Abstract
The goal of glaucoma filtering surgery is to create a low resistance pathway for aqueous outflow. The result is a blister or ‘bleb’ on the conjunctiva, from which fluid drains into the vasculature. Filtering surgery results may be compromised if blebs develop leaks, a problem that surfaces more frequently when antimetabolites are used to control the wound healing response. We investigated the role of tissue remodelling enzymes of the Matrix metalloproteinase (MMP) family in the development of bleb leaks. Our design was a case series. We enrolled glaucoma patients with leaking blebs, glaucoma patients with overhanging blebs and normal eyes. Leaking bleb tissues (n = 11) and bleb leak fluid were collected from patients undergoing bleb revision surgery. Overhanging bleb tissues (from non-leaking blebs, n = 3), normal conjunctiva (n = 8), and aqueous humour (n = 4) were collected for comparison. Samples were analysed for MMP content and proteinase activity by the methods of zymography, westernblotting, immunohistochemistry, and in situ zymography. Our main outcome measures were presence and activity of MMP in sample.
Zymography revealed the presence of a high molecular weight caseinase and a 92-kDa gelatinase of a size appropriate for the proenzyme form of gelatinase B (gelB; MMP-9), in extracts from leaking bleb tissue, but not in bleb leak fluid or aqueous humour samples. In contrast, a 65-kDa gelatinase of a size appropriate for gelatinase A (MMP-2) proenzyme was observed in all samples. All proteinases disappeared when 10 mM EDTA was added to the development buffer, consistent with their identity as MMPs. Western blotting and immunohistochemical analyses confirmed the identity of the 92 kDa proteinase as gelB, and further revealed its absence from extracts of overhanging bleb tissue and normal conjunctiva. In situ zymography demonstrated strong gelatinolytic activity in leaking bleb tissue, but not overhanging bleb tissue or normal conjunctiva.
MMP-g may be involved in the mechanism of formation of bleb leaks. Precise description of the cascade of events leading to bleb leakage may allow the design of therapeutic interventions to prevent, stabilize or reverse bleb leakage.
Keywords: glaucoma, trabeculectomy, matrix metalloproteinase, gelatinase B, filtering surgery, bleb, bleb leak, zymography, western blot, immunohistochemistry, tetracycline
1. Introduction
Glaucoma filtration surgery is performed to control the intraocular pressure (IOP) when medical therapy or other measures fail. The procedure lowers the IOP by creating a fistula between the anterior chamber and the subconjunctival space, creating a filtering bleb (Azuara-Blanco and Katz, 1998). The aqueous humour percolates through the bleb and can then be absorbed through veins and conjunctival lymphatics or pass directly into the tear film in cases where the conjunctiva is thin. The major determinant of glaucoma filtering surgery success is the conjunctival wound healing response. Excessive post-operative fibrosis can lead to filtration failure by resealing of the surgically-created outflow pathways (Cordeiro et al., 2000; Khaw et al., 2001). On the other hand, too little wound healing may eventually lead to bleb leaks, which compromise the ability to maintain an appropriate IOP and provide an avenue for infection (Skuta and Parrish, 1987).
A popular method to control fibrosis is by application of anti-metabolites such as mitomycin C at the time of surgery to the area of conjunctiva destined to become the bleb (Greenfield et al., 1998). This improves the early surgical outcome, but appears to increase the likelihood of late bleb leaks (Lamping et al., 1986; Wilensky, 1992; Chen et al., 1997; Scott et al., 1998). A number of modalities exist to treat bleb leaks (Gehring and Ciccarelli, 1972; Awan and Spaeth, 1974; Joiner et al., 1989; Pederson, 1989; Weber and Baker, 1989), including surgical procedures such as conjunctival patch grafts (Wilson and Kotas-Neumann, 1994), and scleral patch grafts in association with conjunctival advancement (Dunnington, 1950; Melamed et al., 1991; O’Connor et al., 1992; Morris et al., 1998). The reason for the existence of many different procedures to reverse bleb leaks is the fact that none is a cure in all cases. Successful closure of late-onset bleb leaks often requires surgical revision of the bleb (Ritch and Belcher, 1993; Wadhwani et al., 2000). Bleb leaks are the result of conjunctival tissue dissolution, suggesting the involvement of proteinases.
Matrix metalloproteinases (MMPs) are the major effectors of tissue remodelling in vertebrates (Woessner, 1998). Molecular substrates for the MMPs include all classes of extracellular matrix (ECM) proteins, as well as a variety of other molecules involved in determining tissue structure and controlling tissue remodelling (Woessner, 1998; Vu and Werb, 2000). The MMP gelatinase A (gelA; MMP-2) is present in bodily fluids, including the aqueous humour (Ando et al., 1993). GelA can typically be extracted from tissues whether or not they are undergoing remodelling. However, most other MMPs are not present in tissues constitutively, but are synthesized locally by resident cells upon demand, or are released by invading inflammatory cells (Fini et al., 1998a,b). Regulation of MMP expression is very important for controlling MMP activity and inappropriate or over-expression of MMPs causes diverse pathologies across all organ systems (Parks and Mecham, 1998; Chintala et al., 1999). In this study, we investigated the possible role of proteinases in the development of late bleb leaks after glaucoma filtering surgery.
2. Materials and methods
Conjunctival tissue and bleb leak fluid was obtained from patients during bleb revision surgery with the written informed consent of the patients. Bleb leakage was confirmed by Seidel testing. The clinical protocol received institutional review board approval at New England Medical Center Hospitals, Boston, MA. The clinical history of patients from whom tissue specimens were obtained, and the uses made of the tissues in this study, is provided in Table 1. Control conjunctival tissue was also obtained from seven cadaver eyes through the National Disease Research Interchange (NDRI, Philadelphia, PA) and was used for both zymography and western blotting (Table 2).
Table 1.
Clinical findings in surgical specimens
| Patient identifier | Patient age (years/sex) | Type of glaucoma | Samples | Duration between surgery and bleb leak | Duration of leakage | Bleb size | IOP (mmHg) | Antimetabolite treatment | Analyses |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 73/M | POAG | Leaking bleb | 56 Months (11/94–7/99) | 3 Months (10/99) | 2+ | 1 (OS) | Yes | G/C, W, I |
| 2 | 77/M | PXFG | Leaking bleb | 41 Months (8/96–01/00) | 3 Months (4/00) | 1+ | 2 (OD) | Yes | I, IZ |
| 3 | 70/M | POAG | Leaking bleb | 44 Months (6/97–1/00) | 7 Months (8/00) | 2+ | 3 (OD) | Yes | G/C, I |
| 4 | 64/F | POAG | Leaking bleb | 42 Months (6/96–12/99) | 3 Months (2/00) | 3+ | 3 (OS) | Yes | I, W |
| 5 | 65/F | PXFG | Leaking bleb | 58 Months (2/95–4/00) | 4 Months (8/00) | 2+ | 5 (OD) | Yes | G/C |
| 6 | 68/F | PXFG | Leaking bleb | 21 Months (3/00) | 19 Months (10/01) | 1+ | 22 (OS) | Yes | G/C, W |
| 7 | 73/F | POAG | Leaking bleb | 78 Months (3/95–9/01) | 3 Months (12/01) | 2+ | 0 (OD) | No | G/C, W |
| 8 | 64/M | Mixed open angle and angle closure glaucoma | Leaking bleb | 6 Months (6/01–12/01) | 3 Months (3/02) | 2+ | 8 (OD) | Yes | G/C |
| 9 | 72/M | POAG | Leaking bleb | 7 Months (8/95–3/96) | 75 Months (6/02) | 1+ | 13 (OS) | Yes | G/C |
| 10 | 87/F | POAG | Leaking bleb | 27 Months (12/99–05/02) | 1 Month (5/02) | 2+ | 3 (OD) | Yes | G/C |
| 11 | 40/M | Inflammatory glaucoma | Leaking bleb | 1 Month, leaking bleb (4/02) | Bleb revision (4/02) | 2+ | 0 (OS) | Yes | G/C |
| 12 | 79/M | CACG | Overhanging bleb | Trab (7/92), leaking (9/92), recovered (10/92), elevated bleb (1/96) | Bleb revision (8/01) | 3+ | 8 (OS) | No | W, G/C |
| 13 | 63/M | POAG | Overhanging bleb | Trab (6/93), elevated bleb (10/98) | Bleb revision (4/00) | 3+ | 14 (OS) | No | I, W, IZ |
| 14 | 55/F | POAG | Overhanging bleb | Trab (6/92), elevated bleb (8/99) | Bleb revision (4/00) | 3+ | 6 (OD) | No | G, C |
| 15 | 74/M | Normal | Aqueous | N/N | N/A | N/A | 20 (OD) | No | G/C |
| 16 | 73/M | POAG | Aqueous | 56 Months (11/94–7/99) | 3 Months (10/99) | 1 (OS) | Yes | G/C, W, I | |
| 17 | 79/M | Normal | Aqueous | N/A | N/A | N/A | 18 (OD) | No | G/C, W |
| 18 | 70/F | Normal | Aqueous | N/A | N/A | N/A | 25 (OD) | No | W |
| 19 | 71/M | Normal | Conjunctiva | N/A | N/A | N/A | 20 (OD) | No | G/C, I |
G/C, gelatin/casein zymography; I, immunohistochemistry; W, western blot analysis; IZ, in situ zymography; N/A, not available; trab, trabeculectomy; POAG, primary open angle glaucoma; PXFG, pseudoexfoliation glaucoma.
Table 2.
Human cadaver eye specimens
| Eye | Samples | Analyses |
|---|---|---|
| 1 | Conjunctiva | W, G/C |
| 2 | Conjunctiva | W, G/C |
| 3 | Conjunctiva | W, G/C |
| 4 | Conjunctiva | W, G/C |
| 5 | Conjunctiva | W, G/C |
| 6 | Conjunctiva | W, G/C |
| 7 | Conjunctiva | W, G/C |
G/C, gelatin/casein zymography; W, western blot analysis.
2.1. Preparation of tissue extracts
Freshly-collected tissue was placed in a 1.5 ml tube on ice and immediately transferred to the laboratory. To prepare extracts, tissues were homogenized in 40 μl of radioimmunoprecipitation (RIPA) buffer (1% nonidet P40, 20 mM Tris–HCl, 150 mM NaCl, 1 mM Na3VO4, pH 7.4) on ice. Homogenates were centrifuged at 10 000 rpm for 5 min at 4°C and the supernatants were collected. The total protein concentration in each sample was determined using the Bradford assay (Bio-Rad Laboratories, Hercules, CA).
2.2. Zymography
Zymography was used to analyse for the presence of specific proteinase species, including MMPs. This gel electrophoresis technique enables characterization of proteinases by their electrophoretic mobility in the presence of SDS, and their ability to degrade a substrate copolymerized in the gel matrix after removal of SDS. Proenzymes as well as their proteolytically cleaved forms can usually both be visualized because many proenzymes renature into an active configuration after SDS treatment. The procedure is popularly used to detect MMPs, but also detects enzymes of other families. Tissue extracts were fractionated on SDS polyacrylamide gels containing copolymerized gelatin or casein substrates. The procedure was performed according to our standard lab protocol (Fini and Girard, 1990) with minor modifications. Aliquots of tissue extracts containing 20 μg total protein were mixed with SDS gel-loading buffer (Laemmli, 1970) then loaded without reduction or heating onto 10–12% SDS polyacrylamide gels containing 0.1% gelatin or 0.1% beta-casein (Sigma, St Louis, MO). Following electrophoresis, the gels were washed to remove SDS, developed under conditions optimal for MMPs (pH 7.4, 10 mM CaCl2), then stained with Coomassie Brilliant Blue R (Sigma). The location of proteinase species could be easily visualized as clear bands in the blue background of stained substrate. A sample containing a mixture of previously characterized MMPs—the conditioned culture medium from rabbit corneal fibroblasts treated with phorbol myristate acetate (Fina and Girard, 1990) was coelectrophoresed for comparison. In addition, a reduced molecular weight size standard was included on all gels (LifeTechnologies, Gaithersberg, MD).
2.3. Western blot analysis
Equal protein (20 μg) samples of tissue extracts were mixed with sample buffer, reduced by addition of 1% beta-mercaptoethanol, and fractionated on 10–12% SDS-poly-acrylamide gels. After electrophoresis was complete, the proteins were transferred out of the gels to nylon membranes. The membranes were blocked with 10% non-fat dry milk in PBS containing 0.1% Tween-20 (PBS-T), then probed with the same antibodies as used for immunolocalization, described above. After washing with PBS-T, the membrane was incubated with peroxidaseconjugated secondary antibody at room temperature for 1 hr. Finally, the proteins were detected using a chemiluminescence kit (ECL, Amersham Pharmacia Biotech, Piscataway, NJ). Purified human gelB and gelA (from Chemicon, Tamecula, CA) were used as standards.
2.4. Immunohistochemistry
Conjunctival tissue was embedded in OCT compound (Miles, Elkhart, IN). Transverse, 8-μm thick cryostat sections were cut, placed onto super-frost plus slides (Fisher Scientific, Pittsburgh, PA), then fixed by dipping slides in 4% paraformaldehyde at room temperature. Sections were subsequently processed for indirect immunofluorescent localization of MMPs. The primary antibodies were a mouse monoclonal antibody raised against human gelB and a mouse monoclonal antibody raised against human gelA (both from Chemicon, Tamecula, CA). Some experiments also employed a rabbit polyclonal raised against human gelB, a kind gift from Dr Robert M. Senior (Washington University School of Medicine, St Louis, MO). Non-specific binding sites were blocked by incubation with mouse or rabbit serum, respectively. Specific binding of the primary antibodies was visualized using an FITC-labelled secondary antibody. Propidium iodine counterstaining was performed to localize nuclei in the tissue. Staining was visualized by photography through a Nikon microscope equipped for epifluorescence.
2.5. In situ zymography
Zymography, western blotting, and immunolocalization determine the presence of specific proteinase species, but do not provide an assessment of proteinase activity. To determine whether proteolytic activity was present in leaking bleb tissue, in situ zymography was performed on unfixed frozen sections using a gelatin substrate. This technique employs the proteinase substrate, FITC-labelled DQ-gelatin, (Molecular Probes, Eugene, OR), which emits a fluorescent signal when cleaved (Oh et al., 1999). Unfixed frozen sections of tissue were incubated with reaction buffer (0.05 M Tris–HCl, 0.15 M NaCl, 5 mM CaCl2, and 0.2 mM NaN3, pH 7·6) containing 40 μg ml−1 DQ-gelatin overnight. These are conditions that are optimal for detecting MMP activity, but activity due to other neutral proteinases could also be detected. At the end of the incubation period, the fluorescence was observed using a light microscope equipped for epifluorescence (Nikon E400) and captured with a digital camera.
3. Results
Eleven leaking bleb tissues, eight normal conjunctival samples, four samples of bleb leak fluid, and three overhanging bleb tissues were used in this study. A 65-kDa proteinase of a size appropriate to be the proenzyme form of gelA was present in extracts of leaking bleb tissue, bleb leak fluid, and aqueous humour obtained from an individual when undergoing cataract surgery (Fig. 1, gelatin zymogram). In contrast, a 92 kDa gelatinase of a size appropriate to be the proenzyme form of gelB was found only in extracts of leaking bleb tissue. Extracts of leaking bleb tissues also contained higher molecular weight gelatinases. A large amount of a caseinase of slow electrophoretic mobility, and some minor caseinases of more rapid mobility were also seen in extracts of the leaking bleb tissue (Fig. 1, casein zymogram). All evidence of proteinase species was eliminated when developed in the presence of 10 mM EDTA.
Fig. 1.
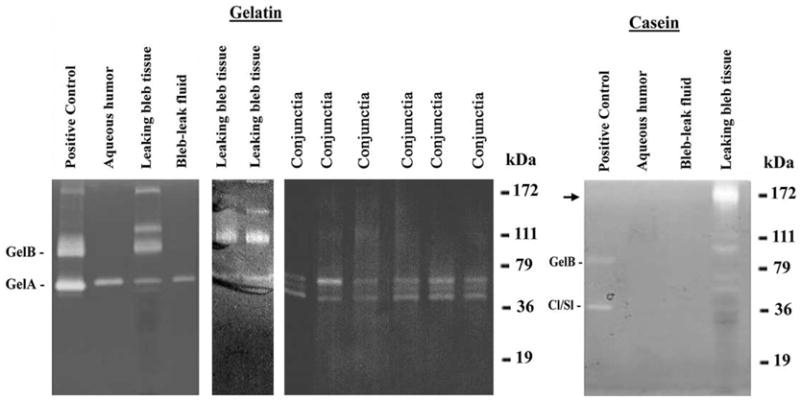
Gelatin and casein zymography of tissue extracts from leaking bleb tissue. Equal protein aliquots of bleb tissue extracts and normal conjunctival extracts were electrophoresed on SDS-polyacrylamide gels containing 0·1% gelatin or casein. Bleb leak fluid and aqueous humour samples were also analysed. Mixed MMPs contained in conditioned medium from rabbit corneal fibroblasts treated with phorbol myristate acetate served as positive control standards. The high molecular weight caseinase is indicated with an arrow. Rabbit gelA is slightly lower in molecular weight than human gelA. GelB was specific for bleb tissue.
Western blot analysis was used to confirm the identity of the 92 kDa gelatinase in leaking bleb tissue as gelB (Fig. 2). This analysis also confirmed that gelB was specific to the leaking bleb tissue. GelB was faintly detectable in only one overhanging bleb sample (Fig. 1). None was detected in bleb leak fluid or normal conjunctiva. Immunolocalization analysis revealed that gelA was distributed throughout the leaking bleb tissue samples, as well as the overhanging bleb samples. GelB was also distributed widely, but was detectable only in the leaking bleb tissue (Fig. 3). In situ zymography revealed strong gelatinolytic activity in leaking bleb tissue. In contrast, overhanging bleb tissue was negative (Fig. 4).
Fig. 2.
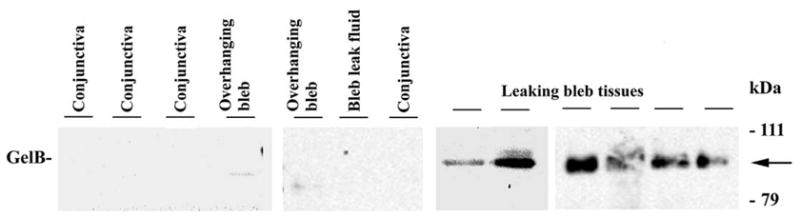
Western blot assay for gelB in leaking bleb tissue. Equal protein aliquots from each tissue were electrophoresed on SDS-polyacrylamide gels, transferred to nylon membranes, and probed with gelB antibody. The migration position of the 92 kDa gelB proenzyme is indicated (arrow). There was strong evidence gelB in all leaking bleb tissue samples, and a faint band detected in only one of the overhanging bleb tissue samples.
Fig. 3.
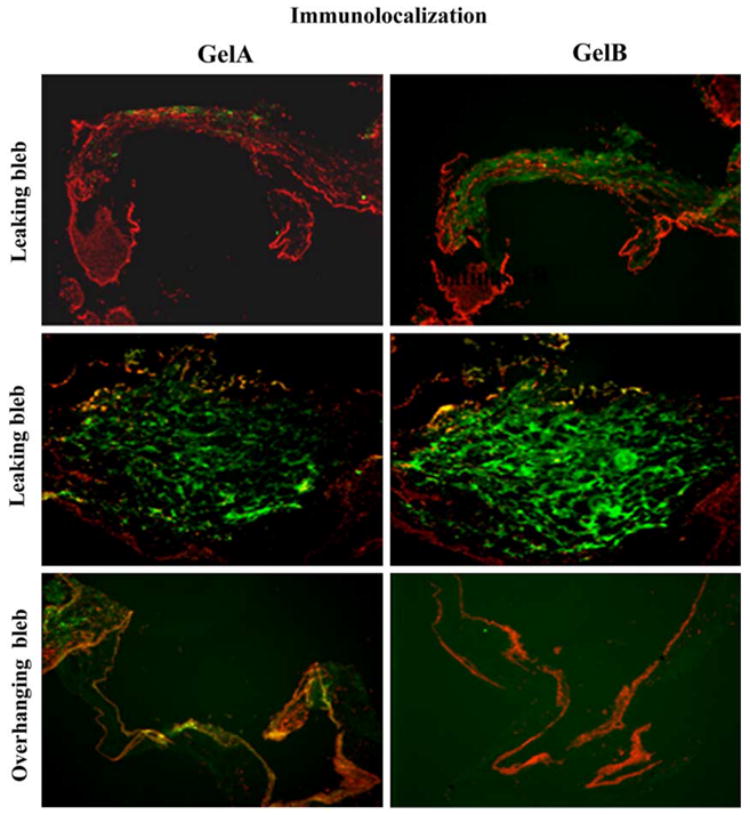
Indirect immunofluorescent localization of gelB and gelA in leaking bleb tissue sections. FITC-conjugated secondary antibody was used to visualize specific binding of primary antibody. Sections were counter-stained with propidium iodide to identify cell nuclei. GelB was specifically localized in leaking bleb tissue.
Fig. 4.
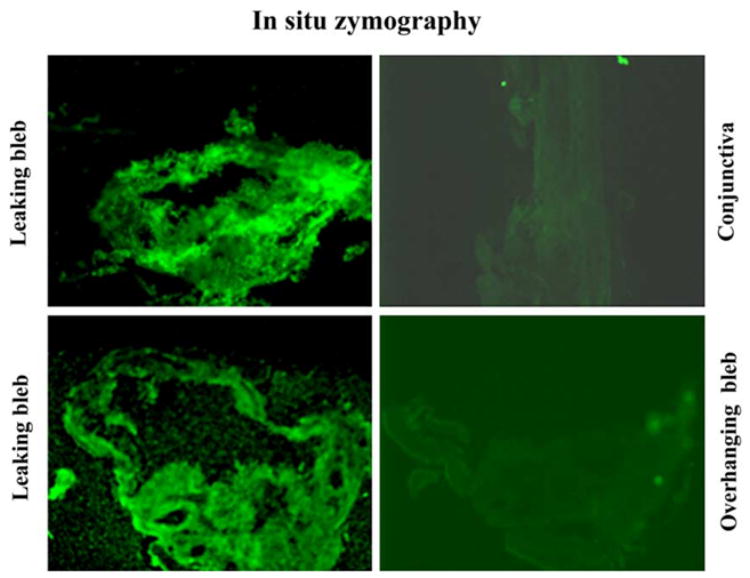
Localization of proteinase activity in leaking bleb tissue by in situ zymography. Frozen bleb-tissue sections were incubated with DQ-gelatin, a substrate which emits a fluorescent signal when cleaved. Gelatinolytic activity was specific for leaking bleb tissue; overhanging bleb tissue was negative.
4. Discussion
GelB (MMp-g) is present in the tissue of leaky blebs, but not in the tissue of overhanging blebs or normal conjunctiva. Extracts of leaking bleb tissues also contained higher molecular weight gelatinases that might represent gelB multimers; conjunctival extracts contained a proteinase species of slightly lower molecular weight than gelA which might represent a cleaved form. All evidence of proteinase species was eliminated when developed in the presence of 10 mM EDTA. This finding is consistent with their identity as MMPs.
Despite many advances in the diagnosis and treatment of glaucoma, IOP remains the major treatable risk factor. Filtration surgery is currently the most definitive method of reducing the IOP and preserving visual function (Skuta and Parrish, 1987; Migdal et al., 1994; Azuara-Blanco and Katz, 1998). Glaucoma filtration surgery differs from most surgical procedures in that inhibition of wound healing is desirable to achieve surgical success. Successful filtration surgery is generally characterized by formation of a filtering bleb, which is a conjunctival ‘blister’ under which aqueous humour accumulates.
Histological examination of functioning blebs reveals loosely arranged connective tissue beneath the conjunctival epithelium. Early onset bleb leaks generally occur within a few months of surgery, and these leaks are largely dependent on the technical aspects of the wound closure. Early bleb failure may be associated with internal obstruction by a blood or fibrinous clot, vitreous, iris, incompletely excised Descemet’s membrane or scleral tissue, or fibroblast proliferation. Rabbit model data suggests that bleb survival may be prolonged and significant complications reduced with the application of antimetabolites (Khaw et al., 1993). In contrast, we defined late failing blebs as those with a history of good bleb function and adequate control of IOP postoperatively for at least 6 months. Although early-onset leaks are usually managed successfully with patching, bandage contact lens or suturing, late-onset leaks are difficult to manage.
The events that lead to late-onset bleb failure are not clearly understood. In this study, we have identified a proteolytic mechanism associated with the pathophysiology of bleb leaks. We further identify a member of the MMP family known as gelatinase B (gelB; MMP-9) as a likely candidate for mediating the proteolysis based on its selective presence in leaking bleb tissue. Given its known substrate specificities, it seems likely that gelB could play a part in increasing the permeability of the bleb tissue, leading to the development of leaks. Although we cannot say whether gelB is the direct cause of bleb leaks, we observed that its presence is associated with a strong gelatinolytic activity that is not found under normal conditions. This is consistent with an earlier study where no gelB was found in normal conjunctiva (Huang et al., 1996). The fact that gelB is found in the uncleaved proenzyme form is not inconsistent with enzymatic activity. The inability to detect cleaved gelB in the presence of enzymatic activity is clearly demonstrated in a number of studies, including a study on repair of the corneal epithelium, a tissue that is continuous with the conjunctiva (Mohan et al., 2002). Cleaved forms of gelB, which might represent activated enzyme, are not typically found in tissues unless massive tissue destruction is occurring, such as in corneal ulceration (Matsubara et al., 1991; Fini et al., 1996). GelB is activated by proteolysis in vitro, but other mechanisms of activation have also been suggested that do not require enzymatic cleavage (Vu and Werb, 2000). Most likely, gelB is activated as needed, in association with cells, and there is little need to accumulate active enzyme. Our observation that gelB is not found in leaking bleb fluid is consistent with its association with cells and extracellular matrix in the bleb tissue. Inappropriate or over-expression of MMPs by resident cells of tissues is associated with many degradative diseases (Parks, 1999). One such condition, corneal ulceration (Williams et al., 1997), offers many parallels to the leaking bleb situation. Thus, leaks occur more commonly in thin avascular blebs surrounded by a vascular ring (Lamping et al., 1986; Wilensky, 1992; Chen et al., 1997), characteristics more common to blebs treated with antimetabolites administered at the time of surgery to inhibit the wound healing response. Similarly, prevascularization of the normally avascular cornea inhibits ulceration (Conn et al., 1980) and a robust wound healing response in the corneal stroma is associated with an arrest in ulcer progression (Fini et al., 1998a,b). Over-expression of gelB by the corneal epithelium is associated with the formation of epithelial defects and stromal ulceration (Matsubara et al., 1991; Fini et al., 1996). In cornea, excessive enzyme expression is probably stimulated by cytokines produced in the inflammatory response associated with healing defects. It is possible that the abnormal situation of aqueous humour in contact with the conjunctival tissue of the bleb has a similar effect; the cytokines and growth factors present in this fluid may stimulate gelB expression by the conjunctival epithelial cells (Kawashima et al., 1998). MMPs such as gelB are also produced by endothelial cells at the tips of invading capillaries (Mohan et al., 1998; Vu and Werb, 2000). Chronic MMP production by capillaries mounting an unsuccessful attempt to vascularize an avascular filtering bleb might result in excessive MMP accumulation. Whatever cells are responsible for gelB production, the abnormal accumulation of the enzyme would gradually cause an increase in tissue permeability, leading to thinning of the bleb tissue and the development of atrophic appearing holes and bleb leaks.
In a rabbit model of corneal injury, it was found that MMP-9 is present in the advancing edge of corneal epithelium during the healing process (Mulholland et al., 2004). Another study in rabbits found that mean bleb survival was significantly improved in animals treated with a broad-spectrum MMP inhibitor, compared to a control group (Wang et al., 2002). The present data suggest that MMP-9 may is present in leaking, but not normal conjunctiva. It is possible that MMP-9 may be directly involved in the degradation of conjunctival tissue associated with bleb failure due to leakage. If future studies support this hypothesis, then a gelB inhibitor may prevent late bleb leaks following glaucoma filtering surgery. Future studies are required to determine if gelB specific inhibitors are effective for the prevention and treatment of late bleb leaks.
Acknowledgments
The authors thank Dr Judah Folkman (Children’s Hospital/Harvard Medical School, Boston, MA) for his assistance with the initial insights that led to this project. Dr Robert Senior (Washington University School of Medicine, St Louis, MO) provided antiserum against human gelatinase B. Drs Surekha Collur, James Heltzer (New England Eye Center, Tufts University School of Medicine, Boston, MA), B. Thomas Hutchinson, A. Robert Bellows and Bradford J. Shingleton (Ophthalmic Consultants of Boston, Boston, MA) assisted with tissue procurement and protocol development.
Footnotes
Grant/Financial support: The National Eye Institute (R01-EY13178, R01-EY12651, R01-EY13643 and P30-EY13078), the Massachusetts Lions Eye Research Fund, Inc., Research to Prevent Blindness, and the Walter G. Ross Foundation.
Contributor Information
M. Elizabeth Fini, Email: efini@med.miami.edu.
Joel S. Schuman, Email: schumanjs@upmc.edu.
References
- Ando H, Twining SS, Yue BY, Zhou X, Fini ME, Kaiya T, Higginbotham EJ, Sugar J. MMPs and proteinase inhibitors in the human aqueous humor. Invest Ophthalmol Vis Sci. 1993;34:3541–3548. [PubMed] [Google Scholar]
- Awan KJ, Spaeth PG. Use of isobutyl-2-cyanoacrylate tissue adhesive in the repair of conjunctival fistula in filtering procedures for glaucoma. Ann Ophthalmol. 1974;6:851–853. [PubMed] [Google Scholar]
- Azuara-Blanco A, Katz LJ. Dysfunctional filtering blebs. Surv Ophthalmol. 1998;43:93–126. doi: 10.1016/s0039-6257(98)00025-3. [DOI] [PubMed] [Google Scholar]
- Chen TC, Wilensky JT, Viana MA. Long-term follow-up of initially successful trabeculectomy. Ophthalmology. 1997;104:1120–1125. doi: 10.1016/s0161-6420(97)30174-2. [DOI] [PubMed] [Google Scholar]
- Chintala SK, Tonn JC, Rao JS. Matrix metalloproteinases and their biological function in human gliomas. Int J Dev Neurosci. 1999;17:495–502. doi: 10.1016/s0736-5748(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Conn H, Berman M, Kenyon K, Langer R, Gage J. Stromal vascularization prevents corneal ulceration. Invest Ophthalmol Vis Sci. 1980;19:362–370. [PubMed] [Google Scholar]
- Cordeiro MF, Siriwardena D, Chang L, Khaw PT. Wound healing modulation after glaucoma surgery. Curr Opin Ophthalmol. 2000;11:121–126. doi: 10.1097/00055735-200004000-00010. [DOI] [PubMed] [Google Scholar]
- Dunnington J. Late fistulization of operative wounds. Diagnosis and treatment. Arch Ophthalmol. 1950;43:407–418. [PMC free article] [PubMed] [Google Scholar]
- Fini ME, Girard MT. The pattern of metalloproteinase expression by corneal fibroblasts is altered by passage in cell culture. J Cell Sci. 1990;97:373–383. doi: 10.1242/jcs.97.2.373. [DOI] [PubMed] [Google Scholar]
- Fini ME, Parks WC, Rinehart WB, Girard MT, Matsubara M, Cook JR, West-Mays JA, Sadow PM, Burgeson RE, Jeffrey JJ, Raizman MB, Krueger RR, Zieske JD. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol. 1996;149:1287–1302. [PMC free article] [PubMed] [Google Scholar]
- Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998a;290:S12–S23. doi: 10.1007/pl00007449. [DOI] [PubMed] [Google Scholar]
- Fini ME, Cook JR, Mohan R, Brinckerhoff CE. Regulation of matrix metalloproteinase gene expression. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. Academic Press; San Diego, CA: 1998b. pp. 299–356. [Google Scholar]
- Gehring JR, Ciccarelli EC. Trichloracetic acid treatment of filtering blebs following cataract extraction. Am J Ophthalmol. 1972;74:622–624. doi: 10.1016/0002-9394(72)90821-5. [DOI] [PubMed] [Google Scholar]
- Greenfield DS, Liebmann JM, Jee J, Ritch R. Late-onset bleb leaks after glaucoma filtering surgery. Arch Ophthalmol. 1998;116:443–447. doi: 10.1001/archopht.116.4.443. [DOI] [PubMed] [Google Scholar]
- Huang SH, Adamis AP, Wiederschain DG, Shima DT, Shing Y, Moses MA. Matrix metalloproteinases and their inhibitors in aqueous humor. Exp Eye Res. 1996;62:481–490. doi: 10.1006/exer.1996.0058. [DOI] [PubMed] [Google Scholar]
- Joiner DW, Liebmann JM, Ritch R. A modification of the use of the glaucoma tamponade shell. Ophthalmic Surg. 1989;20:441–442. [PubMed] [Google Scholar]
- Kawashima Y, Saika S, Yamanaka O, Okada Y, Ohkawa K, Ohnishi Y. Immunolocalization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human subconjunctival tissues. Curr Eye Res. 1998;17:445–451. doi: 10.1080/02713689808951226. [DOI] [PubMed] [Google Scholar]
- Khaw PT, Doyle JW, Sherwood MB, Smith MF, McGorray S. Effects of intraoperative 5-fluorouracil or mitomycin C on glaucoma filtration surgery in the rabbit. Ophthalmology. 1993;100:367–372. doi: 10.1016/s0161-6420(93)31640-4. [DOI] [PubMed] [Google Scholar]
- Khaw PT, Chang L, Wong TT, Mead A, Daniels JT, Cordeiro MF. Modulation of wound healing after glaucoma surgery. Curr Opin Ophthalmol. 2001;12:143–148. doi: 10.1097/00055735-200104000-00011. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamping KA, Bellows AR, Hutchinson BT, Afran SI. Long-term evaluation of initial filtration surgery. Ophthalmology. 1986;93:91–101. doi: 10.1016/s0161-6420(86)33771-0. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Zieske JD, Fini ME. Mechanism of basement membrane dissolution preceding corneal ulceration. Invest Ophthalmol Vis Sci. 1991;32:3221–3237. [PubMed] [Google Scholar]
- Melamed S, Ashkenazi I, Belcher DC, Blumenthal M. Donor scleral graft patching for persistent filtration bleb leak. Ophthalmic Surg. 1991;22:164–165. [PubMed] [Google Scholar]
- Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology. 1994;101:1651–1656. doi: 10.1016/s0161-6420(94)31120-1. [DOI] [PubMed] [Google Scholar]
- Mohan R, Rinehart WB, Bargagna-Mohan P, Fini ME. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during development and injury-related tissue remodeling. J Biol Chem. 1998;273:25903–25914. doi: 10.1074/jbc.273.40.25903. [DOI] [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar W, McCabe F, Russo L, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) regulates and effects epithelial regeneration. J Biol Chem. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Morris DA, Ramocki JM, Shin DH, Glover BK, Kim YY. Use of autologous Tenon’s capsule and scleral patch grafts for repair of excessively draining fistulas with leaking filtering blebs. J Glaucoma. 1998;7:417–419. [PubMed] [Google Scholar]
- Mulholland B, Tuft SJ, Khaw PT. Matrix metalloproteinase distribution during early corneal wound healing. Eye. 2004 August 27; doi: 10.1038/sj.eye.6701557. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- O’Connor DJ, Tressler CS, Caprioli J. A surgical method to repair leaking filtering blebs. Ophthalmic Surg. 1992;23:336–338. [PubMed] [Google Scholar]
- Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- Parks WC, Mecham RP, editors. Matrix Metalloproteinases. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Pederson J. Ocular hypotony. Chapter 13 St Louis: Mosby; 1989. [Google Scholar]
- Ritch RSJ, Belcher CD., III Cases in controversy: management of the leaking filtration bleb. J Glaucoma. 1993;2:114–118. [PubMed] [Google Scholar]
- Scott IU, Greenfield DS, Schiffman J, Nicolela MT, Rueda JC, Tsai JC, Palmberg PF. Outcomes of primary trabeculectomy with the use of adjunctive mitomycin. Arch Ophthalmol. 1998;116:286–291. doi: 10.1001/archopht.116.3.286. [DOI] [PubMed] [Google Scholar]
- Skuta GL, Parrish RK., 2nd Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987;32:149–170. doi: 10.1016/0039-6257(87)90091-9. [DOI] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Wadhwani RA, Bellows AR, Hutchinson BT. Surgical repair of leaking filtering blebs. Ophthalmology. 2000;107:1681–1687. doi: 10.1016/s0161-6420(00)00282-7. [DOI] [PubMed] [Google Scholar]
- Wang TTL, Daniels JT, Mead AL, Murphy G, Khaw PT. ARVO abstract. 2002. [Google Scholar]
- Weber PA, Baker ND. The use of cyanoacrylate adhesive with a collagen shield in leaking filtering blebs. Ophthalmic Surg. 1989;20:284–285. [PubMed] [Google Scholar]
- Wilensky JT. Management of late bleb leaks following glaucoma filtering surgery. Trans Am Ophthalmol Soc. 1992;90:161–168. [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Fini ME, Cousins SW, Pepose JS. Corneal responses to infection. The Cornea. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Fundamentals of Corneas and External Disease. I. Mosby; St Louis, MO: 1997. pp. 129–162. [Google Scholar]
- Wilson MR, Kotas-Neumann R. Free conjunctival patch for repair of persistent late bleb leak. Am J Ophthalmol. 1994;117:569–574. doi: 10.1016/s0002-9394(14)70060-1. [DOI] [PubMed] [Google Scholar]
- Woessner JF. The matrix metalloproteinase family. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. Academic Press: San Diego, CA; 1998. pp. 1–14. [Google Scholar]


