Abstract
Statement of problem
The color of vital craniofacial structures has not been measured accurately.
Purpose
The purpose of this study was to determine the color of vital craniofacial structures and evaluate the validity and test-retest reliability of a noncontacting 45/0-degree optical configuration.
Material and methods
A spectroradiometer and an external light source were configured in a noncontacting 45/0-degree (45-degree illumination and 0-degree observer) optical configuration to measure the color of subjects’ vital craniofacial structures (central and lateral incisor and canine, attached gingiva, lips, and facial skin). The 120 subjects were stratified into 5 age groups with 4 racial categories and balanced for gender. For evaluation of validity, linear regressions and 95% confidence intervals were calculated for ΔL*, Δa*, Δb* [color difference of (CIE) LAB values] between the measured and certified values of the 22 color patches of the DC Color Checker. For test-retest reliability, a random sample of 12 (10%) subjects was remeasured at a second visit. Paired t tests, correlations, and Bland-Altman analyses were performed between the first and second measurements of the 12 pairs of L*, a*, and b* values for the 6 craniofacial structures.
Results
For validity, the mean color difference and linear regression for Commission Internationale d’Eclair-age (CIE) LAB values between measured and certified color of the 22 opaque color patches were ΔE of 1.46 and 0.99 for all regressions, respectively. Only Δa* did not contain zero in its 95% confidence interval. For test-retest reliability, no paired t tests were significantly different from each other, and the Pearson correlation coefficient ranged from 0.9 (9 pairs) to 0.7 (3 pairs). Ten of the 18 Bland-Altman plots showed good reliability.
Conclusion
The spectral reflectance of craniofacial structures can be measured with acceptable validity and test-retest reliability using a noncontacting 45/0-degree optical configuration.
CLINICAL IMPLICATIONS
Given its acceptable validity and reliability, the use of a noncontacting 45/0-degree optical configuration is recommended as a viable alternative to obtained CIELAB values for shade replication in craniofacial prosthetic rehabilitation.
Physical attractiveness is an important factor in an individual’s life,1 with psychological impact extending across a wide range of social and interpersonal situations.2 A prosthodontist’s ability to restore a patient’s teeth3 or facial defect4 with an esthetically pleasing prosthesis is critical to the patient’s emotional well-being. To fabricate an esthetically pleasing prosthesis, information regarding the polychromatic color distribution, translucency, intricate contours, and textures of the adjacent or opposing structures must be provided to the technician. Obtaining an appropriate polychromatic color and translucency match of craniofacial prostheses is an important part of improving prosthesis realism.5 According to the results of clinical studies,6,7 the definitive color match of porcelain crowns to adjacent natural dentition remains problematic. Color replication of the adjacent skin for the craniofacial prosthesis is an even greater challenge because no manufactured standard visual shade guides are available. Thus, shade matching is accomplished often at chairside by mixing dry earth pigments with translucent medical-grade elastomers.8
The color replication process of any restorative material for dental or facial prosthetics can be divided into a shade selection phase, followed by a shade duplication phase.9 Visual shade matching is the most common shade selection method, but unfortunately, it is plagued by unreliable and inconsistent results.10,11 Duplication of the visually selected shade with commercially prepared color specimens is also fraught with errors.12,13
A scientific solution to the present color replication problem requires a proposed change in the paradigm of the color replication process—namely, the use of a color-measuring instrument to obtain quantitative values for the shade matching phase. These values could then be used to model the color of restorative materials during the shade duplication phase, through mixing or layering, to match the intended color of adjacent structures using proven theories.14–16 Recently, the dental industry has initiated this paradigm shift for teeth and ceramic materials, but the outcomes of these efforts have not been validated completely.17 The paradigm shift for color replication of craniofacial structures is in its infancy.18,19
Color determination discrepancies among colorimetric devices with small window apertures20 are attributed to “edge loss,” which occurs with translucent materials such as teeth21 and skin/maxillofacial materials.16 Edge loss occurs when light that scatters through the translucent material, which originally would be seen by the eye, is simply not measured by the instrument due to the configuration of the illuminant, sensor, and aperture. This occurs during conventional reflectance measurements of translucent materials—for instance, skin and teeth—when both the illumination and observation light paths travel through an aperture.
Bolt et al21 have shown that edge loss is wavelength dependent. Thus, small-window color-measuring devices systematically assign incorrect color coordinates for translucent objects. The authors found that the L* value decreases when the window decreases in size from infinity to 3 mm. Similarly, the other color coordinates shift somewhat toward the green along the red-green or a* axis, and more strongly toward the blue along the yellow-blue or b* axis, when the window size decreases. This phenomenon occurs to varying degrees when color measurements are made of translucent materials/structures using the colorimeters or spectrophotometers. Edge loss can be avoided by using a combination of an external light source that does not cause shadowing and a spectroradiometer.21
For traditional color replication, the color of natural dentition has been mapped out using a subjective comparison of paper chips that represent the Munsell color order system.22 In this method of specification, the color of a tooth is compared to a standardized and orderly arrangement of color chips. Using the paradigm shift, objective color measurements of natural dentitions23–28 have been obtained using instruments with small-window apertures. The validity of these measurements is questionable, however, given that small-aperture color-measuring devices will not provide true color measurements for translucent structures. The color of translucent natural dentition has also been measured intraorally using methods that are acceptable to the scientific community—that is, methods addressing the challenges of edge loss.29,30 However, limitations of both studies include the use of a small convenience sample (87 subjects) from a homogenous Japanese population without any report of validity or reliability data.
Several attempts have been made to describe and document the color of craniofacial structures. For instance, one individual’s experiences detailing and describing gingival color has been published.31 Color assessments of the attached gingiva,32,33 papillae,33 and alveolar mucosa33 of humans have been evaluated using Munsell color tabs. The use of a helium-neon gas laser to record the reflectance of the interdental papilla was documented, although no actual color was reported.34 Another study used digital images of gingiva and converted the color of gingiva to wavelength, but also without any documentation of validity or reliability.35
The evolution of skin color among different racial groups has been elaborated in detail.36 Unfortunately, accurate quantitative data of skin color have not been published. The spectral reflectance curves measured with a spectrophotometer of skin has been performed with varying interest in evaluating the contribution of various blood pigments37 or quantifying melanin and hemoglobin in the lips and skin.38 Reflection spectrophotometry was used to collect color data of facial skin for 241 whites, blacks, and Asians as a precursor to computerized selection of pigments for patients requiring maxillofacial prostheses.39 The use of a spectrophotometer for the previously mentioned study required skin contact. Any contact of soft tissue does result in a color change, due to a shift in the blood of the superficial vessels.
To date, no studies have published accurate, objective color data of vital craniofacial structures for a population in the United States using appropriate instruments that do not induce edge loss or require tissue contact. To facilitate the paradigm shift, collecting accurate color data of craniofacial structures is necessary to assess the current status of the color replication process and to ensure that future work can be performed more appropriately within the relevant color space of the craniofacial structures.
The purpose of this descriptive study was to determine the color space of vital craniofacial structures, including vital human dentition, gingiva, lips, and facial skin in a convenience sample of a population that was stratified into 5 age groups and 4 racial categories. The validity and reliability of the instrumental configuration for color measurements of craniofacial structures on human subjects were also evaluated. The research hypothesis was that color measurement using a 45/0-degree optical configuration for measurement of craniofacial structures is valid and reliable.
MATERIAL AND METHODS
A spectroradiometer (PR 705; Photo Research Inc, Chatsworth, Calif) and Xenon arc lamp (300W; Oriel Instruments, Stratford, Conn) were set up in an optical configuration of 0-degree observation and 45-degree illumination for color measurement. This color measurement instrument configuration was evaluated for stability and validity in vitro. This study consisted of measuring the color of the following craniofacial structures: 3 anterior maxillary teeth (central and lateral incisor and canine), the gingiva, lips, and facial skin of a convenience sample of 120 subjects (Institutional Review Board Approval #2003H0001, The Ohio State University). The reliability of the color instrumentation was evaluated on 10% of the total subjects (12 subjects).
A spectroradiometer (PR 705; Photo Research Inc) and fiber optic light cable were fixed on an optical table (Mecom Inc, Rising Sun, Ohio). The fiber optic light cable was connected to the Xenon arc lamp. The spectroradiometer and the optic light cable, positioned at a 45-degree angle inferior to the horizontal plane, provided an optical configuration of 0-degree observation and 45-degree illumination to the object. For all color measurements in this study, spectral reflectance was obtained from 380 to 780 nm with a 2-nm interval (Spectrawin 2.0; Photo Research Inc) and, subsequently, converted to Commission Internationale d’Eclairage L*A*B* (CIELAB) values (D65 illumination and 2-degree observer). The spectroradiometer was standardized to 8 cm to the measured object with a measurement aperture size of 1 mm.
The stability of the light source to be used was evaluated prior to establishing the color measurement protocol for the study. This allowed determination of how much warm-up time was necessary to obtain relative stability prior to color measurement, and the intervals at which recalibration was necessary. To evaluate for light source stability, a smooth and homogenous white tile (SW) was used as the specimen. The measurements were made after turning on the light source and the spectroradiometer. The reflectance spectra of the SW were measured every minute for 2 hours, which resulted in a total of 120 measurements. Each measurement was based on the average of 5 measuring cycles of the spectroradiometer. The initial incident light Ii(λ) was determined using Equation I,40 because the spectral reflectance R(λ) of SW was known, and the reflected light Io(λ) could be measured at time zero. All subsequent measurements of SW were determined in this manner. The reflectance data were then converted to CIELAB values (D65 illumination and 2-degree observer) with respect to the initial incident light at time zero.
| (Equation I) |
The stability of this light source is illustrated in Figure 1. As observed, the ΔE from 1 color measurement to the following SW changed irregularly during the first 30 minutes of color measurement. The maximum ΔE compared with the first measurement was approximately 2.4 ΔE at 14 minutes after the measurement apparatus was turned on. After 30 minutes, the maximum ΔE variation between 2 adjacent measurements was approximately 0.2 ΔE.
Fig. 1.
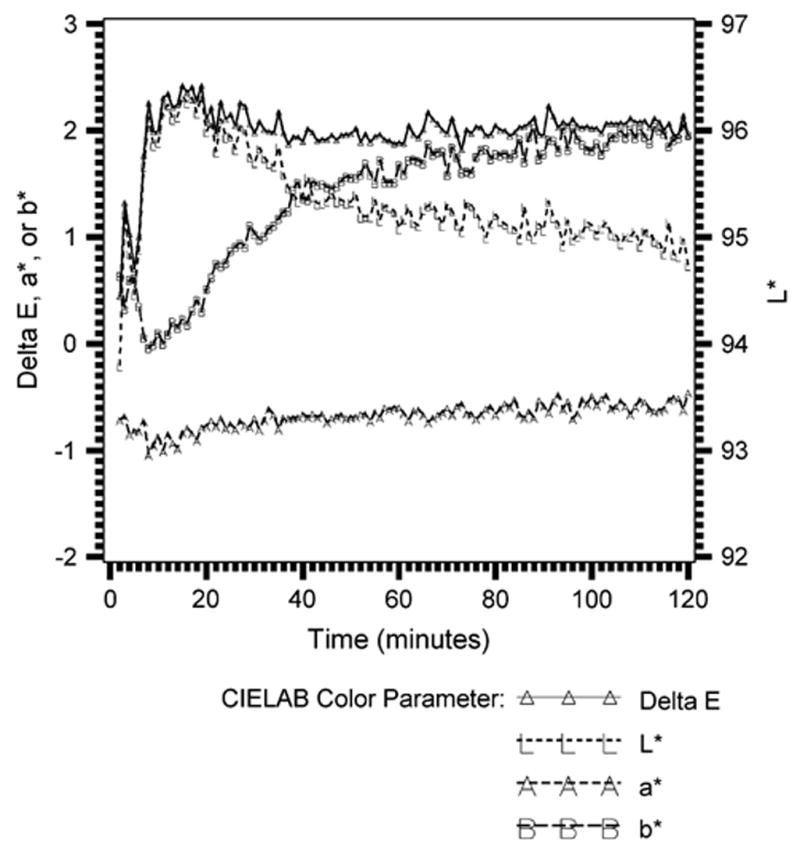
Stability of experimental configuration.
With the information provided from the assessment of the stability of the light source, a color measurement protocol was established. This included turning on the light source for 30 minutes prior to making any measurements. No measuring period ever exceeded 2 hours. Following the 30-minute warm-up period, 2 test measurements were performed before actual measurement of the specimens. To optimize precision of the color measurement configuration, periodic measurements of the reflecting spectrum of SW were made between every subject. This accounted for the average change of the reflected light source between 2 measurements of SW. If the average differences of the spectral reflectance between 2 SW measurements exceeded 0.2% and/or the root mean square (RMS) was larger than 0.003, the spectral data of the specimens between those 2 SW measurements were discarded and redone.
Institutional review board approval was obtained to carry out this study. A total of 120 human subjects over the age of 18 years were recruited for the study through notices posted around The Ohio State University Health Science campus. Six subjects (3 men and 3 women) from 4 racial/ethnic groups (white, black, Asian, Pacific Islander, and other) were recruited from each of the following age groups: 18 to 29 years, 30 to 39 years, 40 to 49 years, 50 to 59 years, and 60 to 85 years.
Potential subjects responded to the advertisements posted near The Ohio State University Medical Center by calling the laboratory and were screened using a telephone screening form. This screening process ensured that potential subjects satisfied the inclusion and exclusion criteria for this study (Table I). Only subjects that were still needed for the study with reference to the stratified recruitment were requested to present for a clinical examination.
Table I.
Inclusion and exclusion criteria for MOCOCS study
| Inclusion criteria |
| Generally healthy subjects between the ages of 18 and 85 years of age |
| Presence of at least 1 maxillary central incisor, 1 lateral incisor, and 1 canine |
| Willingness to brush teeth for 3 minutes prior to color measurement |
| Willingness to spend approximately an hour for this study |
| Sign informed consent and HIPAA forms |
| Exclusion criteria |
| Subjects with any direct or complete coverage restorations on both similar maxillary anterior teeth |
| Subjects with any external surface staining on both similar maxillary anterior teeth |
| Subjects with any intrinsic staining on both similar maxillary anterior teeth—for example, tetracycline stains, or fluorosis |
| Subjects with severe attrition resulting in incisal enamel wear |
| Subjects with spontaneous bleeding from their gingiva due to periodontal disease |
| Pregnant subjects, eliminating the possibility of any misunderstanding that color measurement instrument may cause any harm to the unborn child |
| Psychiatric, cognitive, or social (for example, alcoholism or drug abuse) conditions that would interfere with giving consent and cooperation |
| Prisoners |
The subjects were thoroughly informed about the purpose of this study, the study design, potential harm and benefit, the measures to protect their privacy, and the right to withdraw participation at any point during the project. Any study-related questions were answered, and if the subject expressed interest in participating, the informed consent and Health Information Portability and Accountability Act (HIPAA) forms were signed. Potential subjects were clinically screened to determine whether the 3 specific anterior teeth (maxillary central and lateral incisor and canine) chosen were nonrestored, natural, permanent teeth, and free from external staining or bleaching.
Subjects that qualified for the study were provided with a toothbrush (Butler G.U.M., 411 Soft; John O Butler Co, Chicago, Ill) and toothpaste (Crest; Proctor and Gamble, Cincinnati, Ohio) and were asked to brush their teeth for 3 minutes. Subjects were also asked to remove any facial makeup. The subjects were seated with their lower jaw and forehead resting lightly on a head-frame (similar to what is used during an optometry examination) that was attached to an optical table (Mecom Inc). The spectral reflectance of the maxillary anterior teeth, attached gingiva, lips, and facial skin was measured for each subject, as sequenced and described in Table II. After facial skin and the lips were measured, cheek retractors (Photo Med Intl, Van Nuys, Calif) were used to allow measurements of the attached gingiva and teeth. A total of 360 teeth, 120 gingival, 120 lip, and 120 facial skin measurements were made in this study. Subjects were financially compensated and issued a parking coupon, if necessary, for each color measurement session.
Table II.
Sequence and description of color measurements of subjects’ craniofacial structures
| Order | Maxillofacial structure | Location |
|---|---|---|
| 1 | Skin color | Tip of nose |
| 2 | Lip color | Midline of lower lip color on vermillion border |
| 3 | Attached gingiva color | 3.5 mm apical from free gingival line on central incisor |
| 4 | Maxillary central incisor | Center of buccal surface |
| 5 | Maxillary lateral incisor | Visible midincisal-gingiva and mesial-distal portion of tooth |
| 6 | Maxillary canine | Visible midincisal-gingiva and mesial-distal portion of tooth |
The validity of the color measurement instrument configuration was evaluated after 50% of the 120 subjects (60 subjects) were measured in this study. The maximum and minimum CIELAB values of teeth, lips, skin, and gingiva were generated from the measured colors of the first 60 subjects. The ranges of these CIELAB values were used to select corresponding color patches (DC Color Checker; GretagMacbeth, New Windsor, NY) that provided certified CIELAB values for each of its 240 color opaque patches (5 × 5 cm). A total of 24 of the 240 Color Checker colors had CIELAB values that were within the maximum and minimum range of the subjects’ color data. Validity was defined by the color difference between the 24 selected color patches, measured by the color measurement configuration, and the certified CIELAB values as determined by the following equation41:
| (Equation 2) |
where ΔL*, Δa*, and Δb* were the respective differences between the certified and measured CIELAB values. All color data were expressed in terms of L*, a*, and b* in accordance with CIELAB color space.41 L* refers to the lightness coordinate value (0 for perfect black to 100 for perfect white). The values of a* and b* are the chromaticity coordinates in the red-green axis and the yellow-blue axis, respectively. Positive a* values indicate the red color range and negative values, the green color range. Similarly, positive b* values indicate the yellow color range, whereas negative values indicate the blue color range. To evaluate validity of the color measurements, the following statistical analysis was carried out.
The mean and SDs of the CIELAB differences were then calculated. The mean, SD, and 95% confidence intervals for ΔL*, Δa*, and Δb* values between the measured color and the certified values of the 22 color patches on DC Color Checker were calculated. The 95% confidence interval for ΔL*, Δa*, and Δb* was evaluated if it contained zero. A linear regression was performed at the 95% confidence interval between measured and certified values for ΔL*, Δa*, and Δb*. Twelve subjects (10% of 120 subjects) were requested to return for a second visit to obtain all of the measurements again. The spectral data were converted to CIELAB values. To evaluate test-retest reliability, the following statistical analysis was performed.
Eighteen paired t tests between the first and second measurements of the L*, a*, and b* values for the 6 craniofacial structures (central and lateral incisor and canine, gingiva, lips, and skin) were carried out. A total of 18 correlations between the first and second measurements were also performed for L*, a*, and b* for 4 craniofacial structures measured. Finally, a total of 18 Bland-Altman plots were generated to evaluate whether the differences are, within reason, relative to their mean values. The graph plotted the difference versus the mean of the first and second L*, a*, and b* values for each of the 12 individuals for each of the 6 craniofacial structures.
RESULTS
Data for one of the 360 teeth could not be used (central incisor for A50-1M; 50-year-old Asian male), and thus data for only 359 teeth were usable. The CIELAB values for this unusable data set were L* 112.3, a* 0.8, and b* –10.4. The maximum L* cannot be above 100 for the CIELAB system, and b* should be positive for teeth (in the yellow range).
The mean CIELAB value ranges for the measured craniofacial structures were as follows: Teeth= L* (38.0 to 89.5), a* (0.3 to 12.2), and b*(5.7 to 35.7); Gingiva= L*(27.3 to 80.9), a* (4.2 to 38.2), and b*(5.3 to 26.9); Lips= L*(12.6 to 64.8), a*(7.5 to 34.6), and b*(5.3 to 19.8); and Skin= L*(23.7 to 60.1), a* (12.1 to 27.8), and b*(10.5 to 24.4). The mean, SD, and minimum and maximum CIELAB values of the measured craniofacial structures for this study are elaborated in Table III and diagrammatically represented in Figures 2, 3, and 4.
Table III.
CIELAB values of measured craniofacial structures
| Craniofacial structure | CIELAB | N | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Teeth | L* | 73.3 | 7.7 | 38.0 | 89.5 | |
| a* | 359 | 4.7 | 2.3 | 0.3 | 12.2 | |
| b* | 18.8 | 4.9 | 5.7 | 35.7 | ||
| Gingiva | L* | 52.1 | 8.7 | 27.3 | 80.9 | |
| a* | 120 | 25.9 | 5.2 | 4.2 | 38.2 | |
| b* | 17.8 | 3.3 | 5.3 | 26.9 | ||
| Lips | L* | 41.7 | 8.3 | 12.6 | 64.8 | |
| a* | 120 | 22.7 | 5.4 | 7.5 | 34.6 | |
| b* | 12.0 | 2.5 | 5.3 | 19.8 | ||
| Skin | L* | 46.9 | 8.6 | 23.7 | 60.1 | |
| a* | 120 | 16.9 | 2.5 | 12.1 | 27.8 | |
| b* | 18.5 | 2.7 | 10.5 | 24.4 |
Fig. 2.
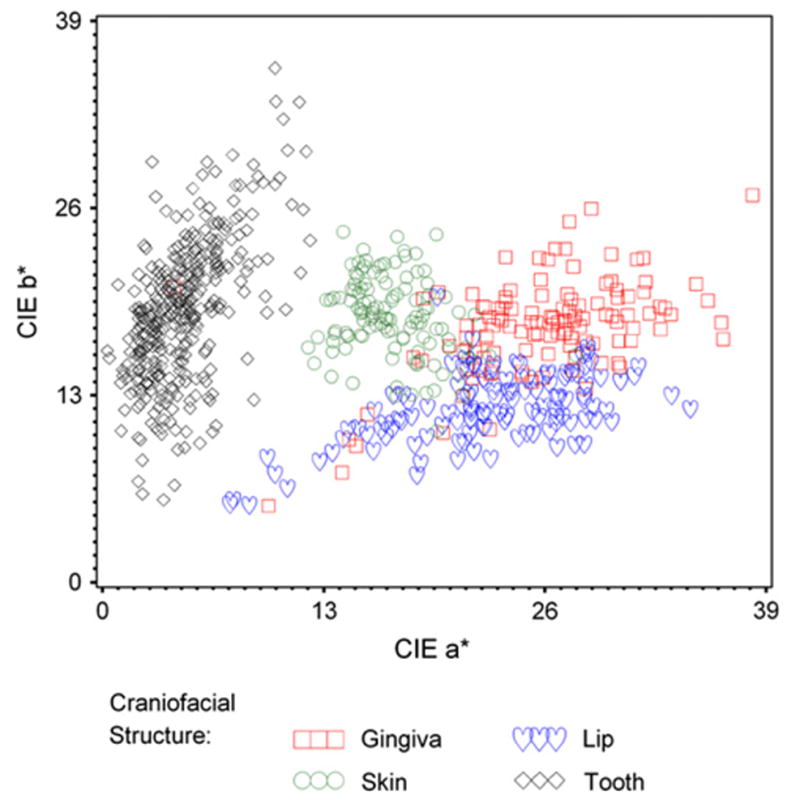
CIE b* versus a* of craniofacial structures.
Fig. 3.
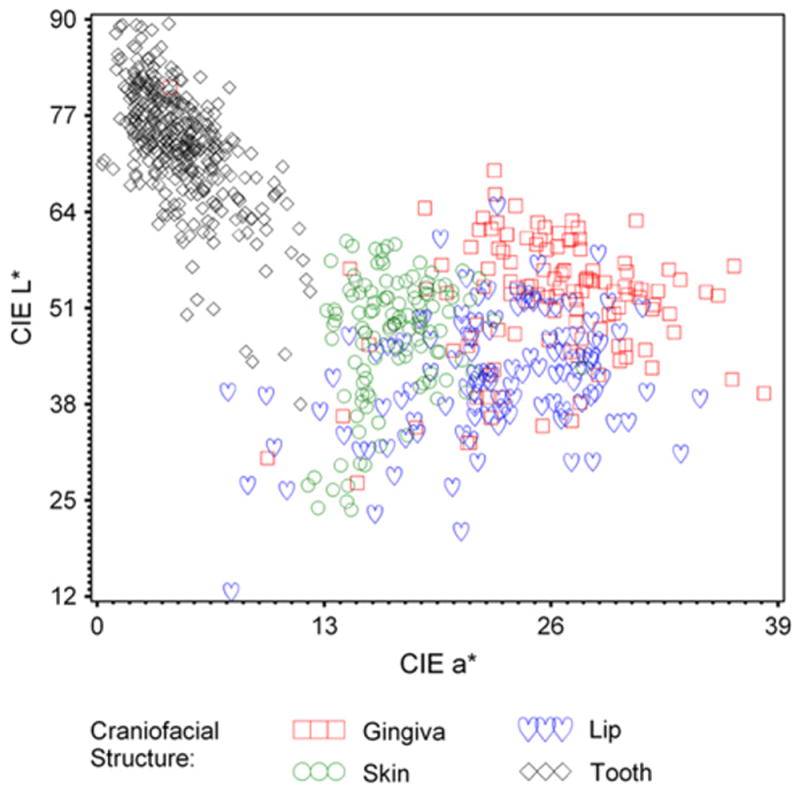
CIE L* versus a* of craniofacial structures.
Fig. 4.
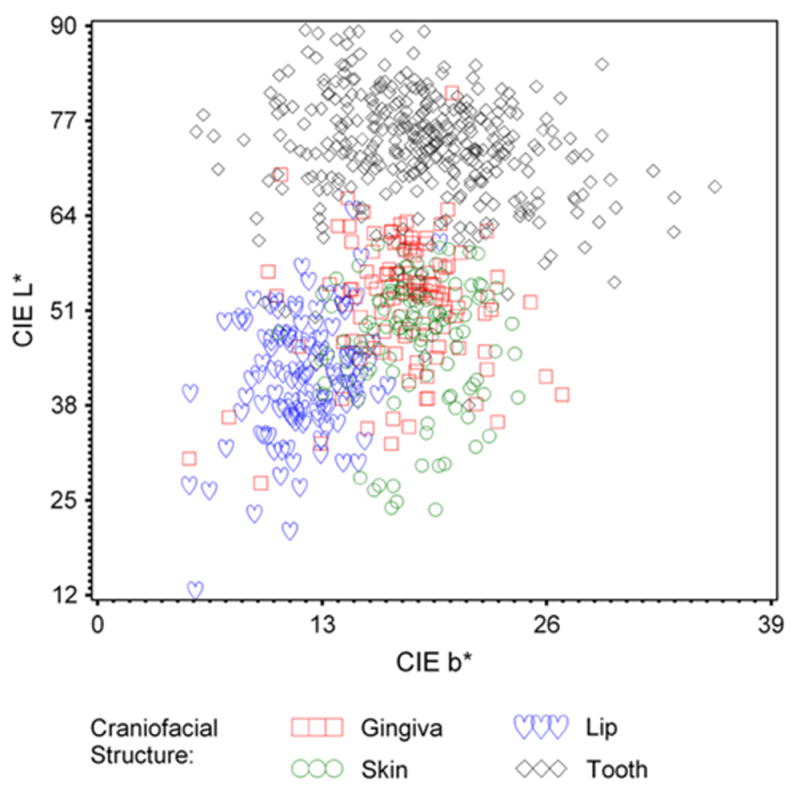
CIE L* versus b* of craniofacial structures.
With respect to validity, all but 2 of the color patches had color differences consistently below 5 ΔE between measured and certified CIELAB values. These 2 patches were measured again, using another color measuring instrument (CR-221, Colorimeter; Minolta, Ramsey, NJ) commonly used in dental research. The L*a*b* values were compared with the measurements made from the spectroradiometer and resulted in a 0.8 ΔE between the 2 instruments. Because both instruments had similar results that differed from the manufacturer, it was postulated that the patches were damaged, resulting in different data than that provided by the manufacturers. The mean average color difference between manufactured CIELAB values of the 22 color patches (DC Color Checker) and measured CIELAB values was 1.46 ΔE, with an SD of 0.88, minimum of 0.24, and maximum of 4.76. The 95% confidence interval of ΔL* and Δb* contained zero, but Δa* did not (Table IV). All the linear regressions had P values of <.0001, and all R2 values for measured versus certified L*, a*, and b* values were 0.99.
Table IV.
ΔL*, Δa* and Δb* values between measured and certified values of 22 color patches on DC Color Checker
| Variable | Mean | Pooled SD | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| ΔL* | −0.01 | 0.73 | −0.33 | 0.31 |
| Δa* | −0.88 | 0.98 | −1.31 | −0.44 |
| Δb* | −0.12 | 0.84 | −0.50 | 0.25 |
For the test-retest reliability, the 18 paired comparisons for the repeated L*, a*, and b* values for the 6 craniofacial structures were not all significantly different from each other, and P values are detailed in Table V. The Pearson correlation coefficients between the 18 pairs are also detailed in Table V. With regard to the correlation coefficients, 9 out of the 18 pairs had a correlation coefficient in the 0.9 range, while 6 pairs were in the 0.8 range, and only 3 pairs were in the 0.7 range. The pairs in the 0.7 range included a* of gingiva, a* of lateral incisor, and b* of gingiva. The Bland-Altman plots of the 18 pairs revealed that only 8 out of 216 observations (6 craniofacial structures, 3 CIELAB values, 12 subject repeats) did not indicate good reliability, as they were outside the ± 2 SD range of the difference. The 8 observations did not indicate good reliability, which included 1 observation of each of the following: L* for a lateral incisor and skin, a* for skin and central incisor, and b* for canine, central incisor, gingiva, and lip.
Table V.
P values of paired t test and Pearson correlation coefficients of CIELAB values between retest measures of craniofacial structures
|
P values
|
Pearson correlation coefficients
|
|||||
|---|---|---|---|---|---|---|
| Craniofacial structures | L* | a* | b* | L* | a* | b* |
| Skin | .8634 | .1880 | .5938 | 0.81 | 0.92 | 0.92 |
| Lip | .1749 | .3129 | .8681 | 0.87 | 0.88 | 0.82 |
| Gingiva | .6134 | .7233 | .5662 | 0.93 | 0.75 | 0.73 |
| Central | .7972 | .1718 | .5454 | 0.97 | 0.83 | 0.96 |
| Lateral | .8124 | .6393 | .9769 | 0.89 | 0.78 | 0.93 |
| Canine | .5339 | .7972 | .5530 | 0.91 | 0.93 | 0.90 |
DISCUSSION
With reference to the results, the hypothesis that color measurements using a 45/0-degree optical configuration for measurement of craniofacial structures are valid and reliable was supported by the data. The mean CIELAB values (Table III) obtained for teeth in this study were similar to that of the study evaluating Japanese teeth using similar technology without edge loss.29 The mean L* values were 73, a* values were 3, and b* values were 17 (mean CIELAB values were extrapolated from the manuscript’s graphs). Although probably not statistically significant, the teeth in the population of the present study measured slightly redder and slightly more yellow compared to the results of the Japanese study.29,30
A study that evaluated the in vivo precision of the colorimeter on 7 subjects26 reported CIELAB values ranging from 46.23 to 60.66 for L*, −0.72 to 2.02 for a*, and 1.92 to 14.70 for b*. The baseline values using the colorimeter for tooth bleaching studies of 2028 and 5227 subjects also reported low ranges for their approximate mean CIELAB values: from 32 to 52 for their L* values, −3 to 0.5 for a* values, and 0 to 7 for b* values. The CIELAB values obtained from studies,26–28 mentioned above, that used a small-area (3 mm) colorimeter were lower than the values from the present study (Table III), due most likely to the effects of edge loss.21
Although no actual instrumental CIELAB values of attached gingiva have been published, the conversion of the visually matched Munsell color tab data could be used as a comparison for the data of the present study.33 The maxillary attached gingiva for 150 subjects was between 8 Munsell color tabs of 7/6 2.5R to 8/4 10R, which when converted to CIELAB values from a table of the same study are the following: L*, 41.2 to 81.4; a*, 15 to 26; and b*, 8.1 to 17.6. The color range of gingiva (Table III) for the present study was generally wider than that of the visually matched Munsell color tab study, with results showing darker (lower L*), more red and green (lower and higher a* range), and more yellow and green (lower and higher b* range). This difference may be due to less reliability when using visual shade selection as compared to the use of a color-measuring instrument.10,11 Although not reported, the 150 subjects may have come from a homogenous sample of white subjects, given that the study was performed in Germany, as compared to the stratified sampling into 4 different racial groups in the present study.
The CIELAB values for skin and lips have not been published. The only quantitative data for skin were reported in values of luminous reflectance, dominant wavelength, and excitation purity for the 241 subjects measured for skin color using a spectrophotometer.39 Because skin contact was necessary for measurement but resulted in a change of skin color, comparison of values with the results of the present study is not appropriate. Also, skin measurements were made on different structures, with color measurements made on the left cheek area of the molar prominence, as compared to measurement of the skin color at the tip of the nose in the present study.
The use of a Xenon arch lamp (Oriel Instruments) did improve the experimental configuration stability in the current study compared to a previous configuration that used tungsten halogen lamps.42 When the tungsten halogen lamp was used, a maximum of 0.4 ΔE between adjacent measurements was found, as compared to 0.2 ΔE in the current study. In both studies, protocols to improve light source stability were used, which included allowing the light to stabilize for 30 minutes prior to use and regular calibration of the SW between each subject. The use of a more stable light source in this study did have an indirect impact on the validity of the color measurement.
The experimental configuration showed above satisfactory validity, as the mean ΔE was 1.46 for the 22 opaque patches measured and compared, which was above the perceptual level for opaque specimen.43 Although the confidence interval of all but Δa* (red-green axis) contained zero, the R2 values for the linear regression were excellent and were all in the 0.99 range. The problem with validity in the Δa* axis may be attributed to the fact that measurements of more reddish structures were particularly more difficult in this study, for example, gingiva and lips. Irregularities on the surface of the structures, especially on the lips and gingiva, may have caused a “micro-specular reflection of light” that produced an error at the time of craniofacial structure measurement. This phenomenon was especially noticeable in the younger population. The other possible errors in the validity of these measurements may be due to the following: (1) the influence of the light stability and (2) the positioning of the spectroradiometer when the required distance between the object to be measured and the instrument was adjusted. A limitation of the study was the inability to validate the experimental configuration with translucent specimens that are within the craniofacial color space. Unfortunately, these specimens are not commercially available. Future studies could attempt to use translucent specimens to validate the measurements with this optical configuration.
The test-retest reliability of this noncontact experimental configuration is acceptable and showed no significant difference between the paired comparison for the L*, a*, and b* for the 6 craniofacial structures, but half of the Pearson correlation coefficients were in the 0.9 range. The spectral reflectance of a contoured surface cannot be duplicated unless the same spot on that surface is measured. Using this optical measurement configuration without contact was a limitation of the study because the method used to standardize the position from the color measurement sensor to the actual structure was not precise. In general, contact color measuring instruments—that is, colorimeters—have shown good reliability of natural color measurements in vitro and in vivo.26 Although these instruments exhibit edge loss, the fact that contact is made with the measured structures ensures that the positioning can be easily repeated. Future studies could evaluate the reliability of the use of a movable pointer so that contact can be made to the structure being measured, with removal of the pointer before actual measurement occurs in order to improve the test-retest reliability.
Other limitations of this study include the small sample size and limited regional sample of the population. Future studies using a random sampling of subjects throughout all of the 50 states may be considered. Also, color measurement of 1 site of the craniofacial structure may not reliably capture the actual color of the subject’s craniofacial structure, nor be uniform across subjects. Future studies could measure the color of several sites from the same structure, such as the attached and nonattached gingiva or several lip or skin locations (for example, ear, forehead, cheeks, and chin) to provide a better sampling of the color of craniofacial structures and to determine which site(s) most accurately capture the color of the structures for subjects.
CONCLUSIONS
The spectral radiance of the craniofacial structures (anterior teeth, gingiva, lips, and facial skin) has been measured with a noncontact instrumental configuration with a 45/0-degree optical configuration. This color measurement configuration was shown to have acceptable test-retest reliability and acceptable validity with opaque specimens.
Acknowledgments
The authors thank Mrs April Logue, former research assistant, for help with subject recruitment and Mr Carl Kipp, restorative laboratory manager, for technical assistance. Appreciation also goes to Ms Jalcyn Laylle for her help in the manuscript submission, and former undergraduate students involved in this study, Mr Corey Valentine, Ms Tiffany Kocol, and Ms Pamela Kamolpechara.
Footnotes
This research was supported in part by the National Eye Institute, National Institutes of Health Grant No. R15 EY013527, and the 2003 Awards and Grants Program of the Editorial Council of The Journal of Prosthetic Dentistry.
References
- 1.Berscheid E, Gangestead S. The social psychological implications of facial physical attractiveness. Clin Plast Surg. 1982;9:289–96. [PubMed] [Google Scholar]
- 2.Bull RHC, Rumsey N. The social psychology of facial appearance. New York: Springer-Verlag; 1988. pp. 9–79. [Google Scholar]
- 3.Davis LG, Ashworth PD, Spriggs LS. Psychological effects of ingival dental treatment. J Dent. 1998;26:547–54. doi: 10.1016/s0300-5712(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 4.Beumer J, Ma T, Roumanas E, Nishimura R. Restoration of facial defects. In: Beumer J, Curtis TA, Marunick MT, editors. Maxillofacial rehabilitation: prosthodontic and surgical considerations. St. Louis: Medico Dental Media Intl; 1996. pp. 387–99. [Google Scholar]
- 5.Taylor TD. Clinical maxillofacial prosthetics. Chicago: Quintessence; 2000. pp. 233–44. [Google Scholar]
- 6.Bergman B, Nilson H, Andersson M. A longitudinal clinical study of Procera ceramic-veneered titanium copings. Int J Prosthodont. 1999;12:135–9. [PubMed] [Google Scholar]
- 7.Haselton DR, Diaz-Arnold AM, Hillis SL. Clinical assessment of high-strength all-ceramic crowns. J Prosthet Dent. 2000;83:396–401. doi: 10.1016/s0022-3913(00)70033-3. [DOI] [PubMed] [Google Scholar]
- 8.Ma T, Hicken SC, Buchanan CR, Deboie RG. Chairside color verification for facial prostheses. J Prosthet Dent. 1988;60:219–21. doi: 10.1016/0022-3913(88)90319-8. [DOI] [PubMed] [Google Scholar]
- 9.Rosenstiel SF, Fujimoto J, Land MF. Contemporary fixed prosthodontics. 4. St Louis: Mosby; 2006. p. 712. [Google Scholar]
- 10.Culpepper WD. A comparative study of shade-matching procedures. J Prosthet Dent. 1970;24:166–73. doi: 10.1016/0022-3913(70)90140-x. [DOI] [PubMed] [Google Scholar]
- 11.Okubo SR, Kanawati A, Richards MW, Childress S. Evaluation of visual and instrument shade matching. J Prosthet Dent. 1998;80:642–8. doi: 10.1016/s0022-3913(98)70049-6. [DOI] [PubMed] [Google Scholar]
- 12.Groh CL, O’Brien WJ, Boenke KM. Differences in color between fired porcelain and shade guides. Int J Prosthodont. 1992;5:510–4. [PubMed] [Google Scholar]
- 13.Douglas RD, Przybylska M. Predicting porcelain thickness required for dental shade matches. J Prosthet Dent. 1999;82:143–9. doi: 10.1016/s0022-3913(99)70147-2. [DOI] [PubMed] [Google Scholar]
- 14.Kubelka P. New contributions to the optics of intensely light-scattering materials. Part 1. J Opt Soc Am. 1948;38:448–57. doi: 10.1364/josa.38.000448. [DOI] [PubMed] [Google Scholar]
- 15.Johnston WM, O’Brien WJ, Tien TY. Concentration additivity of Kubelka-Munk optical coefficients of porcelain mixtures. Color Res Appl. 1986;11:131–7. [Google Scholar]
- 16.Johnston WM, Ma T, Kienle BH. Translucency parameter of colorants for maxillofacial prostheses. Int J Prosthodont. 1995;8:79–86. [PubMed] [Google Scholar]
- 17.Wee AG, Kang EY, Johnston WM, Seghi RR. Evaluating porcelain color match of different porcelain shade-matching systems. J Esthet Dent. 2000;12:271–80. doi: 10.1111/j.1708-8240.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 18.Troppmann RJ, Wolfaardt JF, Grace M, James AS. Spectrophotometry and formulation for coloring facial prosthetic silicone elastomer: a pilot clinic trial. J Facial and Somato Prosthet. 1996;2:85–92. [Google Scholar]
- 19.Cantor R, Webber RL, Stroud L, Ryge G. Methods for evaluating the prosthetic facial materials. J Prosthet Dent. 1969;21:324–32. doi: 10.1016/0022-3913(69)90295-9. [DOI] [PubMed] [Google Scholar]
- 20.Seghi RR, Johnston WM, O’Brien WJ. Performance assessment of colorimetric devices on dental porcelain. J Dent Res. 1989;68:1755–9. doi: 10.1177/00220345890680120701. [DOI] [PubMed] [Google Scholar]
- 21.Bolt RA, Bosch JJ, Coops JC. Influence of window size in small-window color measurement, particularly of teeth. Phys Med Biol. 1994;39:1133–42. doi: 10.1088/0031-9155/39/7/006. [DOI] [PubMed] [Google Scholar]
- 22.Clark EB. The color problem in dentistry. Dent Dig. 1931;37:499–509. [Google Scholar]
- 23.Macentee M, Lakowski R. Instrumental colour measurement of vital and extracted human teeth. J Oral Rehabil. 1981;8:203–8. doi: 10.1111/j.1365-2842.1981.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 24.Goodkind RJ, Schwabacher WB. Use of a fiber-optic colorimeter for in vivo color measurements of 2830 anterior teeth. J Prosthet Dent. 1987;58:535–42. doi: 10.1016/0022-3913(87)90380-5. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstiel SF, Gegauff AG, McCafferty RJ, Johnston WM. In vitro tooth color change with repeated bleaching. Quintessence Int. 1991;22:7–12. [PubMed] [Google Scholar]
- 26.Douglas RD. Precision of in vivo colorimetric assessments of teeth. J Prosthet Dent. 1997;77:464–70. doi: 10.1016/s0022-3913(97)70137-9. [DOI] [PubMed] [Google Scholar]
- 27.Rosenstiel SF, Gegauff AG, Johnston WM. Randomized clinical trial of the efficacy and safety of a home bleaching procedure. Quintessence Int. 1996;27:413–24. [PubMed] [Google Scholar]
- 28.Gegauff AG, Rosenstiel SF, Langhout KJ, Johnston WM. Evaluating tooth color change from carbamide peroxide gel. J Am Dent Assoc. 1993;124:65–72. doi: 10.14219/jada.archive.1993.0143. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa A, Ikeda I, Kawaguchi S. Color and translucency of in vivo natural central incisors. J Prosthet Dent. 2000;83:418–23. doi: 10.1016/s0022-3913(00)70036-9. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa A, Motonomi A, Ikeda I, Kawaguchi S. Color of natural tooth crown in Japanese people. Color Res Appl. 2000;25:43–8. [Google Scholar]
- 31.Dummett CO. Oral pigmentation. J Periodontol. 1960;31:356–60. [Google Scholar]
- 32.Powers JM, Capp JA, Koran A. Color of gingival tissues of blacks and whites. J Dent Res. 1977;56:112–6. doi: 10.1177/00220345770560020301. [DOI] [PubMed] [Google Scholar]
- 33.Heydecke G, Schnitzer S, Turp JC. The color of human ingival and mucosa: visual measurement and description of distribution. Clin Oral Investig. 2005;9:257–65. doi: 10.1007/s00784-005-0006-3. [DOI] [PubMed] [Google Scholar]
- 34.Jones J, McFall WT., Jr A photometric study of the color of health ingival. J Periodontol. 1977;48:21–6. doi: 10.1902/jop.1977.48.1.21. [DOI] [PubMed] [Google Scholar]
- 35.Kleinheinz J, Buchter A, Fillies T, Joos U. Vascular basis of mucosal color. Head Face Med. 2005;1:4. doi: 10.1186/1746-160X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jablonski N, Chaplin G. Skin deep. Sci Am. 2002;287:75–81. doi: 10.1038/scientificamerican1002-74. [DOI] [PubMed] [Google Scholar]
- 37.Edwards EA, Duntley SQ. The pigments and color of living human skin. Am J Anatomy. 1939;65:1–33. [Google Scholar]
- 38.Thibodeau EA, D’Ambrosio JA. Measurement of lip and skin pigmentation using reflectance spectrophotometry. Eur J Oral Sci. 1997;105:373–5. doi: 10.1111/j.1600-0722.1997.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 39.Koran A, Powers JM, Raptis CN, Yu R. Reflection spectrophotometry of facial skin. J Dent Res. 1981;60:979–82. doi: 10.1177/00220345810600061301. [DOI] [PubMed] [Google Scholar]
- 40.Judd DB, Wyszecki G. Color in business, science and industry. 3. New York: John Wiley; 1975. pp. 29–31. [Google Scholar]
- 41.Commission Internationale d’Eclairage (CIE) Colorimetry. CIE Pub No. 15.2. 2. Vienna, Austria: Central Bureau of the CIE; 1986. p. 33. [Google Scholar]
- 42.Wee AG, Lindsey DT, Kuo S, Johnston WM. Color accuracy of commercial digital cameras for use in dentistry. Dent Mater. 2006;22:553–9. doi: 10.1016/j.dental.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuehni RG, Marcus RT. An experiment in visual scaling of small color difference. Color Res Appl. 1979;4:83–91. [Google Scholar]


