Introduction
Every year, traumatic brain injury (TBI) affects over 1.5 million people, claims over 50,000 lives, and imposes costs that exceed 50 billion dollars in the United States alone. Despite the burden of TBI, few treatment options exist, in part because of limited clinically relevant animal models.
Models to study TBI have been developed in several different animal species. Controlled cortical impact (CCI), as a model for focal contusions in the ferret, was first described by Lighthall in 1988, (Lighthall, 1988). CCI was subsequently adapted for other species, including the rat (Dixon et al., 1991) and the mouse (Smith et al., 1995; Fox et al., 1998; Hannay et al., 1999). In the last ten years, mouse models have gained popularity, primarily due to their economy and the ability to use various knockout and targeted overexpression strategies to isolate the role of specific genes and their products in TBI damage and repair mechanisms. Mouse CCI models have been demonstrated to produce contusions that are histopathologically similar to contusion injuries in human TBI patients (Cernak, 2005; Morales et al., 2005). While originally thought to produce focal lesions only, emerging evidence suggests that damage from mouse CCI can extend well beyond the site of the contused tissue (Hall et al., 2005).
Most mouse CCI research has used a “parasagittal cortex” injury location (Smith et al., 1995; Fox et al., 1998; Hannay et al., 1999). This location has been shown to yield pathology in brain areas at, around, and below the strike location, producing deficits in cognitive function, and in some cases, deficits in sensorimotor and visual function. There are two significant challenges with this location: difficulty in discerning primary from secondary damage in the hippocampus, and the scarcity of simple, reliable tests for cognitive performance that are not confounded by sensorimotor or visual impairments. We propose the use of a “sensorimotor cortex” injury location that offers an increased possibility of resolving primary from secondary damage by sparing most of the hippocampal and thalamic structures from direct damage from the initial strike. Furthermore, the sensorimotor injury lends itself to the use of a simple group of behavioral tests to assess the functional consequences of the cortical damage.
Many mouse CCI experiments have used a pneumatically actuated impactor, which has shown good reliability, although posing mechanical challenges in terms of the stroke overshoot, rebound control, and adjustment of contusion time. Electromechanical devices, especially those designed and implemented for high-throughput precision manufacturing uses, have recently been adapted for spinal cord injury models (Narayana et al., 2004; Bilgen, 2005). We now describe the use of an electromechanical injury apparatus adapted for use in mouse brain CCI. This device offers a real-time feedback control system to regulate stroke velocity, depth and time. We have characterized the injury using repeated magnetic resonance imaging (MRI) and behavioral testing as well as traditional histological techniques. The non-invasive nature of MRI provides an opportunity to assess the evolution of damage after CCI injury and provides physiological data concurrent with behavioral assessment.
Our results indicate that a consistent, reliable injury can be achieved with the electromechanical device applied to a sensorimotor cortex location, that lesion cavity volume measurements from MRI are well correlated with measurements made by traditional histological techniques, and that the impact produces lasting sensorimotor deficits.
With this model and characterization method, we seek to extend and add to the value of existing mouse CCI research, by using an electromechanical injury device, by applying the CCI injury to the sensorimotor cortex, by using longitudinal high field MRI, and by assessing sensorimotor impairment and recovery using a simple battery of three behavioral tests.
Materials and Methods
Animals
Adult male C57BL/6 mice (28–30g, 20–25 weeks old) were housed with a 12-hour light-dark cycle, with ad libitum access to food and water. All animal procedures were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. A total of 16 mice were used for this study. Eight animals received CCI injury and eight animals received a sham injury.
CCI Impactor
This device was assembled from commercially available components, as described previously (Narayana et al., 2004; Bilgen, 2005). Briefly, the equipment included a linear motor device (the impactor), power supply and microprocessor controller (Linmot, Zurich, Switzerland), a Plexiglas table, and stand for the linear motor device made with an adjustable manipulator (Kopf, Tujunga, CA) that allowed precise positioning of the impactor. A polished stainless steel tip, which strikes the dura during CCI, was fitted to the end of the impactor slider. The size and shape of the tip, the velocity of the strike, and the contact depth and time, could be varied to achieve contusions of different sizes and severities. In this study, we used a 3.0mm diameter flat face tip with a slightly rounded edge, a 1.5m/s strike velocity, a 1.0mm strike depth, and an 85ms contact time.
Surgical Procedures
Following anesthesia with isoflurane (induction: 2.5%, maintenance: 1.0%), animals were stabilized in a Cunningham stereotaxic frame (Stoelting, Wood Dale, IN), and placed on a heated pad, which maintained core body temperature at 37+/− 1 °C. The scalp and epicranial aponeurosis were retracted, and a 3.5mm diameter circular craniotomy was performed with a burr drill, lateral (right side) to the mid-sagittal suture, with the center at the following coordinates: AP = 0, ML = +2.0 from bregma. The burr and surface of the skull were cooled with periodic application of room temperature saline. Care was taken to avoid the blood vessels coursing along the superior sagittal sinus, and any bleeding from the skull was controlled with bone wax. Once the dural surface was exposed, the position of the impactor and tip was carefully adjusted to be centered within the craniotomy, and angled so the face of the impactor tip was tangential to the dural surface (see Figure 1). The impactor tip was slowly lowered in 0.05mm increments until the tip just contacted the dura (by visual inspection). The cortical impact was initiated through the device graphical user interface of the impactor control software. Firstly, there was a retraction of the tip of 20mm, and then a downward strike of 21mm (20mm retraction plus the 1.0mm programmed injury depth. Given that the injury center was 2.0mm lateral to bregma, the tip contact area included motor (M1, M2) and sensory (S1FL, S1HL) cortical areas. After the impact, the scalp was sutured closed, anesthesia was discontinued, and animal temperature was maintained at 37°C until recovery of locomotion. Sham animals (n=8) received the craniotomy but no impact from the CCI device.
Figure 1.

Drawing of the mouse CCI injury apparatus showing the impactor and part of its mounting system, the craniotomy location, and the orientation of the impactor tip to ensure perpendicularity to the exposed dura and to the surface of the brain.
MRI Scanning
Following induction of anesthesia with 2.5% isoflurane, the animals were positioned in a small plastic cradle attached to a Plexiglas sled. The breathing/anesthesia mask and surface coil were attached and then the sled introduced to the magnet. Animals were monitored for core body temperature and respiration rate throughout the MRI experiments with an MRI-compatible monitoring system (SA Instruments, Stony Brook, NY). Warm humidified air was circulated in the magnet bore to maintain animal temperature at 37 +/1°C, and anesthesia was adjusted, between 1.0 and 1.5% isoflurane, to maintain breathing at a minimum of 20 respirations per minute.
Animals were scanned with a 9.4T Varian INOVA horizontal MRI scanner (Varian Inc., Palo Alto, CA) using a 400mT/m gradient coil set and a 31cm room temperature bore. Given the small size of mouse brains, approximately 10mm x 16mm x 6mm, and our desire for high resolution, high contrast images, surface coils were used. In particular, we used an inductively coupled surface coil, similar to that described previously (Bilgen, 2004), and a simple rectangular detection loop. The inductively coupled coil provided increased signal-to-noise ration and a limited/focused field of view, enabling high spatial resolution.
Scout images were acquired with a gradient echo multislice (GEMS) sequence to ensure precise placement of the brain at the magnet isocenter. Spin-echo multislice (SEMS) images were then acquired, in each of the three planes (TR/TE = 2500/45ms for T2-weighting, data matrix size = 128x128, slice thickness = 1mm, fields of view = 16mm x 10mm (anatomically coronal slices), 20mm x 10mm (sagittal), and 16mm x 20mm (axial), 4 averages per acquisition). The total scan time, including set-up and positioning, was about 90 minutes per animal. For longitudinal data acquisition, we scanned each mouse at 5 time points: at 24 hours, 48 hours, 96 hours, 7 days, and 14 days post injury. The scans at time points 1, 2, 4, and 5 were done immediately following the behavioral assessments. Images were evaluated using the Varian VnmrJ software tools and also by importing the images into NIH ImageJ, version 1.34S.
Injury location measurements for each animal were made from the anatomically horizontal T2-weighted image corresponding to the brain tissue at 0.5 to 1.0mm below the cortical surface, obtained at 24 hours post injury. On this image, two lines were drawn, the first along the sagittal midline and the second, perpendicular to the sagittal midline, just touching the rostral extreme of the olfactory bulbs. A circle that best fit the injury zone, as denoted by image hyperintensity, was then placed on the image, and the center of this circle was considered the injury location. The perpendicular distances from the center of the circle to the two drawn lines were measured using the VnmrJ software tools and these two measurements were the injury location coordinates.
Lesion cavity volume measurements were made from the anatomically coronal T2-weighted images, obtained at 14 days post injury, using the image analysis tools of the MRI scanning system. The closed polygon selection tool was used to delineate the cavity on each slice, with the dorsal aspect of the cavity estimated from a mirror image of the contour of the uninjured hemisphere, and the area function provided the area measurement for each image. The product of area and slice thickness provided the cavity volume for each 1mm thick slice. Measurements were made on successive coronal slices where a cavity was apparent – typically three to five images – and then the volumes were summed to yield the total cavity volume.
Behavioral Tests
Rotarod
The Rotarod has been extensively used in mouse models of TBI, and its potential for high sensitivity to sensorimotor deficits has been demonstrated (Hamm et al., 1994). Rotarod training and measurement were performed using a four-lane Rotarod apparatus (Accuscan, Mentor, OH). Each day for five days prior to injury, animals were trained on the Rotarod at two different speeds (12 and 18 rpm) in the acceleration paradigm and at one speed (8 rpm) in the constant velocity paradigm. Mice were tested using three trials at each speed in each training or measurement session, with a minimum of 120 seconds of rest between trials. We have found this training sufficient to establish a reliable pre-injury baseline performance for each animal. Rotarod speeds were chosen to be sufficiently challenging during training to avoid ceiling effects, but to allow injured animals to complete the task. After surgery, we measured each animal’s performance at five time points: 24, 48, 72 hours, and 7 and 14 days. Post injury scores were normalized using pre-injury means to control for variability in pre-injury performance.
Gridwalk
The gridwalk apparatus was fabricated as described by Baskin (Baskin et al., 2003), using a 1.1cm wire grid of 20cm x 35cm. Animals were allowed to walk on this grid for five minutes, during which their total actual walking time was measured (in real time by stopwatch or afterwards by review of videotape), and the numbers of foot faults for each foot were counted. Foot faults were defined as an instance where the animal attempted to place weight on a foot, which then passed completely through the plane of the wire grid. We observed that after surgery, animals in both age groups tended to walk less, especially at the earlier post injury time points. Since the animals were free to move about the grid, or to remain still and engage in grooming or other stationary behaviors, foot fault data were normalized to actual walking time to account for differences in the degree of locomotion seen in different trials. This was achieved by dividing the total counted foot faults by the total time spent walking to obtain a measure of foot faults per minute of walking. Two measurements were taken pre-injury to establish each animal’s baseline performance, and to allow the animals to become familiar with the apparatus. Post injury measurements were taken beginning at 48 hours post injury and thereafter at the same time points as those for the Rotarod. Walking trials were repeated until at least 90 seconds of walking was observed for each animal at each time point.
Cylinder
The cylinder or spontaneous forelimb test (Schallert and Tillerson, 2000) modified for mouse (Baskin et al., 2003), involves the use of a 10cm diameter transparent cylinder. Each animal was placed in the cylinder, and its spontaneous activity to rear up on its hind limbs and explore the vertical surface with its forelimbs was observed. Animals used either both forelimbs or a single forelimb for an exploration. The number of both, right only, or left only explorations was counted in a five-minute recording interval. Two pre-injury measurements were taken to control for limb preference. The laterality score (Schallert et al., 2000) was computed as follows:
Post injury, measurements were taken at the same time points as for the gridwalk. Animals showed a tendency, in the measurements at 48 hours post injury, to explore the cylinder less frequently and to spend a larger proportion of time engaged in grooming activities. Our approach was to perform additional five minute trials until at least 20 rearing observations were made.
Histology
After the fourteen day post injury behavioral and imaging studies, animals were anesthetized and perfused transcardially with 50 ml of phosphate buffered saline, followed by 100 ml of 4% buffered formaldehyde, delivered via a 23-gauge needle connected to a perfusion pump. The brains were removed, post-fixed in 4% buffered formaldehyde for 12 hours, then transferred to 30% sucrose for cryoprotection before blocking in a coronal mouse brain matrix (Zymed, Pittsburgh, PA), and freezing in 15 x 15mm cryomolds. Frozen coronal sections were cut at 20μm thickness on a Leica CM1850 cryostat (Leica Microsystems, Bannockburn, IL) and stained with 1% thionin, cleared and coverslipped. Sections were visualized with a Nikon inverted-stage microscope at 20x magnification and digital images were captured with a SPOT microscope camera (Diagnostic Instruments, Sterling Heights, MI)
From these histological images, we measured frank tissue loss, i.e. the size of the cavity, with ImageJ software. On each image, the cavity was outlined with the polygon selection tool by visual inspection, with the dorsal aspect of the lesion estimated from the mirror image of the contour of the uninjured hemisphere. A pixel count was obtained with the histogram function in ImageJ. For each animal, the cavity area was measured in 5 to 7 sections, spaced approximately 0.5mm apart, and the total cavity volume was calculated using the formula A1 (0.5X1) + A2(0.5X1+0.5X2)+ An−1(0.5Xn−1+0.5Xn) +An(0.5Xn) where An is the area of the cavity for section (n) and Xn is the distance between sections (n) and (n−1) (Dash et al., 2004).
Statistical Analysis
For the analysis of behavioral data and comparisons between the injured and sham groups, we used the Wilcoxon Signed Rank test, a non-parametric test appropriate for small sized groups where the data distributions are not assumed to be Gaussian. For the comparison of MRI-obtained and histology-obtained lesion cavity volume data, we calculated the Spearman correlation coefficient. All statistical analyses were performed using GraphPad Instat software (GraphPad, San Diego, CA).
Results
MRI: Injury Presentation
Figure 2 shows example T2-weighted spin-echo images from an injured animal at 14 days in the coronal, sagittal and axial planes. These show a strong signal hyperintensity, corresponding to the fluid-filled cavity, where cortical and subcortical tissue has been lost. They also show a well-defined border between the hyperintense and normal-appearing areas, suggesting that by this time the cavity size and shape is stable. The lateral ventricle on the ispilateral side is expanded. There is loss of white matter in the corpus callosum, the ipsilateral cingulum, and the medial portion of the external capsule. The longitudinal cerebral fissure was deflected, towards the injured side, as seen in the anatomically axial images.
Figure 2.

T2-weighted MR images of an injured animal, in the anatomically coronal (top), sagittal (middle) and horizontal (bottom) planes, scanned 14 days after injury. At this time point, the strong signal hyperintensity indicates that a lesion cavity has formed. Arrows denote the cavity. Contiguous slices, TR=2500ms, TE=45ms. Images segmented to show brain only.
Evolution of Injury with Time
Figure 3 shows a time series of 1mm thick anatomically coronal T2-weighted spin-echo images from the same animal, showing the evolution of the injury and the development of the cavity over time. At the first scanning time point – 24 hours – there is typically signal hyperintensity at and around the injury site – and we interpret this as acute edema. By 48 hours, this hyperintensity is diminished, suggesting that the acute edema is resolving. At 7 days, there is typically an area of signal hyperintensity at the center of the injury location, surrounded by a “ring” or “zone” of signal hypointensity. We interpret the central hyperintensity as an area where tissue has been lost and replaced by fluid, and we consider the “ring” as dying tissue (necrotic or apoptotic). At 14 days, there is a continued central hyperintensity and as well as marked hyperintensity in the “ring” region, suggestive of completed tissue loss and replacement by fluid. Also, at 14 days, the border between the cavity and the surrounding normal appearing tissue has become much more distinct than at earlier time points.
Figure 3.
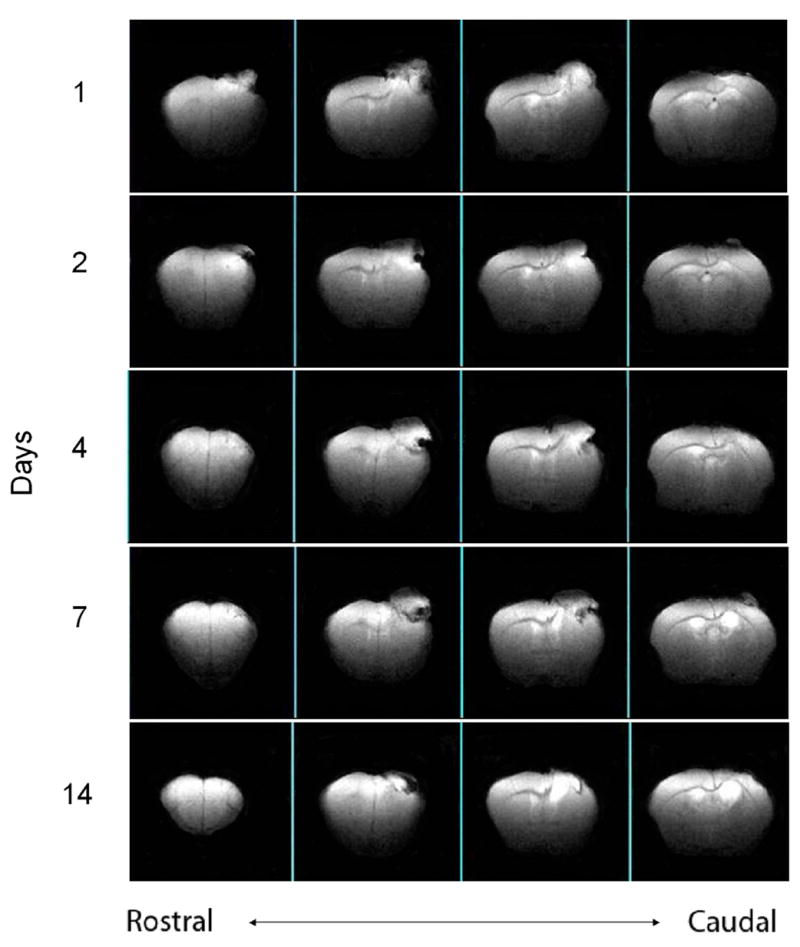
Series of T2-weighted MR images the same injured animal, scanned in the anatomically coronal plane, at 1, 2, 4, 7 and 14 days after injury. Contiguous 1mm slices, TR=2500ms, TE=45ms, field of view: 16mm x10mm. Images segmented to show brain only. This time course shows an early signal hyperintensity, corresponding to acute edema, and a later hyperintensity, consistent with the development of a lesion cavity. At 14 days, there is a well-defined border between the cavity and the adjacent normal-appearing tissue.
Injury Location
MR images were used to generate data on injury location, and to quantify lesion cavity size for comparison with data obtained from histological specimens. Injury location data were derived from anatomically axial MRI images. Figure 4 shows the data on injury location, from T2-weighted axial images at 24 hours post injury. The compact grouping of points on this graph (average distance from the mean location: 0.48mm, min: 0.02mm, max: 0.80mm) demonstrates that the injury location is consistent. Note that the grouping of the points reflects variations due to actual injury location, measurement error, and animal-to-animal differences in brain size.
Figure 4.

Injury location data obtained from T2-weighted anatomically horizontal images, indicating reliable placement of injury and repeatable technique for MRI-based location verification. Squares indicate individual measurements from eight animals, cross indicates mean location.
Behavior
Rotarod results are shown in Figure 5. Post injury mean scores were expressed as a percent of pre-injury score, and error bars indicate standard error of the means. The greatest deficit, for acceleration to 18rpm over 90 seconds, occurred at 48 hours post injury, when injured animals were at 42% and sham animals were at 119% of pre-injury performance, a statistically significant difference (p=0.002, 2-tailed Wilcoxon Sign Rank test). For the injured animals, there was gradual recovery to 72% by 7 days, and 88% of pre-injury performance by 14 days. The results for acceleration to 12rpm, and for constant velocity trials at 12rpm, showed lesser initial deficits, and greater variability, reflecting that these speeds present less challenge to the mice, before and after injury.
Figure 5.

Rotarod latency impairment and recovery data, eight animals with injury compared to eight sham animals, showing that for the injured animals, the greatest deficit was at 24 and 48 hours, with recovery to 88% of pre-injury value by 14 days. Significant differences between injury and sham scores were seen at 24, 48 and 72 hours post injury. ***:p<0.001.
Gridwalk test results, for the left forelimb, are shown in Figure 6. Deficits in the gridwalk test were greatest at 72 hours post injury, reaching a mean of 6.3 footfaults per minute of walking. By 7 days, footfaults occurred at a rate of 4.49 per minute, and by 14 days, the footfaults occurred at a rate of 2.98 per minute. Although some animals did make footfaults with the right (i.e. ipsilateral to the injury) forelimb, especially at the first measurement point at 48 hours post injury, these faults had ceased by 7 days (data not shown).
Figure 6.

Gridwalk impairment and recovery data, presented as left forelimb minus right forelimb footfaults per minutes of walking, showing a peak value in the injured animals of 6.3 at 72 hours, with modest recovery to 3.0 at 14 days., Significant differences between injury and sham scores were seen at all post injury time points. ***:p<0.001.
An analysis of the raw (non-normalized) footfault data reveals similar results to those shown in Figure 6. In preinjury training, only 1 of the 16 animals made more than 2 left front foot faults during observation. At 72 hours post injury and at later time points, only one sham animal made greater than 2 left front footfaults at one time point (7 days), whereas all 8 injured animals made greater than 2 left front footfaults at every time point. Thus, at 72 hours and later, sham behavior was comparable to pre-injury, while injured animals showed marked deficits. Thus analyzing the data with a non-parametric strategy reveals essentially identical results.
Spontaneous forelimb (cylinder) task results are shown in Figure 7. In this task, the mean laterality score of the injured group, which measures preference for the forelimb unaffected by (ipsilateral to) the injury, peaked at 0.25 at 7 days post injury, and was statistically significant compared to the sham group (p=0.031). On this task, normal uninjured performance, for an animal with no preference for right or left forelimb, is at or near zero. Our sham and injured animal groups both showed a slight pre-injury preference for the right side.
Figure 7.

Spontaneous forelimb (cylinder) task impairment and recovery data, showing peak forelimb laterality score at 7 days, with reduction to 66% of peak at 14 days. Significant differences between injured and sham scores were seen at 7 days post injury *:p<0.05.
End-Point Histology and Lesion Cavity Volume
Thionin-stained coronal tissue sections, from brains harvested at 14 days after injury, were used for evaluation of gross pathology and for lesion cavity volume measurement. Examples of thionin-stained sections are shown in Figure 8. Observations of pathology made via light microscopy were qualitatively consistent with MRI findings. At 14 days, there was gross loss of cortical gray matter and subcortical white matter at the injury epicenter, with thinning of the cortical mantle at the margins of the impact zone. The lateral ventricle was expanded on the injured side, and the tissue was deflected at the midline. No obvious hippocampal damage is observed in sections caudal to the impact zone.
Figure 8.

Examples of thionin-stained coronal sections near the injury epicenter. These sections are examples from three different injured animals, at the following AP coordinates with respect to bregma (top to bottom): +0.5, −0.2, −0.5. Scale bars: 1mm.
The MRI-derived and histology-derived lesion cavity volumes are highly correlated (ρ=0.88, p=0.004) as shown in Figure 9. Cavity volumes measured from MRI consistently exceed those measured by histology, by approximately 25%, consistent with other published rodent studies (Kochanek et al., 1995). Figure 9 also shows the data for lesion cavity reproducibility. Measured from MRI images, the mean cavity size was 6.99 mm3 (S.E.M.=0.48), and measured from coronal tissue sections (histology), the mean cavity size was 5.31 mm3 (S.E.M.=0.35).
Figure 9.

Correlation of lesion cavity volume measurements taken from thionin-stained coronal tissue sections with measurements taken from T2-weighted MR images at 14 days.
Discussion
We have characterized a mouse model of lateral sensorimotor CCI, using a linear motor injury device. We used a simple set of behavioral tests sensitive to deficits up to 14 days following injury. Measurements of contusion size using MRI and histology were well correlated and demonstrated a consistent injury size. High field MRI confirmed injury location, and showed acute edema formation and resolution.
The imaging results show some interesting detail of the damage and its evolution. The anatomically axial images demonstrate the value – high resolution, high signal-to-noise ratio – of using a small surface coil for mouse brain imaging. The main tradeoff of this arrangement – a reduction in signal with increasing distance from the coil – is also evident in these images. Given the interest in imaging the lesion and the cortex, the tradeoff is acceptable. The anatomically axial images also show how the brain tissue tends to bulge at the site of the craniotomy, a result of our surgical methods where the skull flap is not replaced after injury. Previous research has shown that replacement of the skull flap after parasagittal CCI can result in greater effective injury severities (Zweckberger et al., 2003). We chose not to replace the skull flap to avoid the complication of causing additional (and potentially variable) damage at surgery time from bringing the sharp piece of bone in contact with the bulging tissue.
Similar to other mouse CCI models, we observed gross loss of cortical and subcortical tissue under the impactor tip contact zone. As expected given the sensorimotor injury location we observed significant deficits in sensorimotor function. For the cylinder test, deficits were significant at 7 days post injury, and for the gridwalk test, animals showed a deficit that persisted at 14 days post injury.
This injury model, based on linear motor device, provides precise injuries, excellent repeatability, real-time strike control, and feedback. It is interesting to compare our results with other models of mouse CCI, particularly in terms of injury severity. We found that reduced strike speed and/or depth resulted in smaller observed lesions on MRI and lesser observed behavioral deficits. Conversely, increased depth caused mortality (unpublished data). It should be noted that the control parameters for this device are different from the parameters used in other devices. Our strike velocity (1.5 m/s), is less than that reported in other studies using pneumatically-actuated impactors (4.0 – 6.0 m/s) (Smith et al., 1995; Fox et al., 1998; Hannay et al., 1999; Hall et al., 2005). One possible explanation is that the linear motor device, via its feedback/servo controller system, maintains tip velocity for the duration of the strike, therefore delivering more energy than a device where the tip begins to decelerate at the instant of impact.
From a behavioral perspective, our gridwalk results are consistent with those of Baskin (Baskin et al., 2003), which showed significant deficits lasting to 4 weeks in a mouse CCI model with a injury lateral to bregma, i.e., in a similar location to ours. However, our cylinder task results, with scores improving after one week post injury, are somewhat in contrast to the results of Baskin, where animals showed a worsening in score from 1 to 4 weeks post injury. It is possible that our injury was slightly less severe than that of Baskin. Unfortunately, there are no published reports of Rotarod scores after sensorimotor CCI in the mouse. In a study of parasagittal CCI in the mouse, Wang found, using a rotarod at 35 rpm – a more challenging speed than ours - that wildtype animals showed their greatest deficit at 24 hours post injury, with recovery to near 50% of pre-injury value by 7 days (Wang et al., 2000). Our Rotarod results show a similar profile, albeit with a shallower initial deficit, perhaps reflecting a less severe injury in our study.
In the context of studies of TBI with animal models, and in particular with genetically modified animals, mouse CCI models have already been shown to be of tremendous value (Longhi et al., 2001). A great majority of these CCI studies have employed the parasagittal injury location, there are very few reports of sensorimotor mouse CCI. Indeed, rigorous characterizations of the sensorimotor injury and its time course have not been published. We believe that our results are a meaningful step in this direction.
This model is well-suited to the study of mechanisms of tissue damage and repair after TBI. Further, the model’s ability to capture longitudinal same-animal information on damage evolution might make it particularly useful in experiments using transgenically modified animals to investigate damage mechanisms or to evaluate potential therapeutic interventions at the pre-clinical stage. With a high correlation between MR and tissue-measured cavity volume, our results illustrate the promise of longitudinal high-field MRI scanning as an important and appropriate technique for mouse CCI studies in vivo.
Acknowledgments
The authors acknowledge the assistance of Thomas Malone with animal handling and MRI scanning and Stanton Fernald with illustrations. Support was provided in part by the National Institutes of Health (R21 AG026482 to NEJB, by R01 NS039123 to WMB, and by core grant P30 HD02528).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- Bilgen M. Simple, low-cost multipurpose RF coil for MR microscopy at 9.4 T. Magn Reson Med. 2004;52:937–940. doi: 10.1002/mrm.20228. [DOI] [PubMed] [Google Scholar]
- Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19:219–226. doi: 10.1177/1545968305278635. [DOI] [PubMed] [Google Scholar]
- Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Moore AN, Moody MR, Treadwell R, Felix JL, Clifton GL. Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J Neurotrauma. 2004;21:1573–1583. doi: 10.1089/neu.2004.21.1573. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Feldman Z, Phan P, Keyani A, Panwar N, Goodman JC, Robertson CS. Validation of a controlled cortical impact model of head injury in mice. J Neurotrauma. 1999;16:1103–1114. doi: 10.1089/neu.1999.16.1103. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Marion DW, Zhang W, Schiding JK, White M, Palmer AM, Clark RS, O'Malley ME, Styren SD, Ho C, et al. Severe controlled cortical impact in rats: assessment of cerebral edema, blood flow, and contusion volume. J Neurotrauma. 1995;12:1015–1025. doi: 10.1089/neu.1995.12.1015. [DOI] [PubMed] [Google Scholar]
- Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- Longhi L, Saatman KE, Raghupathi R, Laurer HL, Lenzlinger PM, Riess P, Neugebauer E, Trojanowski JQ, Lee VMY, Grady MS, Graham DI, McIntosh TK. A Review and Rationale for the Use of Genetically Engineered Animals in the Study of Traumatic Brain Injury. J Cereb Blood Flow Metab. 2001;21:1241–1258. doi: 10.1097/00004647-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H, Maegele M, Neugebauer E, Graham DI, Stocchetti N, McIntosh TK. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Narayana PA, Grill RJ, Chacko T, Vang R. Endogenous recovery of injured spinal cord: longitudinal in vivo magnetic resonance imaging. J Neurosci Res. 2004;78:749–759. doi: 10.1002/jnr.20275. [DOI] [PubMed] [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: optimizing behavioral assessment of outcome. In: Emerich DF, editor. Innovative models of CNS diseases: from molecule to therapy. New Jersey: Humana Press; 2000. pp. 131–151. [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of Matrix Metalloproteinase-9 Gene Knock-Out on Morphological and Motor Outcomes after Traumatic Brain Injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweckberger K, Stoffel M, Baethmann A, Plesnila N. Effect of decompression craniotomy on increase of contusion volume and functional outcome after controlled cortical impact in mice. J Neurotrauma. 2003;20:1307–1314. doi: 10.1089/089771503322686102. [DOI] [PubMed] [Google Scholar]


