A: The lesion volume increased by ∼50% between 1 and 24 hours after cold injury; no further rise in lesion size was found between 24 and 72 hours after cold injury. B: Cells carrying DNA breaks were detected by Klenow fragment of DNA polymerase I-mediated biotin-dATP nick-end labeling (FragEL) in the injured cortex as early as 1 hour after cold injury; their number peaked at 24 hours after injury and then declined. C: In lesioned mice, FragEL-positive cells were also detected in the contralateral hippocampus, starting 24 hours after injury. #P < 0.05, compared with control mice; *P < 0.05, compared with injured mice euthanized 1 hour after cold injury; +P < 0.05, compared with injured mice euthanized 4 hours after cold injury, and §P < 0.05, compared with injured mice euthanized 24 hours after cold injury using unpaired Student’s t-test and Bonferroni corrections for multiple comparisons.
At 24 hours of cold lesioning, all injured mice were hypothermic, lost body weight, and held an altered neurological clinical status, as evidenced by the presence of mono/hemiparesis and impaired motor activity and function (Table 1). Seizures were present in 5 of 13 mice at 24 hours, but in all mice examined at 4 hours and in 0 of six mice examined 72 hours after injury. The mortality rate after cold injury was 31.6%; all cases of death occurred between 4 and 24 hours after placement of cold lesion.
Table 1.
Clinical Parameters of Mice before and at Different Time Points after Cold-Induced Cortical Injury
| Experimental group (n) | Death within the observation period | Clinical score | Seizures | Body temperature (°C) | Weight loss (%) |
|---|---|---|---|---|---|
| Before cold injury (6) | 0 of 6 (0%) | 0 ± 0 | 0 of 6 (0%) | 37.8 ± 0.3 | n.d. |
| 1 hour after injury (6) | 0 of 6 (0%) | n.d. | 0 of 6 (0%) | n.d. | n.d. |
| 4 hours after injury (6) | 0 of 6 (0%) | 9.8 ± 1.9* | 6 of 6 (100%)‡ | 36.7 ± 1.1 | −4.0 ± 1.7 |
| 24 hours after injury (10) | 3 of 10 (30%) | 8.9 ± 1.9* | 1 of 7 (14%) | 34.1 ± 1.6*† | −17.4 ± 3.6*† |
| 72 hours after injury (9) | 3 of 9 (33%) | 6.0 ± 2.6* | 0 of 6 (0%) | 35.0 ± 2.0* | −21.9 ± 7.6*† |
n.d., not detected.
P < 0.05, compared with control mice (examined and euthanized before cold injury) and †P < 0.05, compared with injured mice examined and euthanized 1 hour after cold injury using unpaired Student’s t-test and Bonferroni corrections for multiple comparisons.
P < 0.05, compared with all other groups investigated using 2 × 2 contingency tables and χ2 test.
Temporal Profile of the Expression of Cytokines, Chemokines, and Other Inflammation-Related Factors after Cold-Induced Cortical Injury
The hallmark of injury-induced inflammation is an influx of neutrophils into injured brain tissue.41,42 The presence of neutrophils in the injured brain was indicated by immunohistochemistry using an anti GR-1 antibody (RB6-8C5 Ab). GR-1-positive cells were found in the lesioned cortex at 24 hours (Figure 1) after cold injury but were absent in brains from sham-operated controls. GR-1-positive cells were also not detected or very rare in the contralateral cortex (Figure 1), the ipsi- and contralateral hippocampus (Figure 1), and the ipsi- and contralateral thalamus (not shown).
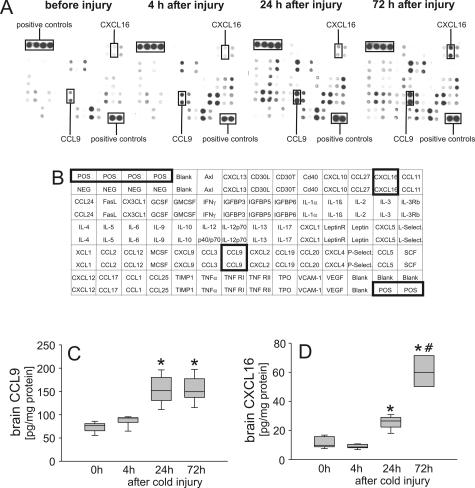
Neutrophil infiltration requires antecedent local expression of cytokines, chemokines, and adhesion molecules. To define the immune factors induced by cold injury, brains were harvested 4, 24, and 72 hours after injury, and the brain expression of 62 cytokines, chemokines, and other inflammation-related factors was profiled by a mouse-specific protein array. Compared with baseline expression in noninjured control brains, 33 of 62 immune factors were induced or up-regulated in mouse brains after cold injury (Figure 3, A and B; Table 2). Time course analysis of protein expression revealed specific expression patterns. The neutrophil chemoattractants KC/CXCL1 and LIX/CXCL5 were rapidly induced after injury. Their expression declined rapidly and reached control levels by 72 hours after cold lesion induction. A similar time course of expression was also found for the cytokine IL-6 as well as the growth factors granulocyte colony-stimulating factor and vascular endothelial growth factor. This group of immune factors may play a specific role in mounting the innate immune response to cold injury. The neutrophil chemoattractant MIP-2/CXCL2; the CXC chemokine CXCL4; the CC chemokines CCL2, CCL5, CCL9, and CCL12; as well as the adhesion molecules P-selectin and L-selectin also peaked within 24 hours after cold injury, but their expression levels were still elevated at 72 hours after cold injury, representing a second pattern of protein expression. The third pattern of expression is represented by that of the cytokines IL-4 and IL-12, the CXC chemokine CXCL16, the chemokine XCL1, and matrix metalloproteinase inhibitor TIMP-1. Their expression increased late after injury reaching maximum levels at 72 hours. To confirm the protein array data, supplemental ELISAs were conducted for the chemokines CCL9 and CXCL16, which were detected for the first time in injured brains. As shown in Figure 3, C and D, results were consistent with protein array data, thus indicating reliability of the latter.
Figure 3.
Temporal profile of cytokine/chemokine expression after cold-induced cortical injury. A: To define the immune factors induced by cold injury, injured brains were harvested 4, 24, and 72 hours after injury, and brain expression of 62 cytokines, chemokines, and other inflammation-related factors was profiled by using a mouse-specific protein array. Compared with baseline expression in noninjured control brains, 33 of 62 immune factors were induced or up-regulated in mouse brains after cold injury (for details, see Table 2). In addition to the positive controls, the chemokines CCL9 and CXCL16 that were additionally measured by ELISA are marked by rectangles. B: The table shows localization of antibodies toward cytokines, chemokines, and other inflammation-related factors on the protein chip. C and D: ELISA experiments revealed increased brain concentrations of both CCL9 (C) and CXCL16 (D) at 24 hours after brain injury. The protein level of CCL9 remained constantly elevated, whereas the expression of CXCL16 further increased up to 72 hours after cortical injury. *P < 0.05, compared with control mice and injured mice euthanized 1 hour after cold injury using unpaired Student’s t-test and Bonferroni corrections for multiple comparisons. #P < 0.05, compared with injured mice euthanized 24 hours after cold injury.
Table 2.
Expression Profiles of Cytokines/Chemokines after Cortical Injury
| Protein name | 4 hours after injury | 24 hours after injury | 72 hours after injury |
|---|---|---|---|
| IL-1β | n.d. | n.d.* | n.d. |
| IL-4 | − | − | + |
| IL-6 | − | Induced | Induced |
| IL-12p40/p70 | − | Induced | Induced |
| IL-12p70 | − | − | Induced |
| TNF-RI | − | − | Induced |
| TNF-RII | − | − | + |
| CXCL1 | − | Induced | − |
| CXCL2 | Induced | Induced | Induced |
| CXCL4 | Induced | Induced | Induced |
| CXCL5 | + | − | − |
| CXCL9 | − | Induced | Induced |
| CXCL12 | − | − | Induced |
| CXCL16 | − | + | ++ |
| CCL2 | − | + | + |
| CCL3 | − | − | Induced |
| CCL5 | − | + | + |
| CCL9 | − | + | + |
| CCL12 | − | Induced | Induced |
| CCL24 | Induced | Induced | Induced |
| CX3CL1 | − | Induced | Induced |
| XCL1 | − | − | + |
| L-selectin | Induced | Induced | Induced |
| P-selectin | Induced | Induced | Induced |
| G-CSF | − | Induced | Induced |
| M-CSF | − | ++ | + |
| VEGF | − | + | − |
| SCF | − | + | − |
| IGFBP3 | + | + | ++ |
| IGFBP5 | − | − | Induced |
| TPO | − | − | + |
| TIMP1 | − | + | + |
| Axl | − | − | + |
| Leptin | + | − | − |
IL, interleukin; TNF-R, tumor necrosis factor receptor; G-CSF, granulocyte colony-stimulating factor; M-CSF, macrophage colony stimulating factor; VEGF, vascular endothelial growth factor; SCF, stem cell factor; IGFBP, insulin-like growth factor binding protein; TPO, thrombopoietin; TIMP, tissue inhibitor of metalloproteinase; n.d., not detectable by protein array analysis.
, Detected by ELISA; −, unchanged (= less than twofold change in the protein expression level compared with noninjured control mice); + and ++, equal or greater than twofold and fourfold change in the protein expression level, respectively.
Impact of MyD88 Deficiency and TLR2/4 Double Deficiency on Inflammation after Cold-Induced Cortical Injury
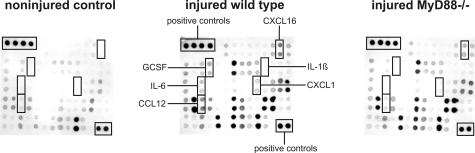
In vitro studies22,23,24,25,26,27 suggest that TLR2 and TLR4 activation by agonists from endogenous sources such as dying cells or disrupted extracellular matrix may be crucial for initiation of an innate immune response to tissue injury. We therefore studied cold injury-induced immune responses in MyD88-deficient mice, which are primarily defective in respect to TLR signaling, as well as in TLR2/4 double-deficient mice. Protein expression levels of 5 of 21 immune factors found to be induced or up-regulated 24 hours after cold lesioning differed in injured MyD88-deficient mice as compared with those in wild-type mice. These factors are IL-6, granulocyte colony-stimulating factor, KC/CXCL1, CXCL16, and CCL12, all of which were expressed at lower levels in brains of MyD88-deficient mice (Figure 4). To validate the array data, expression of IL-1β, IL-6, and CXCL16 were analyzed by ELISA. Consistent with the protein array data, the cold injury-induced increase in brain IL-6 expression was abrogated in MyD88-deficient mice, whereas the levels of CXCL16 were slightly, albeit significantly, reduced in this mouse strain. In contrast to the protein array analysis, we also detected an increase in brain IL-1β concentrations in injured wild-type mice compared with sham-operated controls. Injured MyD88-deficient brains contained substantially lower IL-1β amounts as compared with brains of wild-type mice. The latter result illustrates relatively low-detection sensitivity of protein arrays to specific cytokines (cytokine array sensitivity data are available at http://www.raybiotech.com/mouse_array_sensitivity.pdf).
Figure 4.
Comparative analysis of MyD88 deficiency on cytokine protein expression after cold-induced cortical injury. Mouse cytokine antibody arrays were used to determine the differences in the protein expression of cytokines, chemokines, and other inflammatory factors from sham-operated controls as compared with injured wild-type mice versus injured MyD88-deficient mice. Compared with the protein expression pattern in injured wild-type mice, five immune factors, namely IL-6, granulocyte colony-stimulating factor, KC/CXCL1, CXCL16, and CCL12 (rectangles) were found to be expressed at substantially lower levels in MyD88-deficient mice. To validate the array data, supplemental ELISAs were performed for IL-1β, IL-6, and CXCL16 (see Figure 5).
In contrast to MyD88-deficient mice, protein array analysis did not reveal any differences in brain expression of cytokines, chemokines, and adhesion molecules between TLR2/4 double-deficient mice and wild-type mice 24 hours after cold injury (data not shown). Accordingly, ELISA analyses showed that TLR2/4 double-deficient mice displayed no deficits in the injury-induced production of both IL-1β and CXCL16 (Figure 5). Our data suggest MyD88 dependence but TLR2/4 independence of cold injury-induced immune responses.
Figure 5.
Brain expression of IL-1β, IL-6, and CXCL16 proteins in MyD88- or TLR2/4-deficient mice on cold injury. Cold injury to the brain resulted in a significant increase in brain IL-1β (A), IL-6 (B), and CXCL16 (C) concentrations, compared with sham-operated controls. Whereas injured MyD88-deficient brains contained substantially lower amounts of IL-1β, IL-6, or CXCL16 than brains of wild-type mice, TLR2/4 double-deficient mice showed no deficits in the injury-induced production of both IL-1β and CXCL16. n.d, not determined. *P < 0.05, compared with sham-operated controls. #P < 0.05, compared with injured wild-type mice using one-way analysis of variance and Scheffé’s test.
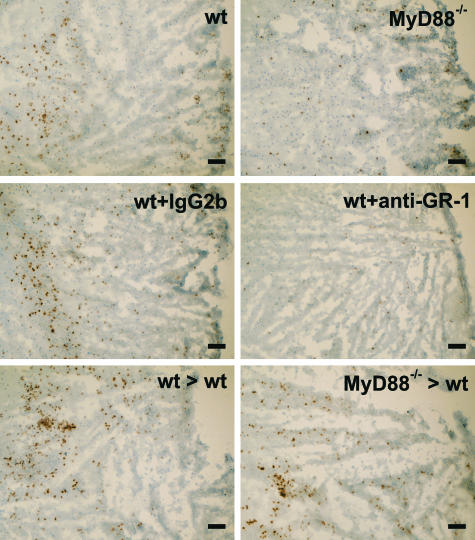
The reduced brain expression of cytokines and chemokines in injured MyD88-deficient mice was paralleled by an attenuated recruitment of GR1-positive cells (neutrophils) to the lesion site (Figure 6). Counting of GR-1-positive cells in the injured cortex revealed an 85% reduction in neutrophil numbers in MyD88-deficient mice (3.7 ± 3.5 cells/mm2) compared with wild-type mice (26.3 ± 6.8 cells/mm2). To determine whether MyD88 signaling in neutrophils and hematopoietic cells might contribute to inflammation after cold-induced cortical injury, wild-type mice were either rendered neutropenic by anti-GR-1 treatment or lethally irradiated and transplanted with MyD88-deficient BM. Whereas GR-1-positive cells were absent in the injured cortex of neutropenic mice (1.5 ± 1.1 versus 28.4 ± 7.8 cells/mm2 in isotype control antibody-treated mice; Figure 6), only a slight reduction in accumulation of GR-1-positive cells was observed in the injured cortex of MyD88 BM chimeras (23.0 ± 10.8 versus 29.0 ± 3.7 cells/mm2 in wild-type BM chimeras; Figure 6). However, neither neutropenic mice nor MyD88 BM chimeras displayed significant differences in expression of cytokines IL-1β and IL-6 as well as the chemokine CXCL16 compared with isotype control antibody-treated injured wild-type mice and wild-type BM chimeras, respectively (Figure 7, A–C). Thus, CNS-resident cells are the most likely source of these cytokines and chemokines after cold-induced cortical injury.
Figure 6.
Impact of MyD88 deficiency and neutrophil depletion on neutrophil infiltration into the lesion site on cold injury. The presence of neutrophils in injured brains was analyzed by immunohistochemistry using an anti GR-1 antibody. Substantial numbers of GR-1-positive cells (stained brown) were found in the lesioned cortex at 24 hours after cold injury in wild-type mice that were untreated (wt; top left image), pretreated with isotype control antibodies (wt + IgG2b; middle left image), or lethally irradiated and transplanted with wild-type BM (wt > wt; bottom left image). Although MyD88 deficiency resulted in an attenuated recruitment of GR1-positive cells to the lesion site (MyD88−/−; top right image), GR-1-positive cells were nearly absent in the injured cortex of neutropenic mice (wt + anti-GR-1; middle right image). Only a slight reduction in accumulation of GR-1-positive cells was observed in the injured cortex of MyD88 BM chimeras (MyD88−/− < wt; bottom right image). Scale bars = 100 μm.
Figure 7.
Brain expression of IL-1β, IL-6, and CXCL16 proteins in neutropenic mice and BM chimeras. Neither neutropenic mice (wt + anti-GR-1) nor MyD88 BM chimeras (MyD88−/− > wt) displayed significant differences in expression of cytokines IL-1β (A) and IL-6 (B) as well as the chemokine CXCL16 (C) compared with isotype control antibody-treated injured wild-type mice (wt + IgG2b) and wild-type BM chimeras (wt > wt). *P < 0.05, compared with sham-operated controls using one-way analysis of variance and Scheffé’s test.
Impact of MyD88 Deficiency and TLR2/4 Double Deficiency on Lesion Development and Clinical Outcome
Because inflammation is thought to contribute substantially to secondary brain damage after acute physical injury,5,6 we also assessed the impact of MyD88 deficiency and TLR2/4 double deficiency on lesion development and clinical status. The lesion volume produced by cold injury was 25% smaller in MyD88-deficient mice than in wild-type mice (Figure 8A). In addition, the number of cells containing broken DNA was significantly lower in injured parietal cortex of MyD88-deficient mice (475 ± 301 cells/×35 field) than in wild-type mice (964 ± 319 cells/×35 field). The number of FragEL-positive cells was also lower in all other brain structures affected by cold injury, including the lesion-remote hippocampus and thalamus (for example: contralateral hippocampus, 107 ± 74 cells/×35 field in MyD88-deficient mice versus 261 ± 106 cells/×35 field in wild-type mice). Reduction of brain damage observed in MyD88-deficient mice was associated to an improved neurological status (Figure 8B). In contrast to MyD88-deficient mice, lesion sizes and numbers of injured cells did not differ in TLR2/4 double-deficient mice and wild-type mice. Moreover, TLR2/4 double deficiency had no detectable impact on the neurological status (Figure 8, A and B). In line with unaltered cytokine and chemokine production was the lack of influence of both neutropenia and MyD88 BM chimerism on lesion development and clinical status (Figure 8, C and D).
Figure 8.
Impact of MyD88 deficiency, TLR2/4 double deficiency, and neutropenia on lesion development and neurological outcome on cold injury. Lack of MyD88 led to a significant reduction in lesion size (A) and to an improved neurological status (B), whereas TLR2/4 double deficiency had no effect on cold injury-induced CNS damage and the clinical outcome. Neither neutropenia (wt + anti-GR-1) nor the MyD88 BM chimerism (MyD88−/− > wt) influenced lesion development and the clinical status, compared with isotype control antibody-treated injured wild-type mice (wt + IgG2b) and wild-type BM chimeras (wt > wt) (C and D). #P < 0.05, compared with injured wild-type mice using one-way analysis of variance and Scheffé’s test.
Discussion
Sterile injury induces symptoms that are reminiscent of inflammation on infection. TLRs mediate their action via MyD88 cell activation not only on microbial or viral challenge but also if discontinuity of regular homeostasis goes along with cell and cell matrix disruption. Therefore, we analyzed MyD88, TLR2, and TLR4 for their potential roles in early responses to acute brain injury.
First, we investigated cytokine/chemokine expression profiles in murine brains at different time points after cold-induced cortical injury. The results of our respective protein array analysis clearly demonstrated that physical injury to the brain resulted in an immediate activation of the innate immune response. Accordingly, expression of 33 of 62 immune factors analyzed, including the cytokines IL-6; the neutrophil chemoattractants KC/CXCL1, MIP-2/CXCL2, and LIX/CXCL5; the monocyte chemoattractants CCL2, CCL5, and CCL12; as well as the adhesion molecules P- and L-selectin, was induced or enhanced to a substantial degree. Up-regulation of several immune factors reported here is in line with recent reports on cytokine and chemokine expression after brain trauma in humans and animal models using other technologies. For instance, early up-regulation of IL-6, MIP-2/CXCL2, and CCL2 expression after cold-induced brain injury was evident. These findings comply with early up-regulation of these factors in the brain after experimental brain trauma as found by immunoblot analysis, ELISA, and/or immunocytochemical techniques as reported previously.43,44,45 In contrast to recent observations (for review, see Schmidt et al6), however, our protein array analysis did not reveal induction of the proinflammatory cytokines IL-1β and tumor necrosis factor-α after cortical injury. This finding might be attributable to low sensitivity of the protein array to these cytokines because we found increased IL-1β levels in the brain after cold injury in supplemental ELISA analysis. In addition to expression levels of previously implicated immune factors, those of further immune factors having not been implicated earlier were found to be altered in response to acute brain injury. Among these newly implicated chemokines are the CC chemokine CCL9 and the CXCL chemokine CXCL16. CCL9 is a member of the macrophage inflammatory protein (MIP)-1 CC chemokine subfamily.46 CCL9 induces chemotaxis of CD4+ and CD8+ T cells and dendritic cells,47,48 as well as potently suppresses activity of the colony formation of BM-derived myeloid progenitor cells.49 In the brain, increased CCL9 expression has been recently observed in mouse models of Staphylococcus aureus-induced brain abscess formation50 and pneumococcal meningitis.51 However, its functional role in the pathogenesis of infection and trauma of the CNS remains to be clarified. CXCL16 is the second transmembrane chemokine identified to date, bearing significant structural homology to fractalkine/CX3CL1.52 When expressed on the cell surface of antigen-presenting cells, CXCL16 facilitates uptake of oxidized low-density lipoproteins53 and phagocytosis of bacteria.54 Membrane-bound CXCL16 acts as an adhesion molecule for CXCR6-expressing leukocytes.55 On cleavage from the cell surface, the soluble CXCL16 molecule has chemoattractant activity for activated CXCR6-expressing CD4+ and CD8+ T cells.55,56,57 Most recently, up-regulation of CXCL16 expression in the CNS has been demonstrated in experimental autoimmune encephalomyelitis58 and pneumococcal meningitis.59 In the experimental autoimmune encephalomyelitis model, CXCL16 has been found to contribute to mononuclear cell trafficking into the spinal cord in the course of this inflammatory disease.58 The role of CXCL16 in brain trauma, however, needs to be determined. Overall, our protein array and ELISA data indicate rapid and sustained up-regulation of cytokine and chemokine expression after aseptic cold injury-induced brain injury. Among the most rapidly induced factors were the CXC chemokines KC/CXCL1 and LIX/CXCL5. Both chemokines are strong candidates for recruiting neutrophils to injured brain areas. CNS-specific overexpression of KC/CXCL-1 produced substantial neutrophil infiltration into perivascular, meningeal, and parenchymal sites,60 and neutralization of LIX/CXCL5 significantly inhibited neutrophil influx into the cornea during LPS keratitis.61 Our immunohistochemical findings together with previous reports41,42,62 imply that leukocyte infiltration is a relatively delayed phenomenon in the pathogenesis of aseptic brain injury, occurring later than 4 hours and reaching its maximum between 24 and 48 hours after injury. Thus, CNS resident cells are the most likely source of cytokines and chemokines like KC/CXCL1 and LIX/CXCL5 at early time points after injury.
Growing evidence indicates a role of TLRs not only in immune responses to infection, but also in pathogen-independent inflammation on sensing of host-derived danger signals such as HSP60, HSP70, GP96, HMGB-1, monosodium urate, and hyaluronan fragments, which have been shown to activate TLR2 and/or TLR4.22,23,24,25,26,27 Activation of TLR4 initiates both MyD88-dependent and -independent signaling cascades, whereas TLR2 signaling is fully dependent on the intracellular adaptor protein MyD88.33,34 We found that loss of MyD88 caused multiple defects in cytokine/chemokine expression and reduced neutrophil infiltration on physical injury to the brain. This observation is consistent with an important role of MyD88 in innate immune responses under noninfectious conditions as has been reported earlier. Accordingly, lack of MyD88 expression correlated with reduction of atherosclerosis through a decrease of macrophage recruitment to the artery wall and reduced chemokine levels.63,64 Myd88 deficiency also resulted in suppression of neutrophil accumulation and cytokine production after injection of the endogenous danger signal uric acid14 in subcutaneous air pouches.26 Moreover, in a mouse experimental autoimmune encephalomyelitis model, MyD88-deficient mice displayed no histologically apparent inflammation.40 In this model, the absence of MyD88 expression from either the radio-sensitive hematopoietic or the radio-resistant CNS compartment caused decreased mononuclear infiltration of the CNS.40 MyD88 expression in hematopoietic cells, however, seems not be required for immune activation after cold-induced brain injury because wild-type mice irradiated and transplanted with MyD88-deficient BM displayed similar up-regulation of cytokines/chemokines and neutrophil infiltration as both wild-type BM chimeras and normal wild-type mice.
To assess the potential contribution of TLR2 and TLR4 to effects mediated by MyD88, we also subjected TLR2/4 double-deficient mice to cold injury. Surprisingly, the lack of TLR2 and TLR4 did not affect injury-induced cytokine/chemokine production. In contrast, published data demonstrated involvement of TLR2 and TLR4 in uric acid-induced, MyD88-dependent inflammation26 and contribution of both TLRs to MyD88-dependent immune activation in atherosclerosis.64,65 Moreover, TLR2 has also been reported to serve a proinflammatory role in renal ischemia-reperfusion injury,66 whereas TLR4 has been implicated in initiating the inflammatory response in hemorrhagic shock67 and in ischemia-reperfusion injury of the heart and liver.68,69 Presently, data on the role of TLRs in noninfectious CNS disease are limited and inconclusive. TLR2 (but not TLR4) deficiency was associated with transient, selective reductions in lesion-induced cytokine/chemokine expression in denervated zones of the hippocampus after transection of axons in the entorhinal cortex,70 whereas loss of functional TLR4 resulted in diminished brain cytokine levels after cerebral ischemia-reperfusion injury71 and lack of TLR2 had no effect on leukocyte infiltration in experimental autoimmune encephalomyelitis.40 Reasons for the discrepancy among studies might be attributable to differences in experimental models. Invasive surgical procedures necessary to transect the entorhinal cortex or to create cerebral ischemia/reperfusion injury bear the risk of contamination with microbial products. Thus challenge with the TLR4 ligand LPS exacerbated neurodegeneration in mouse models of cerebral hypoxia/ischemia72 and amyotrophic lateral sclerosis,73 certainly by promoting an innate immune response. Furthermore, different types of injury and/or by different tissues might cause release of different and specific sets of danger signals.
Tissue injury might result in release of self-DNA and self-RNA from dying cells, both of which have been suggested to act as immunostimulatory factors.16,74 Recently, single-stranded RNA within small nuclear ribonucleoprotein particles was found to activate innate immune cells through TLR7 and TLR8.75 Moreover, when formulated with cationic lipids, mammalian DNA was shown to stimulate cytokine production and immune cell activation in a partly TLR9-dependent manner. Because signaling through TLR7, TLR8, and TLR9 depends on MyD88, their activation by endogenous nucleic acids may contribute to the MyD88-mediated immune activation in our model of cold-induced cortical injury. This hypothesis is strengthened by a recent report on delayed and diminished inflammatory responses in a mouse model of multiple sclerosis if TLR9 expression was lacking.40 However, it is also conceivable that disruption of IL-1β or/and IL-18 signaling in MyD88−/− mice is causative for diminished cytokine/chemokine production after cold lesioning. This concept is supported by recent demonstration of an attenuated production of proinflammatory cytokines in IL-1R-deficient mice subjected to either penetrating cortical injury76 or cerebral hypoxia/ischemia.77 In summary, our data strongly suggest that MyD88 signaling in aseptic brain injury is independent of TLR2 and TLR4. Apart from MyD88 signaling, other signal transduction pathways seem to contribute to immune activation on acute brain tissue damage. For instance, extracellular ATP released from damaged cells have been demonstrated to mediate a rapid microglia response toward injury78 and to induce the release of cytokines like IL-1β, tumor necrosis factor-α, and IL-6 from microglia by P2 receptor activation.79
Efforts during the past years have provided evidence that the profound inflammatory response after physical brain injury contributes to secondary brain damage.6 Accordingly, we found that an increase in lesion size between 1 and 24 hours after cold injury was paralleled by innate immune activation and an attenuated immune response observed in MyD88-deficient mice was associated with reduction of CNS injury, whereas the lack of TLR2 and TLR4 had no effect on both neuroinflammation and lesion development. We further demonstrated that MyD88-dependent activation of radio-resistant CNS cells contributes to brain damage secondary to cold lesioning, whereas invaded neutrophils did not affect acute lesion development. The latter finding is compatible with several reports on lack of infarction size reduction through depletion of neutrophils or interference with neutrophil-endothelial interactions after physical brain injury.80,81 Although the mechanism underlying the neuroprotective effect of MyD88 deficiency remains to be elucidated in detail, our data and those of others suggest involvement of the IL-1R-MyD88 pathway. We observed here that injury-induced up-regulation of IL-1β expression was abrogated in MyD88-deficient mice. Recent studies have demonstrated that inhibition of IL-1β generation and/or IL-1 receptor antagonism attenuated tissue damage after traumatic brain injury.82,83 Similar results have been obtained in models of cold-induced brain injury. Thus, inhibition of caspase-1, which converts pro-IL-1β into its active form, has been shown to lead to a significant reduction of the infarction volume after aseptic cryogenic injury.84 Treatment with an IL-1 receptor antagonist has also been reported to diminish lesion size in mice subjected to cold-induced cortical injury.85 Finally, our observation of just partial decrease of cold injury-induced cortical injury if MyD88 expression is absent indicates involvement of further MyD88-independent inflammatory and/or noninflammatory mechanisms of secondary brain damage after lesioning. MyD88-independent immunostimulatory factors may include ATP and its P2 receptors as well as the complement system,86,87 whereas oxidative stress and glutamate excitotoxicity seem to be major noninflammatory mediators of secondary brain damage.88,89
In summary, we have characterized the time course of cytokine/chemokine expression pattern changes after acute physical injury to the brain. Expression of specific cytokines/chemokines depended on MyD88 signaling on radio-resistant CNS cells, but neither on activation of TLR2 and TLR4, two pattern recognition receptors that have been implicated in the recognition of endogenous danger signals, nor on invaded neutrophils. Moreover, we have shown that loss of MyD88 resulted in reduced acute brain injury and an improved neurological status. This finding emphasizes a therapeutic potential of MyD88 targeting for improvement of acute physical brain injury outcome.
Acknowledgments
We thank Ms. J. Benson for copyediting the manuscript.
Footnotes
Address reprint requests to U. Koedel, Department of Neurology, Klinikum Grosshadern, Ludwig Maximilians-University, Marchioninistr. 15, Munich, D-81377, Germany. E-mail: uwe.koedel@med.uni-muenchen.de.
Supported by the Deutsche Forschungsgemeinschaft (grant SFB576/A5 to U.K. and grants SFB576/A1 and TR22/A5 to C.J.K.).
References
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CAJ. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-Like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury—an inflammatory disease? Brain Res Brain Res Rev. 2005;48:388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167:4844–4852. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Suzuki K, Coban C, Takeshita F, Itoh Y, Matoba H, Kohn LD, Klinman DM. Genomic DNA released by dying cells induces the maturation of APCs. J Immunol. 2001;167:2602–2607. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52:2656–2665. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- Skoberne M, Beignon AS, Bhardwaj N. Danger signals: a time and space continuum. Trends Mol Med. 2004;10:251–257. doi: 10.1016/j.molmed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52:2936–2946. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- Dutcher SA, Underwood BD, Walker PD, Diaz FG, Michael DB. Patterns of heat-shock protein 70 biosynthesis following human traumatic brain injury. J Neurotrauma. 1998;15:411–420. doi: 10.1089/neu.1998.15.411. [DOI] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash P. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Brain Res Mol Brain Res. 2002;104:148–158. doi: 10.1016/s0169-328x(02)00331-5. [DOI] [PubMed] [Google Scholar]
- Tayag EC, Nair SN, Wahhab S, Katsetos CD, Lighthall JW, Lehmann JC. Cerebral uric acid increases following experimental traumatic brain injury in rat. Brain Res. 1996;733:287–291. doi: 10.1016/0006-8993(96)00669-5. [DOI] [PubMed] [Google Scholar]
- Conti A, Sanchez-Ruiz Y, Bachi A, Beretta L, Grandi E, Beltramo M, Alessio M. Proteome study of human cerebrospinal fluid following traumatic brain injury indicates fibrin(ogen) degradation products as trauma-associated markers. J Neurotrauma. 2004;21:854–863. doi: 10.1089/0897715041526212. [DOI] [PubMed] [Google Scholar]
- Iseki K, Hagino S, Mori T, Zhang Y, Yokoya S, Takaki H, Tase C, Murakawa M, Wanaka A. Increased syndecan expression by pleiotrophin and FGF receptor-expressing astrocytes in injured brain tissue. Glia. 2002;39:1–9. doi: 10.1002/glia.10078. [DOI] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Yang G, Chen SF, Morita-Fujimura Y, Chan PH. Cold injury in mice: a model to study mechanisms of brain edema and neuronal apoptosis. Prog Neurobiol. 1999;57:289–299. doi: 10.1016/s0301-0082(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann DL, Bresnahan JC, Beattie MS, Shah BR. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J Neurotrauma. 1992;9:197–217. doi: 10.1089/neu.1992.9.197. [DOI] [PubMed] [Google Scholar]
- Leker RR, Gai N, Mechoulam R, Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke. 2003;34:2000–2006. doi: 10.1161/01.STR.0000079817.68944.1E. [DOI] [PubMed] [Google Scholar]
- Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, Piesche M, Schroers R, Weiss E, Kirschning CJ, Rochford CD, Bruck W, Becher B. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma. 1994;11:499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- Royo NC, Wahl F, Stutzmann JM. Kinetics of polymorphonuclear neutrophil infiltration after a traumatic brain injury in rat. Neuroreport. 1999;10:1363–1367. doi: 10.1097/00001756-199904260-00038. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Banos MA, Herregodts P, Hooghe R, Hooghe-Peters EL. Expression of interleukin (IL)-1beta, IL-6 and their respective receptors in the normal rat brain and after injury. Eur J Immunol. 1992;22:2963–2971. doi: 10.1002/eji.1830221131. [DOI] [PubMed] [Google Scholar]
- Otto VI, Stahel PF, Rancan M, Kariya K, Shohami E, Yatsiv I, Eugster HP, Kossmann T, Trentz O, Morganti-Kossmann MC. Regulation of chemokines and chemokine receptors after experimental closed head injury. Neuroreport. 2001;12:2059–2064. doi: 10.1097/00001756-200107030-00053. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, Poltorak AN, Bergstressor PR, Beutler B, Takashima A. Dendritic cells produce macrophage inflammatory protein-1 gamma, a new member of the CC chemokine family. J Immunol. 1996;156:3102–3106. [PubMed] [Google Scholar]
- Zhao X, Sato A, Dela CC, Linehan M, Luegering A, Kucharzik T, Shirakawa AK, Marquez G, Farber JM, Williams I, Iwasaki A. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b+ dendritic cells. J Immunol. 2003;171:2797–2803. doi: 10.4049/jimmunol.171.6.2797. [DOI] [PubMed] [Google Scholar]
- Youn BS, Jang IK, Broxmeyer HE, Cooper S, Jenkins NA, Gilbert DJ, Copeland NG, Elick TA, Fraser MJJ, Kwon BS. A novel chemokine, macrophage inflammatory protein-related protein-2, inhibits colony formation of bone marrow myeloid progenitors. J Immunol. 1995;155:2661–2667. [PubMed] [Google Scholar]
- Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- Rupprecht TA, Angele B, Klein M, Heesemann J, Pfister HW, Botto M, Koedel U. Complement C1q and C3 are critical for the innate immune response to Streptococcus pneumoniae in the central nervous system. J Immunol. 2007;178:1861–1869. doi: 10.4049/jimmunol.178.3.1861. [DOI] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Wuttge DM, Zhou X, Sheikine Y, Wagsater D, Stemme V, Hedin U, Stemme S, Hansson GK, Sirsjo A. CXCL16/SR-PSOX is an interferon-gamma-regulated chemokine and scavenger receptor expressed in atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2004;24:750–755. doi: 10.1161/01.ATV.0000124102.11472.36. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Nakayama T, Kume N, Takahashi S, Yamaguchi J, Minami M, Hayashida K, Kita T, Ohsumi J, Yoshie O, Yonehara S. Cutting edge: SR-PSOX/CXC chemokine ligand 16 mediates bacterial phagocytosis by APCs through its chemokine domain. J Immunol. 2003;171:1647–1651. doi: 10.4049/jimmunol.171.4.1647. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–1140. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- Fukumoto N, Shimaoka T, Fujimura H, Sakoda S, Tanaka M, Kita T, Yonehara S. Critical roles of CXC chemokine ligand 16/scavenger receptor that binds phosphatidylserine and oxidized lipoprotein in the pathogenesis of both acute and adoptive transfer experimental autoimmune encephalomyelitis. J Immunol. 2004;173:1620–1627. doi: 10.4049/jimmunol.173.3.1620. [DOI] [PubMed] [Google Scholar]
- Klein M, Paul R, Angele B, Popp B, Pfister HW, Koedel U. Protein expression pattern in experimental pneumococcal meningitis. Microbes Infect. 2006;8:974–983. doi: 10.1016/j.micinf.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Tani M, Fuentes ME, Peterson JW, Trapp BD, Durham SK, Loy JK, Bravo R, Ransohoff RM, Lira SA. Neutrophil infiltration, glial reaction, and neurological disease in transgenic mice expressing the chemokine N51/KC in oligodendrocytes. J Clin Invest. 1996;98:529–539. doi: 10.1172/JCI118821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are produced selectively by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol. 2007;81:786–792. doi: 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, Billiar TR. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202:407–417. doi: 10.1016/j.jamcollsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Oyama J, Blais CJ, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, Millward JM, Landmann R, Rivest S, Finsen B, Owens T. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. 2006;26:12826–12837. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, D’Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Capodici J, Cannon G, Communi D, Boeynaems JM, Kariko K, Weissman D. Extracellular mRNA induces dendritic cell activation by stimulating tumor necrosis factor-alpha secretion and signaling through a nucleotide receptor. J Biol Chem. 2002;277:12689–12696. doi: 10.1074/jbc.M110729200. [DOI] [PubMed] [Google Scholar]
- Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Krady JK, O’Malley M, Styren SD, DeKosky ST, Levison SW. The type 1 interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury. J Neurosci. 2002;22:6071–6082. doi: 10.1523/JNEUROSCI.22-14-06071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Lazovic J, Krady JK, Mauger DT, Rothstein RP, Smith MB, Levison SW. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J Cereb Blood Flow Metab. 2005;25:17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl MW, Biagas KV, Grundl PD, Barmada MA, Schiding JK, Nemoto EM, Kochanek PM. Effects of neutropenia on edema, histology, and cerebral blood flow after traumatic brain injury in rats. J Neurotrauma. 1994;11:303–315. doi: 10.1089/neu.1994.11.303. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Dixon CE, Robichaud P, Clark RS, Marion DW, Kochanek PM. Reduced brain edema after traumatic brain injury in mice deficient in P-selectin and intercellular adhesion molecule-1. J Leukoc Biol. 2000;67:160–168. doi: 10.1002/jlb.67.2.160. [DOI] [PubMed] [Google Scholar]
- Sanderson KL, Raghupathi R, Saatman KE, Martin D, Miller G, McIntosh TK. Interleukin-1 receptor antagonist attenuates regional neuronal cell death and cognitive dysfunction after experimental brain injury. J Cereb Blood Flow Metab. 1999;19:1118–1125. doi: 10.1097/00004647-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Tehranian R, Andell-Jonsson S, Beni SM, Yatsiv I, Shohami E, Bartfai T, Lundkvist J, Iverfeldt K. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma. 2002;19:939–951. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- Morita-Fujimura Y, Fujimura M, Kawase M, Murakami K, Kim GW, Chan PH. Inhibition of interleukin-1beta converting enzyme family proteases (caspases) reduces cold injury-induced brain trauma and DNA fragmentation in mice. J Cereb Blood Flow Metab. 1999;19:634–642. doi: 10.1097/00004647-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Jones NC, Prior MJ, Burden-Teh E, Marsden CA, Morris PG, Murphy S. Antagonism of the interleukin-1 receptor following traumatic brain injury in the mouse reduces the number of nitric oxide synthase-2-positive cells and improves anatomical and functional outcomes. Eur J Neurosci. 2005;22:72–78. doi: 10.1111/j.1460-9568.2005.04221.x. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- Elward K, Gasque P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kochanek PM, Kagan VE. Oxidative stress in immature brain after traumatic brain injury. Dev Neurosci. 2006;28:420–431. doi: 10.1159/000094168. [DOI] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]