Abstract
Interleukin (IL)-6 acts via a receptor complex consisting of the cognate IL-6 receptor (IL-6R) or the soluble IL-6 receptor (sIL-6R) and glycoprotein 130 (gp130). Here, we investigated the role of these IL-6R components in hypertension and vascular hypertrophy in mice. Angiotensin (Ang) II (1.1 mg/kg/day) caused hypertension and cardiac/aortic hypertrophy in wild-type, but not IL-6−/−, mice throughout 7 days. A recombinant dimeric soluble gp130 (sgp130Fc; 50 to 100 μg, i.p.) blocked Ang II hypertension but not hypertrophy in wild-type mice. Cognate IL-6R was detected in aortic smooth muscle, but its levels and those of plasma sIL-6R were ∼50% decreased in IL-6−/− mice. Ang II infusion activated signal transducer and activator of transcription-3 in heart of WT and decreased Ang II receptor 1 (ATR1) expression in aorta. Both responses were unaffected by sgp130Fc and absent in IL-6−/− mice. In summary, we show that IL-6 trans-signaling is required for Ang II-dependent hypertension, but that hypertrophy, down-regulation of AT1R, and cardiac signal transducer and activator of transcription-3 activation are mediated via cognate IL-6R. These data show that IL-6 responses in a single disease context are governed by both modes of IL-6 signaling, with each pathway eliciting different outcomes. Inhibition of IL-6 signaling is suggested as a potential therapy for hypertension and cardiac hypertrophy.
There is emerging evidence for a role of interleukin (IL)-6 and its related cytokines in angiotensin (Ang) II-dependent vascular dysfunction. Plasma levels of IL-6 are strongly associated with hypertension in humans and can be decreased by administration of Ang II receptor antagonists.1,2,3,4,5,6 In animal models of hypertension and volume overload-induced hypertrophy, IL-6 and its related cytokines, leukemia-inhibitory factor and cardiotrophin-1, are elevated.7,8,9,10 In addition, Ang II can increase IL-6 synthesis in cultured cells via extracellular signal-regulated kinase, mitogen-activated protein kinase, and cAMP response element-binding protein.11,12
Ang II has potent and diverse actions throughout the cardiovascular system including vasoconstriction, induction of vascular smooth muscle proliferation, cardiac hypertrophy, reactive oxygen species, and aldosterone production. Ang II responses are mediated predominantly via AT1 receptor activation (AT1R), a G protein-coupled receptor expressed on endothelial cells, monocytes, and vascular smooth muscle cells. However, whether IL-6 can mediate the biological and vascular damaging effects of Ang II is unknown.
Independently of Ang II signaling, an involvement of the IL-6 signal-transducing receptor gp130 in pressure overload and salt-sensitive models of hypertrophy has been found, whereas in vitro leukemia inhibitory factor and cardiotrophin-1 can stimulate cardiomyocyte hypertrophy via signal transducer and activator of transcription-3 (STAT3) and gp130.10,13,14,15 However, no studies to date have shown that IL-6 itself can cause vascular or cardiac hypertrophy either in vitro or in vivo. In addition, whether Ang II acting via IL-6 can activate STAT3 and gp130 signaling is unknown. Finally, the relative roles of the two IL-6 receptor isoforms, soluble (sIL-6R) and cognate IL-6R, in controlling the various Ang II responses have not been delineated.
The mechanisms by which IL-6 potentially affects hypertension or hypertrophy are unknown. To establish the relationship between Ang II and IL-6 activity, it is therefore essential to consider how IL-6 responses are governed in vivo. In the classic sense, IL-6 binds a membrane-bound cognate receptor (IL-6R) that then couples to gp130 to transmit its signal. This response can, however, be mimicked through IL-6 binding to its soluble receptor. The resulting IL-6/sIL-6R is then able to directly activate gp130 through a process termed IL-6 trans-signaling. The in vivo significance of this alternative mode of IL-6 activation is only fully appreciated when you consider the cellular distribution of both the cognate IL-6R and gp130. Although IL-6R displays a restricted expression profile, gp130 is ubiquitously expressed. IL-6 trans-signaling therefore affords IL-6 with the capacity to activate responses in cells that would inherently remain unresponsive to IL-6 itself. At present, the significance of this dual mode of activation remains unclear.
Herein, we examined in vivo responses to low-dose Ang II, which causes hypertension and hypertrophy during a 7-day period and reveals that IL-6−/− mice are very well protected against both responses in vivo. Mechanistic studies using soluble gp130Fc (sgp130Fc), an inhibitor of trans-signaling generated by linking sgp130 to the Fc domain of IgG1, were conducted to establish the mechanisms involved. The results provide insight into mechanisms of Ang II signaling in vascular disease and propose IL-6 as a potential target for treatment of hypertension, cardiac hypertrophy, and heart failure.
Materials and Methods
Materials
Rabbit anti-human AT1-R, normal rabbit or goat IgG, and rabbit serum were from Santa Cruz Biotechnology (Santa Cruz, CA), goat anti-mouse IL-6R was from R&D Systems (Oxon, UK). Anti-rabbit IgG-Alexa 568 and anti-goat IgG-Alexa 568 were from Molecular Probes (Eugene, OR). Recombinant sgp130Fc was purified from the supernatant of stably transfected Chinese hamster ovary cells as described.16 Unless otherwise stated, all compounds were from Sigma (Poole, UK).
Animal Studies
All animal experiments were performed in accordance with the United Kingdom Home Office Animals (Scientific Procedures) Act of 1986. Experiments were performed using male C57BL/6J IL-6-deficient (IL-6−/−) mice.17 Age-matched wild-type (WT) male C57BL/6 mice (25 to 30 g) were obtained from Charles River, Margate, Kent, UK. All mice were kept in constant-temperature cages (20 to 22°C) and given free access to water and standard chow.
Hypertension Studies
Male 10- to 12-week-old WT and IL-6−/− mice were anesthetized via inhalation of 2% isoflurane (98% oxygen). Osmotic minipumps (Alzet model 1002; Durect Corp., Cupertino, CA) containing Ang II (infusion rate, 1.1 mg/kg/day) or vehicle were implanted subcutaneously in the midscapular area. Systolic blood pressure (BP) was monitored daily for 3 days before implantation (training period) and up to 7 days after implantation via tail cuff plethysmography (World Precision Instruments, Stevenage, Hertfordshire, UK) in conscious mice. Soluble gp130 was administered via intraperitoneal injection of sgp130Fc [100 μg/mouse−1 (3.33 mg/kg) days −1 and +1, 50 μg/mouse−1 (1.67 mg/kg) days +3 and +5].
Assessment of Heart/Body Weight Ratio, Aortic Medial Area, and Immunohistochemistry
Seven days after implantation, mice were sacrificed by cervical dislocation and heart and body weights recorded. The descending thoracic aorta was removed, cleaned of adipose tissue, and fixed in paraffin wax. For measurement of medial area, 15-μm sections were taken using a cryostat, placed on glass slides, fixed using acetone, and stained with hematoxylin. Images were acquired using a ×10 air lens, with an Axiovert S100TV microscope and Hamamatsu Orca Digital Camera (Hamamatsu Corporation, Bridgewater, NJ), and medial area was calculated using Scion Image (Scion Corp., Frederick, MD). For each aorta, three separate sections were analyzed and averaged. For immunohistochemistry, 10-μmol/L transverse sections were methanol-fixed on glass slides, permeabilized using 0.1% (w/v) Triton X-100/phosphate-buffered saline (PBS), and blocked using 1% (w/v) bovine serum albumin/PBS (AT1R) or 10% rabbit serum (IL-6R). Negative controls used equivalent concentrations of isotype control IgG. Imaging was performed on an Axiovert S100TV inverted microscope connected to a Bio-Rad MRC 1024ES laser-scanning system (Bio-Rad Microscience, Hemel Hempstead, UK) and using standard analysis software (Lasersharp 2000; Bio-Rad Microscience). Images were acquired using a ×10 air lens, with excitation at 568 nm and emission 595/35 nm. Quantification of receptor expression was determined by averaging pixel intensity across the area of antigen expression using Lasersharp 2000. Each group consisted of four to five separate animals. For each animal, three separate aortic sections were quantified with three to four separate determinations per section. Postacquisition processing was performed using Adobe Photoshop (Mountain View, CA).
Determination of Plasma sIL-6R
Seven days after implantation, mice were sacrificed by cervical dislocation and blood collected by cardiac puncture and chilled on ice. Whole blood was centrifuged (4°C, 3000 rpm, 10 minutes) to recover plasma supernatant. Soluble IL-6R (sIL-6R) expression was quantified using enzyme-linked immunosorbent assay with goat anti-mouse IL-6R (R&D Systems).
Electrophoretic Mobility Shift Assays for STAT1 and STAT3 Activation
Mouse hearts were ground to a fine powder in liquid nitrogen using a pestle and mortar. Cytosolic and nuclear extracts were then prepared from the powder using a rapid technique for the extraction of nuclear proteins.18 In brief, the powder was resuspended in ice-cold buffer A (10 mmol/L HEPES, pH 8, 1.5 mmol/L MgCl2, and 10 mmol/L KCl) containing 0.5 mmol/L dithiothreitol, 0.2 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L sodium orthovanadate, 50 mmol/L NaF, and proteinase inhibitors (diluted 1:1000; Sigma) and incubated on ice for 30 minutes. The nuclei were pelleted by centrifugation, and the cytosolic fraction was removed. The nuclear pellet was resuspended in ice-cold buffer C (20 mmol/L HEPES, pH 8, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L ethylenediaminetetraacetic acid, pH 8, and 25% glycerol) containing 0.5 mmol/L dithiothreitol, 0.2 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L sodium orthovanadate, 50 mmol/L NaF, and proteinase inhibitors (diluted 1:1000; Sigma) and incubated on ice for 30 minutes to allow for high-salt extraction. Cellular debris was removed by brief high-speed centrifugation, and the resulting supernatants (nuclear extract) were collected. Protein concentrations were determined using the Bradford method. Electrophoretic mobility shift assays were performed using 10 μg of nuclear extract as previously described.19 Oligonucleotides containing the SIE STAT-binding GAS elements were annealed and labeled with [α32P]dTTP (Amersham Pharmacia) using the Klenow fragment of DNA polymerase I. The composition of STAT-DNA binding complexes was determined by supershift assays using rabbit polyclonal antibodies specific to STAT1 (M22, Sc-592) and STAT3 (C20, Sc-483) (both from Santa Cruz Biotechnology). Densitometry analysis was performed using the ImageJ 1.34s software (http://rsb.info.nih.gov/ij/). Alterations in STAT DNA-binding activity were expressed relative to control levels (equal to 1).
Isometric Tension Functional Studies
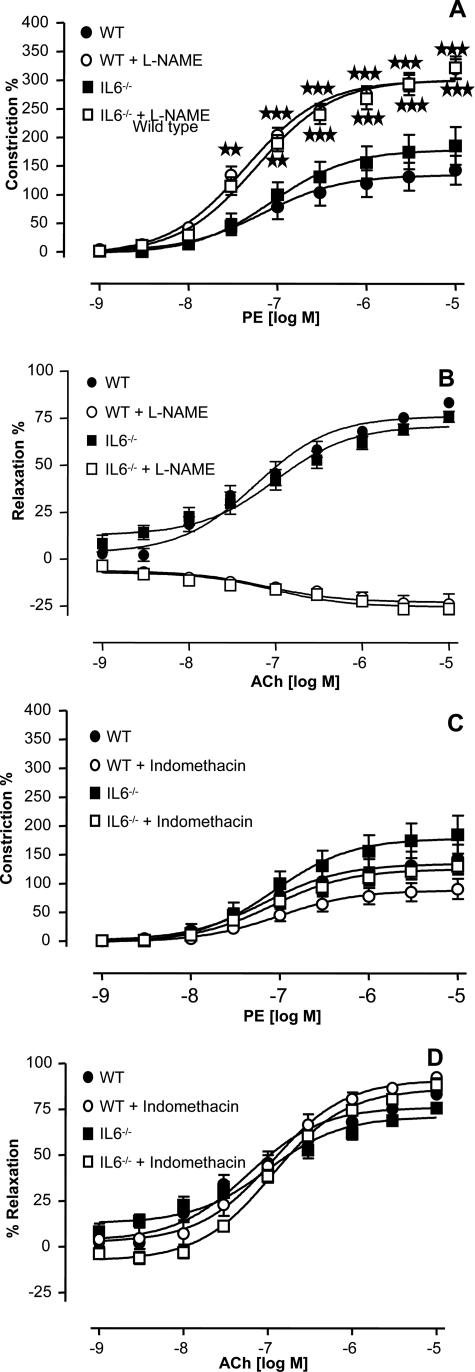
Male WT or IL-6−/− mice (25 to 30 g) were sacrificed by cervical dislocation. The thoracic aorta was removed and placed in Krebs-Henseleit buffer (120 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4·7H2O, 24 mmol/L NaHCO3, 1.1 mmol/L KH2PO4, 10 mmol/L glucose, and 2.5 mmol/L CaCl2·2H2O). The aorta was dissected free of adipose tissue, taking care to retain adventitia, cut into rings (2 to 3 mm), and suspended in an isometric tension myograph (model 610; DMT, Aarhuis, Denmark) containing Krebs buffer at 37°C and gassed with 5% CO2/95% O2. A resting tension of 3 mN was maintained, and changes in isometric tension recorded via Myodaq software (DMT). After a 60-minute equilibration period, vessels were primed with 48 mmol/L KCl and rested for a further 30 minutes before a concentration (1 μmol/L) of phenylephrine (PE) producing ∼75% contraction was added. Once the response stabilized, 1 μmol/L acetylcholine (ACh) was added to assess endothelial integrity. Any rings that did not maintain contraction or relaxed <50% of the PE-induced tone were discarded. Rings were washed for 30 minutes, after which a cumulative concentration-response curve to PE was constructed (1 nmol/L to 10 μmol/L) to assess vasoconstrictor activity. The tissues were then washed for 60 minutes to restore basal tone, after which the vessels were constricted to ∼80% using 1 μmol/L PE. Once a stable response to PE was observed, cumulative concentration-response curves were constructed to 1 nmol/L to 10 μmol/L ACh to assess endothelium-dependent and -independent relaxations, respectively. Experiments were repeated in the presence of 300 μmol/L l-nitroarginine-methyl ester (l-NAME), 10 μmol/L indomethacin, or 1 μmol/L Ang II, which were added for 15 minutes before the end of wash periods. Constriction and dilation curves were then constructed as described previously. In some experiments the constriction response to a bolus dose of Ang II was examined. Responses were expressed as a percentage of either baseline tension (vasoconstriction) or contracted tension (vasodilation). ED50 for ACh relaxation is defined as the concentration of agonist giving half-maximal relaxation and was determined from experimental dose-response curves. Responses from patent rings of each animal (three to four rings) were combined to produce an average for each sample (n).
Statistical Analysis
Data are expressed as mean ± SEM. Groups are compared with WT group using two-way analysis of variance with Bonferroni’s posttest to isolate differences between groups. P < 0.05 or less was considered statistically significant.
Results
Systolic BP Elevations to Ang II Are Attenuated in IL-6−/− Mice
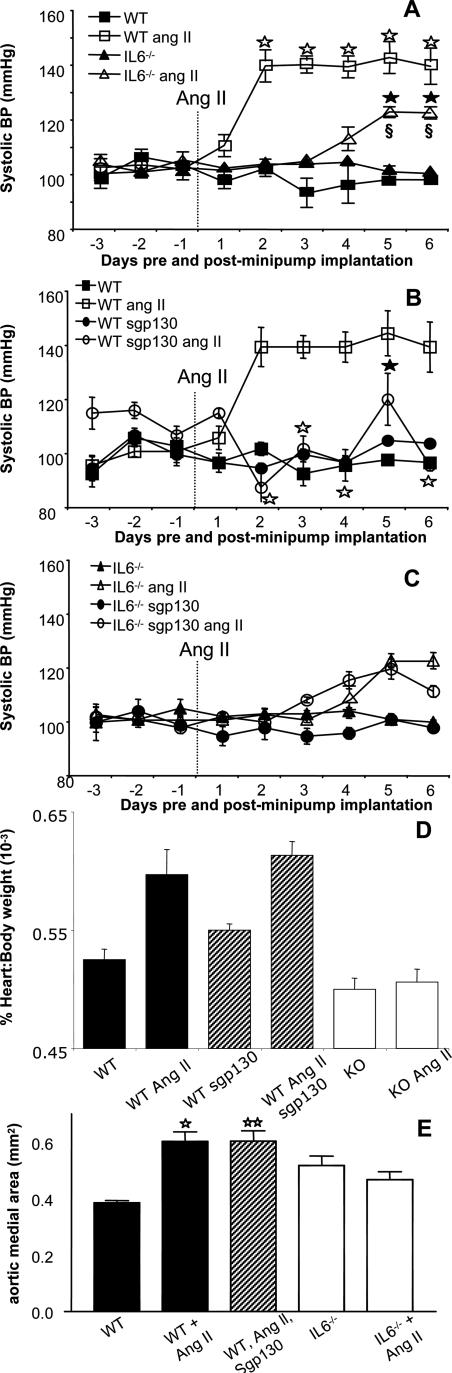
No significant difference was noted between basal BP of WT and IL-6−/− mice (101.7 ± 1.4 mm Hg, n = 14, versus 102.2 ± 0.6 mm Hg, n = 14, for WT and IL-6−/−, respectively, mean ± SEM; Figure 1A, days −3 to −1), and this remained unaltered after subcutaneous infusion of vehicle (saline) (Figure 1A). Infusion of Ang II (1.1 mg/kg−1/day−1) significantly increased systolic BP in WT mice from day +2 to +7 compared with WT controls (Figure 1A, mean maximal pressure 139.4 ± 9.1 mm Hg days +2 to +6 versus 97 ± 0.93 mm Hg days +2 to +6, P < 0.001, compare with day 0). In contrast, the BP increase observed in IL-6−/− mice was significantly reduced (P < 0.01 compare days +5 to +6) when compared with WT mice. Further, in the IL-6−/− group there was no BP increase until day +4. These data demonstrate Ang II-dependent hypertension is reduced both in severity and onset in vivo in IL-6−/− mice.
Figure 1.
Ang II-dependent hypertension and hypertrophy is attenuated in IL-6−/− mice, and IL-6 trans-signaling is required for BP elevations in WT. A: BP responses to Ang II infusion in WT and IL-6−/− mice. Ang II (1.1 mg/kg per day) was infused into male, 10 to 12 weeks old, WT and IL-6−/− mice by osmotic minipump. Systolic BP was monitored daily for 3 days (training) before implantation and 7 days after implantation via tail cuff plethysmography. ▪, WT sham group (n = 6); □, WT Ang II-infused group (n = 8); ▴, IL-6−/− sham group (n = 6); Δ, IL-6−/− Ang II-infused group (n = 8). ⋆P < 0.001 compared with WT control group; ★P < 0.0001 compared with IL-6−/− group; §P < 0.01 compared with WT Ang II-infused group; using two-way analysis of variance with Bonferroni’s posttest (mean ± SEM). B: Administration (intraperitoneally) of sgp130Fc inhibits Ang II-dependent hypertension in WT mice. Ang II (1.1 mg/kg per day) was infused into male, 10 to 12 weeks old, WT mice by osmotic minipump with or without administration of sgp130Fc (intraperitoneal injection on days −1 and +1 at 100 μg/mouse and days +3 and +5 at 50 μg/mouse), and BP was monitored as in A. ▪, WT group (n = 6); □, WT Ang II-infused group (n = 8); •, WT sgp130-treated (n = 5); and ○, WT Ang II-infused sgp130-treated (n = 6). ⋆P < 0.0001 compared with WT Ang II-infused group; ★P < 0.05 compared with WT Ang II-infused group using two-way analysis of variance with Bonferroni’s posttest (mean ± SEM). C: Administration of sgp130Fc in IL-6−/− mice. Ang II (1.1 mg/kg per day) was infused into male, 10 to 12 weeks old, IL-6−/− mice by osmotic minipump with or without administration of sgp130Fc (intraperitoneal injection on days −1 and +1 at 100 μg/mouse and days +3 and +5 at 50 μg/mouse), and BP was monitored as in A. ▴, IL-6−/− group (n = 6); Δ, IL-6−/− Ang II-infused group (n = 8); •, IL-6−/− sgp130-treated (n = 5); and ○, IL-6−/− Ang II-infused sgp130-treated (n = 6). D: Cardiac hypertrophy after Ang II infusion in WT and IL-6−/− mice. Heart and body weight was recorded and compared for each group at the end of the Ang II infusion period (n = 12, mean ± SEM; ⋆P < 0.001, ⋆⋆P < 0.05, compared with WT group; unpaired Student’s t-test). WT mice were treated with sgp130Fc as described in B. E: Aortic medial area was determined as described in Materials and Methods. For each animal, three aortic sections were analyzed and averaged (n = 6 separate animals, mean ± SEM; ⋆P < 0.001 compared with WT group. ⋆⋆P < 0.05, compared with WT group; unpaired Student’s t-test).
Effect of sgp130Fc on Ang II-Induced Hypertension in WT Mice
To examine the mechanisms of IL-6 involvement in Ang II-dependent hypertension, BP was measured in WT mice after administration of sgp130Fc, a selective inhibitor of IL-6 trans-signaling (100 μg/mouse−1 (approx 3.3 mg/kg−1) days −1 and +1, 50 μg/mouse−1 (∼1.7 mg/kg−1) days +3 and +5). In WT mice, BP increases to Ang II were significantly suppressed by sgp130Fc (P < 0.0001; P < 0.05 compare days +2 to +6), when compared with WT Ang II-treated mice (Figure 1B). There was no effect of sgp130Fc administered alone to WT mice (Figure 1B). These data demonstrate that IL-6 trans-signaling is required for the pressor effects of low-dose Ang II. Because small changes in food intake may alter salt intake, food consumption and body weight gain was monitored during sgp130Fc administration in WT and IL-6−/− mice. The data showed that neither parameter was affected during a 7-day period either before or during 7 days of sgp130Fc administration (data not shown).
Effect of sgp130Fc on Ang II-Induced Hypertension in IL-6−/− Mice
From days +4 to +6, a small rise in BP was noted in IL-6−/− mice with Ang II that appeared less than when sgp130Fc was administered to WT with Ang II. This was unexpected because complete blockade of IL-6 signaling as in the knockout should be at least as effective as inhibition of trans-signaling using sgp130Fc. Therefore, to examine this further, sgp130 was administered to IL-6−/− mice and BP examined during Ang II infusion. The data shows that sgp130 does not potentiate the effect of IL-6 deficiency, because a small increase in BP toward the end of the time course, similar to the response of IL-6−/− alone is noted (Figure 1C). Therefore, sgp130Fc is more effective at preventing Ang II-dependent hypertension in WT mice than the combination of sgp130Fc/IL-6−/−. One potential explanation is that, in the absence of IL-6, signaling by alternative gp130-activating cytokines, such as leukemia inhibitory factor, Oncostatin-M, or cardiotrophin-1, is up-regulated to compensate and that this would not inhibited by sgp130Fc because of its significantly greater kd for IL-6/sIL-6R than that of other IL-6-related cytokine receptors.20,21 Alternatively, the idea that sgp130Fc administration is more effective than IL-6 deficiency is primarily based on one data point (day 6), and analysis of variance analysis of WT/Ang II/sgp130Fc compared with IL-6−/−/Ang II, or IL-6−/−/Ang II/sgp130Fc finds no significant differences between these data sets (not shown).
IL-6 Deficiency Prevents Ang II-Induced Cardiac and Aortic Hypertrophy
Subcutaneous infusion of Ang II significantly increased heart:body weight ratio and aortic medial area in WT mice; however, no increases were observed in IL-6−/− mice (Figure 1, C and D). Furthermore, infusion of sgp130Fc did not prevent Ang II-induced hypertrophy in WT mice (Figure 1, D and E). These data suggest that Ang II-induced cardiac and aortic hypertrophy require IL-6 but that trans-signaling is not involved.
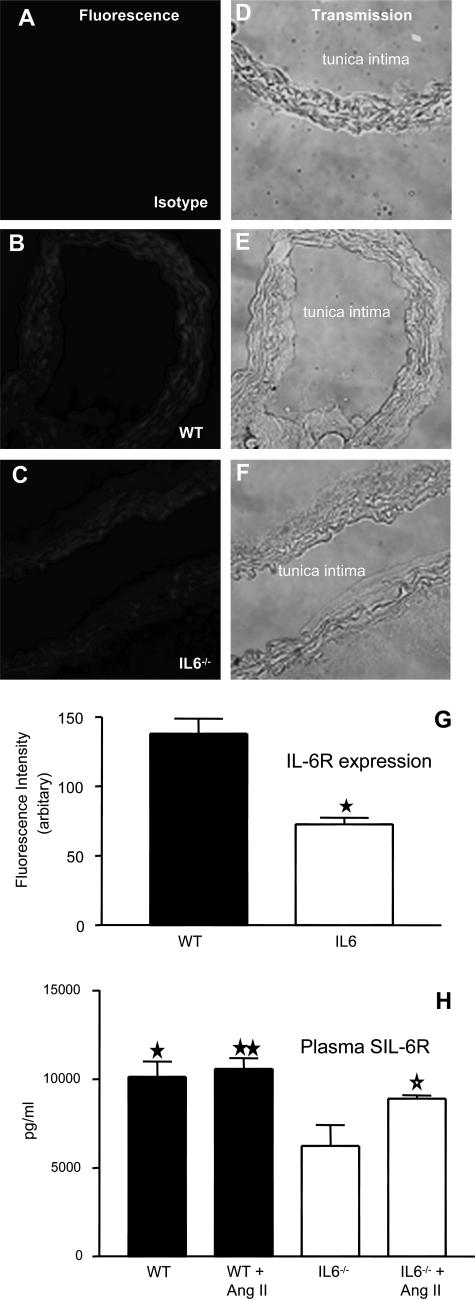
Expression of IL-6R in Aortic Tissue and Plasma Levels of sIL-6R Are Decreased in IL-6−/− Mice
The inability of sgp130Fc to influence changes in Ang II-induced cardiac and aortic hypertrophy suggests that regulation of these events is governed by classic IL-6R signaling. Consequently, expression of the cognate IL-6R was examined in aortic sections using immunohistochemistry. As shown, staining was apparent in aortae from both WT and IL-6−/− mice; however, the level of expression was markedly reduced (∼50%) in IL-6−/− vessels (Figure 2, A–G). Similarly, plasma levels of sIL-6R were ∼50% decreased in IL-6−/− mice versus WT (Figure 2H). Ang II infusion in the WT group did not change plasma sIL-6R or elevate IL-6, but in IL-6−/− mice its levels were significantly increased (P < 0.05; Figure 2H and data not shown). These data indicate that IL-6 deficiency results in significant losses in circulating and tissue IL-6R. Furthermore, expression of cognate IL-6R on aortic tissue provides a mechanism for IL-6 regulation of hypertrophy, which is independent of IL-6 trans-signaling, in which circulating or tissue IL-6 directly activates vascular IL-6R.
Figure 2.
Aortic IL-6R and plasma sIL-6R is significantly decreased in IL-6−/− mice. Aortic sections from WT and IL-6−/− mice were sectioned and stained for IL-6R. Representative sections are shown for each condition. A–C: IL-6R fluorescence; D–F: corresponding phase-contrast images. G: Mean pixel intensity was determined after fluorescence staining of multiple aortic sections (n = 6, separate aortae, mean ± SEM; ★P < 0.0002, compared with WT controls, unpaired Student’s t-test). H: Effect of Ang II infusion on plasma sIL-6R levels, determined by enzyme-linked immunosorbent assay (n = 6 per group, mean ± SEM; ⋆P < 0.05, IL-6−/− group compared with IL-6−/− Ang II-infused group, ★P < 0.02, ★★P < 0.005, IL-6 −/− group compared with WT and WT Ang II-infused group; unpaired Student’s t-test).
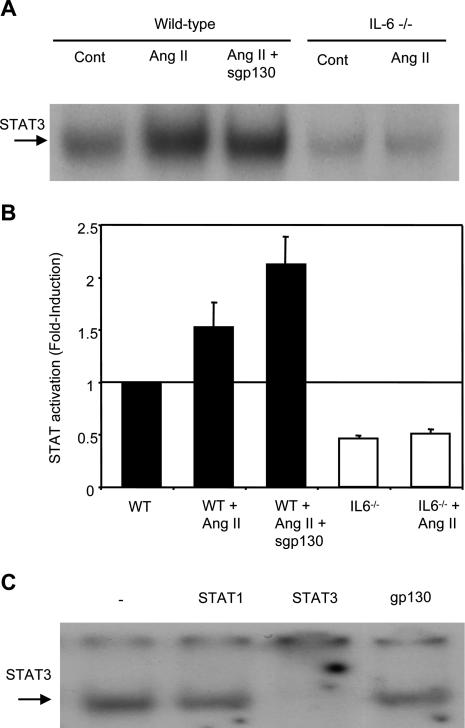
Ang II Infusion Induces STAT3 Activation in Heart Tissue from WT and WT sgp130Fc-Treated, but Not in IL-6−/−, Mice
To substantiate signaling via the classic model of IL-6 activation, studies examined the regulation of gp130-mediated STAT3 activation in cardiac tissue. After nuclear extraction from heart tissue, STAT activation levels were examined using electrophoretic mobility shift assay. The data show a twofold increase in STAT activation with Ang II infusion in WT that is not inhibited by sgp130Fc infusion and is absent in IL-6−/− mice (Figure 3, A and B). Although there was a greater STAT activation with sgp130Fc, this difference was not statistically significant from Ang II infusion alone. Supershift analysis subsequently showed that the composition of this DNA/protein complex consisted predominantly of STAT3 (Figure 3C). These data show that Ang II infusion in vivo leads to an IL-6-dependent activation of STAT3 in heart that is not mediated by IL-6 trans-signaling and is probably involved in the hypertrophic response.
Figure 3.
STAT3 is activated in hearts from Ang II-infused WT, but not IL-6−/−, mice and is not inhibited in WT by sgp130Fc. A: STAT binding in representative samples. Nuclear STAT DNA-binding activity was analyzed by electrophoretic mobility shift assays with 32P-labeled SIE probe in the hearts of WT and IL-6−/− mice, with or without Ang II and sgp130Fc. B: Densitometric analysis of STAT DNA binding. The mean fold activation from three separate experiments is presented. All values are equated to the mean of the WT control sample (equal to 1) (n = 3, mean ± SEM). C: Supershift analysis of nuclear extracts from WT mouse heart treated with Ang II. Control samples without the addition of antibody are shown, with samples containing antibodies against STAT1, STAT3, and gp130 (negative control) (n = 3, mean ± SEM).
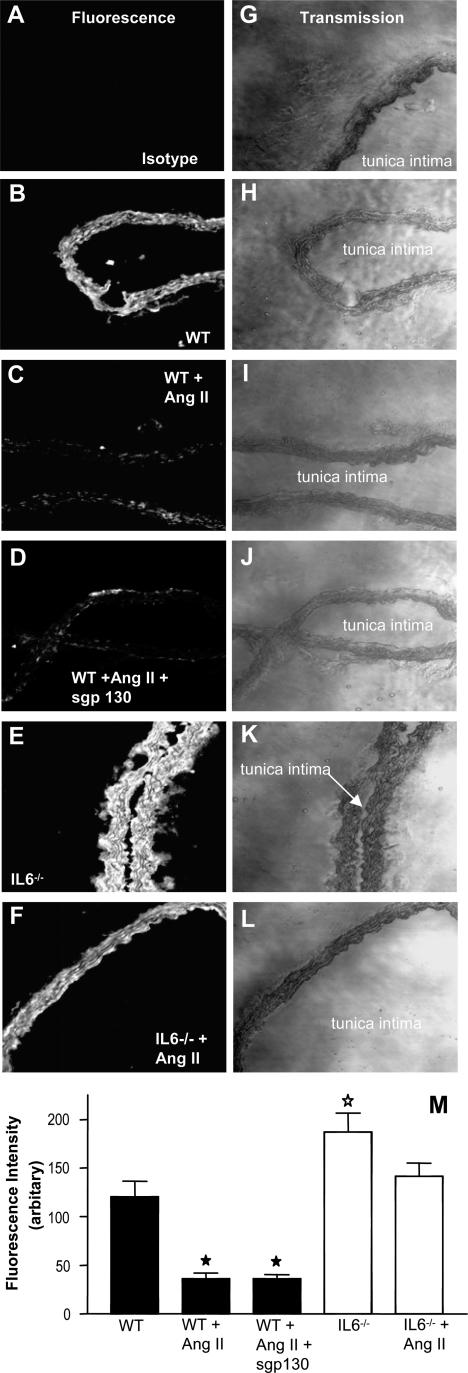
Expression of the AT1R in Aortae of WT and IL-6−/− Mice
To examine whether IL-6 deficiency altered Ang II signaling via changes in Ang II receptor expression, AT1R was examined by immunohistochemistry in aortic sections. Comparisons of pixel intensity in several sections showed that AT1R expression in IL-6−/− mice was significantly greater than in WT mice (P < 0.03, Figure 4). After Ang II infusion, AT1R expression in both WT and sgp130Fc-treated WT mice, but not IL-6−/− mice, was significantly decreased (P < 0.0005, Figure 4). These data indicate that IL-6 suppresses expression of AT1R, both basally and after Ang II infusion, and that this occurs via cognate IL-6R signaling.
Figure 4.
AT1R expression is decreased in aortae from WT Ang II-infused mice with or without sgp130Fc but elevated in IL-6−/−. Aortae were sectioned and stained for AT1R. A–F: Fluorescence images for isotype control (A), WT (B), WT + Ang II (C), WT + Ang II + sgp 130 (D), IL-6−/− (E), and IL-6−/− + Ang II (F). G–L: Corresponding phase-contrast images for A–F, respectively. M: Mean pixel intensity was determined after fluorescence staining of aortic sections (n = 6, separate aortae, mean ± SEM; ★P < 0.0005 ⋆P < 0.03, compared with WT controls, unpaired Student’s t-test).
In Vitro Studies on Vascular Reactivity of WT and IL-6−/− Aortic Rings
To examine whether IL-6 deficiency alters the mechanisms of vessel tone regulation in mice, the constriction/relaxation responses of thoracic aortic rings to the α-receptor agonist PE and endothelial-dependent relaxant ACh were characterized in the presence and absence of the nitric-oxide synthase inhibitor l-NAME or the cyclo-oxygenase inhibitor indomethacin. Phenylephrine causeda concentration-dependent constriction in rings from WT and IL-6−/− mice, with no significant differences observed between the groups, either in the time to develop force or the ability to maintain constriction (Figure 5A). Similarly, no differences were found in vasodilation responses to ACh between strains, with ED50 for relaxation 8.5 × 10−8 or 9 × 10−8 mol/L for WT and IL-6−/− mice, respectively (Figure 5B). Vasorelaxation can be mediated by either nitric oxide (NO) or prostacyclin (PGI). To examine for differences in bioactivity of these mediators in IL-6 deficiency, responses were re-examined in the presence of either l-NAME or indomethacin to inhibit generation of NO or PGI, respectively. As expected from our previous studies with WT aortae,22 inclusion of l-NAME potentiated constriction and inhibited relaxation because of inhibition of eNOS, whereas indomethacin had a small inhibitory effect on constriction (Figure 5). Identical effects were found in rings from IL-6−/− mice, indicating that a similar balance of NO and PGI is required to sustain tone (Figure 5). Finally, no differences in DETA NONOate-dependent dilation were found between the WT and IL-6−/− rings, indicating that smooth muscle guanylate cyclase and cGMP signaling is preserved after IL-6 deletion (not shown). Collectively, these data indicate that the bioactivity of NO and PGI and the ability of vessels to maintain tone in response to physiological stimuli is unchanged in IL-6 deficiency.
Figure 5.
Characterization of in vitro regulation of the vessel tone in IL-6−/− aortic rings. Aortic ring functional responses were determined as described in Materials and Methods. A: PE constriction dose response in aortic rings in the presence or absence of 300 μmol/L l-NAME (n = 8 to 9). B: ACh-relaxation dose response in PE-preconstricted aortic rings in the presence or absence of 300 μmol/L l-NAME (n = 8 to 9). C: Constriction dose response to PE in aortic rings in the presence or absence of 10 μmol/L indomethacin (n = 8 to 9). D: Relaxation dose response to ACh in PE-preconstricted aortic rings in the presence or absence of 10 μmol/L indomethacin (n = 8 to 9). Data are expressed as mean ± SEM. ★★P < 0.01 compared with WT group; ★★★P < 0.001 compared with WT group using two-way analysis of variance with Bonferroni’s posttest to isolate differences between groups.
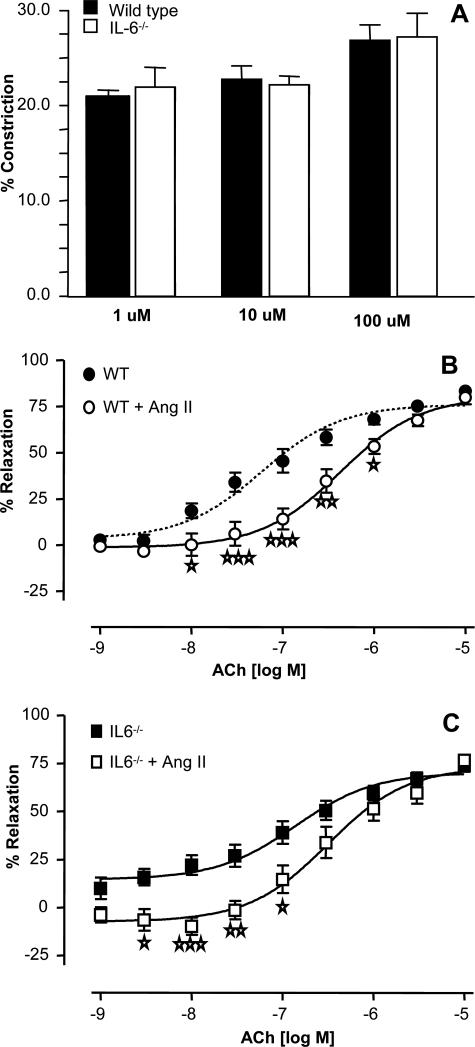
Ang II Signaling in Aortic Rings Is Unchanged in IL-6−/−
Ang II has direct vasoconstrictive effects in aortic vessels that could participate in the hypertensive response in vivo. To examine whether vascular Ang II signaling is altered in IL-6−/−, in vitro signaling by Ang II in WT and IL-6−/− aortae was characterized. First, direct vasoconstriction to three boluses of Ang II was measured and found to be identical in both groups (Figure 6A). It was not possible to construct a dose-response curve to Ang II because low doses appeared to induce desensitization to higher doses of the drug. Next, rings were preincubated with 1 μmol/L Ang II for 15 minutes before PE constriction and ACh relaxation. Preincubation with Ang II caused no significant change in vasoconstriction responses in either group (not shown) but led to a significant right-shift in the relaxation dose-response curve to lower concentrations of ACh (Figure 6, B, and C). This response in WT aortae, characterized as induction of endothelial dysfunction in vitro,23 appears to be unaffected by IL-6 deficiency with ED50 for relaxation at 7.5 × 10−7 or 6.5 × 10−7 for WT and IL-6−/−, respectively. These data indicate that IL-6 is not required for the acute vasoconstrictive effects of Ang II in aortae in vitro and furthermore suggest that this in vitro response may not be related to in vivo hypertensive responses of Ang II, which we have shown herein to require IL-6 trans-signaling.
Figure 6.
IL-6−/− aortae constrict to Ang II and show endothelial dysfunction, similar to WT. Aortic ring functional responses were determined as described in Materials and Methods. A: Constriction responses in unconstricted WT or IL-6−/− aortic rings to bolus doses of Ang II (n = 6). B: Relaxation dose response to ACh in PE-preconstricted WT aortic rings after 30 minutes of preincubation with 1 μmol/L Ang II (n = 8). C: Relaxation dose response to ACh in PE-preconstricted IL-6−/− aortic rings after preincubation with 1 μmol/L Ang II (n = 8). ⋆P < 0.05, ⋆⋆P < 0.01, and ⋆⋆⋆P < 0.001 compared with IL-6−/− control group using two-way analysis of variance with Bonferroni’s posttest. Data are expressed as mean ± SEM.
Discussion
IL-6 belongs to a family of cytokines that promote cellular responses through a receptor complex consisting of at least one subunit of the signal-transducing glycoprotein gp130. Although gp130 activation classically occurs through IL-6 binding to a membrane-bound cognate receptor (IL-6R), many of the biological activities assigned to IL-6 are mediated via a naturally occurring soluble IL-6 receptor (sIL-6R). This soluble receptor forms an agonistic complex with IL-6 that binds gp130 to trigger cellular responses. This activity is termed “IL-6 trans-signaling” and it can be selectively blocked by sgp130Fc, a recombinant protein that leaves classical IL-6R signaling unabated.20 Consequently, IL-6 trans-signaling affords IL-6 with the capacity to trigger responses in cell types that would remain unresponsive to IL-6 itself. Although several recent reports have begun to unearth the physiological and pathophysiological properties of IL-6 trans-signaling,24 it remains primarily unclear how both classic IL-6 signaling and IL-6 trans-signaling harmonize to affect the overall biological activities of IL-6. The rationale for such thinking is derived from two recent studies that highlight that a balance between these two modes of activation affect distinct T-cell responses to influence their recruitment and activation.19,25 The current report extends this concept and provides evidence that such a relationship may affect individual activities within a given disease process. Consequently, highlighting distinctions between the outcomes of IL-6 trans-signaling and classic IL-6 signaling has implications that affect the modern day thinking associated with this inflammatory cytokine. Previous studies measuring plasma levels of IL-6 and its related cytokines have suggested a role for this pathway in hypertension and vascular hypertrophy, including one involving Ang II.1,2,3,5,6,7,9,10 However, it was not known whether IL-6 participated directly as an effector of Ang II signaling and whether antagonizing IL-6 could attenuate these responses in vivo. In addition, the molecular mechanisms by which IL-6 might participate in Ang II-mediated responses via its receptor-dependent signaling remained to be elucidated. Here, we show that IL-6 is an effector of Ang II signaling in vivo but that hypertension and hypertrophy are mediated by distinct IL-6R-dependent mechanisms. Specifically, we suggest that trans-signaling by sIL-6R mediates hypertension, whereas classic IL-6 signaling via cognate IL-6R expressed in vascular tissue is required for cardiac and aortic hypertrophy.
To understand the contribution of these two pathways of IL-6R signaling in Ang II responses, we used sgp130Fc. With this approach, treatment of WT mice with sgp130Fc during the Ang II infusion selectively blocked hypertension (Figure 1B). In contrast, cardiac and aortic hypertrophy was unaffected, suggesting a role for IL-6 signaling through the cognate IL-6R. Although sgp130 can also inhibit leukemia inhibitory factor or cardiotrophin-1, the kd for interaction of sgp130 with sIL-6R is 100- to 1000-fold lower than that of sgp130’s interaction with other IL-6-related cytokines.20,21 In addition, the anti-hypertensive effect of sgp130 was mimicked by IL-6 deficiency. Therefore, the most likely effect to account for the bioactivity of sgp130 is inhibition of IL-6/sIL-6R signaling. These data indicate that both forms of IL-6 signaling participate in Ang II vascular effects and, furthermore, show that IL-6 signaling for hypertrophy is independent of its pressor effects.
A role for IL-6 in hypertension in vivo has been suggested by a study comparing BP responses in WT or IL-6−/− mice given high-dose Ang II (5.2 mg/kg per day, approximately fivefold higher than in our study), in combination with a high-salt diet.26 In contrast to our findings, deletion of IL-6 did not influence the pressor response to high-dose Ang II on a normal salt diet.27 Here, considerably lower Ang II doses that are still sufficient to generate pressor responses were used, and it was found that deletion of IL-6 was fully sufficient to prevent BP elevations throughout the first 4 days (Figure 1A). These data indicate that the mechanisms of Ang II-hypertension vary with dose, with high-dose Ang II elevating BP by IL-6-independent mechanisms. We found that aortae from IL-6−/− mice constrict to Ang II and display endothelial dysfunction when preincubated with Ang II, similar to WT vessels (Figure 6). This indicates that the direct vascular constrictive effects of Ang II are preserved with IL-6 deletion and are therefore unlikely to participate in the pressor response to low-dose Ang II. Perhaps at higher doses of Ang II, direct vasoconstriction by Ang II contributes to the rise in BP, negating the impact of IL-6.
Here, we show that that IL-6 deletion prevents both aortic and cardiac hypertrophy in vivo, in response to low-dose Ang II (Figure 1, C and D). IL-6 signals are coordinated after its binding to the membrane-bound cognate IL-6 receptor. This protein is expressed on the surface of a number of cells, including neutrophils, monocytes, hepatocytes, endothelial cells, adrenal cells, and lymphocytes. Expression of IL-6R in the vascular wall was found, consistent with localization in smooth muscle (Figure 2). Activation of gp130 on the cells would then mediate intracellular activation of Src and JAK, which in turn phosphorylate STAT3 and mediate gene induction.28,29,30,31 Indeed, STAT3 activation in cardiac tissue after Ang II infusion was found, which was absent in IL-6−/− and insensitive to sgp130Fc (Figure 3). These data propose the cognate IL-6R as a potential therapeutic target for treatment of Ang II-dependent cardiac hypertrophy and heart failure.
A study of the downstream regulation of receptors for both pathways (AT1R, IL-6R) by both Ang II and IL-6 in vivo showed that Ang II elevated plasma sIL-6R in IL-6−/− but not WT mice (Figure 2G). In addition, IL-6 deficiency was associated with decreased expression of both cognate IL-6R and plasma sIL-6R, suggesting that IL-6 regulates expression of IL-6R at the gene level (Figure 2). These data show that Ang II regulates sIL-6R in a manner that is antagonized by IL-6. On the other hand, before Ang II infusion, expression of AT1R was higher in IL-6−/− aortae, indicating a compensatory up-regulation, as previously shown.32 Interestingly, even with a twofold elevation of AT1R, responses to Ang II in this strain were either lower (BP/hypertrophy/STAT3 activation) or unaffected (in vitro constriction) (Figures 1 and 6). The elevation in AT1R could represent an attempt to compensate for attenuated Ang II responses in IL-6 deficiency, eg, by up-regulating components of the Ang II-signaling pathway, but this is not clarified herein. However, Ang II significantly decreased AT1R expression in WT but not IL-6−/−, and furthermore, this was not blocked by sgp130Fc (Figure 4M). These data indicate that IL-6 acting via the cognate IL-6R mediates Ang II-dependent down-regulation of AT1R in vivo. A novel mechanism of Ang II and IL-6 interaction in vivo, via a complex interdependence in receptor regulation is demonstrated. Exactly how Ang II stimulates IL-6 signaling is unclear. We found no increases in either circulating sIL-6R or plasma IL-6 (undetectable) after Ang II infusion (Figure 2A and data not shown). Therefore, we propose that Ang II may regulate IL-6 signaling intracellularly, downstream of receptor/gp130 triggering (Figure 7). A further question regards the site of action where sIL-6R signaling mediates hypertension. Possibilities that will need to be addressed in future experiments include resistance arteries, where most of the vasoconstrictive effect of Ang II takes place, and the kidney, where sodium retention is an important contributor to hypertension in this model.
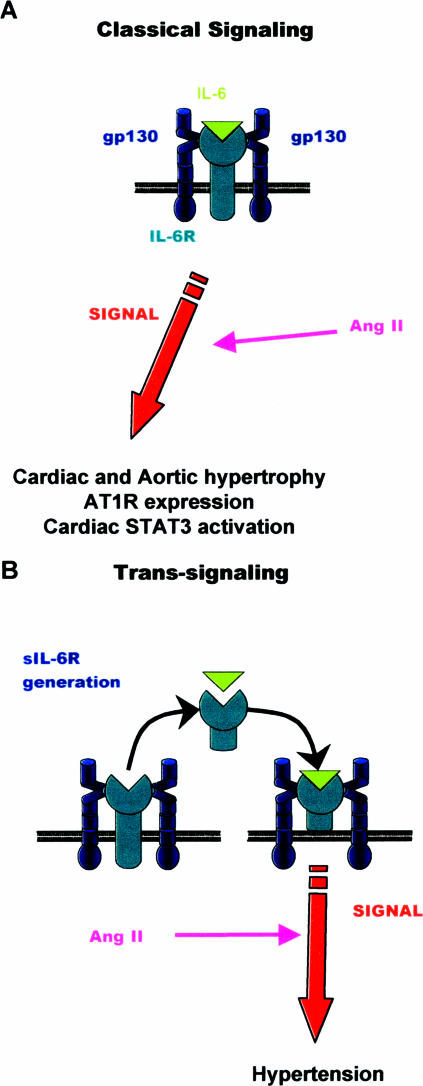
Figure 7.
Illustration of how the different modes of IL-6 signaling influence Ang II-dependent responses in vivo. A: Classic signaling involves the binding of IL-6 to the cognate IL-6R, which couples to gp130 in the cell membrane. This was found to be responsible for cardiac and aortic hypertrophy, down-regulated AT1R expression, and STAT3 activation. B: IL-6 trans-signaling uses a soluble IL-6 receptor and stimulates hypertension.
In summary, our study shows that Ang II-dependent hypertension and hypertrophy are mediated by different IL-6R signaling pathways with IL-6 trans-signaling controlling Ang II-dependent hypertension but classic IL-6R signaling regulating hypertrophy, down-regulation of AT1R, and cardiac STAT3 activation (Figure 7). A hitherto unidentified relationship between IL-6-mediated STAT3 activation and Ang II signaling is established. Inhibition of IL-6 signaling is therefore suggested as a potential therapy for hypertension or cardiac hypertrophy.
Footnotes
Address reprint requests to Valerie B. O’Donnell, Ph.D., Dept of Medical Biochemistry and Immunology, Heath Park, Cardiff, CF14 4XN, UK. E-mail: o-donnellvb@cardiff.ac.uk.
Supported by the Wellcome Trust (to V.B.O., S.A.J., and B.C.), the Deutsche Forschungsgemeinschaft, Bonn, Germany (grants SFB415 and TP-B5 to S.R.J. and J.S.), and Kidney Research UK (KRUK) (to C.A.F.).
References
- Vázquez-Oliva G, Fernandez-Real JM, Zamora A, Vilaseca M, Badimon L. Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J Hum Hypertens. 2005;19:457–462. doi: 10.1038/sj.jhh.1001845. [DOI] [PubMed] [Google Scholar]
- Manabe S, Okura T, Watanabe S, Fukuoka T, Higaki J. Effects of angiotensin II receptor blockade with valsartan on pro-inflammatory cytokines in patients with essential hypertension. J Cardiovasc Pharmacol. 2005;46:735–739. doi: 10.1097/01.fjc.0000185783.00391.60. [DOI] [PubMed] [Google Scholar]
- Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- Stumpf C, John S, Jukic J, Yilmaz A, Raaz D, Schmieder RE, Daniel WG, Garlichs CD. Enhanced levels of platelet P-selectin and circulating cytokines in young patients with mild arterial hypertension. J Hypertens. 2005;23:995–1000. doi: 10.1097/01.hjh.0000166840.63312.12. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- Kurdi M, Randon J, Cerutti C, Bricca G. Increased expression of IL-6 and LIF in the hypertrophied left ventricle of TGR(mRen2)27 and SHR rats. Mol Cell Biochem. 2005;269:95–101. doi: 10.1007/s11010-005-3085-1. [DOI] [PubMed] [Google Scholar]
- Dai RP, Dheen ST, He BP, Tay SS. Differential expression of cytokines in the rat heart in response to sustained volume overload. Eur J Heart Fail. 2004;6:693–703. doi: 10.1016/j.ejheart.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Briest W, Rassler B, Deten A, Leicht M, Morwinski R, Neichel D, Wallukat G, Ziegelhoffer T, Zimmer HG. Norepinephrine-induced interleukin-6 increase in rat hearts: differential signal transduction in myocytes and non-myocytes. Pflugers Arch. 2003;446:437–446. doi: 10.1007/s00424-003-1043-x. [DOI] [PubMed] [Google Scholar]
- Takimoto Y, Aoyama T, Iwanaga Y, Izumi T, Kihara Y, Pennica D, Sasayama S. Increased expression of cardiotrophin-1 during ventricular remodeling in hypertensive rats. Am J Physiol. 2002;282:H896–H901. doi: 10.1152/ajpheart.00591.2001. [DOI] [PubMed] [Google Scholar]
- Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, Koyasu S, Matsui H, Yamauchi-Takihara K, Harada M, Saito Y, Ogawa S. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 2001;89:661–669. doi: 10.1161/hh2001.098873. [DOI] [PubMed] [Google Scholar]
- Funakoshi Y, Ichiki T, Takeda K, Tokuno T, Iino N, Takeshita A. Critical role of cAMP-response element-binding protein for angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 2002;277:18710–18717. doi: 10.1074/jbc.M110430200. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, Sugiyama S, Matsuda S, Koyasu S, Yamauchi-Takihara K, Hirano T, Kawase I, Hirota H. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003;93:221–229. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- Ancey C, Menet E, Corbi P, Fredj S, Garcia M, Rucker-Martin C, Bescond J, Morel F, Wijdenes J, Lecron JC, Potreau D. Human cardiomyocyte hypertrophy induced in vitro by gp130 stimulation. Cardiovasc Res. 2003;59:78–85. doi: 10.1016/s0008-6363(03)00346-8. [DOI] [PubMed] [Google Scholar]
- Uozumi H, Hiroi Y, Zou Y, Takimoto E, Toko H, Niu P, Shimoyama M, Yazaki Y, Nagai R, Komuro I. gp130 plays a critical role in pressure overload-induced cardiac hypertrophy. J Biol Chem. 2001;276:23115–23119. doi: 10.1074/jbc.M100814200. [DOI] [PubMed] [Google Scholar]
- Schroers A, Hecht O, Kallen KJ, Pachta M, Rose-John S, Grotzinger J. Dynamics of the gp130 cytokine complex: a model for assembly on the cellular membrane. Protein Sci. 2005;14:783–790. doi: 10.1110/ps.041117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin RM, Hurst SM, Nowell MA, Harris DA, Horiuchi S, Morgan LW, Wilkinson TS, Yamamoto N, Topley N, Jones SA. Differential regulation of neutrophil-activating chemokines by IL-6 and its soluble receptor isoforms. J Immunol. 2004;172:5676–5683. doi: 10.4049/jimmunol.172.9.5676. [DOI] [PubMed] [Google Scholar]
- Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- Richards PJ, Nowell MA, Horiuchi S, McLoughlin RM, Fielding CA, Grau S, Yamamoto N, Ehrmann M, Rose-John S, Williams AS, Topley N, Jones SA. Functional characterization of a soluble gp130 isoform and its therapeutic capacity in an experimental model of inflammatory arthritis. Arthritis Rheum. 2006;54:1662–1672. doi: 10.1002/art.21818. [DOI] [PubMed] [Google Scholar]
- Anning PB, Coles B, Bermudez-Fajardo A, Martin PE, Levison BS, Hazen SL, Funk CD, Kuhn H, O’Donnell VB. Elevated endothelial nitric oxide bioactivity and resistance to angiotensin-dependent hypertension in 12/15-lipoxygenase knockout mice. Am J Pathol. 2005;166:653–662. doi: 10.1016/S0002-9440(10)62287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JM, Wheatcroft S, Fan LM, Kearney MT, Shah AM. Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2− production, vascular tone, and mitogen-activated protein kinase activation. Circulation. 2004;109:1307–1313. doi: 10.1161/01.CIR.0000118463.23388.B9. [DOI] [PubMed] [Google Scholar]
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad el B, Lehr HA, Schmitt E, Bopp T, Kallen KJ, Herz U, Schmitt S, Luft C, Hecht O, Hohlfeld JM, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Renz H, Neurath MF, Galle PR, Finotto S. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- Lee DL, Leite R, Fleming C, Pollock JS, Webb RC, Brands MW. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension. 2004;44:259–263. doi: 10.1161/01.HYP.0000139913.56461.fb. [DOI] [PubMed] [Google Scholar]
- Schuringa JJ, Dekker LV, Vellenga E, Kruijer W. Sequential activation of Rac-1, SEK-1/MKK-4, and protein kinase Cdelta is required for interleukin-6-induced STAT3 Ser-727 phosphorylation and transactivation. J Biol Chem. 2001;276:27709–27715. doi: 10.1074/jbc.M009821200. [DOI] [PubMed] [Google Scholar]
- Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T, Akira S. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci USA. 1996;93:3963–3966. doi: 10.1073/pnas.93.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- Novotny-Diermayr V, Zhang T, Gu L, Cao X. Protein kinase C delta associates with the interleukin-6 receptor subunit glycoprotein (gp) 130 via Stat3 and enhances Stat3-gp130 interaction. J Biol Chem. 2002;277:49134–49142. doi: 10.1074/jbc.M206727200. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Hilfiker A, Kaminski K, Hilfiker-Kleiner D, Guener Z, Klein G, Podewski E, Schieffer B, Rose-John S, Drexler H. Role of interleukin-6 for LV remodeling and survival after experimental myocardial infarction. FASEB J. 2003;17:2118–2120. doi: 10.1096/fj.03-0331fje. [DOI] [PubMed] [Google Scholar]