Abstract
During an inflammatory state, functional myeloperoxidase (MPO) is released into the vessel as a result of intravascular neutrophil degradation. One mechanism of resulting cellular injury involves endothelial internalization of MPO, which causes oxidative damage and impairs endothelial signaling. We report the discovery of a protein that facilitates MPO internalization, cytokeratin 1 (CK1), identified using affinity chromatography and mass spectrometry. CK1 interacts with MPO in vitro, even in the presence of 100% human plasma, thus substantiating biological relevance. Immunofluorescent microscopy confirmed that MPO added to endothelial cells can co-localize with endogenously expressed CK1. CK1 acts as a scaffolding protein for the assembly of the vasoregulatory plasma kallikrein-kinin system; thus we explored whether MPO and high molecular weight kininogen (HK) reside on CK1 together or whether they compete for binding. The data support cooperative binding of MPO and HK on cells such that MPO masked the plasma kallikrein cleavage site on HK, and MPO-generated oxidants caused inactivation of both HK and kallikrein. Collectively, interactions between MPO and the components of the plasma kallikrein-kinin system resulted in decreased bradykinin production. This study identifies CK1 as a facilitator of MPO-mediated vascular responses and thus provides a new paradigm by which MPO affects vasoregulatory systems.
Vascular injury due to inflammation is caused by the release of injurious radicals and proteins from granule components of neutrophils and monocytes into the extracellular space. MPO is one of the principal enzymes stored in azurophilic granules of neutrophils and peroxisome-positive lysosomes of monocytes and comprises 5 and 1%, respectively, of the cells’ total protein.1 MPO imparts its antimicrobial properties by converting hydrogen peroxide, generated by neutrophils during a respiratory burst, into hypochlorous acid, a powerful oxidant that readily reacts with adjacent thiol, disulfide, and amino acid residues.2
Increased levels of MPO are detected in serum of patients with inflammatory disease, suggesting that neutrophils aberrantly degranulate in the vascular lumen.3,4,5,6,7 Free MPO spilled into the vasculature interacts with endothelial cells and contributes to endothelial dysfunction.8,9,10 Our previous explorations of this process indicated that MPO is internalized by endothelial cells with a resultant rise in intracellular oxidant radicals.11 Others have extended these findings by demonstrating that MPO transcytoses through the endothelium and concentrates in the subendothelial matrix.12 Internalized MPO modulates vascular signaling and vasodilatory functions by decreasing the bioavailability of nitric oxide via multiple mechanisms.6,8 For example, hypochlorous acid can chlorinate l-arginine, thus reducing functional substrate for endothelial nitric-oxide synthetase, and substrate radicals produced by MPO can catalytically consume nitric oxide.6,8
As a continuation of our previous work, our aim was to identify proteins on the endothelial cell surface that specifically interacted with MPO, with the goal of finding proteins that facilitate its internalization. We have discovered that cytokeratin 1 (CK1) is an endothelial binding partner for MPO. CK1 is part of a possible endothelial receptor complex containing urokinase-like plasminogen activator receptor (uPAR) and the receptor for the globular head of complement 1q protein (gC1qR). This multiprotein receptor complex is a platform for assembly and activation of the vasoregulatory plasma kallikrein-kinin system.13,14 Circulating high molecular weight kininogen (HK) binds to this complex and serves as the endothelial receptor for the zymogen prekallikrein. An endothelial membrane- and matrix-associated enzyme, prolylcarboxypeptidase, activates prekallikrein, which then cleaves the nonapeptide bradykinin from HK.15 Because both MPO and HK interact with CK1, MPO might interfere with the functionality of the kallikrein-kininogen system.
The results herein provide a novel layer of interaction between MPO and endothelial vasoregulatory systems that could potentiate vascular injury during inflammation. Functional studies indicate that CK1 can facilitate internalization of MPO, and moreover, this interaction has the potential to disrupt normal endothelial processes.
Materials and Methods
Cell Culture and Reagents
Cells
Human umbilical vein endothelial cells (HUVECs) and endothelial growth medium (EGM) basal media with Singlequot supplements were purchased from Cambrex (East Rutherford, NJ). Cells were typically used between passages 5 and 10. EA.hy926, an immortalized endothelial hybridoma cell line, was kindly provided by Cora-Jean Edgell, Ph.D. (University of North Carolina at Chapel Hill, Chapel Hill, NC) and cultured in Gibco/Invitrogen (Carlsbad, CA) Dulbecco’s modified Eagle medium with penicillin-streptomycin and 10% fetal bovine serum. Both cell lines were grown in a 5% CO2 humidified incubator. EA.hy926 cells were used in addition to HUVECs in immunofluorescent co-localization studies and in internalization blocking experiments. Reproducibility of the data using two different endothelial cell lines lends confidence in its validity and implies generality in endothelial systems.
Antibodies
Mouse and rabbit anti-human MPO antibodies were purchased from DAKO (Carpinteria, CA). An additional mouse anti-human MPO antibody was purchased from Abcam (Cambridge, MA). Mouse anti-proteinase 3 antibodies were purchased from Lab Vision Neomarkers (Fremont, CA) and Research Diagostics, Inc. (Concord, MA). Rabbit anti-CK1 antibody was purchased from Covance Research Products (Berkley, CA), and the mouse pan-cytokeratin antibody (clone PCK-26) was from Sigma (St. Louis, MO). Affinity-purified goat anti-CK1 antibody was prepared as previously characterized.16,17 Normal total IgG of all species was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-conjugated secondary antibodies were purchased from Chemicon (Temecula, CA), and Alexa Fluor 488- and 569-conjugated secondary antibodies were purchased from Invitrogen (Carlsbad, CA). Alkaline phosphatase-conjugated streptavidin was purchased from Pierce (Rockford, IL). Alkaline phosphatase-conjugated Affinipure F(ab′)2 fragment donkey anti-mouse IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). The alkaline phosphatase substrate kit was either from Bio-Rad Laboratories (Hercules, CA) or Pierce.
Purified Proteins
MPO and catalase were purchased from Calbiochem (San Diego, CA). Proteinase 3 was purchased from Weislab AB (Lund, Sweden). Recombinant CK1 (rCK131) was prepared as previously reported.17 Pooled human cytokeratins and l-methionine were purchased from Sigma. High molecular weight kininogen, plasma kallikrein, factor XII (SA 27.77 U/mg), and high molecular weight kininogen-deficient human plasma were purchased from Enzyme Research Laboratories (South Bend, IN). PilB, a methionine sulfoxide reductase derived from Neisseria gonorrhoeae, was generously provided by Dr. Nathan Brot at Cornell University, Joan and Sanford I. Weill Medical College, New York, NY.
Immunoprecipitations of MPO and CK1
HUVEC lysates were prepared as either whole cell or membrane fraction lysates. To prepare the whole cell lysate, HUVECs were washed with ice-cold phosphate-buffered saline (PBS) and scraped in lysis buffer [25 mmol/L HEPES, 12.5 mmol/L MgCl2, 150 mmol/L KCl, 0.5% Nonidet P-40, 1 mmol/L sodium orthovanadate, 50 mmol/L sodium fluoride, and Roche (Almeda, CA) complete ethylenediaminetetraacetic acid proteinase inhibitor cocktail] at 4°C. Glycerol was added to 10%. Lysates were pooled and split evenly into three tubes. Insoluble debris was centrifuged and separated from lysate. Membrane fraction lysate was prepared using Pierce’s MEM-PER isolation kit as instructed with the addition of sodium fluoride, sodium orthovanadate, and the Roche protease inhibitor cocktail. The pooled membrane fraction was dialyzed in PBS + 1% Nonidet P-40 overnight for 3 nights, changing the dialysate each night. Glycerol was added to 10%, and the pooled lysate split evenly into three tubes. Protein A/G beads (Pierce) and normal mouse IgG were added to each tube for 1 hour at 4°C and then centrifuged to preclear the lysate. Ten μg of purified proteinase 3 or MPO was added to the lysates for 5 hours at 4°C, and mouse anti-MPO or anti-PR3 antibodies were added to the appropriate tubes overnight. Protein A/G beads were added to the tubes for 1 hour at 4°C. The beads were washed with lysis buffer and PBS, and any protein complexes were eluted from the beads directly in Laemmli buffer. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then stained with Coomassie Brilliant Blue R-250. Unique bands in the MPO immuno-precipitation lane were excised and analyzed by matrix-assisted laser desorption ionization/time of flight at the University of North Carolina’s Proteomics Core Facility using methods published by Parker and colleagues.18
Mouse anti-MPO antibody (100 μg) was immobilized on Pierce’s AminoLink Plus coupling gel as instructed. HUVECs were washed with ice-cold Hanks’ balanced salt solution and scraped at 4°C into lysis buffer (formulation above) containing 0.5% Triton X-100 and 10% glycerol. The lysate was sheared with a 22-gauge needle and the insoluble debris centrifuged and separated. The lysate was split into two tubes, one of which received 2.5 μg of purified MPO. The tubes were rocked at 4°C for 3 hours, after which immobilized mouse anti-MPO was added to the tubes overnight. The gel-containing lysates were spun and washed in Pierce’s Handee Cup spin columns with 10 column volumes of lysis buffer and eluted using the AminoLink plus kit. Laemmli buffer was added to each elution fraction and the fractions were subjected to SDS-PAGE and Western blot analysis.
Two μg of purified MPO was mixed with either 2 μg of recombinant CK1, 2 μg of recombinant control protein, or nothing in IP buffer (25 mmol/L HEPES, 12.5 mmol/L MgCl2, 150 mmol/L KCl, 0.5% Nonidet P-40, and 10% glycerol) overnight at 4°C with constant mixing. Four μg of either mouse anti-MPO or mouse anti-pan CK antibody was added to the mix, as well as antibody alone in IP buffer, and allowed to mix overnight at 4°C. Protein A/G beads (Pierce) were added for 1 hour and washed, and protein complexes were eluted directly in Laemmli buffer. Elutions were subject to SDS-PAGE and Western blot analysis.
Enzyme-Linked Immunosorbent Assays (ELISAs) Demonstrating MPO Binding to CK1 and HK
ELISA plates were coated with 5 μg/ml of either rabbit anti-CK1 or normal rabbit IgG overnight at 4°C. Wells were blocked with a 0.2% fish gelatin buffer (150 mmol/L NaCl, 50 mmol/L Tris base, 0.05% Tween 20, 0.2% fish gelatin, and 0.02% sodium azide, pH 7.6) for 4 hours at room temperature, and all subsequent steps were performed in this blocking solution. CK1 was captured overnight at 4°C from a mix of solubilized human keratins (10 μg/ml) derived from human epidermis. Purified MPO was presented to the captured CK1 at 5 μg/ml at room temperature for 4.5 hours. The wells were exposed to mouse anti-MPO antibody at a 1:100 dilution and each of three sera from patients with circulating MPO-specific anti-neutrophil cytoplasmic autoantibodies diluted at 1:100 in duplicate overnight at 4°C. The CK1-MPO complexes were detected by alkaline phosphatase-conjugated secondary antibody incubation for 1 hour at room temperature followed by the addition of substrate. Plates were read at 405 nm after 1 hour using a VERSAmax plate reader (Molecular Devices, Sunnyvale, CA).
ELISA plates were coated with either rabbit anti-CK1 antibody or normal rabbit IgG at 5 μg/ml overnight at 4°C. The wells were blocked in a 1% fish gelatin buffer for 4 hours at room temperature and then exposed to a 10 μg/ml solution of solubilized human keratins in blocking buffer overnight at 4°C to capture CK1. Plasma was obtained from healthy patients in Vacutainer tubes K3 ethylenediaminetetraacetic acid 12 mg (purple top) (Becton Dickinson, Franklin Lakes, NJ). Solutions of 0, 25, 50, 75, and 100% plasma were prepared with or without the addition of 5 μg/ml MPO. The plasma solutions were prepared using 1% fish gelatin buffer that was drawn into the Vacutainer tubes to keep the ethylenediaminetetraacetic acid concentration constant throughout this stage of the experiment. The plasma solutions were placed in the wells for 4 hours at room temperature. The captured MPO was labeled with the DAKO mouse anti-MPO antibody at a 1:100 dilution at 4°C overnight. The next day, secondary antibody was applied for 1 hour at room temperature followed by substrate. The plates were read at 405 nm 3 hours after the addition of substrate.
ELISA plates were coated either with rabbit or mouse anti-MPO antibody or with normal rabbit or mouse IgG at 5 μg/ml overnight at 4°C. The wells were blocked with 0.2% fish gelatin buffer for 1 hour at room temperature, and all subsequent steps were performed in the blocking buffer. MPO was captured at 5 μg/ml for 1 hour at room temperature. Wells were washed thoroughly, and 2 μg/ml biotinylated HK was added with or without a 75-fold excess of unlabeled HK in duplicate. Unlabeled HK only was also added to wells in duplicate. After 1 hour at room temperature, the wells were washed, and alkaline phosphatase-conjugated streptavidin was added for an additional hour. After the addition of substrate for 1 hour, the plate was read at 405 nm. Similar experiments were conducted using ELISA plates directly coated with either MPO or bovine serum albumin.
ELISA plates were coated with either rabbit anti-MPO antibody or normal rabbit IgG (5 μg/ml) overnight at 4°C. The wells were blocked with 1% fish gelatin buffer for 2 hours at room temperature. HK-deficient plasma was replenished with a physiological concentration (80 μg/ml) of biotinylated high molecular weight kininogen (bHK). The HK-deficient plasma was added to the wells with or without bHK overnight at 4°C. After thorough washing, alkaline phosphatase-conjugated streptavidin was added to the wells for 1 hour at room temperature. Subsequently, substrate was applied, and the plate was placed at 4°C overnight. Spectrophotometer readings were taken at 405 nm after resting the plates at room temperature for 6 hours.
Immunofluorescent Co-Localization of MPO and CK1
EA.hy926 cells were plated on glass coverslips, grown to ∼90% confluence, washed, and treated with MPO (10 μg/ml) for 10 minutes in serum-free Dulbecco’s modified Eagle’s medium. The cells were washed in PBS, fixed in 4% paraformaldehyde, and permeabilized with acetone. Goat serum was used as a block (at 10% in Tris-buffered saline with 0.05% Triton X-100) and antibody dilution buffer (at 1% in Tris-buffered saline with 0.05% Triton X-100), and the cells were labeled with rabbit anti-CK1 and mouse anti-MPO antibodies. Alexa Fluor-conjugated secondary antibodies were used for detection: the Alexa Fluor 488 was goat anti-rabbit, and the Alexa Fluor 568 was goat anti-mouse. The coverslips were mounted on slides with anti-fade and analyzed using a LSM5 Pascal confocal laser-scanning microscope (Zeiss, Thornwood, NY) with the accompanying software suite.
HUVECs were plated to ∼90% confluence (140,000 cells per well) and allowed to attach overnight to 0.1% gelatin-coated glass coverslips in 12-well culture dishes. Cells were washed twice with prewarmed, phenol red-free EGM basal media with or without 2% fetal bovine serum, and MPO was added at 15 μg/ml for 12 minutes at 37°C. Cells were then placed on ice and washed in ice-cold PBS and fixed in ice-cold 2% paraformaldehyde for 45 minutes on ice. The wells were then washed and permeabilized with ice-cold 1:1 methanol/acetic acid on ice for 10 minutes. Goat serum was used as the blocking agent (at 10% in Tris-buffered saline with 0.05% Triton X-100) and the antibody dilution buffer (at 1% in Tris-buffered saline with 0.05% Triton X-100). The coverslips were blocked for 1 hour at room temperature. The cells were then labeled with the Abcam mouse anti-MPO antibody (1:250 dilution) for 1 hour at room temperature, followed by rabbit anti-CK1 (1:80 dilution) in the same conditions. Alexa Fluor-conjugated secondary antibodies were used for detection: the Alexa Fluor 488 was goat anti-rabbit, and the Alexa Fluor 568 was goat anti-mouse. The secondary antibodies were prepared at a 1:500 dilution and applied simultaneously. The coverslips were mounted on slides with anti-fade and analyzed using a Zeiss LSM5 Pascal confocal laser-scanning microscope with the accompanying software suite.
Antibody Blocking Experiments
EA.hy926 cells grown in 12-well culture dishes, or HUVECs grown in six-well dishes, were washed with serum-free, phenol red-free Dulbecco’s modified Eagle’s medium. Cells were pretreated in the serum-free media with blocking antibodies. One experiment used a blocking mix consisting of 16.67 μg/ml goat-anti-CK1 (which blocks HK binding to endothelial cells) and a 50-fold molar excess of factor XII (which also binds CK1) for 45 minutes at 37°C.16 Other experiments used the pan-cytokeratin antibody or normal mouse IgG at 16.67 μg/ml for 45 minutes at 37°C. MPO (0.5 to 2 μg/ml) was added subsequently to the cells in the presence of the blocking antibodies for 10 minutes. The cells were washed with the serum-free media and trypsinized. When the cells released from the plate, either trypsin-neutralizing solution (Cambrex) or serum-containing medium was added, and the cells were collected. The cells were processed using the Caltag Laboratories Fix and Perm kit (Invitrogen) and stained for MPO using the rabbit anti-MPO antibody in conjunction with an Alexa Fluor 488 chicken anti-rabbit secondary antibody. The stained cells were analyzed using a FACScan flow cytometer linked to a CellQuest software system (Becton-Dickinson Immunocytometry Systems, San Jose, CA).
HK-Binding Studies on HUVECs
HUVECs were plated to confluence in EGM basal media (without the Singlequot supplement) in 96-well tissue culture wells that were precoated with a 1% gelatin solution. Eighteen to twenty-four hours after plating, the cells were washed with a HEPES-carbonate buffer (154 mmol/L NaCl, 5.6 mmol/L KCl, 3.4 mmol/L NaHCO3, 5.5 mmol/L dextrose, 5 mmol/L HEPES, 2 mmol/L CaCl2, 1 mmol/L MgCl2, and 0.1% gelatin, pH 7.4), and biotinylated HK was added to the cells at 20 nmol/L in HEPES-carbonate buffer either alone or in the presence of a 50-fold molar excess of unlabeled HK or MPO for 30 minutes at 37°C. Unbound proteins were washed away, and the cells were treated with alkaline phosphatase-conjugated streptavidin at room temperature for 1 hour. The cells were washed, the substrate was applied, and the absorbance was read at 405 nm after 1 hour at room temperature.
Immunofluorescent Co-Localization of MPO and HK on Endothelial Cells
HUVECs were grown to 80% confluence on 0.1% gelatin-coated glass coverslips. Biotinylated HK (50 nmol/L) was premixed with a fivefold molar excess of MPO in EGM basal medium. The cells were gently washed with the EGM basal medium and the protein mix added for 30 minutes at 37°C. Control groups included each protein added individually or no protein. The cells were placed on ice, washed thoroughly in ice-cold PBS, and then fixed in 1.5% paraformaldehyde for 40 minutes at room temperature. Some coverslips were dipped in acetone to permeabilize the cells. The coverslips were blocked in 10% chicken serum for 45 minutes. Subsequent steps were performed in 1% chicken serum for 1 hour at room temperature in the order listed with thorough PBS washes in between each step: Alexa Fluor 488-conjugated streptavidin diluted 1:500, rabbit anti-MPO at a 1:2500 dilution, and Alexa Fluor 569 chicken anti-rabbit secondary antibody diluted 1:1500. The coverslips were then mounted in anti-fade medium.
ELISA Measuring the Effects of MPO on Bradykinin Production
To quantitate bradykinin released in the presence of MPO, HK (50 nmol/L) was incubated with 5, 50, or 500 nmol/L MPO in PBS for 20 minutes at room temperature in microcentrifuge tubes. Kallikrein (50 nmol/L) was added to the mix for 20 minutes at room temperature, and the samples were frozen at −80°C for future analysis. Samples were thawed and analyzed for bradykinin release using Dainippon Pharmaceutical’s MARKIT-M bradykinin ELISA kit (Osaka, Japan). Our experimental samples were handled for analysis per the manufacturer’s instructions specific to urine specimens. Amount of reagents added was adjusted to accommodate smaller volumes.
To measure the effect of the MPO-H2O2-Cl− system activity on bradykinin production, HK (50 nmol/L) was incubated with MPO (10, 50, or 250 nmol/L) and 0.45 μmol/L H2O2 in PBS in the presence or absence of catalase (10-fold molar excess to MPO) for 20 minutes at room temperature in microcentrifuge tubes. l-Methionine (100 μmol/L) was added to the mix for 20 minutes to quench HOCl activity. Kallikrein (50 nmol/L) was then added for 20 minutes at room temperature, and the samples were frozen at −80°C for future analysis. Samples were thawed and analyzed for bradykinin release using the aforementioned bradykinin ELISA kit.
To determine the direct effects of HOCl on bradykinin production, HK (50 nmol/L) was pretreated with increasing concentrations of HOCl in PBS at room temperature for 20 minutes in microcentrifuge tubes. A 20-fold excess of l-methionine (compared with HOCl concentration) was added to the mix for an additional 20 minutes. Kallikrein was subsequently added to 50 nmol/L for 20 minutes at room temperature, and the samples were frozen at −80°C for further analysis. Bradykinin release was measured in the samples using the bradykinin ELISA kit. The unknown values were determined using a seven-point standard curve with an R2 value of 0.95.
Kallikrein Protease Activity Assay
Kallikrein (50 nmol/L) was treated with HOCl or HOCl that was preincubated with a 20-fold excess of l-methionine (to quench oxidative properties) at varying concentrations in microcentrifuge tubes for 15 minutes. l-Methionine was subsequently added to those tubes that received nonquenched HOCl. Samples were transferred to a 96-well plate, and Chromogenix S-2303 plasma kallikrein-specific chromogenic substrate (Diapharma, Westchester, OH) was added and mixed, and OD 405 nm was read after 30 minutes of rocking at room temperature. Values were adjusted by subtracting background OD values of HOCl + l-methionine without kallikrein.
Methionine Sulfoxide Reductase Assay
HK (50 nmol/L) was pretreated with 25 μmol/L HOCl in PBS for 20 minutes at room temperature, and unreacted HOCl was quenched with 500 μmol/L l-methionine (a 20-fold molar excess) for an additional 20 minutes. PilB (50 nmol/L) was added to the reaction mix in the presence of 15 mmol/L dithiothreitol and 30 mmol/L Tris-HCl, pH 7.4, for 1.5 hours at 37°C. After cooling the tubes to room temperature, plasma kallikrein was added to the reaction at 50 nmol/L for 25 minutes, and kallikrein activity was stopped with 0.5 μmol/L di-isopropylfluorophosphate. The samples were deproteinized using reagents provided with the Dainippon MARKIT-M bradykinin ELISA kit and analyzed for bradykinin release using Bachem Peninsula Laboratory’s (San Carlos, CA) bradykinin EIA kit as per the manufacturer’s instructions. The unknown values were determined using a five-point standard curve with an R2 value of 0.90.
Results
Identification of CK1 and CK9 as Endothelial MPO-Binding Proteins
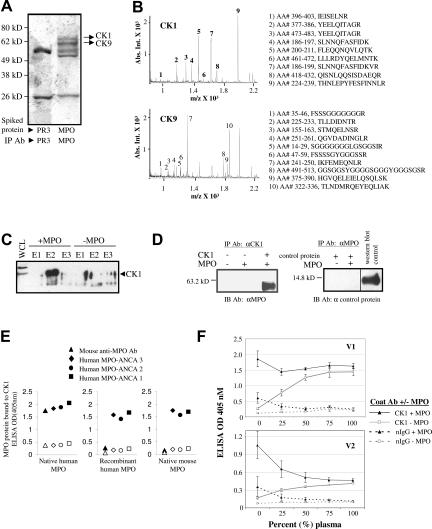
To identify endothelial cell surface proteins that bind MPO, purified MPO was incubated with HUVEC membrane lysates, and subsequent complexes were immunopurified using an anti-MPO antibody. For comparison, proteinase 3, also a cationic neutrophil granule protein, was added to an aliquot of endothelial lysates and analyzed for binding partners in parallel. Two proteins were identified that specifically bound MPO and not proteinase 3 under these conditions (Figure 1A). Matrix-assisted laser desorption ionization/time of flight analysis de-termined these to be CK1 (67 kd) and CK9 (62 kd) (Figure 1B).
Figure 1.
CK1 and CK9 specifically bind MPO. A: Coomassie-stained gel of immunopurified MPO or proteinase 3 (PR3) complexes resolved by SDS-PAGE. Mass spectrometry analysis of the unique bands in the MPO-spiked membrane fraction identified CK1 and CK9. The spectra are shown in B. The tryptic peptides are numbered and displayed with the corresponding sequences. C: Western blot analysis verified that CK1 binds to MPO. HUVEC whole cell lysates were incubated with purified MPO and passed over a monoclonal MPO-specific antibody immobilized on Aminolink Plus coupling gel. The proteins eluted from the beads were subjected to SDS-PAGE and Western blot analysis using CK1-specific antibody. E1, E2, and E3 are sequential elution fractions from the beads. D: In vitro mixing of purified proteins resulted in the binding of MPO to CK1 but not to a control recombinant protein (BMP7). Western blot analysis (IB) of immunopurified complexes (IP) indicated that an anti-CK1 antibody (Ab) immunoprecipitated MPO but only when complexed with CK1. MPO does not bind the control recombinant protein demonstrating the specificity of the MPO-CK1 interaction. E: Sandwich ELISAs: the different shapes represent the antibody used to detect MPO binding. Solid black indicates the ELISA well was coated with a CK1 antibody, and open shape indicates the well was coated with normal IgG. Antibody-captured CK1 was incubated with purified MPO, recombinant MPO, or purified mouse MPO. Results show that purified MPO was detected by the monoclonal MPO antibody, but this antibody did not react with recombinant MPO or mouse MPO. Anti-MPO antibodies from three patients with anti-neutrophil cytoplasmic autoantibody disease (MPO-ANCA) recognized the CK1-MPO complex with all three forms of MPO protein. F: CK1 captures MPO directly from human plasma. ELISA wells were coated with either anti-CK1 antibody or normal IgG. CK1 was then captured and incubated with or without MPO in increasing amounts of plasma drawn from two healthy volunteers (V1 and V2).
CK1 was verified to physically bind MPO using an anti-MPO affinity column to capture CK1 in complex with MPO in the context of HUVEC protein lysates. Western blot analysis showed CK1 specifically eluted with MPO (Figure 1C). Mixing of purified MPO and CK1 further confirmed the specificity of this interaction (Figure 1D). MPO specifically bound CK1 whereas a negative-control recombinant protein did not.
We next examined whether the interaction of MPO and CK1 was limited to native human MPO and, moreover, whether the complex was detectable using human anti-MPO antibodies from patients with MPO-specific autoantibodies.19 Sandwich ELISAs demonstrated that CK1 complexed with human native MPO, recombinant human MPO, and native mouse MPO. MPO was detected only when an anti-CK1 antibody was present to capture CK1 as compared with when a normal rabbit IgG was used as the capture antibody (Figure 1E). The mouse anti-human MPO antibody reacted with only the native MPO protein.
MPO interacts with several blood proteins such as ceruloplasmin (its natural inhibitor), albumin, and the lipoprotein apo-a1.20,21,22 The above studies were performed in total cell lysates or in serum-free buffer to test specificity. We asked if the MPO-CK1 interaction was influenced by the presence of plasma proteins, as found in an in vivo environment. In a competition assay, purified MPO binding to immobilized CK1 was slightly competed away by addition of plasma from volunteer 1 and saturated at 25% plasma (Figure 1F). This effect was more pronounced in volunteer 2 but saturated at 50%. In parallel, plasma was added to immobilized CK1 without exogenously added MPO to determine whether circulating plasma MPO would bind. Free MPO in plasma bound CK1, and interestingly, binding was maximal at the concentration coinciding with the saturation point with excess added MPO, indicating all available CK1 was complexed.
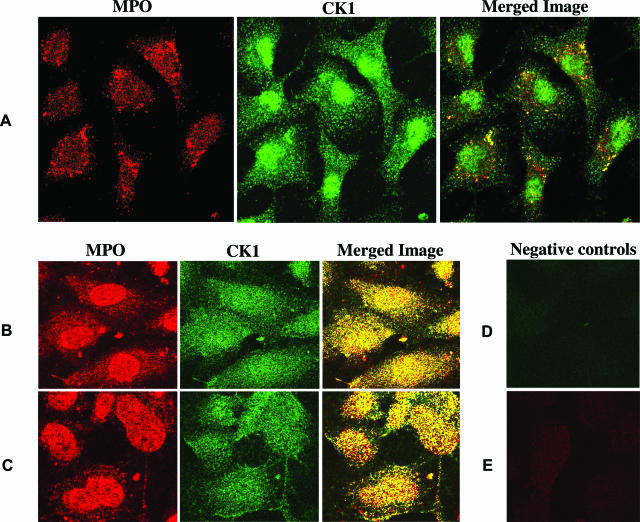
To test this interaction at the cellular level, cells expressing surface CK1 were treated with MPO in the absence or presence of plasma and analyzed for the ability of these proteins to co-localize. By immunofluorescence microscopy, MPO co-localized with CK1 expressed on EA.hy926 cells and on HUVECs (Figure 2). Widespread co-localization was noted in HUVECs on the cell surface and intracellularly in both the absence and presence of serum (2%). Based on this data, MPO binding to CK1 is highly likely to occur in vessels.
Figure 2.
MPO and CK1 co-localize in living cells. A: EA.hy926 cells were incubated with MPO in serum-free medium. MPO was detected with the DAKO mouse anti-MPO antibody, and CK1 was detected using a polyclonal anti-CK1 antibody. The yellow foci in the merged image indicate areas of co-localized CK1 and MPO. B and C: HUVECs were also exposed to MPO in serum-free (B) or serum-containing (C) medium. MPO was labeled in this experiment with a mouse MPO antibody from Abcam. CK1 was labeled with the same polyclonal antibody used in A. Widespread co-localization was noted in both conditions. D: Negative control for the CK1 label on HUVECs (anti-rabbit secondary antibody only). E: Negative control for the MPO label on HUVECs (cells not treated with MPO but exposed to the anti-MPO antibody and its corresponding secondary antibody). Original magnifications, ×60.
Internalization of MPO by Endothelial Cells Is Blocked by Interfering with the MPO-CK1 Interaction
To test whether CK1 participates in the uptake of MPO, we performed a blocking experiment using anti-CK1 antibodies. After antibody treatment, cells were analyzed for uptake of added MPO by flow cytometry. Experimental design included a trypsinization step to remove residual MPO not internalized by the cells. MPO uptake was reduced in the presence of anti-CK1 antibodies but not IgG controls (Figure 3). Blocking of MPO internalization was concentration-dependent, ie, saturation of the system with MPO overwhelmed the antibody blocking effects (Figure 3A). A blocking mix consisting of a polyclonal goat anti-CK1 antibody and FXII reduced the percentage of MPO-positive EA.hy926 cells from 42.1 to 6.1% when the cells were treated with 2 μg/ml MPO but did not block when treated with 5 μg/ml MPO. Similar results were obtained when a monoclonal antibody specific for type II cytokeratins was used as a blocking antibody on HUVECs. Pretreatment with this antibody resulted in more than a 50% decrease in the number of MPO-positive cells (Figure 3B). This monoclonal antibody was used to block CK1 on EA.hy926 cells; however, in this experiment normal mouse IgG was tested to assure that the mouse IgG, in general, was not responsible for blocking MPO. The CK antibody decreased the number of MPO-positive cells from 33% (with the normal mouse IgG) to 12.7%.
Figure 3.
Internalization of MPO by endothelial cells is reduced by blocking the MPO and CK1 interaction. Experiments are flow cytometry analyses of endothelial cells labeled with a polyclonal anti-MPO antibody. A: EA.hy926 cells were pretreated with a CK1-blocking mix consisting of factor XII (FXII) and a goat anti-CK1 antibody (CK Ab) raised against a kininogen-binding site of CK1. Cells were then exposed to 2 or 5 μg/ml MPO for 10 minutes. B: HUVECs were pretreated with a pan-CK (pCK Ab) antibody and then 2 μg/ml MPO was added for 10 minutes. C: EA.hy926 cells were pretreated with either a pan-CK (pCK Ab) antibody or normal mouse IgG before 0.5 μg/ml MPO was added for 10 minutes. FITC, fluorescein isothiocyanate; FSC, forward scatter.
MPO Directly Interacts with HK
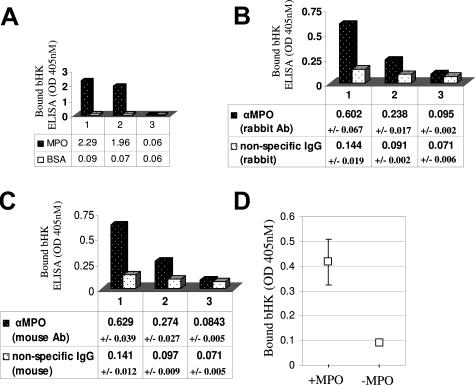
To investigate potential effects of MPO binding to CK1 on vascular processes, we asked if MPO would compete with HK for binding to CK1, because HK is known to also bind CK1.23 Rather than compete for CK1 binding, a more than fivefold increase in binding of biotinylated HK was observed in the presence of MPO (50-fold excess), whereas the controls using a 50-fold excess of unlabeled HK blocked biotinylated HK binding by 40% (Figure 4A). Moreover, by immunofluorescence microscopy, MPO and HK were found to co-localize at the surface membrane and in the cytoplasm of endothelial cells (Figure 4B). We next asked if enhanced binding could be attributable to a direct interaction between HK and MPO. Data from a direct ELISA (Figure 5A) and from a capture ELISA (Figure 5, B and C) verify that MPO and HK have the capability to physically interact. Importantly, we also showed that immobilized MPO bound kininogen from plasma (Figure 5D), thus demonstrating that this interaction is favored in a physiological matrix.
Figure 4.
MPO enhances kininogen binding to endothelial cells and the proteins co-localize on the endothelial cell surface and intracellularly. A: MPO enhances kininogen binding. HUVECs were treated with biotinylated high molecular weight kininogen (bHK) either alone or in the presence of a 50-fold molar excess of unlabeled high molecular weight kininogen (uHK) or MPO for 30 minutes at 37°C. Bound bHK was detected using alkaline phosphatase-conjugated streptavidin. Unlabeled HK competed with bHK binding resulting in 50% less bHK protein bound. MPO enhanced bHK binding by 5.35-fold. B–E: MPO and kininogen co-localize in/on endothelial cells. HUVECs were either treated with bHK and purified MPO (B–D) or protein-free medium. The cells were labeled using both Alexa Fluor 488-conjugated streptavidin (B) and an anti-MPO antibody with corresponding Alexa Fluor 568 secondary antibody (C). D: The merged image of B and C showing a high degree of co-localization between MPO and bHK. The merged image of cells that were treated with protein-free medium is shown in E. Original magnifications, ×60.
Figure 5.
MPO binds kininogen. A: Direct ELISA: column 1: biotinylated kininogen (bHK) binds immobilized MPO but not bovine serum albumin as detected by alkaline phosphatase-conjugated streptavidin; column 2: bHK binding is reduced in the presence of excess unlabeled kininogen (uHK); column 3: unlabeled kininogen alone was negative. B: Sandwich ELISA: further validation of the direct interaction between MPO and kininogen. Column 1: monoclonal MPO antibody-captured MPO binds bHK as detected by alkaline phosphatase-conjugated streptavidin; column 2: competition of bHK binding in the presence of excess uHK; column 3: unlabeled kininogen alone was negative. Shown are the averaged results of three independent trials ± SDs. C: Sandwich ELISA: validation using a second MPO antibody. Column 1: polyclonal MPO antibody-captured MPO binds bHK as detected by alkaline phosphatase-conjugated streptavidin; column 2: competition of bHK binding in the presence of excess uHK; column 3: unlabeled kininogen alone was negative. Shown are the results of three independent trials ± SDs. D: MPO captures bHK from plasma. HK-depleted plasma was replenished with bHK to physiological levels. Immobilized MPO was used as bait to capture bHK from plasma. Shown are the results (with SE bars) of three independent experiments.
MPO Interferes with the Plasma Kallikrein-Kinin System
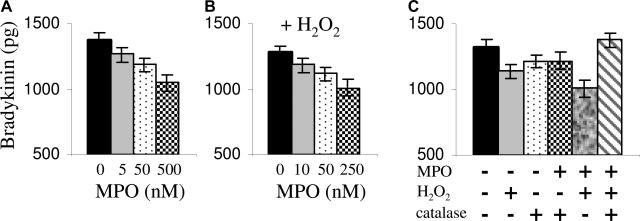
To further explore MPO-mediated perturbations of the plasma kallikrein-kinin pathway, we tested whether MPO affected bradykinin production. We hypothesized that MPO binding to HK would interfere with its cleavage by kallikrein to produce bradykinin. Bradykinin production from HK by plasma kallikrein was monitored in an in vitro system with and without MPO. The results indicate that a 10-fold molar excess of MPO (500 nmol/L) to HK reduced bradykinin production by 30% compared with equimolar concentrations (50 nmol/L) (Figure 6A). The data indicate that MPO binding to HK physically hinders the accessibility of the kallikrein cleavage site on HK. We next asked if MPO’s peroxidase function would influence bradykinin production. The data indicate that when hydrogen peroxide (H2O2), the substrate of MPO, is present, inhibition of bradykinin production is enhanced showing a significant reduction at 250 nmol/L (Figure 6B) versus 500 nmol/L MPO without H2O2. Supporting that the conversion of H2O2 and Cl− to hypochlorous acid by MPO was involved, the addition of catalase, an H2O2 scavenger, reversed this effect (Figure 6C).
Figure 6.
MPO interferes with the plasma kallikrein-kininogen system: bradykinin production is diminished. A: MPO interfered with bradykinin production through steric hindrance at high concentrations. Shown are the results of four independent experiments measuring the bradykinin liberated from 50 nmol/L HK in the presence of MPO. B: Provided with its substrate (H2O2), MPO affects bradykinin production at lower concentrations. Shown are the results of three independent experiments. C: The enzymatic activity of MPO (conversion of H2O2 to HOCl) hinders bradykinin production, which is rescued in the presence of catalase. Shown are the results of three independent experiments.
Effects of HOCl on Kallikrein and HK
HOCl, an antimicrobial oxidant produced by MPO, has long been implicated in the pathology of inflammation because it readily reacts with, and alters, proteins and their functions.2 Thus, proteins in close proximity to MPO could be affected by MPO-produced HOCl. We first evaluated the effects of HOCl on the protease activity of plasma kallikrein, using a colorimetric activity assay. Kallikrein activity was inhibited 50% at an 80-fold molar excess of HOCl to kallikrein and completely inhibited at a 200-fold excess (Figure 7A). A 20-fold molar excess of l-methionine to HOCl quenched its oxidant activity2 because thiols and thioether groups of methionine are the most reactive substrates for HOCl oxidation.
Figure 7.
Hypochlorous acid (HOCl) inhibits bradykinin production. A: The proteolytic activity of kallikrein is abolished by HOCl dose-dependently but is protected when HOCl is quenched with l-methionine (20-fold molar excess). Shown are the results (with SDs) of three separate experiments. B: HK is oxidized by HOCl, resulting in a product uncleavable by active kallikrein. HK was pretreated with increasing concentrations of HOCl. A 20-fold excess of l-methionine was added (20 minutes) before the addition of active kallikrein. Bradykinin production was assessed by ELISA. Shown are the results from four separate experiments. C: HOCl inactivates kininogen by oxidizing a critical methionine residue. HOCl-oxidized HK was treated with a methionine sulfoxide reductase (PilB), and the inhibition of bradykinin production was reversed. DTT, dithiothreitol.
Kininogens oxidized by chloramines (stable breakdown products of HOCl) cannot bind prekallikrein or be cleaved by kallikrein, an effect attributed to a modification of the critical Met-361 residue adjacent to the internal kinin sequence in kininogen to methionine sulfoxide.24,25 HOCl can mediate this same methionine-specific chemistry; thus we hypothesized HOCl would oxidize HK preventing bradykinin release by kallikrein.26,27 The addition of HOCl to HK inhibits bradykinin production in a dose-dependent manner (Figure 7B). The addition of PilB, a methionine sulfoxide reductase, reversed the decrease in bradykinin production and verified that an HOCl-oxidized methionine was responsible for this reduction.
Discussion
MPO has long been viewed to function primarily as a bactericidal enzyme. A new paradigm presented here extends this perspective and suggests that MPO is profoundly involved in the regulation of cellular homeostasis, and may play a central role in the regulation of vasodilatation through direct interactions with components of the kallikrein-kininogen system (diagrammed in Figure 8). We propose that in the inflammatory microenvironment, MPO is internalized by endothelial cells through a direct interaction with the endothelial surface protein CK1 (Figure 8A), known as a scaffold for the plasma kallikrein-kinin system.13,16 CK1 seems to tether MPO into close proximity of HK, and the two proteins are internalized in complex (Figure 8C). Moreover, MPO and HK form a complex such that blocks the HK site normally cleaved by kallikrein (Figure 8D). In addition, MPO’s peroxidase activity causes oxidation and inactivation of HK protein (Figure 8E) and inactivates plasma kallikrein’s activity (Figure 8F). The end result is a reduction in bradykinin liberation from HK (Figure 8G). Manipulations of bradykinin levels at sites of inflammation could have profound effects on vascular tone and other mediator functions of bradykinin.
Figure 8.
Proposed schematic for the interactions of MPO with endothelial cells and the plasma kallikrein-kininogen system. When a neutrophil releases its granule constituents and oxygen radicals at sites of inflammation, MPO can leak into the lumen of the vessel. A: Endothelial cells bind and internalize MPO, in part through interactions with the cell-surface protein CK1; MPO and CK1 enter the cells in complex. B: MPO can also enter cells through other mechanisms that have yet to be fully characterized. C–F: MPO can modulate the action of the plasma kallikrein-kininogen system. c: MPO associates directly with high molecular weight kininogen (HK) and increases the amount of HK bound to the cells; this complex appears to internalize. D: When MPO and kininogen are coupled, kallikrein is unable to cleave HK. MPO uses the hydrogen peroxide generated during a neutrophil’s respiratory burst to oxidize chloride and produce hypochlorous acid (represented by the yellow asterisk). E: Hypochlorous acid can oxidize and inactivate HK by altering kallikrein’s cleavage site (F), as well as abrogate the protease activity of kallikrein. G: In the absence of MPO, endothelial surface proteins including CK1 bind circulating high molecular weight kininogen. Plasma kallikrein subsequently cleaves bradykinin (small red circle with B) from kininogen; bradykinin then binds to specific endothelial receptors to induce nitric oxide generation.
Of central importance is the finding that CK1 facilitates MPO internalization by endothelial cells. This is in concordance with reports that MPO uptake is energy-dependent, a characteristic of receptor-mediated endocytosis.12 Cytokeratins are typically thought of as structural intermediate filaments, although descriptions of the functions of CK1 in endothelial cells imply that it is more than merely structural. CK1 functions as part of the cells’ environmental response pathways sensitized to oxidative stress; endothelial CK1 surface protein expression is up-regulated in such conditions.16,28 Although this is the first report of a specific endothelial MPO-binding protein, multiple groups have investigated the entry of free MPO into endothelial cells. In our study, internalized MPO was not exclusively localized to CK1, suggesting other possible mechanisms for cell association and trafficking of MPO. Cell surface glycosaminoglycans have been implicated in MPO internalization.12 In addition, MPO binds to albumin through both sequence- and charge-specific mechanisms; the albumin-bound MPO is nonspecifically internalized by albumin-binding proteins.21 However, our group and others have observed substantial MPO internalization in albumin-free conditions, supporting CK1 binding as a mode of entry.
MPO, being highly cationic, is often argued to interact with the endothelium solely through charge-based interactions. The mechanism through which CK1 and MPO interact is yet to be determined. In light of the reports that HK is not internalized when bound to CK1 through its cationic domain 5, we would argue that internalization of CK1-bound MPO is unique and specific.29,30 This would be fundamentally different from mechanisms mediating MPO’s adherence to endothelial cell-surface glycosaminoglycans or its internalization coupled to albumin.
We questioned whether MPO and HK reside on CK1 together or whether they compete for binding. The data support cooperative binding on cells, which would tether MPO and HK into close proximity. For reasons yet to be determined, MPO caused a substantial increase in cell-bound HK. One speculation is that MPO is transporting HK to additional sites for binding; MPO can bind through both CK1-dependent and -independent interactions. It is tempting to speculate that investigators will find additional situations in which MPO and HK functionally overlap. For example, it was reported that MPO and HK individually bind CD11b/CD18 integrin, and interestingly, the outcomes were functionally opposing.31,32,33
The ultimate biological mediator produced by the plasma kallikrein-kinin system is the vasoactive peptide bradykinin, which is cleaved from HK by kallikrein. Bradykinin binds at least two endothelial receptors to induce a hypotensive and anti-thrombotic state by stimulating endothelial nitric oxide production, prostacyclin synthesis, and tissue plasminogen activator release.23,34,35 Although MPO increases HK bound to endothelial cells, we do not believe this augmentation correlates with increased bradykinin production based on our studies. It may be that MPO binding and internalization of HK serves to sequester HK from kallikrein and hinder HK’s effector functions in the inflammatory milieu. Beyond the physical interference of MPO with the HK-kallikrein interaction, there was strong evidence that protein damage caused by the methionine-specific oxidizing agents produced by MPO could be responsible for decreased bradykinin production.26,27 There was a direct effect of biologically relevant concentrations of HOCl on kallikrein protease activity, and the oxidation of HK rendered it resistant to kallikrein proteolysis. HOCl concentrations less than 15 μmol/L are sublethal, whereas activation of the endothelial apoptosis machinery begins at a 30 to 50 μmol/L dose, and oncotic cell death is provoked at more than 100 μmol/L.36 The data show that even sublethal concentrations of HOCl overtly affect the ability of this system to produce bradykinin, and doses beyond 25 μmol/L totally inhibited bradykinin production (not shown). HOCl is a highly reactive oxidant, so the proximity of MPO to HK resulting from their direct interaction and their shared interaction with CK1 could be very important when considering these experimental results.2
During an acute and chronic inflammatory state, MPO is released into the vessel and is functional, based on published studies demonstrating chlorotyrosine in vessel walls.37 Only MPO can mediate this chemistry under physiological conditions. Given the large amount of MPO per neutrophil (5% of the dry weight), the large number of neutrophils infiltrating during acute infection and inflammation, and the altered flow in vessels associated with procoagulant activity, we propose that concentrations of MPO in the inflammatory milieu can approach the 500 mmol/L range used in these studies.38 Furthermore, in this microenvironment the data indicate the potential for MPO-kininogen interactions to interfere with the kininogen-kallikrein process. The effects of MPO’s enzymatic activity could be even more pronounced than we are reporting if in fact nitration of tyrosine residues affects kininogen processing.12,39 MPO can mediate tyrosine nitration in inflammatory conditions through a reaction with nitrite, a stable end-product of nitric oxide metabolism. A hydrogen peroxide-dependent reaction, nitrite is oxidized by MPO to form the nitrogen dioxide radical, a species capable of nitrating both free and protein-bound tyrosines.40,41 Such reactions have been associated with the inactivation of endothelial angiotensin-converting enzyme as well as the structural alteration of endothelial matrix-associated fibronectin.12,41,42
Previous studies showed that MPO remains active after internalization and catalytically consumes NO, thereby limiting the endothelial vasodilatory response.8 In comparison, our work unveils a mechanism whereby MPO may prevent endothelial NO generation in the inflammatory milieu by limiting the production of bradykinin, the most potent known inducer of endothelial NO.
A limitation of these studies is the lack of an in vivo or ex vivo model to demonstrate that MPO can interfere with bradykinin production and thus impair vascular function. Although observed in a cellular system, it is difficult to extrapolate the consequences of the MPO and CK1 interaction to the inflamed tissue environment. This question will be a focus of future studies. Another point that needs further exploration is the composition of the internalized CK1, MPO, and/or HK complexes. Because of limitations in available reagents, this could not be performed herein.
In summary, CK1 is an endothelial receptor that aids in the internalization of MPO. MPO also directly interacts with HK, which also binds CK1 protein. The products of MPO’s enzymatic activity can oxidize and inactivate both HK and plasma kallikrein. The collective interactions between MPO and the plasma kallikrein-kinin system result in a decrease in bradykinin production. Whether the paradigm discovered here is a normal process of an inflammatory response or whether it is a pathophysiological mediator of organ injury is yet to be determined. It may be that leakage of small amounts of MPO localized at the vessel wall play a role that is ultimately beneficial to the host. The converse may be true when MPO is released in excess quantities during systemic inflammation. Regardless, the importance of MPO in vascular biology is further demonstrated by these studies.
Acknowledgments
We thank Dr. Robert Bagnell and Victoria Madden in the University of North Carolina Microscopy Services Laboratory for their expertise and advice, Drs. Herb Weissbach and Nathan Brot for providing reagents and excellent experimental guidance, Hyunsook Chin and Susan Hogan for providing assistance with data analysis, and Nirmal Khandoobhai for helping to prepare the manuscript.
Footnotes
Address reprint requests to Joshua Astern, CB# 7155, 5009 Burnett-Womack, UNC Kidney Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7155. E-mail: jastern@med.unc.edu.
Supported by the National Institutes of Health (grants DK-58335-01 and grant HL052779-10 to A.H.S.).
References
- Nauseef WM, Malech HL. Analysis of the peptide subunits of human neutrophil myeloperoxidase. Blood. 1986;67:1504–1507. [PubMed] [Google Scholar]
- Pullar JM, Vissers MC, Winterbourn CC. Living with a killer: the effects of hypochlorous acid on mammalian cells. IUBMB Life. 2000;50:259–266. doi: 10.1080/713803731. [DOI] [PubMed] [Google Scholar]
- Arimura Y, Minoshima S, Kamiya Y, Tanaka U, Nakabayashi K, Kitamoto K, Nagasawa T, Sasaki T, Suzuki K. Serum myeloperoxidase and serum cytokines in anti-myeloperoxidase antibody-associated glomerulonephritis. Clin Nephrol. 1993;40:256–264. [PubMed] [Google Scholar]
- Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- Zhang C, Patel R, Eiserich JP, Zhou F, Kelpke S, Ma W, Parks DA, Darley-Usmar V, White CR. Endothelial dysfunction is induced by proinflammatory oxidant hypochlorous acid. Am J Physiol. 2001;281:H1469–H1475. doi: 10.1152/ajpheart.2001.281.4.H1469. [DOI] [PubMed] [Google Scholar]
- Baldus S, Heitzer T, Eiserich JP, Lau D, Mollnau H, Ortak M, Petri S, Goldmann B, Duchstein HJ, Berger J, Helmchen U, Freeman BA, Meinertz T, Munzel T. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic Biol Med. 2004;37:902–911. doi: 10.1016/j.freeradbiomed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Preston GA, Pendergraft WF, Segelmark M, Heeringa P, Hogan SL, Jennette JC, Falk RJ. Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants. Am J Pathol. 2001;158:581–592. doi: 10.1016/S0002-9440(10)64000-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph K, Tholanikunnel BG, Ghebrehiwet B, Kaplan AP. Interaction of high molecular weight kininogen binding proteins on endothelial cells. Thromb Haemost. 2004;91:61–70. doi: 10.1160/TH03-07-0471. [DOI] [PubMed] [Google Scholar]
- Mahdi F, Shariat-Madar Z, Todd RF, III, Figueroa CD, Schmaier AH. Expression and colocalization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood. 2001;97:2342–2350. doi: 10.1182/blood.v97.8.2342. [DOI] [PubMed] [Google Scholar]
- Shariat-Madar Z, Mahdi F, Schmaier AH. Recombinant prolylcarboxypeptidase activates plasma prekallikrein. Blood. 2004;103:4554–4561. doi: 10.1182/blood-2003-07-2510. [DOI] [PubMed] [Google Scholar]
- Hasan AA, Zisman T, Schmaier AH. Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells. Proc Natl Acad Sci USA. 1998;95:3615–3620. doi: 10.1073/pnas.95.7.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariat-Madar Z, Mahdi F, Schmaier AH. Mapping binding domains of kininogens on endothelial cell cytokeratin 1. J Biol Chem. 1999;274:7137–7145. doi: 10.1074/jbc.274.11.7137. [DOI] [PubMed] [Google Scholar]
- Parker CE, Warren MR, Loiselle DR, Dicheva NN, Borchers CH. Identification of components of protein complexes. Patterson C, Cyr DM, editors. Totowa: Humana Press,; Methods in Molecular Biology, Ubiquitin–Proteasome Protocols. 2005:pp 117–171. doi: 10.1385/1-59259-895-1:117. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Segelmark M, Persson B, Hellmark T, Wieslander J. Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin Exp Immunol. 1997;108:167–174. doi: 10.1046/j.1365-2249.1997.d01-992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci USA. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Qiu Q, Mahdi F, Shariat-Madar Z, Rojkjaer R, Schmaier AH. Assembly and activation of HK-PK complex on endothelial cells results in bradykinin liberation and NO formation. Am J Physiol. 2001;280:H1821–H1829. doi: 10.1152/ajpheart.2001.280.4.H1821. [DOI] [PubMed] [Google Scholar]
- Kozik A, Moore RB, Potempa J, Imamura T, Rapala-Kozik M, Travis J. A novel mechanism for bradykinin production at inflammatory sites. Diverse effects of a mixture of neutrophil elastase and mast cell tryptase versus tissue and plasma kallikreins on native and oxidized kininogens. J Biol Chem. 1998;273:33224–33229. doi: 10.1074/jbc.273.50.33224. [DOI] [PubMed] [Google Scholar]
- Nieziołek M, Kot M, Pyka K, Mak P, Kozik A. Properties of chemically oxidized kininogens. Acta Biochim Pol. 2003;50:753–763. [PubMed] [Google Scholar]
- Khor HK, Fisher MT, Schoneich C. Potential role of methionine sulfoxide in the inactivation of the chaperone GroEL by hypochlorous acid (HOCl) and peroxynitrite (ONOO−). J Biol Chem. 2004;279:19486–19493. doi: 10.1074/jbc.M310045200. [DOI] [PubMed] [Google Scholar]
- Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J Biol Chem. 2003;278:28403–28409. doi: 10.1074/jbc.M304739200. [DOI] [PubMed] [Google Scholar]
- Collard CD, Montalto MC, Reenstra WR, Buras JA, Stahl GL. Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am J Pathol. 2001;159:1045–1054. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan AA, Cines DB, Ngaiza JR, Jaffe EA, Schmaier AH. High-molecular-weight kininogen is exclusively membrane bound on endothelial cells to influence activation of vascular endothelium. Blood. 1995;85:3134–3143. [PubMed] [Google Scholar]
- Shariat-Madar Z, Schmaier AH. Kininogen-cytokeratin 1 interactions in endothelial cell biology. Trends Cardiovasc Med. 1999;9:238–244. doi: 10.1016/s1050-1738(00)00028-1. [DOI] [PubMed] [Google Scholar]
- Johansson MW, Patarroyo M, Oberg F, Siegbahn A, Nilsson K. Myeloperoxidase mediates cell adhesion via the alpha M beta 2 integrin (Mac-1, CD11b/CD18). J Cell Sci. 1997;110:1133–1139. doi: 10.1242/jcs.110.9.1133. [DOI] [PubMed] [Google Scholar]
- Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brummer J, Rudolph V, Munzel T, Heitzer T, Meinertz T, Baldus S. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci USA. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng N, Fairbanks MB, Heinrikson RL, Canziani G, Chaiken IM, Mosser DM, Zhang H, Colman RW. Cleaved high molecular weight kininogen binds directly to the integrin CD11b/CD18 (Mac-1) and blocks adhesion to fibrinogen and ICAM-1. Blood. 2000;95:3788–3795. [PubMed] [Google Scholar]
- Brown NJ, Gainer JV, Murphey LJ, Vaughan DE. Bradykinin stimulates tissue plasminogen activator release from human forearm vasculature through B(2) receptor-dependent, NO synthase-independent, and cyclooxygenase-independent pathway. Circulation. 2000;102:2190–2196. doi: 10.1161/01.cir.102.18.2190. [DOI] [PubMed] [Google Scholar]
- Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3:845–852. doi: 10.1038/nrd1522. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1309–1314. doi: 10.1161/01.ATV.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM. Insights into myeloperoxidase biosynthesis from its inherited deficiency. J Mol Med. 1998;76:661–668. doi: 10.1007/s001090050265. [DOI] [PubMed] [Google Scholar]
- Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- Mohiuddin I, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Nitrotyrosine and chlorotyrosine: clinical significance and biological functions in the vascular system. J Surg Res. 2006;133:143–149. doi: 10.1016/j.jss.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- Baldus S, Eiserich JP, Brennan ML, Jackson RM, Alexander CB, Freeman BA. Spatial mapping of pulmonary and vascular nitrotyrosine reveals the pivotal role of myeloperoxidase as a catalyst for tyrosine nitration in inflammatory diseases. Free Radic Biol Med. 2002;33:1010–1019. doi: 10.1016/s0891-5849(02)00993-0. [DOI] [PubMed] [Google Scholar]