Abstract
We used a multiple channel, single unit recording technique to investigate the neural activity in different corticolimbic and basal ganglia regions in freely moving rats before and during generalized amygdala kindled seizures. Neural activity was recorded simultaneously in the sensorimotor cortex (Ctx), hippocampus, amygdala, substantia nigra pars reticulata (SNr) and the subthalamic nucleus (STN). We observed massive synchronized activity among neurons of different brain regions during seizure episodes. Neurons in the kindled amygdala led other regions in synchronized firing, revealed by time lags of neurons in other regions in crosscorrelogram analysis. While there was no obvious time lag between Ctx and SNr, the STN and hippocampus did lag behind the Ctx and SNr in correlated firing. Activity in the amygdala and SNr contralateral to the kindling stimulation site lagged behind their ipsilateral counterparts. However no time lag was found between the kindling and contralateral sides of Ctx, hippocampus and STN. Our data confirm that the amygdala is an epileptic focus that emits ictal discharges to other brain regions. The observed temporal pattern indicates that ictal discharges from the amygdala arrive first at Ctx and SNr, and then spread to the hippocampus and STN. The simultaneous activation of both sides of the Ctx suggests that the neocortex participates in kindled seizures as a unisonant entity to provoke the clonic motor seizures. Early activation of the SNr (before the STN and hippocampus) points to an important role of the SNr in amygdala kindled seizures and supports the view that different SNr manipulations may be effective ways to control seizures.
Keywords: hippocampus, substantia nigra pars reticulata, epilepsy, rats, synchronization, single unit recording
1. Introduction
Kindling is a widely used animal model to study basic mechanisms underlying epileptic seizures. Kindled seizures initiated in limbic structures share several characteristics of temporal lobe epilepsy (TLE), the most common and intractable form of human epilepsy (Engel, 1996). Elucidating how epileptiform activity is initiated and how it propagates is essential for our understanding of epileptogenic processes and the state of epilepsy.
Lesion studies have implicated various brain structures in the expression of kindled seizures, such as the prefrontal and orbital cortices, the stria terminalis, the hippocampus, brain stem and basal ganglia structures (McIntyre 1975; Corcoran et al., 1975; Engel and Katzman 1977; Racine et al., 1988).
The amygdala and hippocampus are believed to be key structures involved in TLE. Indeed epileptiform paroxysms in both structures were found in patients with complex partial seizures and many of these patients remained seizure-free for years following selective amygdala/hippocampectomies or temporal lobectomy (Feindel and Rasmussen, 1991; Degado-Escueta and Walsh, 1985; Quesney 1986). Accordingly, manipulations of the amygdala and hippocampus have served as useful experimental models for epilepsy research, including kindling, kainic acid and tetanus toxin models (Racine 1978; Bertram, 1997; Tremblay et al., 1983; Bragin et al., 1999; Hawkins and Mellanby, 1987).
The basal ganglia have been hypothesized to be involved in the transition from limbic to generalized motor seizures (McNamara et al., 1986). Most studies have focused on the substantia nigra pars reticulata (SNr), the output nucleus of the basal ganglia system. Gale and Iadarola (1980) first described the existence of nigral control of an epilepsy system. SNr activation is thought to inhibit a “dorsal midbrain anticonvulsant zone” and thereby to facilitate seizure occurrence (Redgrave et al., 1992a,b). Other basal ganglia structures, either directly or indirectly connected to the SNr, may also be involved in the modulation of seizures. For example, microinjection of GABA antagonists into the striatum has been shown to protect against amygdala-kindling seizures in the rat (Cavalheiro et al., 1987). A number of studies have recently focused on the role of the subthalamic nucleus (STN) in epileptic seizures. Injection of GABA agonists into the STN significantly reduced motor seizures, probably due to decreased excitatory input to the SNr (Veliskova et al., 1996; Deransart et al., 1998). And, finally, the frontal cortex is believed to be the route seizures take from subcortical limbic structures to the brainstem and spinal cord, where the convulsive components are manifested (Corcoran et al., 1975; Kelly et al. 1999; McIntyre and Gilby, 2005).
A critical question concerning the pathophysiology of epileptic seizures is how the ictal discharges propagate within epileptic networks. In the present study, taking advantage of the high temporal and spatial resolution of single unit recording, we sought to determine the sequence of propagation of ictal discharges within the corticolimbic basal ganglia network in amygdala kindled rats.
2. Materials and Methods
2.1 Animals
Fifteen adult male Sprague-Dawley rats weighing 350 to 400 g at the time of surgery were used in this study. Animals were housed individually under a reversed dark-light cycle (lights off from 7:00 to 19:00) for seven days before surgery. Animals were treated in accordance with the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals and the experimental protocol was approved by the Animal Care and Use Committee of Wake Forest University School of Medicine.
2.2 Surgery
Rats were anesthetized with ketamine (100 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.). Two types of microwire arrays were used. For single unit recording only, six arrays of eight stainless steel teflon-insulated microwires (50 μm diameter, Biographics Inc. Winston-Salem, NC), soldered onto connecting pins on a headstage, were stereotaxically lowered bilaterally into the motor cortex (Ctx), SNr and either CA1 region of the dorsal hippocampus or STN. For simultaneous recording and stimulation, two arrays of 10 microwires were used; the additional two microwires (same as recording microwires) were used to deliver kindling stimulation. These arrays were implanted in the basal lateral amygdale (Figure 1). Coordinates for these regions were obtained from the atlas of Paxinos and Watson (1986) as follows: 1.0 mm anterior to the bregma (A), 2.8 mm lateral to the midline (L), 1.7 mm ventral to the dorsal surface of the brain (V) for the Ctx; −2.8 mm (A), 4.5 mm (L) and 7.5 mm (V) for the basolateral amygdala; −3 mm (A), 1.5 mm L and 2.2-2.5 mm (V) for the CA1 region of dorsal hippocampus; −3.8 mm (A), 2.5 mm (L) and 7.3 mm (V) for the STN; and −5.4 mm (A), 2.0 mm (L) and 7.8 mm (V) for the SNr. Since only four sites could be targeted, the Ctx, amygdala and SNr were recorded in every rat. For the fourth target, either the hippocampus or STN was selected. Due to this limitation there are no simultaneous recordings of hippocampal and STN neurons. The headstage was secured onto the rat's cranium with dental cement using six anchoring screws (1 mm diameter) surrounding the surgery area. Animals received ampicillin (60,000 U, i.m.) before surgery to prevent infection. Animals were housed individually and allowed to recover from surgery for at least 14 days before being subjected to the experiment.
Figure 1.
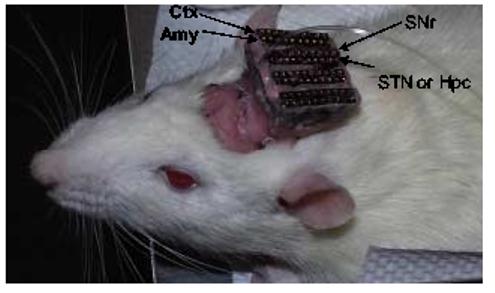
Photograph showing the configuration of electrode arrays. Eight electrodes arrays were implanted into the Ctx, Amy, SNr, STN or Hpc bilaterally. Arrows indicted the arrays implanted in different regions in the right side of the brain, same arrangement was made in the left side of the brain.
2.3 Experimental procedures
Kindling stimulation was delivered daily into one amygdala (8 right side and 7 left side) using a 1 s stimulation train at 60 Hz, 1 ms pulse width, at 100-300 μA. Rats were placed in a chamber with dim light. Rats were more active and performed better during the dark cycle in operant tasks. Stimulation was initiated when the rat was at rest, usually about 10 min into the experimental session. Daily kindling gradually induced progressive symptoms of seizures as classified by Racine (1972). Initially head bobbing and jaw movement were observed, followed by facial clonus, rearing, forelimb clonus and finally stage 5 kindled convulsive seizures were apparent with 1 to 2 weeks of kindling. Animal behaviors were recorded with a digital video camera that was synchronized with a data acquisition system with 30-ms time resolution (Biographic Inc. Winston-Salem NC).
Extracellular recording of the four selected brain areas were performed by connecting a head stage plug with unity gain preamplifiers and a lightweight cable between a motor-assisted commutator and the implanted microwire assembly. The commutator was free to turn as necessary, permitting unrestricted movement of the rat. Neuro-electric signals were passed from the headset assemblies to programmable amplifiers, filters (0.5 and 5 kHz) and a multi-channel spike-sorting device (Plexon Inc., Dallas, TX). Valid spikes (signal to noise ratio > 3) were selected using amplitude and duration thresholds. And waveforms were recorded during experimental sessions.
As many as 62 neurons from the Ctx, amygdala, SNr and the STN or hippocampus were monitored simultaneously from 64 microelectrodes. Spike activity before and during amygdala kindled seizures were recorded (1 kHz sample rate) and monitored with Magnet data acquisition software (Biographics Inc., Winston-Salem, NC). Spike train activity was analyzed offline with the PC-based programs Stranger (Biographics Inc., Winston-Salem, NC) and Nex (Plexon Inc., Dallas, TX).
2.4 Data Analysis
Cross-correlation analysis was performed to reveal the temporal sequence of synchronized firing (Chang et al., 2000). Cross-correlation histograms were created with the Nex program. One neuron was selected as the reference neuron and all other neurons recorded within that same session were defined as partner neurons for the cross-correlation analysis. The time of occurrence of spikes from the reference neuron was set at 0 s and the partner neuron's firing 0.2-0.5 s before and after each reference neuron's spike was plotted using a 1-ms bin size. The spikes appearing on the plus side of the time scale represent the firing of a partner neuron after the reference neuron, and the spikes on the minus side of the time scale represent the partner neuron's firing before the reference neuron. The significance level of the cross-correlograms was tested using 99% confidence intervals in the Nex program. The numeric results were then exported to Matlab as a matrix, peaks above the 99% confidence level were detected by Matlab and the distance (called Peak latency) between the peak and 0 point were calculated in Matlab for each significant pair of the cross-correlation. Each calculated 0 point to peak distance was transformed to a time lag value using the following formula:
Depending on the duration of the seizure episode, a 60 – 398 s period immediately following kindling stimulation were selected for cross-correlogram analysis. This period covered the whole seizure episode including all stages of that particular seizure. In some cases, the early stage of a seizure lasted for a very short period of time (< 5 s), which prevented us from analyzing different stages of seizures due to an insufficient number of spikes. Thus the data described below can be thought of as representing the total expression of a fully generalized stage 5 seizures. Student's t test was used to compare the time lags between brain regions. In all cases p < 0.05 was considered significant.
2.5 Histology
At the conclusion of the final experimental session, each animal was subjected to the same anesthesia as in surgery. A positive current of 10 – 20 μA was passed through selected microwires for 10 – 20 s to deposit iron ions. Animals were then sacrificed and perfused intra-cardially with a 4% paraformaldehyde solution. Coronal sections (45 μm thick) were cut through the Ctx, hippocampus, amygdala, STN and SNr and mounted on glass slides. Incubation of the mounted sections in a solution containing 5% potassium ferricyanide/10% HCl revealed the iron deposits (recording sites) in the form of blue dots. Boundaries of the four brain areas were assessed with reference to the rat brain atlas of Paxinos and Watson (1986).
3. Results
3.1 Histology and data selection
The data from all kindling and recording sites were confirmed to be located in the desired structures (Figure 2). The cross-correlograms were taken from fully kindled rats, which had previously experienced no less than stage 5 seizures. The average kindling rate to the first stage 5 seizure was 8.9±2.1 stimulations.
Figure 2.
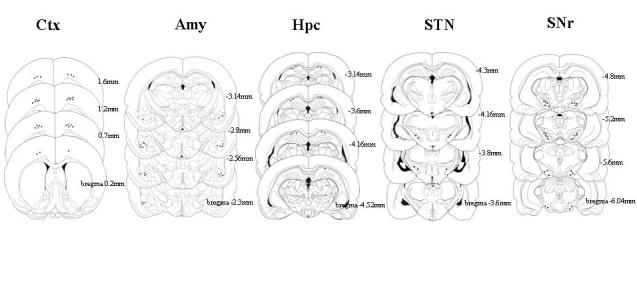
Histological localization of recording electrodes in the Ctx, Amy, Hpc, STN and SNr.
3.2 Synchronization of neural activity during amygdala kindled seizures
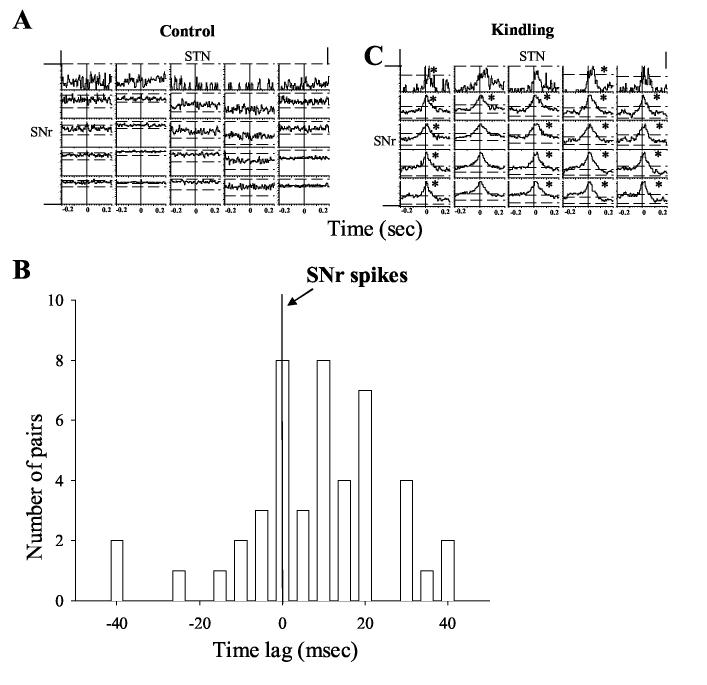
Pairwise cross-correlograms were created for all sets of simultaneously recorded neurons. A total of 8,216 pairs of cross-correlograms from 485 recorded neurons (146 Ctx, 114 basolateral amygdala, 31 STN, 83 hippocampus and 111 SNr neurons) were analyzed in kindled rats expressing stage 5 seizures. Depending on the duration of seizure episode, periods of 60 to 398 s (186 ± 29 s) immediately following the kindling stimulus were selected to be analyzed. These periods covered all stages of kindled seizures in each case. A matched period before the kindling stimulation in the same session was selected as the control period. Example of single neural activity before and during kindled seizures is shown in Figure 3. Figure 4 demonstrates crosscorrelogram of pairs of neurons in different brain regions before and during kindled seizures. Synchronized activity between different neurons was rarely found before the kindling stimulation (Figure 4 A, C). However, amygdala stimulation in the kindled rats triggered immediate seizures with massive synchronized activity (Figure 4 B, D), as well as behavioral seizures, beginning with jaw movements, followed by nodding of the head and ultimately stage 5 clonic motor convulsions. Table 1 shows the percentage of significantly cross-correlated pairs of neurons between different and within the same brain regions. The degree of synchronization within the same structure, in terms of percentage of significantly correlated pairs of neurons, surprisingly was lowest in the kindled focus, the amygdala (27%), and highest in the cerebral cortex (64%).
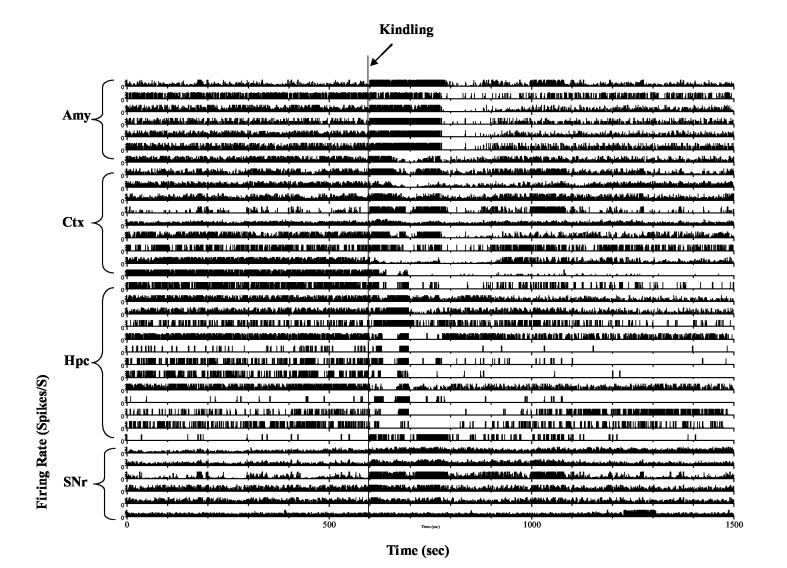
Figure 3.
Example of single unit activity recorded simultaneously from the amygdala (Amy), cortex (Ctx), hippocampus (Hpc) and substantia nigra pars reticulata (SNr) during kindled seizures. Kindling stimulation was delivered at 600 s as indicated by arrow. Notice a variety of neural responses took place immediately following kindling stimulation. Rate meter was plotted using 2 times of local mean as maximum firing rate.
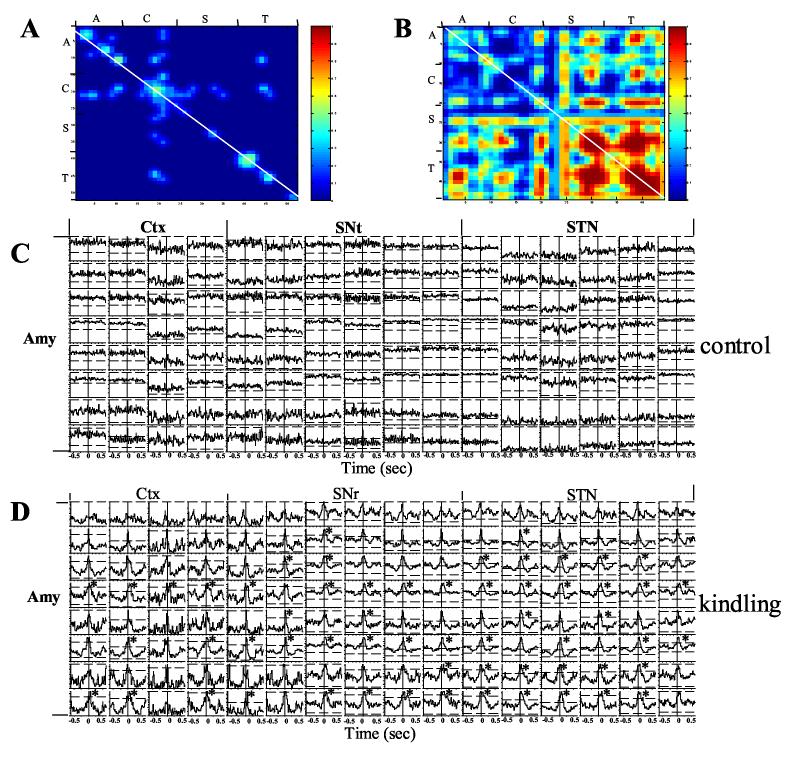
Figure 4.
Synchronized activity between simultaneously recorded neurons from the Amy, Ctx, STN and SNr before and during amygdala kindled seizures. A) Color coded significant cross-correlation level (99% confidence level shown as red) between neurons in different brain regions before amygdala kindling. Note that there was negligible cross-correlation between different neurons. B) During amygdala kindled seizures, massively synchronized firing appeared between neurons within the same and across different brain regions. The white diagonal line represents autocorrelation. C) Cross-correlogram analysis of synchronized activity between the neurons in the amygdala kindling site and other regions before amygdala kindling. Note that there was no obvious cross-correlation between the neurons. D) During amygdala kindled seizure, however, massive synchronization took place between neurons as demonstrated by peaks above 99% confidence lines. Many of the cross-correlations revealed a time lag of synchronized activity peaks. Note that most of the synchronized peaks lay on the plus side of crosscorrelogram plot, indicating a delay following spikes in the amygdala kindling site (indicated by * signs. A = amygdala, C = cortex, S = SNr and T = STN).
Table 1.
Percent of cross-correlated pairs between neurons in different brain regions. Ak = amygdala kindling site, ck = cortex kindling side, hk = hippocampus kindling side, sk = SNr kindling side, tk = STN kindling side, an = amygdala non-kindling side, cn = cortex non-kindling side, hn = hippocampus non-kindling side, sn = SNr non-kindling side, tn = STN non-kindling side.
| ak | ck | hk | sk | tk | An | Cn | hn | sn | tn | |
|---|---|---|---|---|---|---|---|---|---|---|
| ak | 27.5 | 24.3 | 15.8 | 43.7 | 45.3 | 18.8 | 23.2 | 8.5 | 41.8 | 20.0 |
| ck | 64.3 | 22.5 | 46.3 | 37.0 | 28.9 | 50.5 | 9.6 | 38.9 | 17.3 | |
| hk | 39.9 | 44.0 | * | 18.0 | 27.3 | 21.5 | 19.7 | * | ||
| sk | 40.0 | 46.9 | 21.9 | 35.8 | 27.6 | 34.3 | 37.3 | |||
| tk | 56.1 | 14.3 | 34.7 | * | 50.0 | 36.1 | ||||
| an | 32.5 | 21.9 | 9.7 | 24.6 | 14.3 | |||||
| cn | 51.8 | 10.9 | 29.3 | 38.5 | ||||||
| hn | 30.4 | 10.9 | * | |||||||
| sn | 44.7 | 27.0 | ||||||||
| tn | 50.0 |
data not available since STN and Hpc were not recorded simultaneously.
3.3 Spread of ictal discharges in the ipsilateral side of corticolimbic basal ganglia system
3.3.1 Temporal relationship between amygdala kindling site and other brain regions
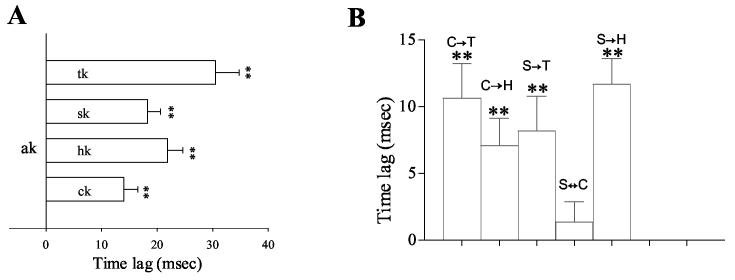
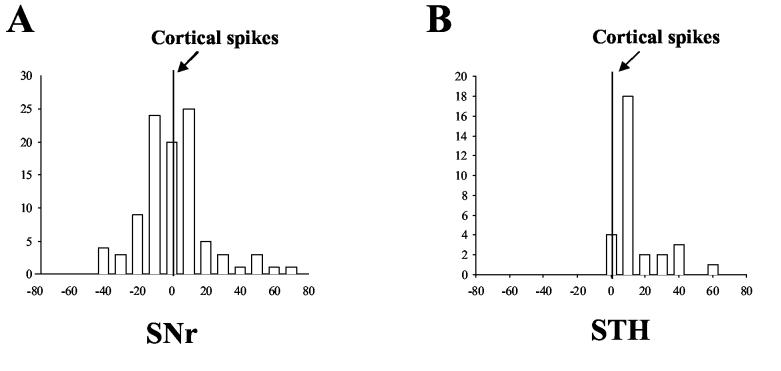
Detailed analysis of time lag in the cross-correlogram provides the sequence of spread of ictal discharges within the meso-cortical and basal ganglia system. Activity in the amygdala kindled site led activity in all of the other regions in the cross-correlation analysis. The time lag from the amygdala spikes ranged from 14 ms in the Ctx to 30 ms in the STN (Figure 5A). Figure 6 shows the distribution of synchronized peaks of different brain regions in relation to spikes within the amygdala kindled site. Peaks of neurons in the hippocampus and the STN never preceded the zero point set for the amygdala reference spikes in the cross-correlogram. That is, STN and hippocampal neurons consistently fired on the ‘plus side’ of amygdala neurons. This finding indicates that the amygdala generally led the STN and hippocampus in synchronized firing. In the SNr and Ctx, there were a few peaks of correlated firing that appeared before the amygdala spikes (on the minus side), however, most of peaks occurred after the amygdala spikes, indicating a leading position of amygdala cells in synchronized firing through the entire seizure epoch.
Figure 5.
Time lags between the amygdala kindling site and other regions in the kindling side of the brain during amygdala kindled seizures. A) Amygdala neurons led cortical, hippocampal, SNr and STN neurons in synchronized firing, ranging from 14 ± 2.5 ms for cortical neurons to 30.5 ± 4.2 ms for STN neurons. B) Time lags between the neurons of different regions in the kindling side. A = amygdala, C = cortex, H = hippocampus, S = SNr and T = STN. The arrow indicates the direction of the temporal sequence (student's t test, ** p < 0.01 vs. reference spike).
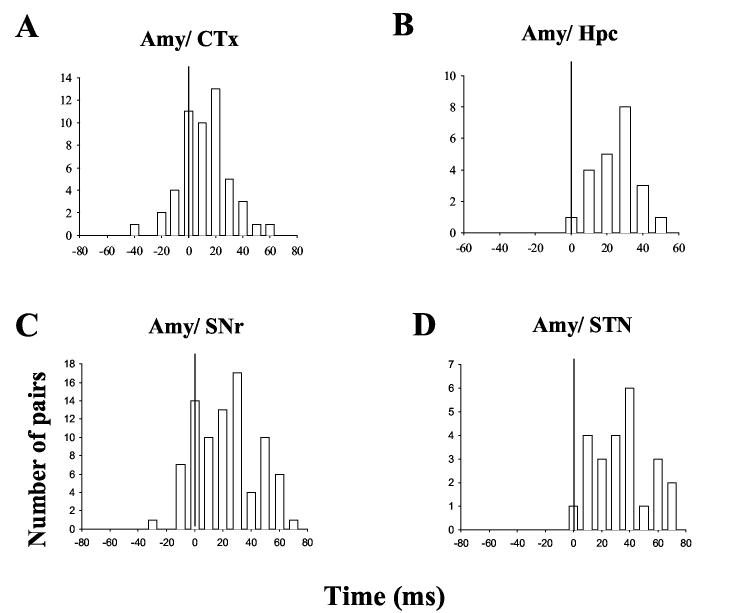
Figure 6.
Distribution of synchronized activity peaks between neurons in the amygdala kindling site and other regions ipsilateral to the kindled site. The numbers of neuron pairs (ordinate) that exhibited different time lags (abscissa) are shown. Note that most of correlation peaks in the Ctx and SNr and all of the peaks in the hippocampus and STN occurred in the plus side of the plots, following amygdala spikes, indicating that amygdala neurons led the other regions in synchronized firing.
3.3.2 Temporal relationship between basal ganglia structures
Two basal ganglia sites, the STN and SNr, were examined in the present study. These two sites have been implicated in seizure control (Gale and Iadarola 1980; Depaulis et al., 1994; Veliskova et al., 1996). The STN sends a major glutamatergic projection to the SNr, by which it regulates basal ganglia output. Unexpectedly, we found that the STN usually lagged the SNr in synchronized firing during kindled seizures. Figure 7A demonstrates cross-correlogram plot using SNr spike as a reference event in one representative rat before and during amygdala kindled seizures. No synchronized firing was observed before amygdala kindling when the rat was behaving normally (left panel). All of these neurons exhibited synchronized firing during amygdala kindled seizures (right panel). Most of STN synchronized spikes appeared after SNr spikes (on the plus side). Figure 7 B shows the distribution of time lags of all cross-correlated pairs between the SNr and STN. As can be seen, most of the synchronized spikes of the STN laid on the plus side of the plot, indicating a time lag behind SNr spikes. The mean time lag of STN spikes was 8.2 ± 2.6 (p<0.01, student's t test, STN lagged behind SNr spikes, Figure 5 B).
Figure 7.
Temporal relationship between STN and SNr neurons during amygdala kindled seizures. A) Cross-correlogram plots from selected STN and SNr neurons in the kindling side before (left panel) and during amygdala kindled seizures (right panel). SNr neurons were used as reference neurons. Synchronization occurred in these neurons only during amygdala kindled seizure (displayed in the right panel). Most STN neurons lagged behind SNr neurons in synchronized firing (Marked by * signs). B) Distribution of time lags between synchronized firing of STN and SNr neurons (SNr neurons were reference neurons). Most of the STN spike peaks followed SNr spikes with time lags up to 40 ms.
3.3.3 Temporal relationship between basal ganglia and cortex
No obvious time lag was found between the SNr and Ctx. As demonstrated in figure 8 A, the correlated peaks were evenly distributed around the 0 s point in the cross-correlation plot with a mean time lag of 1.4 ± 1.5 ms (p>0.05 student's t test, Figure 4). The STN neurons, on the other hand, consistently lagged Ctx neurons with a mean delay of 10.6 ± 2.6 ms (p<0.01, student's t test, Figure 5B and Figure 8 B).
Figure 8.
Temporal relationship of correlated firing between Ctx and basal ganglia neurons in the kindling side during amygdala kindled seizures. A) Distribution of correlated spikes between cortical and SNr neurons using cortical neurons as a reference. Synchronized SNr spikes were distributed evenly around the cortical spikes at the 0 s reference point with an average time lag of −1.4 ±1.5 ms. B) Distribution of correlated spikes between cortical and STN neurons. All correlated STN spikes were located on the plus side of the time lag with a peak at 10 ms.
3.3.4 Temporal relationship between hippocampus and cerebral cortex/basal ganglia
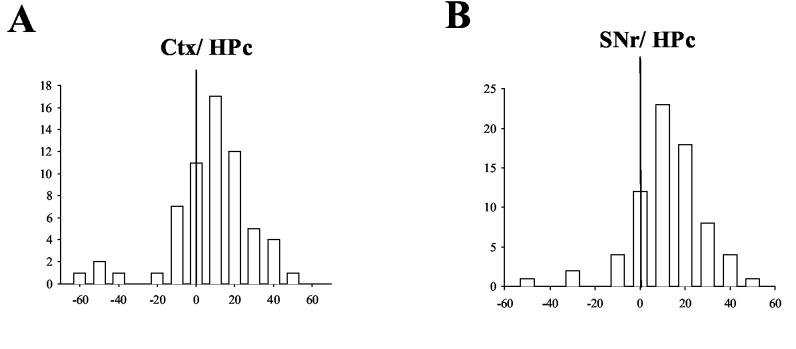
Figure 9A shows the distribution of synchronized hippocampal spikes in relation to cortical neurons during amygdala kindled seizures. Many correlated spikes in the hippocampus occurred 10 ms after cortical spikes in the cross-correlogram plot (Figure 9A). Few hippocampal peaks appeared before cortical spikes, and the biggest hippocampal peak appeared at 10 ms. Overall, hippocampal cells lagged cortical cells in synchronized firing with a mean time lag of 7.1 ± 2.0 ms (Figure 5B, significant difference from 0 ms, student's t test P < 0.01).
Figure 9.
Temporal relationship of correlated spikes between hippocampal and cortical\nigral neurons in the kindling side during amygdala kindled seizures. A) Distribution of correlated spikes of hippocampal neurons in relation to cortical neurons. The majority of correlated spikes of hippocampal neurons were on the plus side of the plot with a mean time lag of 4.8 ± 3.3 ms. B) Distribution of correlated spikes of hippocampal neurons in relation to SNr neurons. Most of correlation spikes were on the plus side of plot, with the mean lag time being 11.6 ± 1.9 ms.
Hippocampal cells also lagged SNr cells in synchronized firing. Figure 9 B shows the distribution of synchronized hippocampal spikes around SNr spikes. Most of the hippocampal spikes occurred on the plus side of SNr spikes with mean time lag of 11.6 ± 1.9 ms (p<0.01, student's t test, Figure 5B).
3.4 Spread of ictal discharges between ipsilateral and contralateral regions
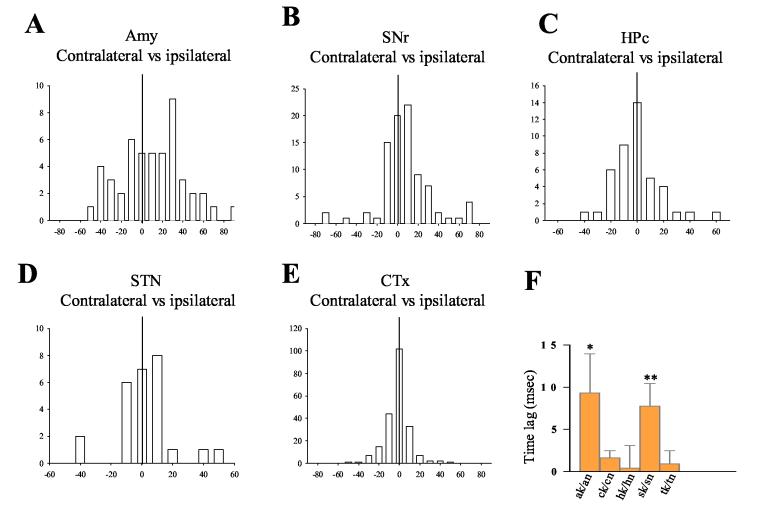
As ictal discharges spread globally and caused massive synchronization among widespread brain regions in both hemispheres, the temporal sequence of synchronized activity between the analogous regions in different sides of the brain can provide additional information about the organization of epileptic network underpinning amygdala kindled seizures. Cross-correlogram analysis revealed that the contralateral amygdala lagged the kindled amygdala in synchronized firing during seizures (Figure 10A). The mean lag time between the ipsilateral and contralateral amygdala was about 10 ms (p<0.05, student's t test, Figure 10A and F). A similar time lag, ∼8 ms, existed between the ipsilateral and contralateral SNr (p<0.01, student's t test, Figure 10 B and F). For the Ctx, hippocampus and STN, synchronized spikes in the contralateral side were evenly distributed around ipsilateral spikes with time lags less than 2 ms, which were not significantly different from the 0 s reference point (Figure 10 C, D, E and F). Thus our data indicate that there is simultaneous synchronized firing between the ipsilateral and contralateral sides of these regions during the majority of the period encompassing the totality of the amygdala kindled seizures.
Figure 10.
Temporal relationship of correlated spikes between the same region ipsilateral and contralateral to the kindling site. A) Distribution of correlation spikes in the amygdala contralateral to the kindling site. The contralateral amygdala neuronal spikes occurred with a 9.7 ± 4.7 ms delay following the spikes of neurons within the kindled amygdala. B) Distribution of correlation spikes of contralateral SNr in relation to SNr ipsilateral to the kindling site. Contralateral SNr neurons fired with a delay of 7.8 ± 2.6 ms relative to SNr neurons ipsilateral to the kindling site. C), D) and E) Distributions of correlation spikes of neurons in the Ctx (C), hippocampus (D) and STN (E) contralateral to the kindling, relative to neurons in the same structures ipsilateral to the kindling. No time lag was found between the opposite sides in these structures. F) Summary of time lag data between contralateral and ipsilateral sides of the same regions. A significant time lag was found in the amygdala and SNr regions only (*p < 0.05, **p < 0.01, student's ttest).
3.5 Synchronized activity within the same brain region during amygdala kindled seizures
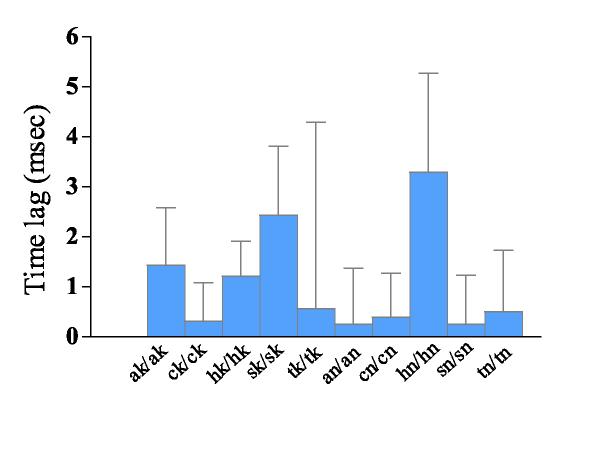
Cross-correlogram analysis of neurons co-localized within the same regions during amygdala kindled seizures showed no significant time lags. Figure 11 depicts the time lags of synchronized activity within the 5 recording regions. The time lags ranged from 0.2 ms in the contralateral SNr to 3.3 ms in the contralateral hippocampus. None of them differed significantly from the 0 point when the reference neurons fired.
Figure 11.
Time lags between synchronized activity of neurons within the same recording region during amygdala kindled seizures. No significant difference between the time lag and reference spikes (at 0 s point) was found in any of the recording regions.
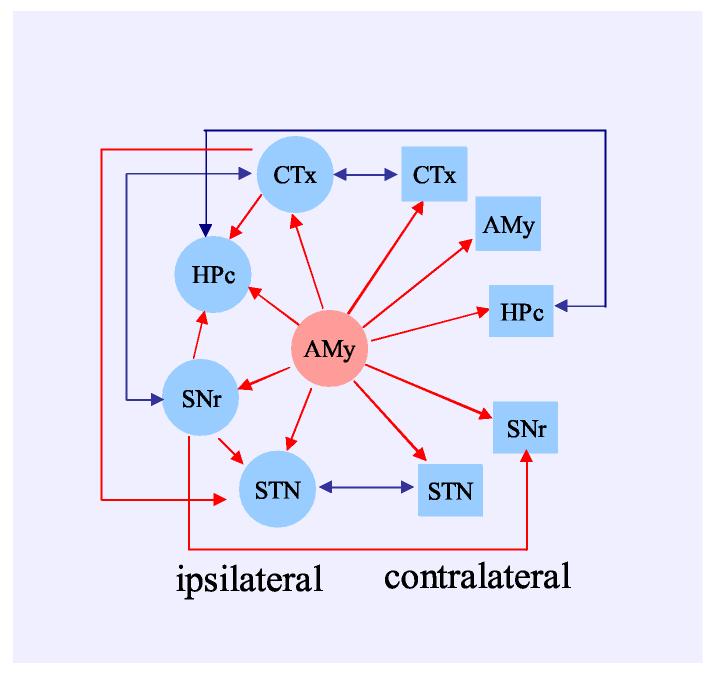
The schematic plot depicted in Figure 12 shows the temporal relationship within the corticolimbic and basal ganglia systems during amygdala kindled seizures. The ictal spikes clearly originated in the amygdala kindling site and spread to other regions of the system. In the ipsilateral side, they reached Ctx and SNr first. The ictal discharges then further traveled to the STN and hippocampus. During most of the seizure period, synchronized spikes fired simultaneously between ipsilateral and contralateral cortex, hippocampus and STN. Meanwhile synchronized firing in the amygdala and SNr contralateral to the kindling site did lag spikes in the homotopic structures on the kindled side.
Figure 12.
Schematic summery of ictal discharge propagation within the corticolimbic basal ganglia system. Kindling initiates ictal discharges in the amygdala kindling site. Synchronized activity is sequentially propagated to other corticolimbic basal ganglia regions; it reaches the cortical and SNr regions first and than propagates to the hippocampus and STN. Simultaneous spikes were detected between ipsilateral and contralateral cortex, STN and SNr while a time lag occured between spikes in the ipsilateral and contralateral amygdala and SNr neurons. The red line indicates the existence of a time lag in the direction of the arrow. The blue line indicates the simultaneous activation of synchronized firing.
4. Discussion
Of great interest in epileptic research is the temporal sequence of ictal discharge propagation during epileptic seizures (Faingold 2004; Morimoto et al., 2004; Blumenfeld 2005). 2-deoxyglucose uptake and Fos-like immunoreactivity have been employed to measure the activity of different structures related to seizure expressions. The early stages of seizures are likely to involve the amygdala and amygdalo-hippocampal regions in both status and amygdala kindling induced seizures. Activity spreads to other brain regions, including basal ganglia, thalamic nuclei, neocortex as the seizure progress into later stages of generalized motor seizures (Engel et al., 1978; Clark et al., 1991; McIntyre et al., 1991; White and Price 1993 a,b; Handforth and Ackermann 1995; Veliskova et al., 2005). Owing to their low temporal resolution (in the order of minutes), these methods can only measure brain activities involved in certain stage of seizures, it can not detect the propagation of discharge within epileptic networks during the ictal state. The major advantages of single unit recording are the high temporal (ms range) and spatial (mm range) resolutions. When large scale, multiple region recording is employed, as in this study, the temporal sequence of propagation of ictal discharges within the epileptic network can be determined precisely.
Amygdala kindling is a desirable model to study the spread of ictal discharges for several reasons. Firstly, the amygdala is one of the most epileptogenic regions in the brain and kindled seizures can be readily developed and triggered with intra-amygdala stimulation. Secondly, the epileptic focus (amygdala) is clearly known to the investigators and can be used as a control for validating analysis procedures. Thirdly, widespread propagation of ictal discharges into different brain regions allows us to study the broad neural network associated the triggered epileptic seizures. And last but not least, this model is relevant to one of the most common clinically observed and intractable forms epilepsy, TLE (Sato et al., 1990; Coulter et al., 2002; Loscher 2002).
One of the most interesting findings of this study is the early activation of the SNr by the ictal discharges originating from the amygdala. It was unexpected that the SNr would be activated even earlier than the hippocampus, which has reciprocal connections with the amygdala (Amaral et al., 1992; Van Groen and Wyss, 1990; Pikkarainen et al., 1999; Braak et al., 1996), and than the STN, which sends a glutamatergic projection to the SNr (Hamani et al., 2004). The anatomic substrate for such early SNr activation is likely to be a direct projection from the amygdala to the SNr (Bunny and Aghajanian, 1976), which could provide orthodromic activation of SNr cells. Early activation of the SNr supports the idea that it might play an important role in seizure control. As a basal ganglia output site, the SNr regulates epileptic seizures through three pathways, the nigrothalamic pathway connecting to the cerebral cortex (Alexander et al., 1990), the nigrotectal pathway regulating the “dorsal midbrain anticonvulsant zone” (Redgrave at al., 1992a, b) and the pedunculopontine pathways projected to the pons and spinal cord (Depaulis et al., 1994; Mena-Segovia et al., 2004; Nolte et al., 2006). Several lines of evidence have indicated the SNr plays a key role in mediating epileptic seizures. 1. Endogenous GABA concentrations were decreased in amygdala kindled seizures (Loscher and Schwark 1987). 2. Microinjection of GABA and its agonists into the SNr blocked the seizure induced by chemical stimulation (Iadarola and Gale 1982; Zhang et al., 1989), kindling (Loscher et al., 1987; McNamara et al., 1984), electroshock (Mirski et al., 1986), audiogenic stimulation (Gonzalez and Hettinger 1984) and genetic manipulations (Depaulis et al.,1988). 3. Deep brain stimulation (DBS) of the SNr, presumably via local inhibition, can block amygdala kindled (Morimoto and Goddard, 1987; Shi et al., 2006) and drug induced seizures (Velisek et al., 2002). These findings suggest that the SNr is strategically located in a pivotal position such that it can regulate the propagation of ictal discharges during amygdala kindled seizures (Mosh and Garant 1996).
It has been reported that anterior and posterior parts of the SNr were differentially involved in seizure control (Moshe et al., 1994). Recent study by Veliskova et al. (2005) found that the SNr posterior was activated during pre-clonic period of flurothyl induced generalized seizures while the anterior part of SNr was not involved till pre-tonic clonic seizure. During the ictal state, both sites increased their activities. Anticonvulsant effect of deep brain stimulation was found only in the anterior part of SNr during flurothyl induced seizures (Velisek et al., 2002). Our recent study demonstrated antiepileptogenic effects of DBS of the anterior part of SNr in the amygdala kindling model (Shi et al., 2006). Based on these observations, electrodes were implanted in the anterior part of the SNr in the present study (Figure 2).
The hippocampus is a key structure involved in TLE. In spite of the existence of abundant reciprocal connections between the hippocampus and amygdala (Amaral et al., 1992; Van Groen and Wyss 1990; Pikkarainen et al., 1999), we found that the ictal discharges reached the hippocampus later than the Ctx and SNr. The delayed activation of hippocampal neurons does not necessarily indicate that cortical or SNr activity drives hippocampal synchronized firing. The delay may be a result of the trisynaptic circuit and dentate filtering within the hippocampal complex (Coulter, 2000), whereby the signals traveling from the amygdala and entorhinal cortex to the dentate gyrus, and from there to the CA3 and CA1 regions, experience delays at each step (Anderson et al., 1971; Swanson et al., 1978). On the other hand, tightly coupled synchronization between both sides of the hippocampus was found in the present study. This finding of bilateral hippocampal synchronization was consistent with previous research in which a similar finding was obtained during the clonic stage of amygdala kindled seizures (Pijn et al., 1991), and suggests that there is inter-hippocampal synchronized oscillation during kindled seizures. This may explain why the kindling of one hippocampus kindles in parallel the contralateral hippocampus (McIntyre and Edson, 1987).
In spite of the important role of the hippocampus in TLE, hippocampal inactivation had only a limited effect on retardation of amygdala kindling (Tanaka et al., 1991) or no effect at all (Racine et al., 1988). Furthermore, Sato et al., (1990) proposed that the hippocampus and amygdala might act as a mutually antagonistic circuit during seizure expression. The constant lag of hippocampus firing behind not only the amygdala kindling site, but also the Ctx and SNr, together with the smaller percent of neuron pairs with synchronized firing between the amygdala kindling site and the hippocampus (Table 1), supports the notion that the hippocampus may not be a critical structure in mediating amygdala kindled seizures. The hippocampus also appears not to be involved during the spontaneous convulsive seizures that occur days to weeks follow exposure to pilocarpine induced status epileptics (Harvey and Sloviter, 2005).
It is clear, however, that the cerebral cortex is an important structure mediating amygdala kindling, especially during stage 5 convulsive seizures. Cortical regions send abundant projections to the hippocampus, STN and amygdala and are essential for the manifestation of motor seizures (Braak et al., 1996; McDonald 1998; Hamani et al., 2004). Kelly et al. (1999) used cortical spread depression, a reversible lesion method to inactivate frontal motor cortex, and found that it could block the convulsive expression of amygdala kindled seizures. The authors emphasized that the cortex mediates a crucial role in clonic motor seizures by transferring the ictal discharges to the brain stem and spinal cord. Early activation of the cerebral cortex during synchronized firing supports the view that the cerebral cortex plays an important role in amygdala kindled seizures. The reciprocal connections between the cerebral cortex and amygdala (Mascagni et al., 1993; McDonald and Mascagni 1996; McDonald et al., 1996) provide anatomic substrates for such synchronized activity. The lack of a time lag between the two cortical hemispheres may reflect a global activation of cortical circuits during the majority of the clonic motor seizures.
The STN is a pivotal structure in the basal ganglia system, which receives abundant cortical and pallidal inputs, and projects to basal ganglia output nuclei, such as the globus pallidus internal and the SNr. In addition, it also sends projections to the pedunculopontine nucleus and other brain stem structures. The STN thus is positioned to play a critical role in motor and other functions (Hamani et al., 2004). Paz et al. (2005) reported a synchronized, rhythmic burst of STN activity that was associated with cortical EEG spike and wave discharges in genetic absence epileptic rats. The authors suggested that such discharges may convey signals to the basal ganglia output nuclei and contribute to the thalamocortical spike and wave discharges.
Several recent studies have tried to use DBS in the STN to reduce epileptic seizures in both animal models and epilepsy patients (Bingaman et al., 2000; Chabardes, 2002. Dinner, 2002; Shon et al., 2004; Usui et al., 2005). The effect of DBS of the STN is presumably to induce local inhibition in the STN, which in turn reduces excitatory input to the SNr. Thus, the conceptual framework of both Paz's study and the DBS experiments is based on the activity of STN-SNr projections. However, our results demonstrated unexpectedly that the STN lags behind the SNr in synchronized firing during generalized amygdala kindled seizures. Again, time lag per se does not necessarily indicate a drive-follow relationship between pairs of neurons; it is possible that both cells follow the same driving signal, in this case ictal discharge from the amygdala via different pathways. It is possible that ictal discharge may pass through the cortex before reaching the STN, the possibility was supported by the fact that the STN lagged behind the Ctx in synchronized firing. A recent study by Sharott et al. (2005) revealed similar SNr to STN signal transfer directions during a period with 1 Hz slow wave cortical activity, whereas the direction was reversed during 15 Hz high frequency oscillations. A low frequency oscillation (around 2 Hz) was found during stage 5 amygdala kindled seizures in our study (Woodward et al., 2003), suggesting that there may be a common mechanism underlying SNr-STN signal transfer direction in both studies.
In summary, the present study provides a detailed account of the temporal sequence of propagation of ictal discharges during amygdala kindled seizures. The observed early activation of the cerebral cortex and SNr highlights the important role of these structures in generalized amygdala kindled seizures. Similar analyses can be used to explore possible different pathways subserving other types of epileptic seizures, and help us to further understand the mechanisms of epilepsy and to design new therapeutic strategies.
Acknowledgements
This study was supported by NIH grants NS-43441 and NS 40628, TW-006144 to JYC and NS-19608 to DJW, National Natural Science Foundation of China (30170307, 30370461) to FL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Crutcher MD, Delong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculootor, ‘prefrontal’ and ‘limbic’ functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael T. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala. Wiley-Liss; New York, Chichester, Brisbane, Toronoto, Singapore: 1992. pp. 1–66. [Google Scholar]
- Anderson P, Bliss TV, Skrede KK. Lamellar organization of hippocampal pathways. Exp. Brain Res. 1971;13:222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- Bertram EH. Functional anatomy of spontaneous seizures in a rat model of limbic epilepsy. Epilepsia. 1997;38:95–105. doi: 10.1111/j.1528-1157.1997.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Bingaman WE, Hadar EJ, Najm IM, Montagomery E, Luders HO. Chronic stimulation of the subthalamic nucleus for the treatment of medically intractable seizures: a case report. Epilepsia. 2000;41(suppl 7):149. [Google Scholar]
- Blumenfeld H. Cellular and Network Mechanisms of Spike-Wave Seizures. Epilepsia. 2005;46:21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, Bohl J. Functional anatomy of human hippocampal formation and related structures. J. Child. Neurology. 1996;11:265–275. doi: 10.1177/088307389601100402. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Wilson CL, Vizentin E, Mathern GW. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999;40:1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK. The precise localization of nigral afferents in the rat as determined by a retrograde tracing technique. Brain Res. 1976;117:423–435. doi: 10.1016/0006-8993(76)90751-4. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Bortolotto ZA, Turski L. Microinjections of the gamma-aminobutyrate antagonist, bicuculline methiodide, into the caudate-putamen prevent amygdala-kindled seizures in rats. Brain Res. 1987;411:370–372. doi: 10.1016/0006-8993(87)91089-4. [DOI] [PubMed] [Google Scholar]
- Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disorders. 2002;4:S83–S93. [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience. 2000;99:433–443. doi: 10.1016/s0306-4522(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Clark M, Post RM, Weiss SR, Cain CJ, Nakajima T. Regional expression of c-fos mRNA in rat brain during the evolution of amygdala kindled seizures. Brain Res. Mol Brain Res. 1991;11:55–64. doi: 10.1016/0169-328x(91)90021-o. [DOI] [PubMed] [Google Scholar]
- Corcoran ME, Urstad H, McCaughran JA, Jr., Wada JA. Frontal lobe and kindling in the rat. Can. J. Neurol. Sci. 1975;2:501–508. doi: 10.1017/s0317167100020643. [DOI] [PubMed] [Google Scholar]
- Coulter D,A. Mossy fiber zinc and temporal lobe epilepsy: pathological association with altered “epileptic” gamma-aminobutyric acid A receptors in dentate granule cells. Epilepsia. 2000;41(Suppl 6):S96–99. doi: 10.1111/j.1528-1157.2000.tb01565.x. 2000. [DOI] [PubMed] [Google Scholar]
- Coulter DA, McIntyre DC, Loscher W. Animal models of limbic epilepsies: What can they tell us? Brain Pathology. 2002;12:240–256. doi: 10.1111/j.1750-3639.2002.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degado-Escueta AV, Walsh GO. Type I complex partial seizures of hippocampal origin: excellent results of anterior temporal lobectomy. Neurology. 1985;35:143–154. doi: 10.1212/wnl.35.2.143. [DOI] [PubMed] [Google Scholar]
- Depaulis A, VERGNES M, Marescaux C, Lannes B, Warter JM. Evidence that activation of GABA receptors in the substantia nigra suppresses spontaneous spike-and-wave discharges in the rat. Brain Res. 1988;448:20–29. doi: 10.1016/0006-8993(88)91097-9. [DOI] [PubMed] [Google Scholar]
- Depaulis A, VERGNES M, Marescaux C. Endogenous control of epilepsy: the nigral inhibitory system. Prog. Neurobiol. 1994;42:33–52. doi: 10.1016/0301-0082(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Deransart C, Le BT, Marescaux C, Depaulis A. Role of the subthalamo-nigral input in the control of amygdala-kindled seizures in the rat. Brain Res. 1998;807:78–83. doi: 10.1016/s0006-8993(98)00745-8. [DOI] [PubMed] [Google Scholar]
- Dinner DS, Neme S, Nair D, Montgomery EB, Baker KB, Rezai A, Luders HO. EEG and evoked potential recording from the subthalamic nucleus for deep brain stimulation of intractable epilepsy. Clin. Neurophysiol. 2002;113:1391–1402. doi: 10.1016/s1388-2457(02)00185-2. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr., Katzman R. Facilitation of amygdaloid kindling by lesions of the stria terminalis. Brain Res. 1977;122:137–142. doi: 10.1016/0006-8993(77)90669-2. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr., Wolfson L, Brown L. Anatomical correlates of electrical and behavioral events related to amygdaloid kindling. Ann. Neurol. 1978;3:538–544. doi: 10.1002/ana.410030615. [DOI] [PubMed] [Google Scholar]
- Engel J. Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- Faingold CL. Emergent properties of CNS neuronal networks as targets for pharmacology: application to anticonvulsant drug action. Prog. Neurobiol. 2004;72:55–85. doi: 10.1016/j.pneurobio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Feindel W, Rasmussen T. Temporal lobectomy with amygdalectomy and minimal hippocampal resection: review of 100 cases. Can. J. Neurol. Sci. 1991;18:603–605. doi: 10.1017/s0317167100032807. [DOI] [PubMed] [Google Scholar]
- Gale K, Iadarola MJ. Seizure protection and increased nerve-terminal GABA: delayed effects of GABA transaminase inhibition. Science. 1980;208:288–291. doi: 10.1126/science.6768130. [DOI] [PubMed] [Google Scholar]
- Gonzalez LP, Hettinger MK. Intranigral muscimol suppresses ethanol withdrawal seizures. Brain Res. 1984;298:163–166. doi: 10.1016/0006-8993(84)91162-4. [DOI] [PubMed] [Google Scholar]
- Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- Handforth A, Ackermann RF. Mapping of limbic seizure progressions utilizing the electrogenic status epilepticus model and the 14C-2-deoxyglucose method. Brain Res. Rev. 1995;20:1–23. doi: 10.1016/0165-0173(94)00003-8. [DOI] [PubMed] [Google Scholar]
- Harvey BD, Sloviter RS. Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats: Implications for hippocampal epileptogenesis. J. Comp. Neurol. 2005;488:442–463. doi: 10.1002/cne.20594. [DOI] [PubMed] [Google Scholar]
- Hawkins CA, Mellanby JH. Limbic epilepsy induced by tetanus toxin: a longitudinal electroencephalographic study. Epilepsia. 1987;28:431–444. doi: 10.1111/j.1528-1157.1987.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Cellular compartments of GABA in brain and their relationship to anticonvulsant activity. Mol. Cell. Biochem. 1981;39:305–329. doi: 10.1007/BF00232582. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Battye RA, McIntyre DC. Cortical spreading depression reversibly disrupts convulsive motor seizure expression in amygdala-kindled rats. Neuroscience. 1999;91:305–313. doi: 10.1016/s0306-4522(98)00656-3. [DOI] [PubMed] [Google Scholar]
- Loscher W, Schwark WS. Further evidence for abnormal GABAergic circuits in amygdala-kindled rats. Brain Res. 1987;420:385–390. doi: 10.1016/0006-8993(87)91262-5. [DOI] [PubMed] [Google Scholar]
- Loscher W, Czuczwar SJ, Jackel R, Schwarz M. Effect of microinjections of gamma-vinyl GABA or isoniazid into substantia nigra on the development of amygdala kindling in rats. Exp. Neurol. 1987;95:622–638. doi: 10.1016/0014-4886(87)90304-9. [DOI] [PubMed] [Google Scholar]
- Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ, Coleman JR. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1993;57:697–715. doi: 10.1016/0306-4522(93)90016-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Cortico-cortical and cortico-amygdaloid projections of the rat occipital cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1996;71:37–54. doi: 10.1016/0306-4522(95)00416-5. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McIntyre DC. Split-brain rat: transfer and interference of kindled amygdala convulsions. Can. J. Neurol. Sci. 1975;2:429–437. doi: 10.1017/s0317167100020576. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Edson N. Facilitation of secondary site kindling in the dorsal hippocampus following forebrain bisection. Exp. Neurol. 1987;96:569–579. doi: 10.1016/0014-4886(87)90219-6. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Don JC, Edson N. Distribution of [14C]2-deoxyglucose after various forms and durations of status epilepticus induced by stimulation of a kindled amygdala focus in rats. Epilepsy Res. 1991;10:119–133. doi: 10.1016/0920-1211(91)90004-y. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Gilby KL. Parahippocampal Networks, Intractability and the Chronic Epilepsy of Kindling. In: Blume W, editor. Intractable Epilepsy Conference, Advances in Neurology. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 77–83. [PubMed] [Google Scholar]
- McNamara JO, Galloway MT, Rigsbee LC, Shin C. Evidence implicating substantia nigra in regulation of kindled seizure threshold. J. Neurosci. 1984;4:2410–2417. doi: 10.1523/JNEUROSCI.04-09-02410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO, Bonhaus DW, Shin C. Role of the substantia nigra in the kindling model of limbic epilepsy. Adv. Exp. Med. Biol. 1986;203:139–146. doi: 10.1007/978-1-4684-7971-3_10. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosc. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Mirski MA, McKeon AC, Ferrendelli JA. Anterior thalamus and substantia nigra: two distinct structures mediating experimental generalized seizures. Brain Res. 1986;397:377–380. doi: 10.1016/0006-8993(86)90642-6. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Goddard GV. The substantia nigra is an important site for the containment of seizure generalization in the kindling model of epilepsy. Epilepsia. 1987;28:1–10. doi: 10.1111/j.1528-1157.1987.tb03613.x. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Moshe SL, Brown LL, Kubova H, Veliskova J, Zukin RS, Sperber EF. Maturation and segregation of brain networks that modify seizures. Brain. Res. 1994;665:141–146. doi: 10.1016/0006-8993(94)91164-9. [DOI] [PubMed] [Google Scholar]
- Moshe SL, Garant DS. Substantia nigra GABA receptors can mediate anticonvulsant or proconvulsant effects. Epilepsy Res. Suppl. 1996;12:247–256. [PubMed] [Google Scholar]
- Nolte MW, Loscher W, Gernert M. Pedunculopontine neurons are involved in network changes in the kindling model of temporal lobe epilepsy. Neurobiol Dis. 2006 doi: 10.1016/j.nbd.2006.03.003. In press. [DOI] [PubMed] [Google Scholar]
- Paxinos GWC. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Paz JT, Deniau JM, Charpier S. Rhythmic bursting in the cortico-subthalamopallidal network during spontaneous genetically determined spike and wave discharges. J. Neurosci. 2005;25:2092–2101. doi: 10.1523/JNEUROSCI.4689-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijn JP, Van Neerven J, Noest A, Lopes da Silva FH. Chaos or noise in EEG signals; dependence on state and brain site. Electroencephalogr Clin. Neurophysiol. 1991;79:371–381. doi: 10.1016/0013-4694(91)90202-f. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J. Comp. Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Quesney LF. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27(Suppl 2):S27–S45. doi: 10.1111/j.1528-1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Racine R. Kindling: the first decade. Neurosurgery. 1978;3:234–252. doi: 10.1227/00006123-197809000-00018. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Racine RJ, Paxinos G, Mosher JM, Kairiss EW. The effects of various lesions and knife-cuts on septal and amygdala kindling in the rat. Brain Res. 1988;454:264–274. doi: 10.1016/0006-8993(88)90826-8. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Simkins M, overton P, Dean P. Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy--I. Mapping of dorsal midbrain with bicuculline. Neuroscience. 1992a;46:379–390. doi: 10.1016/0306-4522(92)90059-b. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Marrow LP, Dean P. Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy--II. Pathways from substantia nigra pars lateralis and adjacent peripeduncular area to the dorsal midbrain. Neuroscience. 1992b;46:391–406. doi: 10.1016/0306-4522(92)90060-f. [DOI] [PubMed] [Google Scholar]
- Sato M, Racine RJ, McIntyre DC. Kindling: basic mechanisms and clinical validity. Electroencephalogr. Clin. Neurophysiol. 1990;76:459–472. doi: 10.1016/0013-4694(90)90099-6. [DOI] [PubMed] [Google Scholar]
- Sharott A, Magill PJ, Bolam JP, Brown P. Directional analysis of coherent oscillatory field potentials in the cerebral cortex and basal ganglia of the rat. J. Physiol.-London. 2005;562:951–963. doi: 10.1113/jphysiol.2004.073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. Deep brain stimulation of the substantia nigra pars reticulata exerts long lasting suppression of amygdala kindled seizures. Brain Res. 2006 doi: 10.1016/j.brainres.2006.03.050. In press. [DOI] [PubMed] [Google Scholar]
- Shon YM, Kim YI, Yang DW. Effect of deep brain stimulation of the subthalamic nucleus in lithium-pilocarpine status epilepticus of rats: The functional anatomy using fos immunohistochemistry. Epilepsia. 2004;45:49–50. [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J. Comp. Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kondo S, Hori T, Tanaka S, Yonemasu Y. Various hippocampal lesions induced by multi-fractional ibotenic acid injections and amygdala kindling in rats. Brain Res. 1991;559:154–158. doi: 10.1016/0006-8993(91)90299-b. [DOI] [PubMed] [Google Scholar]
- Tremblay E, Ottersen OP, Rovira C, Ben-Ari Y. Intra-amygdaloid injections of kainic acid: regional metabolic changes and their relation to the pathological alterations. Neuroscience. 1983;8:299–315. doi: 10.1016/0306-4522(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Usui N, Maesawa S, Kajita Y, Endo O, Takebayashi S, Yoshida J. Suppression of secondary generalization of limbic seizures by stimulation of subthalamic nucleus in rats. J. Neurosurgery. 2005;102:1122–1129. doi: 10.3171/jns.2005.102.6.1122. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J. Comp. Neurol. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Velisek L, Veliskova J, Moshe SL. Electrical stimulation of substantia nigra pars reticulata is anticonvulsant in adult and young male rats. Exp. Neurol. 2002;173:145–152. doi: 10.1006/exnr.2001.7830. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velsek L, Moshe SL. Subthalamic nucleus: a new anticonvulsant site in the brain. Neuroreport. 1996;7:1786–1788. [PubMed] [Google Scholar]
- Veliskova J, Miller AM, Nunes ML, Brown LL. Regional neural activity within the substantia nigra during peri-ictal flurothyl generalized seizure stages. Neurobiol. Dis. 2005;20:752–759. doi: 10.1016/j.nbd.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. II. The effects of focal deactivation. J. Neurosci. 1993;13:4810–4830. doi: 10.1523/JNEUROSCI.13-11-04810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. I. Patterns of 14C-2-deoxyglucose uptake and Fos immunocytochemistry. J. Neurosci. 1993;13:4787–4809. doi: 10.1523/JNEUROSCI.13-11-04787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DJ, Shi LH, Luo F, Chang JY. Neural network underlying amygdala kindled seizures characterized by large scale, single unit recording in freely moving rats. Soc. Neurosci. Abstr. 2003 2303.17. [Google Scholar]
- Zhang H, Rosenberg HC, Tietz EI. Injection of benzodiazepines but not GABA or muscimol into pars reticulata substantia nigra suppresses pentylenetetrazol seizures. Brain Res. 1989;488:73–79. doi: 10.1016/0006-8993(89)90694-x. [DOI] [PubMed] [Google Scholar]