Summary
We investigated the thermodynamic basis of HK97 assembly by scanning calorimetry and cryo-electron microscopy. This pathway involves self-assembly of hexamers and pentamers of the precursor capsid protein gp5 into procapsids; proteolysis of their N-terminal Δdomains; expansion - a major conformational change; and covalent crosslinking. The thermal denaturation parameters convey the changes in stability at successive steps in assembly, and afford estimates of the corresponding changes in free energy. The procapsid represents a kinetically accessible local minimum of free energy. In maturation, it progresses to lower minima in a cascade punctuated by irreversible processes ("locks") – i.e. proteolysis and crosslinking - that lower kinetic barriers and prevent regression. We infer that Δ-domains not only guide assembly but also restrain the procapsid from premature expansion; their removal by proteolysis is conducive to initiating expansion and to its proceeding to completion. We also analyzed the mutant E219K, whose capsomers reassemble in vitro into procapsids with vacant vertices called "whiffleballs". E219K assemblies all have markedly reduced stability compared to wildtype gp5 (ΔTp ~ −7 to −10°C; where Tp is the denaturation temperature). As the mutated residue is buried in the core of gp5, we attribute the observed reduction in stability to steric and electrostatic perturbations of the packing of sidechains in the subunit interior. To explain the whiffleball phenotype, we suggest that these effects propagate to the capsomer periphery in such a way as to differentially affect the stability or solubility of dissociated pentamers, leaving only hexamers to reassemble.
Keywords: Differential scanning calorimetry, cryo-electron microscopy, virus capsid structure, morphogenesis, conformational changes
Introduction
To assemble a viral capsid, hundreds of protein subunits must be coordinated precisely in three dimensions to produce a shell of predetermined size, shape, and complexity. For the smallest and simplest viruses, all the information needed for assembly is encoded in the subunit fold. Such particles may be passed through cycles of assembly and disassembly in vitro 1. For more elaborate viruses, larger numbers of subunits are involved and transient assembly factors – chaperones, scaffolding proteins, and a maturational protease – may be required 2. Viruses of this kind include the tailed bacteriophages and the herpesviruses which, despite their very different hosts, follow similar pathways of capsid assembly 3; 4;5. In these systems, assembly proceeds in two stages: formation of a precursor particle (procapsid) and its maturation 6; 7. Maturation renders the capsid so robust that its subunits may only be dissociated, if at all, with strong denaturants and, typically, the dissociation products are unable to refold and reassemble. At least some procapsids are capable of disassembly/ reassembly reactions, but maturation is an essentially irreversible process.
Mechanisms that govern capsid assembly are likely to recur in the assembly of macromolecular complexes in general 8. HK97 affords an advantageous system in which to study them. Its pathway (Fig. 1A) has been extensively characterized. The capsid protein precursor, gp5, is a fusion of the scaffolding protein - its Δ-domain (residues 2 to 103) - with the major capsid protein, gp5* (residues 104 to 385). Gp5 forms pentameric and hexameric capsomers that self-assemble in vivo or in vitro into a T=7l procapsid called Prohead I. If the viral protease gp4 is co-expressed with gp5, ~ 50 copies are incorporated into Prohead I. The protease is not needed for assembly but is required for maturation which begins with proteolysis of the Δ-domains. The resulting particle, Prohead II, is metastable and undergoes a major structural transformation – "expansion" 9; 10 - that is triggered in vivo by DNA packaging and may be induced in vitro by acidification.
Figure 1. HK97 capsid assembly with and without the maturational protease.

The pathway (A) is for the expressed capsid protein in the absence of the portal/connector protein and DNA packaging. Pathway (B) refers to in vitro assembly of Prohead I and how this assembly pathway is altered in the mutant E219K which reassembles into whiffleballs.
Expansion proceeds to the final Head II state via three intermediates, called Expansion Intermediates I, II and III 11 (Fig. 1A). Conversion of Prohead II to Head II involves large rotations (~ 40°) and translations (~ 40 Å) of the subunit cores that radically alter the capsid structure 12; 13. (We use the term "core" for the portion of the molecule that appears to move as a single rigid body throughout capsid assembly. It consists of the A- and P-domains but not two protruding motifs - the E-loop and the N-arm – see Fig. 6A). These movements are accompanied by remodeling of the E-loop, which is a β-hairpin, and the N-arm, which has an extended conformation in Head II but is disordered in Prohead II. Expansion is accompanied by the formation of a network of covalent crosslinks, connecting residue K169 in the tip of the E-loop with N356 on a neighboring subunit 14; 15. Crosslinking is sterically controlled by the subunit rotations that bring crosslinkable residues, initially far apart, within reach. The first crosslinks form at the EI-II stage and the final ones in the last step to Head II. These interactions effect an essential stabilization of the capsid 16.
Figure 6. Location and surroundings of residue E219 mutated to K in the "whiffleball" mutant.

(A) The gp5* subunit (conformer B in the refined Head II structure 27 is shown in a ribbon diagram, as viewed from the capsid interior. The side-chains of E219 and its nearest neighbors are highlighted in space-filling mode (E219, red; R135, blue; E364, orange; L132, A216 and L366, green; and I183, grey). The wedge-shaped A-domains pack around the central axis of a capsomer, while the P-domains form its periphery: see the pentamer model in (C) and (D). The A- and P-domains together form the structural core of the subunit that moves as single rigid body throughout the transition from Prohead II to Head II. Residue E219 is buried within this core. (B) The network of charged residues close to and presumably interacting electrostatically with E219 mostly lie near the capsid inner surface. Side view (C) and bottom view (D) of a Prohead II pentamer from the cryo-EM-derived Cα model (PDB ID 1IF0; 12) are shown as ribbon diagrams, with E219 (in red) and four other residues highlighted. The positions of the residues, which were approximated by threading the Head II side-chains through the Prohead II model, illustrate possible connections of E219 with the inter-capsomer interface. Residues E364 (orange) and L366 are neighbors of E219 on β-strand J, while residues E363 (magenta) and R365 are adjacent, on the other side of β-strand J and form part of the hexamer-pentamer interface.
Differential scanning calorimetry (DSC) affords a quantitative method to assess the stability and energetics of interactions in macromolecular systems 17. In a previous study, we used DSC in combination with cryo-EM and mutagenesis to examine the effects of crosslinking and expansion on HK97 capsid stability 16. We found that crosslinking is entirely responsible for the greater stability of the mature capsid; expansion allows crosslinking to take place, with the added benefit of doubling the capsid volume and hence its capacity for DNA. Here, we follow a similar approach to investigate the role of proteolysis in capsid assembly and a point mutation with altered assembly properties. Specifically, we analyze the distinct assembly states of uncleaved gp5 - unassembled capsomers, Prohead I, and capsids that were induced to expand without prior cleavage by treatment with urea - and compare them with earlier data on gp5*-containing particles. In Prohead I, the capsomers are shaped like parachutes whose canopies, comprising gp5* plus the C-terminal segments of the Δ-domains, form the capsid's contiguous shell, while the underlying gondolas are composed of the remaining portions of the Δ-domains 10. We also investigate the morphogenic mutation, E219K. Whereas capsomers of wild-type gp5 reassemble in vitro into Prohead I, E219K capsomers reassemble into "whiffleballs" lacking pentamers at their vertices 18 (Fig. 1B). The present study complements and extends earlier calorimetric work on the capsids of T4 19; 20 and P22 21; 22. It was undertaken because, although T4 and P22 subscribe to the same paradigm as HK97 and their capsid proteins are now known to have similar folds 23; 24; 25, there are also significant differences between the respective systems. P22 maturation is not accompanied by proteolysis. With T4, unlike HK97, capsid expansion per se effects a sufficient stabilization of the capsid 26. An additional incentive to analyze the HK97 system in this way was the availability of a crystal structure for Head II 23; 27 as well as detailed structural information on other states 12; 13 that allow the thermodynamic data to be considered in terms of inter- and intramolecular interactions.
Results
As a macromolecular complex is heated, the interactions that stabilize it are disrupted at temperatures that are characteristic for a given specimen. In DSC, samples are heated at a constant rate and the input of energy required to effect such transitions is recorded in the thermogram (e.g. Fig. 2A). With proteins, the major event is denaturation and it may be preceded by minor events that represent thermally induced conformational changes. If a specimen has multiple conformations – as in sequential stages of capsid assembly - their energetics may be compared from the thermograms.
Figure 2. Calorimetry of various assembly states of wild-type HK97 capsid protein and the E219K mutant.

(A) Thermograms of five states of wild-type gp5 or the cleaved form gp5* in the case of Prohead II. P-I = Prohead I, etc. Capsomers prepared by dissociating Prohead I are predominantly (> 80%) hexamers and the remainder, pentamers. In pentamer preparations, the proportions are reversed. (B) Thermograms of four assembly states of the mutant capsid protein, gp5 (E219K). The minor peak at 57° in the whiffleball curve is contributed by unassembled capsomers. (C–E) Negative staining electron microscopy of a gp5 "pentamer" preparation confirms that the majority of these oligomers are indeed pentamers, but a minority (~ 15%) of hexamers is also present. (C) a field of oligomers. Bar = 500 Å. (D) left - a typical single pentamer; middle - averaged pentamer after rotational and translational alignment; right - averaged pentamer after 5-fold symmetrization; (E) left - a typical single hexamer; middle - averaged hexamer after rotational and translational alignment; right - averaged hexamer after 2-fold symmetrization. Since hexamers are fewer than pentamers, less averaging was done, so these images are noisier. Bar = 50 Å.
Thermodynamic parameters measured from these scans are compiled in Table I. Thermal stability is measured by Tp, the peak temperature of a denaturation endotherm. The enthalpy change accompanying denaturation, ΔHm, is the area under the peak and represents the energy required to disrupt interactions within and between subunits. The entropy change, ΔSm, is given by ΔHm/Tp. The breadth of a thermal event is expressed as ΔTFWHH, its full width at half-height. For a two-state transition, this parameter represents cooperativity. Thermodynamic stability, ΔG°, is the difference in free energy between the folded and unfolded forms. This quantity may be calculated from the denaturation parameters obtained by calorimetry (see Methods). The effect of a structural transition or a mutation on thermodynamic stability may be expressed in terms of ΔΔG°.
Table I.
Thermodynamic parameters from calorimetric scans of HK97 capsids
| Capsid | Tp (°C) | ΔTFWHH (°C) | ΔHm kcal/mol | ΔSm cal/mol/K |
|---|---|---|---|---|
| capsomers wt | 63.3 | 3.9 | 159 | 473 |
| pentamers wt a | 63.2 | 4.5 | ND | ND |
| capsomers (E219K) | 56.3 | 3.6 | 131 | 398 |
| P- I wt | 81.7
54.2 (r) |
6.3
2.5 |
190
14 |
535
43 |
| P- I wta (reassembled) | 81.6
54.2 (r) |
6.0
2.7 |
ND
ND |
ND
ND |
| P- I (E219K) | 71.5 | 2.0 | 192 | 557 |
| E219K “whiffleballs” | 67.4 | 2.0 | 145 | 426 |
| P- II wtb | 86.3 | 6.6 | 188 | 523 |
| P- II (E219K) | 74.6 | 4.1 | 175 | 503 |
| Expanded P- I wt | 91.6 | 3.9 | 308 | 844 |
| Expanded P- I (K169Y)a | 77.6 | 5.5 | ND | ND |
For each entry, four scans at 120°C/h were performed, giving average uncertainties (expressed as ± two standard errors of the mean) of ± 0.2 °C for Tp, and ± 3.5 % for ΔHm and ΔSm. ΔHm and ΔSm are expressed per mole of gp5 or gp5* protein subunits of which there are 420 copies arranged as hexamers and pentamers on the T = 7l lattice of the icosahedral HK97 capsid. (r) denotes a reversible event; all other event are irreversible.
These runs were carried out at 90 °C/h and the value of Tp has been adjusted upward based upon the observed changes in Tp for capsomers and wt Prohead I at different scan rates of 60–120 °C/h. Values of ΔHm and ΔSm are not reported (ND) because these were single experiments.
from Ross et al. 16.
Assembly of wild-type capsomers into Prohead I enhances their thermal stability by ~ 20°C
To assess the contribution of inter-capsomer interactions to stabilizing Prohead I, we compared thermograms recorded for unassembled capsomers with those of assembled particles (Fig. 2A). Capsomers were prepared by dissociating Prohead I in high ionic strength at alkaline pH, and then dialyzing back into the original buffer to eliminate any effect of solution conditions on the comparison. Material prepared in this way is a mixture of hexamers and pentamers, mainly hexamers 28. The resulting thermograms (e.g. Fig. 2A, 4th curve) exhibit a single, quite sharp, endotherm with a Tp of 63°C. In contrast, native Prohead I (i.e. capsids isolated directly from cells in which gp5 was expressed) denatures at 82°C, almost 20° higher; moreover, this event is substantially broader (Fig. 2A, 3rd curve).
To confirm the assembly competence of these capsomers as well as the authenticity of Prohead I reassembled in vitro, we prepared the latter particles and analyzed them by calorimetrỹ The resulting thermograms (e.g. Fig. 2A, 2nd curve) were indistinguishable from those of native Prohead I except for a vestigial event at 62°C attributable to a small amount of unassembled capsomers.
Prohead I, but not capsomers, exhibits a minor thermal event at 54°
This event, well separated from the denaturation endotherm and involving about 8% as much enthalpy (Fig. 2A, 2nd and 3rd curves), is exhibited by both native and reassembled Prohead I but not by capsomers. Unlike thermal denaturation, this transition is reversible (data not shown). Since the capsids remain intact after this transition, its structural basis may be tested by cryo-EM of Prohead I that is heated past 54°C and then rapidly frozen before the structure can revert (Conway et al., Ms in submission).
Conversion of Prohead I to Prohead II involves a structural change in gp5*
As visualized by cryo-EM, the cleaved and uncleaved procapsids are similar in size and structure 10. Evident differences are confined to the inner surface where a "gondola" of density underlies each Prohead I capsomer (Fig. 4A). The gondolas are absent from Prohead II, implying that they are clusters of Δ-domains. The Tp of Prohead II is 4 - 5° higher than that of Prohead I, while these denaturation events are of similar width (Fig. 2A, 1st curve; cf. 16). The simple “excision-of-Δ-domains” scenario should not result in enhanced stability, i.e. an increased Tp, whence we infer that conversion of Prohead I to Prohead II is accompanied by a subtle revision of the interactions in the contiguous shell (see Discussion).
Figure 4. Structural comparison of Expanded Prohead I with native Prohead I and Head II.

Cryo-EM reconstructions are shown of (A) Prohead I and (B) Expanded Prohead I. The representation of Head II (C) was calculated by limiting the crystal structure 23 to the same resolution as the cryo-EM data, i.e. 11 Å. In each case, renderings of the outer surface (left) and inner surface (middle) are shown, together with a central section (right) in which protein is dark. The insert between (A) and (B) shows Prohead I at lower magnification and the lower resolution of ~ 25 Å under which conditions, the T=7 levo surface lattice and the skewing of the hexamers are more evident. The Δ-domain-associated gondolas of density underlying each capsomer are colored yellow in (A) – middle panel. This density is rather diffuse – (A), right panel, yellow arrows, indicative of some disorder. Expanded Prohead I has the same diameter as Head II but its facets are less flat (black arrows, right panel in (B)). The white arrows in (A), left and middle panels, mark protruding E-loops. The shell of Expanded Prohead I is thicker than that of Head II and it has additional density on the outer surface (empty arrowheads) which we interpret as translocated Δ-domains.
Prohead I may be expanded (without cleavage) by treatment with 5M urea
In the normal course of events, degradation of the Δ-domains is a prerequisite for expansion and crosslinking to occur. Cleavage of gp5 primes Prohead II for expansion, which can be triggered in vitro by acidification to pH 4 9. The same treatment does not cause Prohead I to expand (R.L.D., unpublished observations). However, a transition akin to expansion, as judged by mobility in non-denaturing gels, is induced by treating Prohead I with 5M urea (Fig. 3A). Consistent with this inference, the transition is accompanied by substantial crosslinking, as evidenced by SDS-PAGE (Fig. 3B). We call these particles, putatively expanded but retaining their Δ-domains, Expanded Prohead I.
Figure 3. Gel electrophoresis and calorimetric analysis of Expanded Prohead I.

(A) shows that the mobility of Prohead I in native agarose gels, after treatment with 5M urea (Expanded Prohead I), is markedly altered and perceptibly different from that of unassembled capsomers. (B) SDS-PAGE of Expanded Protein I shows a ladder of bands with mobilities corresponding to multiples of 42 kDa, indicative of a substantial degree of crosslinking of the uncleaved gp5. Also shown are a ladder of partially crosslinked gp5* (bands at multiples of 31 kDa), and gp5 monomers (not crosslinked). (C) Thermograms showing calorimetric scans of Expanded Prohead I (2nd curve) with wild-type gp5 which is partially crosslinked (see Fig. 3B) and the non-crosslinking mutant, K169Y (3rd curve). To allow direct comparison, the corresponding thermograms of Head II and native Prohead I are reproduced from 16 and Figure 2, respectively.
To characterize them further, we performed cryo-EM and image reconstruction. Expanded Prohead I (Fig. 4B) is compared with Prohead I (uncleaved, unexpanded; Fig. 4A) and Head II (cleaved, expanded; Fig. 4C) at the same resolution: it is indeed almost fully expanded, having a vertex-to-vertex diameter of 600 Å, essentially the same as Head II. Also, like Head II, Expanded Prohead I has rather flat facets, although they are buckled in the middle, not planar (cf. right-hand panels in Fig. 4).
The shell of Expanded Prohead I is thicker and has a more pronounced surface relief than that of Head II. However, lacking the Δ-domain gondolas (yellow in Fig. 4A, middle panel), it is markedly thinner than the shell of Prohead I. Compared with Head II, Expanded Prohead I has additional densities on both the outer and inner surfaces (arrows and arrowheads in Fig. 4B, right panel), suggesting that the Δ-domains are partly translocated to the outer surface. However, it appears that these domains are too bulky to be fully translocated, so that the rotations undergone by the gp5* cores en route to the Head II state 12 cannot go to completion. Nevertheless, the transition proceeds far enough for crosslinking to take place. Consistent with these data, the E-loops which engage in the crosslinks and protrude outwards on unexpanded capsids (white arrows in Fig. 4B, left and middle panels) are less prominent in Expanded Prohead I, except around the pentamers. A similar effect whereby the penton-proximal E-loops are the last to remain protruding was observed on EI-III capsids 15.
Expanded Prohead I is partially stabilized by crosslinking
In Figure 3C, a thermogram of Expanded Prohead I (2nd curve) is compared with those of Prohead I (4th curve) and Head II (1st curve). Its Tp is 10° higher than for Prohead I. The width of its denaturation endotherm resembles that of Head II, but its Tp is 5° lower. This thermogram lacks the reversible minor event at 80° exhibited by Head II (Fig. 3C, 1st curve) and the 54° event of Prohead I. Since expanding Prohead II in the absence of crosslinking does not enhance its thermal stability 16, we attribute the increased Tp of Expanded Prohead I to crosslinking.
To test this hypothesis, we prepared Expanded Prohead I with the mutant K169Y which is unable to crosslink, and confirmed by cryo-EM that these particles were morphologically similar to wild-type Expanded Prohead I (data not shown). Their thermogram is shown in Fig. 3C (3rd curve). Expanded Prohead I (K169Y) melts at 77°, ~ 4° lower than Prohead I (wild-type). If we allow a 2° decrease in Tp for the K169Y substitution 16, it follows that inducing Prohead I to expand without prior cleavage results in a 2°C reduction in its Tp, i.e. a net destabilization. However, when wild-type Prohead I is expanded, most crosslinks form (Fig. 3B), resulting in a ΔTp of +12°C; this increment is the same as that effected by crosslinking the cleaved and expanded surface lattice.
Capsomers and Proheads of the whiffleball mutant E219K have reduced stability
The E219K mutation was initially identified in a screen for mutant gp5s with altered assembly properties. As outlined above (Introduction), E219K capsomers – which appear to be exclusively hexamers after dissociation from Prohead I - reassemble only into whiffleballs. To investigate the thermodynamic consequences of this amino acid substitution, we scanned capsomers, Prohead I, and Prohead II of the E219K mutant protein (Fig. 2B). These data show a similar trend as with wild-type gp5 (Fig. 2A), but there are some differences. First, there is a systematic reduction in thermal stability. The Tp's are displaced to lower temperatures, by 7 – 10°, depending on the state of assembly. Second, the E219K Prohead I endotherm is much sharper than for wild-type, i.e. ΔTFWHH ~ 2° vs. 6° (Table 1); in contrast, the wild-type and E219K capsomer endotherms have the same width, ΔTFWHH ~ 3.5°. Third, the minor 54° event of wild-type Prohead I is absent.
To investigate the consequences of the absence of gp5 pentamers at the procapsid vertices, we assembled whiffleballs according to Li et al. 18 and scanned them also (Fig. 2B, 2nd curve). Their denaturation endotherm is as sharp (ΔTFWHH ~ 2°) as that of Prohead I (E219K), but their Tp is 4° lower. This thermogram also has an event at 56° that reflects the presence of some unassembled capsomers. From the size of this peak, they should account for ~ 25% of the gp5 present.
We conclude that the E219K mutation results in a general destabilization relative to assemblies of wild-type gp5. Nevertheless, the mutant Prohead I (with pentamers at its vertices) is more stable than mutant capsomers by about the same margin in Tp (+ 15°–18°) as with wild-type gp5. Despite lacking pentons, whiffleballs are only slightly less stable than Prohead I (E219K), their Tp's differing by 4°.
With wild-type gp5, pentamers are no less stable than hexamers
The failure to recover pentamers from the dissociation products of Prohead I (E219K) 18 suggests that the mutant pentamers may be less stable than hexamers. Since E219K assemblies are generally less stable than wild-type (cf. Figs. 2A and 2C), it may be that E219K pentamers, once dissociated, simply fall apart. Alternatively, they may precipitate or even reorganize into hexamers. In fact, Xie and Hendrix 28 found that wild-type capsomers may be converted from mostly hexamers to mostly pentamers under appropriate solvent conditions, (they were able to distinguish the two kinds of capsomers on the basis of their differing mobilities in non-denaturing gel electrophoresis).
To test the "labile pentamer" hypothesis, we sought to compare pentamers with hexamers by calorimetry; however, the E219K protein precipitates under the buffer conditions that favor pentamers 18. Nevertheless, we were able to produce wild-type pentamers. To confirm their oligomeric status, we examined this material by negative staining EM and image analysis (Fig. 2C-E) and, in this way, estimated that > 80% of these oligomers were indeed pentamers. Calorimetrically, this preparation was indistinguishable from capsomers from dissociated Prohead I, which are mostly hexamers (cf. curves 5 and 4 in Fig. 2A). Thus, we conclude that any differential lability of pentamers should be specific to the E219K mutation.
Discussion
The progressive stabilization of the maturing HK97 capsid is plotted in Fig. 5A in terms of Tp, the denaturation temperature. Assembly of capsomers into Prohead I enhances their thermal stability by 20°C. Prohead I is already a robust particle whose Tp (82°) is comparable to some proteins of thermophiles. Conversion to Prohead II effects a further stabilization (ΔTp, +4.5°), reflecting a subtle structural change in the contiguous shell (Fig. 5B). Assembly of the E219K mutant up to the Prohead II stage follows a similar course, but at each stage the mutant particle is ~ 10° less stable than wild-type. When Prohead II expands to Head II, further stabilization to a Tp of 96° is conferred by crosslinking (Fig. 5A). Unlike Prohead II, which may be induced to expand by relatively mild stimuli, Prohead I expands only when exposed to a strong denaturant, i.e. 5M urea, but this transition also results in substantial crosslinking and a concomitant stabilization.
Figure 5. Summary of changes in thermal stability and structure between various assembly states of HK97 capsid protein.
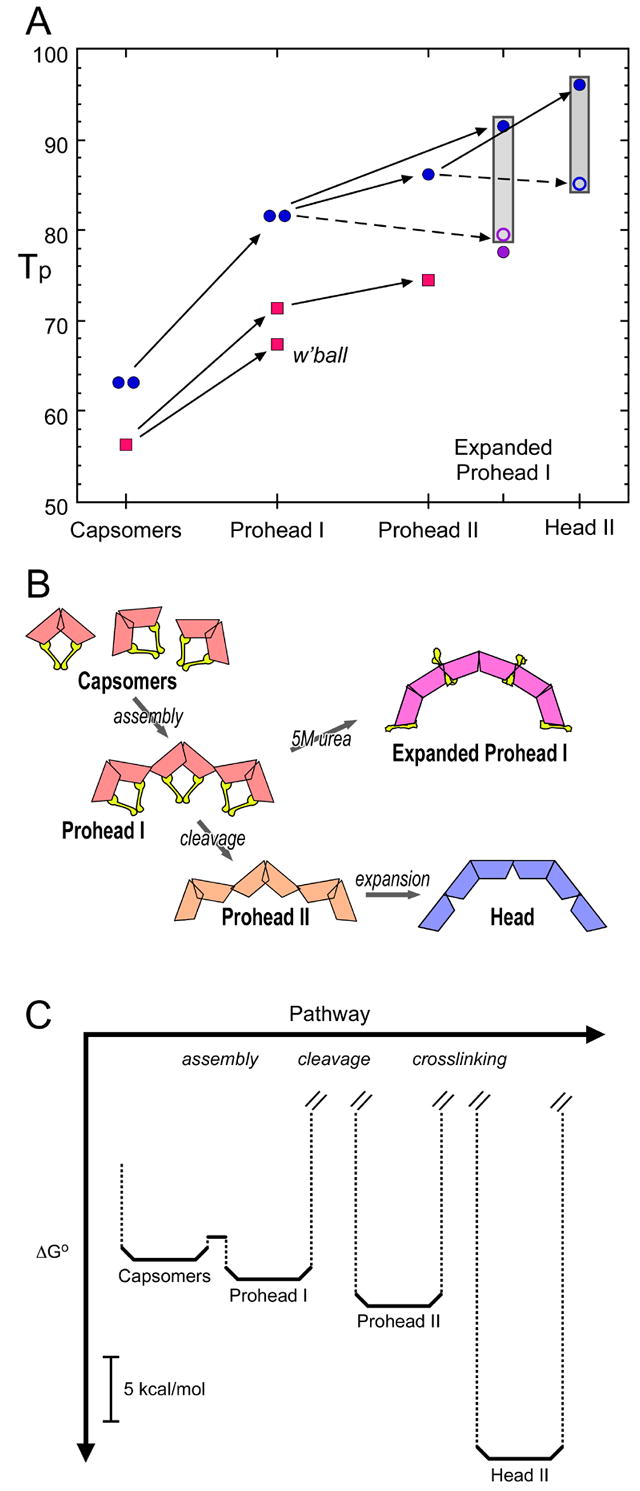
A – Summary of denaturation temperatures (Tp). Wild-type gp5 (blue), the E219K mutant (red), and the K169Y mutant (purple). The Tp's of wild-type capsomers (predominantly hexamers – left data point) and pentamers (right data point) are the same, 63°. Wild-type capsomers reassemble in vitro into Prohead I (right data point) which is calorimetrically indistinguishable from native Prohead I (left data point). Cleavage of Prohead I produces Prohead II, the precursor capsid primed for expansion. Expansion per se results in slightly reduced thermal stability but crosslinking confers progressively greater stability (gray columns), +12° with complete crosslinking. A similar stabilization is effected with both cleaved and uncleaved gp5. The open ring at the bottom of each gray column refers to a hypothetical particle of wild-type gp5/gp5* that is expanded but has no crosslinking. At each stage of assembly from capsomers to Prohead II, the E219K mutant is ~ 10° less stable than wild-type. (B) Schematic representation of the HK97 capsid assembly pathway, showing the inferred position of the Δ-domains (yellow), if present; and using different colors to convey different configurations of the gp5 subunit cores in capsomers of assembled surface lattices. (C) Major steps in the free energy cascade that drives HK97 assembly. At 37°C, the ensemble of capsid protein undergoes a net reduction in free energy at each step, as indicated. The heights of the kinetic barriers between successive states are not known but the one between capsomers and Prohead I is presumably low as assembly procedes readily in vivo and in vitro. The kinetic barriers between Prohead I and Prohead II and between Prohead II and Head II are presumably high as the initial states in both cases exhibit a negligibly small rate of spontaneous conversion; however, these barriers are circumvented by proteolysis and crosslinking (or, in vivo, DNA packaging), respectively.
Cooperation among capsomers lends stability to Prohead I
Capsomers and pentamers denature in single endotherms (Fig. 2) whose asymmetry is characteristic of oligomeric dissociation processes 29: the best fits obtained for these curves were for hexamer and pentamer dissociation processes, respectively (data not shown). With wild-type gp5, we observed no significant difference between hexamers and pentamers (Fig. 2). Prohead I differs from unassembled capsomers in (i) a 20° increment in Tp; and (ii) the appearance of a minor endotherm at 54°. A similar assembly-related stabilization was observed with the enzyme, riboflavin synthase, for which, assembly of pentamers into paired pentamers was found to be accompanied by a 20° increase in Tp 30. In the case of gp5, the stabilization of capsomers assembling into Prohead I corresponds to a ΔΔG° of ~ 1.5 kcal/mol (of monomer) at 37°C. This appears to represent a packing effect whereby isolated capsomers may have a tendency to undergo local unfolding around their peripheries that then leads to complete conformational breakdown; in procapsids, this tendency is suppressed by inter-capsomer interactions. We call this effect cooperation to distinguish it from cooperativity.
The assembly-related stabilization of gp5 capsomers does not reflect enhanced cooperativity because the width of the endotherm increases (Fig. 2). If the transitions are interpreted as simple two-state processes (folded/unfolded), the observed broadening would indicate a decrease in cooperativity. However, thermal denaturation of protein assemblies involves the disruption of multiple interactions – within subunits, between subunits, and between capsomers. If these interactions are similarly but not equally resistant to thermal stress, they should be disrupted in a single composite endotherm. Thus, the broadening of the denaturation endotherm when capsomers assemble into Prohead I reflects a widening of the distribution of Tp's of the various subprocesses. Similarly, we attribute the threefold difference in ΔTFWHH between wild-type and E219K Prohead I to the respective endotherms being composed of sets of more (E219K) or less (wild-type) coincident sub-endotherms.
Role of Δ-domain cleavage in facilitating expansion
As Prohead II matures to Head II, the gp5* subunit cores undergo rigid-body rotations and the N-arm and E-loop are refolded (Introduction). Do comparable structural changes take place earlier in the pathway, and, in particular, how are the Δ-domain and the N-arm affected? In Prohead I, the Δ-domain moiety adjoining the N-arm lines the inner rim of a capsomer 10, and the N-arm itself is presumably folded and in this vicinity, i.e. on the inner surface. As Prohead I expands and the subunit cores rotate, the Δ-domains must also be drawn into this region where they impede the formation of inter-capsomer contacts (Fig. 5B). Thus we attribute the reduced stability of the Expanded Prohead I conformation vis-à-vis the Head conformation to this effect. To make such a comparison without the complication of crosslinking, we refer to the K169Y mutant: the Tp of Expanded Prohead I (K169Y) is 77° vs. 83° for Head I, the fully expanded (and cleaved) K169Y capsid. Thus, the incomplete expansion (Fig. 4B) and crosslinking (Fig. 3B) of Expanded Prohead I (wild-type) probably have similar origins. It follows that one function of cleavage in the HK97 system is to remove this “chock” - the Δ-domain - so that expansion and crosslinking may go to completion.
The major capsid protein of T4 also has a sizable Δ-domain, 65 residues 26. In the T4 system, expansion has been studied on tubular capsid analogs called polyheads. Uncleaved polyheads may be induced to expand by the relatively mild treatment of exposure to 0.2M guanidine-HCl, giving rise to a variety of partially expanded structures 31. Here, also, incomplete expansion was attributed to the trapping of Δ-domains at inter-subunit interfaces. Morover, it has been shown by immuno-gold electron microscopy that the T4 Δ-domains, if still present, are translocated from the inner to the outer surface in expansion 32, as we infer to be the case for HK97 (Figs. 4B & 5B).
In Expanded Prohead I, the gondolas disappear (cf. Figs. 4A & 4B) and it appears likely that the corresponding portions of the Δ-domains – putatively 10, residues 1 to ~ 85 - are dissociated and, perhaps, unfolded. To investigate the Δ-domain alone, we expressed and purified it, and subjected to calorimetry, whereupon we observed a very broad endotherm between 20°C and 50°C (data not shown). Its low temperature and the absence of such an event with Prohead I or capsomers suggest that isolated Δ-domains are not fully folded and these domains only assume their proper conformation and oligomeric state(s) in the context of folded and assembled capsomers.
Role of the Δ-domain in promoting assembly and stabilizing Prohead I
Structural information on isolated capsomers is limited to negatively stained projections (Fig. 2B) which indicate that the capsomers have essentially the same arrangements as in Prohead I, although the hexamer image is noisier, being based on fewer particles. The stain-excluding features at the centers of both capsomers presumably represent Δ-domain gondolas. We posit that the propensity of Δ-domains to self-associate in clusters of five and six (a double trimer – see Fig. 4A) helps to guide the gp5 subunits in forming assembly-competent capsomers; moreover, as long as these interactions between Δ-domains are maintained, they restrain the gp5* moieties from switching to their Prohead II state. When the Δ-domains are proteolyzed, this constraint is eliminated and the contiguous shell undergoes a subtle structural change that results in a ΔTp of + 4.5° when Prohead I is converted to Prohead II and a stabilization of ΔΔG° ~ −2.5 kcal/mol. Presumably, exposing Prohead I to 5M urea has a similar effect, disrupting the Δdomain interactions and driving the capsid as far along the expansion pathway as the still-attached Δ-domains allow. In the P22 and HSV systems, which have separate scaffolding proteins, a similar restraining effect has been attributed to the scaffold–capsid protein interaction in the procapsid 33; 34.
Successive states of the N-arm during capsid maturation
Our working hypothesis for the structural basis of HK97 maturation envisages the following conformational history for the N-arm. In capsomers and Prohead I, it should have a definite conformation: otherwise, it is hard to see how the Δ-domain could influence aggregation of the gp5* core, if connected to it only by a disordered polypeptide. This putative conformation is probably context-specific as the N-arm is rather short (33 amino acids) to fold into a separate domain. In Prohead II, the N-arm is disordered (12 and I. Gertsman, L. Gan & J.E. Johnson, personal communication), and the switch undergone by the contiguous shell between the Prohead I and II states presumably involves unfolding of the N-arm (see also 35). In Head II, it again assumes a definite structure – in this case, an extended one that is stabilized only by interactions with other subunits 27. This arrangement is reminiscent of the C-terminal arm of the SV40 capsid protein invading neighboring capsomers 36, and represents a manifestation of the principle of domain-swapping 37 in higher-order structures.
Destabilizing effects of the E219K mutation
How does this amino acid substitution reduce the stability of procapsids? Essentially the same value for ΔΔG° between mutant and wild-type (5.5 kcal/mol) was obtained for Prohead I and Prohead II. Similarly, E219K capsomers are less stable than wild-type by 3.7 kcal/mol. Since the denaturational enthalpy ΔHm is essentially the same for wild-type and mutant Proheads (Table 1), it follows that the observed destabilization of the mutant capsids is mainly if not wholly entropic.
The Tp of whiffleballs is 4° lower than that of Prohead I (E219K). That this change reflects their lack of pentamers and not a different packing of hexamers after reassembly is implied by the fact that native and reassembled Prohead I (wild-type) have identical thermal denaturation profiles (Fig. 2A). Moreover, the hexameric frameworks of whiffleballs and Prohead I are superimposable 18. We attribute the observation that the same hexamers in whiffleballs melt 4° earlier than in Prohead I to the absence of inter-capsomer "cooperation" around the whiffleball vertices.
Assembly-competence and capsomer stability
Is it possible to rationalize the effects of the E219K substitution on assembly and stability in terms of this residue's location in the capsid? Since the gp5* subunit structure from Head II fits snugly into the cryo-EM density map of Prohead II 12, and the contiguous shell of Prohead I closely resembles that of Prohead II, we take the latter model as the best current guide for interpreting the effects of the mutation. The E219 side-chain is buried inside the subunit core, between the major α-helix and the capsid-interior surface (Fig. 6A). There, it is saltbridge-linked to R135 and close enough to E364 to form a hydrogen bond, and thus appears to be part of a network of charged residues (Fig. 6B). Changing E219 to K should induce re-arrangements in side-chain packing arising from the extra bulk of lysine and the charge reversal at this position. In general terms, therefore, the decreased stability of E219K Proheads and capsomers appear to arise from an inability to achieve a comparably stable packing of side-chains in the subunit interior. The inferred remodeling of E219's electrostatic network may be an important factor in the observed decrease in stability, as electrostatic networks - and salt bridges in particular - appear to contribute to the increased stability of proteins from thermophiles as compared to their homologs from ambient-temperature organisms 38.
In Prohead I capsomers, residue E219 is far from subunit interfaces (Fig. 6C & D); however, mutation-induced side-chain rearrangements around E219 may propagate outward to affect more distant residues and may thus be responsible for the altered reassembly properties of the mutant. For example, the two close E219 neighbors, E364 and L366, are on β-sheet J in the P-domain on the peripheries of both hexamers and pentamers. On the opposite side of this strand, residues E363 and R365 appear to contact neighboring capsomers (Fig. 6C & D). We speculate that rearrangements of the peripheral residues E363 and R365 in E219K pentamers alter their properties sufficiently to account for the absence of pentamers in the dissociation products of Prohead I and hence, the vacant vertices of whiffleballs. Such rearrangements could also explain why E219K Prohead I and whiffleballs have an altered electrophoretic mobility, relative to wild-type Prohead I and why whiffleballs shift towards normal electrophoretic mobility when wild-type capsomers are added 18.
Thermodynamic forces that drive capsid assembly
To estimate the free energy differences between states of assembly at the physiological temperature of 37°C, which is far from the observed denaturation temperatures, the full form of the Gibbs-Helmholtz equation must be used (Methods). The resulting free energy curves indicate that wild-type capsids have maximum stability in the physiological temperature range (Supp. Figure 1). At 37°C, the difference in free energy between gp5 capsomers and Prohead I, amounting to about 2 kcal/mol (of monomers) - Fig. 5C - is quite small in view of the large difference in Tp (Table 1). However, although subject to substantial experimental uncertainty (Supp. Table 1), it is similar to the value obtained by other, quite different methods, for the change in free energy when dimers of the hepatitis B virus capsid protein assemble into capsids 39; 40. There is a similar decrement, i.e. ~ 2 kcal/mol, between Prohead I and Prohead II. On the other hand, a massive stabilization of ~ 13 kcal/mol accompanies the transition of Prohead II to Head II (Fig. 5C). This large change is attributable to the network of crosslinks, as there is little change in free energy associated with HK97 capsid expansion in the absence of crosslinking (16 and this study).
Materials & Methods
Preparation of Proheads
Proheads were made using plasmids which express HK97 gene 5 (or genes 4 and 5) under the control of the T7 φ10 promoter, and purified by a combination of differential centrifugation, polyethlyene glycol precipitation and velocity sedimentation in glycerol gradients, as described previously 18; 41. Plasmid pV0 (pT7-5Hd2.9 41) was used to produce Prohead II. Preparations of Prohead I were made using protease-knockout plasmids pVB (pT7-5Hd2.9(fsBstB1) 41) or pVK 18. Derivative plasmid pVK-E219K was used to make E219K Prohead I and plasmid pV0-E219K was used to make E219K Prohead II (both contain the mutation E219K: codon 219 changed from gaa (Glu) to aaa (Lys) 18. E. coli strain BL21(DE3)pLysS was used as host. Cells were induced for ~ 16 hrs at 28°C and used immediately.
Prohead I purification steps prior to chromatography were done using TKG buffer (20 mM Tris HCl, pH 7.5, 100 mM potassium glutamate). Proheads were further purified by adsorption to Poros HQ20 quaternary amine ion exchange columns (ABI, Foster City CA) in 20 mM tris(hydroxymethyl)amino-methane hydrochloride (Tris HCl)- bis-tris-propane buffer (Sigma) at pH 7.5 with 20 mM NaCl and elution with a linear gradient of NaCl to 0.5 M at 13-33 ml/min using a BioCAD chromatography system (ABI, Foster City CA). Prohead samples were concentrated by ultracentrifugation, resuspended in a small volume, and dialyzed into a buffer appropriate for the next step.
The E219K Prohead II-producing strain actually yields a mixture of Prohead I and Prohead II. Accordingly, E219K Prohead II was purified by dissociating the unwanted Prohead I using the glucose method described below. After Prohead I dissociation, the E219K Prohead II was separated from bulk capsomers by pelleting via ultracentrifugation and then repurified using Poros HQ20 anion exchange chromatography as described above.
Expansion of Prohead I
Prohead I was induced to expand by a 2 hr treatment treated with 5 M urea in 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES) - KOH buffer at pH 5.5. After two hrs, the buffer was exchanged to TAMg (40 mM Tris base, 20 mM acetic acid pH 8.1, and 1 mM magnesium sulfate) using centrifugal gel filtration in small spin columns. 0.2 ml samples were loaded onto 2.2 ml columns of washed, pre-equilibrated and pre-centrifuged Sephadex G25 (coarse) (Pharmacia), and centrifuged for 2 min at 1000 rpm in the swinging bucket rotor of a large clinical centrifuge (International PR-J).
Dissociation of Prohead I into capsomers
Wild-type capsomers were made by dissociating Prohead I by treatment at room temperature with 2 M KCl in 20 mM Tris HCl pH 9.5 for ~16 hrs, followed by dialysis into 5 mM Tris HCl pH 7.5. E219K Prohead I was dissociated by treatment with 40% glucose in 20 mM Tris HCl pH 9.5 for ~16 hrs. The extent of dissociation was checked using agarose gel electrophoresis. Before use, capsomer preparations were dialyzed into our standard calorimetry buffer (below).
Purification of capsomers and re-assembly into Whiffleballs
Capsomers produced by dissociation of E219K Prohead I were dialyzed to remove glucose and purified using Poros HQ20 anion exchange chromatography as described above. Capsomers (which elute before proheads) were assembled into whiffleballs by concentrating the protein about 100-fold and transferring to PBS buffer as described previously 18. Purified E219K hexamers were precipitated with 70% (w/v) (NH4)2SO4 at 4°C, resuspended at ~ 35 mg/ml and dialyzed against PBS, containing 80 g NaCl, 2 g KCl, 11.5 g Na2HPO4 • 7H2O, 2 g KH2PO4 per liter.
Assessment of purity
In a final step, all capsid and capsomer samples were dialyzed into calorimetry buffer. Purity and homogeneity were assessed by SDS-PAGE and agarose gel electrophoresis. Many samples were also checked by electron microscopy. Native agarose gels were run in DNA mini-gel equipment using TAMg buffer and stained for protein after drying 41. SDS-polyacrylamide gel electrophoresis methods were modified from 42 using a low-crosslinker stock (33.5% acrylamide plus 0.3% methylene bis acrylamide); all samples were TCA-precipitated prior to heating in SDS 41.
Differential Scanning Calorimetry
We analyzed various assemblies of uncleaved gp5 under standard conditions, i.e. in our "calorimetry buffer" (17.2 mM K2HPO4, 2.8 mM KH2PO4, 0.1 M KCl, pH 7.5 at a protein concentration of ~ 1 mg/ml, and a heating rate of 120 deg/hr. Protein concentrations were determined and calorimetry was performed as described previously 16, using a Micro-Cal (Springfield, MA) MC-2 calorimeter equipped with 0.6 ml cells and operated at a scan rate of 1.98 K/min. Scans of buffer vs. buffer were subtracted to minimize systematic differences between the cells. The Tps observed have some dependence on scan rate. In this context, we found the dependencies of capsomers, Prohead I and Prohead II, both wild-type and mutant, to be very similar (data not shown). Accordingly, the ΔTp values that we recorded are a very close approximation to the values that would be obtained at zero scan rate where kinetic effects would be absent.
Determination of Changes in Free Energy
ΔG °, the difference in standard free energy of the folded and unfolded forms of a capsid, may be calculated for any temperature, T, from the thermodynamic parameters of denaturation evaluated by DSC (i.e. the denaturational enthalpy, entropy and heat capacity - ΔHm, ΔSm, and ΔCp), with the integrated form of the Gibbs-Helmholtz equation
| (1) |
Tm is the median temperature of the denaturation endotherm which, except for highly asymmetric transitions, is very close to the peak temperature, Tp. Accordingly, we substituted the determined values of Tp for Tm in the calculations. The values used for ΔCp and the resulting values of ΔH, ΔS at 37°C are given in Supplementary Table 1. For the difference in the free energy of unfolding between a reference state (state A, e.g. a wild-type (wt) capsid) and an altered state (state B, e.g. a later conformation or a mutant) evaluated at TA, the Tp of state A, is denoted by ΔΔG °(TA; AB). For our data, the ΔCp term of Eqn. (1) does not make a significant contribution for temperatures within ~ 15° of TA. Accordingly, in this region, ΔΔG° may be evaluated using the following approximation 43,
| (2) |
It may be seen from Eqn. (2) that because ΔSm, B is a constant, ΔΔG° scales with ΔTp, the difference in Tp. In this approximation, the thermal stability of a particular capsid, given by its Tp, is also indicative of its thermodynamic stability, providing the rationale for Fig. 5A.
Cryo-EM and Image Reconstruction
Expanded Prohead I was imaged at 50,000x magnification on a CM200-FEG electron microscope (FEI, Mahwah, NJ) equipped with a Gatan 626 cryo-holder 44. 4 focal pairs, selected on the basis of their optical diffraction patterns, were digitized on a SCAI scanner (Z/I Imaging, Huntsville, AL) at a step size of 7 μm, corresponding to 1.4 Å pixels. Image reconstruction, including contrast transfer function (CTF) correction, was performed as described 45. The micrographs yielded 1724 particles (focal pairs), of which 1156 were included in the final map. Its resolution, assessed in terms of the Fourier Shell Correlation coefficient 46 with a threshold of 0.3, was 11.1 Å. Analyses were performed on Macintosh G5 computers (Apple Computer, Cupertino CA), and panels in Figure 4 were prepared on Linux workstations (Dell, Austin TX) with Amira 3.1 software (Mercury Computer Systems/3D Viz group, San Diego CA and Merignac, France).
Negative staining of pentamers
A sample was negatively stained with uranyl acetate and imaged on a CM200-FEG electron microscope at 120 keV and 66,000x magnification. Micrographs were digitized as described above. From these fields, round-appearing particles were extracted as representing axial views. These were sorted, initially by visual criteria, into pentamers (N = 190) or hexamers (N=35) The proportion of pentamers, ~ 85%, was subsequently confirmed by a quantitative, iterative, correlation-based classification procedure. In each cycle, all the data were compared against the current average pentamer and hexamer images and re-assigned according to which gave the higher correlation coefficient.
Supplementary Material
Acknowledgments
We thank Drs G. Piszczek, E. Fodor, and A. Ginsburg for some DSC scans, Drs L. Liljas and R. Nussinov for helpful discussions, and Drs K. Lee and U. Baxa and Ms C. Moyer for comments on the manuscript. This work was supported in part by the Intramural Research Programs of NIAMS and NIDDK and by NIH grant R01 GM47795 to RWH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bancroft JB. The self-assembly of spherical plant viruses. Adv Virus Res. 1970;16:99–134. doi: 10.1016/s0065-3527(08)60022-6. [DOI] [PubMed] [Google Scholar]
- 2.Benson SD, Bamford JK, Bamford DH, Burnett RM. Does common architecture reveal a viral lineage spanning all three domains of life? Mol Cell. 2004;16:673–85. doi: 10.1016/j.molcel.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Steven AC, Spear PG. Herpesvirus capsid assembly and envelopment. In: Chiu W, Burnett RM, Garcea RL, editors. Structural Biology of Viruses. Oxford Univ. Press; New York: 1997. pp. 312–351. [Google Scholar]
- 4.Baker ML, Jiang W, Rixon FJ, Chiu W. Common ancestry of herpesviruses and tailed DNA bacteriophages. J Virol. 2005;79:14967–70. doi: 10.1128/JVI.79.23.14967-14970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duda RL, Hendrix RW, Huang WM, Conway JF. Shared architecture of bacteriophage SPO1 and herpesvirus capsids. Curr Biol. 2006;16:R11–3. doi: 10.1016/j.cub.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 6.King J, Chiu W. The procapsid-to-capsid transition in double-stranded DNA bacteriophages. In: Chiu W, Burnett RM, Garcea R, editors. Structural Biology of Viruses. Oxford University Press; New York: 1997. pp. 288–311. [Google Scholar]
- 7.Steven AC, Heymann JB, Cheng N, Trus BL, Conway JF. Virus maturation: dynamics and mechanism of a stabilizing structural transition that leads to infectivity. Curr Opin Struct Biol. 2005;15:227–36. doi: 10.1016/j.sbi.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendrix RW, Duda RL. Bacteriophage HK97 head assembly: a protein ballet. Adv Virus Res. 1998;50:235–88. doi: 10.1016/s0065-3527(08)60810-6. [DOI] [PubMed] [Google Scholar]
- 9.Duda RL, Hempel J, Michel H, Shabanowitz J, Hunt D, Hendrix RW. Structural transitions during bacteriophage HK97 head assembly. J Mol Biol. 1995;247:618–635. doi: 10.1006/jmbi.1995.0168. [DOI] [PubMed] [Google Scholar]
- 10.Conway JF, Duda RL, Cheng N, Hendrix RW, Steven AC. Proteolytic and conformational control of virus capsid maturation: the bacteriophage HK97 system. J Mol Biol. 1995;253:86–99. doi: 10.1006/jmbi.1995.0538. [DOI] [PubMed] [Google Scholar]
- 11.Lata R, Conway JF, Cheng N, Duda RL, Hendrix RW, Wikoff WR, Johnson JE, Tsuruta H, Steven AC. Maturation dynamics of a viral capsid: visualization of transitional intermediate states. Cell. 2000;100:253–63. doi: 10.1016/s0092-8674(00)81563-9. [DOI] [PubMed] [Google Scholar]
- 12.Conway JF, Wikoff WR, Cheng N, Duda RL, Hendrix RW, Johnson JE, Steven AC. Virus maturation involving large subunit rotations and local refolding. Science. 2001;292:744–8. doi: 10.1126/science.1058069. [DOI] [PubMed] [Google Scholar]
- 13.Wikoff WR, Conway JF, Tang J, Lee KK, Gan L, Cheng N, Duda RL, Hendrix RW, Steven AC, Johnson JE. Time-resolved molecular dynamics of bacteriophage HK97 capsid maturation interpreted by electron cryo-microscopy and X-ray crystallography. J Struct Biol. 2006;153:300–6. doi: 10.1016/j.jsb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Duda RL. Protein chainmail: catenated protein in viral capsids. Cell. 1998;94:55–60. doi: 10.1016/s0092-8674(00)81221-0. [DOI] [PubMed] [Google Scholar]
- 15.Gan L, Conway JF, Firek BA, Cheng N, Hendrix RW, Steven AC, Johnson JE, Duda RL. Control of crosslinking by quaternary structure changes during bacteriophage HK97 maturation. Mol Cell. 2004;14:559–69. doi: 10.1016/j.molcel.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Ross PD, Cheng N, Conway JF, Firek BA, Hendrix RW, Duda RL, Steven AC. Crosslinking renders bacteriophage HK97 capsid maturation irreversible and effects an essential stabilization. EMBO J. 2005;24:1352–1363. doi: 10.1038/sj.emboj.7600613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makhatadze GI, Privalov PL. Energetics of protein structure. Adv Protein Chem. 1995;47:307–425. doi: 10.1016/s0065-3233(08)60548-3. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Conway JF, Cheng N, Steven AC, Hendrix RW, Duda RL. Control of virus assembly: HK97 "Whiffleball" mutant capsids without pentons. J Mol Biol. 2005;348:167–82. doi: 10.1016/j.jmb.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 19.Ross PD, Black LW, Bisher ME, Steven AC. Assembly-dependent conformational changes in a viral capsid protein. Calorimetric comparison of successive conformational states of the gp23 surface lattice of bacteriophage T4. J Mol Biol. 1985;183:353–364. doi: 10.1016/0022-2836(85)90006-3. [DOI] [PubMed] [Google Scholar]
- 20.Steven AC, Greenstone HL, Booy FP, Black LW, Ross PD. Conformational changes of a viral capsid protein Thermodynamic rationale for proteolytic regulation of bacteriophage T4 capsid expansion, cooperativity, and super-stabilization by soc binding. J Mol Biol. 1992;228:870–884. doi: 10.1016/0022-2836(92)90871-g. [DOI] [PubMed] [Google Scholar]
- 21.Galisteo ML, King J. Conformational transformations in the protein lattice of phage P22 procapsids. Biophys J. 1993;65:227–35. doi: 10.1016/S0006-3495(93)81073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galisteo ML, Gordon CL, King J. Stability of wild-type and temperature-sensitive protein subunits of the phage P22 capsid. J Biol Chem. 1995;270:16595–16601. doi: 10.1074/jbc.270.28.16595. [DOI] [PubMed] [Google Scholar]
- 23.Wikoff WR, Liljas L, Duda RL, Tsuruta H, Hendrix RW, Johnson JE. Topologically linked rings of covalently joined protein subunits form the dsDNS bacteriophage HK97 capsid. Science. 2000;289:2129–2133. doi: 10.1126/science.289.5487.2129. [DOI] [PubMed] [Google Scholar]
- 24.Fokine A, Leiman PG, Shneider MM, Ahvazi B, Boeshans KM, Steven AC, Black LW, Mesyanzhinov VV, Rossmann MG. Structural and functional similarities between the capsid proteins of bacteriophages T4 and HK97 point to a common ancestry. Proc Natl Acad Sci USA. 2005;102:7163–8. doi: 10.1073/pnas.0502164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Li Z, Zhang Z, Baker ML, Prevelige PE, Jr, Chiu W. Coat protein fold and maturation transition of bacteriophage P22 seen at subnanometer resolutions. Nat Struct Biol. 2003;10:131–5. doi: 10.1038/nsb891. [DOI] [PubMed] [Google Scholar]
- 26.Black LW, Showe MK, Steven AC. Morphogenesis of the T4 head. In: Karam J, editor. Molecular Biology of Bacteriophage T4. Am Soc Microbiol; Washington,D.C.: 1994. pp. 218–258. [Google Scholar]
- 27.Helgstrand C, Wikoff WR, Duda RL, Hendrix RW, Johnson JE, Liljas L. The refined structure of a protein catenane: the HK97 bacteriophage capsid at 3.44 A resolution. J Mol Biol. 2003;334:885–99. doi: 10.1016/j.jmb.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Xie Z, Hendrix RW. Assembly in vitro of bacteriophage HK97 proheads. J Mol Biol. 1995;253:74–85. doi: 10.1006/jmbi.1995.0537. [DOI] [PubMed] [Google Scholar]
- 29.Sturtevant JM. Biochemical applications of differential scanning calorimetry. AnnRevPhysChem. 1987;38:463–488. [Google Scholar]
- 30.Zylberman V, Craig PO, Klinke S, Braden BC, Cauerhff A, Goldbaum FA. High order quaternary arrangement confers increased structural stability to Brucella sp lumazine synthase. J Biol Chem. 2004;279:8093–101. doi: 10.1074/jbc.M312035200. [DOI] [PubMed] [Google Scholar]
- 31.Kocsis E, Greenstone HL, Locke EG, Kessel M, Steven AC. Multiple conformational states of the bacteriophage T4 capsid surface lattice induced when expansion occurs without prior cleavage. J Struct Biol. 1997;118:73–82. doi: 10.1006/jsbi.1996.3833. [DOI] [PubMed] [Google Scholar]
- 32.Steven AC, Bauer AC, Bisher ME, Robey FA, Black LW. The maturation-dependent conformational change of phage T4 capsid involves the translocation of specific epitopes between the inner and the outer capsid surfaces. J Struct Biol. 1991;106:221–236. doi: 10.1016/1047-8477(91)90072-5. [DOI] [PubMed] [Google Scholar]
- 33.Thuman-Commike PA, Greene B, Jakana J, McGough A, Prevelige PE, Chiu W. Identification of additional coat-scaffolding interactions in a bacteriophage P22 mutant defective in maturation. J Virol. 2000;74:3871–3. doi: 10.1128/jvi.74.8.3871-3873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymann JB, Cheng N, Newcomb WW, Trus BL, Brown JC, Steven AC. Dynamics of herpes simplex virus capsid maturation visualized by time-lapse cryo-electron microscopy. Nat Struct Biol. 2003;10:334–41. doi: 10.1038/nsb922. [DOI] [PubMed] [Google Scholar]
- 35.Lee KK, Gan L, Tsuruta H, Hendrix RW, Duda RL, Johnson JE. Evidence that a local refolding event triggers maturation of HK97 bacteriophage capsid. J Mol Biol. 2004;340:419–33. doi: 10.1016/j.jmb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Liddington RC, Yan Y, Moulai J, Sahli R, Benjamin TL, Harrison SC. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991;354:278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 37.Bennett MJ, Schlunegger MP, Eisenberg D. 3D domain swapping: a mechanism for oligomer assembly. Protein Sci. 1995;4:2455–68. doi: 10.1002/pro.5560041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Nussinov R. How do thermophilic proteins deal with heat? Cell Mol Life Sci. 2001;58:1216–33. doi: 10.1007/PL00000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceres P, Zlotnick A. Weak protein-protein interactions are sufficient to drive assembly of hepatitis B virus capsids. Biochemistry. 2002;41:11525–31. doi: 10.1021/bi0261645. [DOI] [PubMed] [Google Scholar]
- 40.Zlotnick A. Theoretical aspects of virus capsid assembly. J Mol Recognit. 2005;18:479–90. doi: 10.1002/jmr.754. [DOI] [PubMed] [Google Scholar]
- 41.Duda RL, Martincic K, Hendrix RW. Genetic basis of bacteriophage HK97 prohead assembly. J Mol Biol. 1995;247:636–47. doi: 10.1006/jmbi.1994.0169. [DOI] [PubMed] [Google Scholar]
- 42.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 43.Becktel WJ, Schellman JA. Protein stability curves. Biopolymers. 1987;26:1859–1891. doi: 10.1002/bip.360261104. [DOI] [PubMed] [Google Scholar]
- 44.Cheng N, Conway JF, Watts NR, Hainfeld JF, Joshi V, Powell RD, Stahl SJ, Wingfield PE, Steven AC. Tetrairidium, a four-atom cluster, is readily visible as a density label in three-dimensional cryo-EM maps of proteins at 10–25 A resolution. J Struct Biol. 1999;127:169–176. doi: 10.1006/jsbi.1999.4120. [DOI] [PubMed] [Google Scholar]
- 45.Conway JF, Steven AC. Methods for reconstructing density maps of "single particles" from cryoelectron micrographs to subnanometer resolution. J Struct Biol. 1999;128:106–118. doi: 10.1006/jsbi.1999.4168. [DOI] [PubMed] [Google Scholar]
- 46.Saxton WO, Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982;127 (Pt 2):127–38. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


