Abstract
The viral RNA-dependent RNA polymerases show a conserved structure where the fingers domain interacts with the top of the thumb domain to create a tunnel through which nucleotide triphosphates reach the active site. We have solved the crystal structures of poliovirus polymerase (3Dpol) in complex with all four NTPs, showing that they all bind in a common preinsertion site where the phosphates are not yet positioned over the active site. The NTPs interact with both the fingers and palm domains, forming bridging interactions that explain the increased thermal stability of 3Dpol in the presence of NTPs. We have also examined the importance of the fingers-thumb domain interaction for the function and structural stability of 3Dpol. Results from thermal denaturation experiments using circular dichroism and 2-aniliino-6-napthaline-sulfonate (ANS) fluorescence show that 3Dpol has a melting temperature of only ∼40°C. NTP binding stabilizes the protein and increases the melting by 5-6 °C while mutations in the fingers-thumb domain interface destabilize the protein and reduce the melting point by as much as 6 °C. In particular, the burial of Phe30 and Phe34 from the tip of the index finger into a pocket at the top of the thumb and the presence of Trp403 on the thumb domain are key interactions required to maintain the structural integrity of the polymerase. The data suggest the fingers domain has significant conformational flexibility and exists in a highly dynamic molten globule state at physiological temperature. The role of the enclosed active site motif as a structural scaffold for constraining the fingers domain and accommodating conformational changes in 3Dpol and other viral polymerases during the catalytic cycle is discussed.
Introduction
Poliovirus is the prototypical member of the Picornaviridae family of viruses that also includes coxsackie, foot-and-mouth disease, hepatitis A, and the rhinoviruses. These highly homologous viruses contain ∼7500-nt positive-sense single-stranded RNA genomes encoding a single large polyprotein that is cleaved by viral proteases into about a dozen different proteins required for viral propagation. The last protein within the polyprotein is 3Dpol, a RNA-dependent RNA polymerase (RdRP). This enzyme is responsible for replicating the infecting positive-sense genome into minus-sense complements and then using these as templates for the synthesis of positive-sense genomes that are used for translation and packaging into new virions.
The three-dimensional structure of 3Dpol consists of thumb, palm, and fingers domains according to the usual right hand analogy used to describe polymerase structures (Figure 1(a)). Because the fingers domain forms four tightly intertwined finger structures, we have expanded this analogy by describing the domain using index, middle, ring, and pinky fingers (Figure (b))1. Like other RdRPs, 3Dpol adopts an enclosed conformation where extensive interactions between the thumb and fingers domains completely encircle the active site and create an NTP entry tunnel from the back of the polymerase and an RNA binding site at the front. In contrast with DNA polymerase and DNA-templated RNA polymerase structures, this enclosed feature is emerging as a common structural feature of RdRPs and it is found in all available viral RdRP structures 2. These include polymerases from three serotypes of human rhinovirus 3 and foot-and-mouth disease virus 4 that are also in the Picornaviridae family, rabbit hemorrhagic disease 5 and Norwalk 6 viruses from the Caliciviridae family, hepatitis C virus 7; 8; 9; 10 and bovine viral diarrhea virus 11 representing members of the Flaviviridae family, reovirus λ3 12, and the double-stranded RNA bacteriophage φ6 13.
Figure 1.
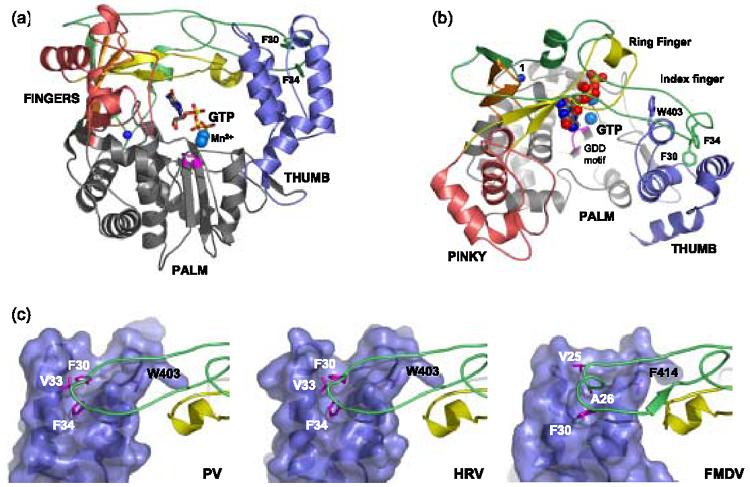
Poliovirus 3Dpol-GTP structure and Picornaviral Finger-Thumb Interactions. (a) Front and (b) top views of the 3Dpol-GTP complex highlighting the various domains of the polymerase. The thumb domain is blue and the palm is colored grey with the conserved catalytic center colored in magenta. The fingers domain can be subdivided into individual digits where the index finger is shown in green, the middle finger in orange, the ring finger in yellow, and the pinky finger in pink. The buried N-terminus is shown with a blue sphere and several of the aromatic residues important for the interdomain interaction between the fingers and thumb domains (F30, F34, W403) are depicted with sticks. (c) Detailed view of homologous interactions at the top of the three picornaviral polymerase thumb domains (surface representation) to illustrate the hydrophobic crevasse into which hydrophobic residues from the index fingertip are inserted. Polymerases are from poliovirus (PV1), human rhinovirus (HRV3), and foot-and-mouth disease virus (FMDV4).
Structural and biochemical analyses of these enzymes suggest that the interdomain interaction between the tip of the index finger and the top of the thumb domain plays a significant role in the proper function of these polymerases. In poliovirus 3Dpol the interaction is dominated by the insertion of Phe30 and Phe34 from the tip of the index finger into hydrophobic pockets at the top of the thumb domain, and essentially identical structures are seen in other picornaviral polymerases (Figure 1(c)). This appears to be a fairly strong interaction in that it remains intact despite the unfolding of the entire fingers domain in the wildtype poliovirus 3Dpol structure14, an event which is likely the result of a steric clash from crystal packing contacts1. Biochemical studies further indicate the index finger-thumb interaction is important for the proper functioning of the protein; when Phe30 was mutated to an alanine there was a complete loss of in vitro activity and mutation of Val33 and Phe34 to alanines resulted in the loss of in vivo viral infectivity 15. Similar results have been observed for hepatitis C virus polymerase, where a single mutation of Leu30 from the λ1-loop of HCV polymerase to the polar residues Arg or Ser was sufficient to inactivate the polymerase 16. The enclosed active site topology of the RdRPs also plays a key role in nucleotide triphosphate binding by creating a tunnel through which these enter the active site. Studies of poliovirus 3Dpol have shown a gradual loss of polymerase activity over time that is greatly accelerated by incubation at physiological temperatures, but can be overcome by incubating the enzyme with NTPs17; 18. Based on our previously determined structure of a 3Dpol-GTP complex1, we hypothesized that this protection from thermal inactivation is due to interactions with the bound NTP that bridge the palm domain and the ring finger, thus stabilizing the native 3Dpol structure (Figure 1).
In this study we have solved the structures of 3Dpol in complex with all four NTPs and show that they all bind in a common site between the fingers and palm domains. Using 2-anilino-6-naphthalene-sulfonate (ANS) fluorescence, we further demonstrate that all four NTPs significantly increase the thermal unfolding temperature of 3Dpol, providing a molecular explanation for the protection from thermal inactivation. Interestingly, we observe a significant pre-melting transition increase in ANS fluorescence that suggests a portion of the polymerase exists in a molten globule state at physiological temperature. Characterized by a loose association of stable secondary structures, molten globules structures are known to be highly dynamic and flexible structures that are often found in proteins and enzymes undergoing significant conformational changes when carrying out their function19. In combination with our structural data, the ANS melting data suggests that the 3Dpol fingers domain is the portion of the polymerase responsible for the molten globule type behavior. This led us to investigate the importance of the enclosed active site motif that creates the NTP binding site for stabilizing the structure of the protein. We find that perturbations of the index finger-thumb interaction reduce the melting point of the protein and dramatically reduce in vitro elongation activity. Together, these data show that poliovirus polymerase is inherently unstable at physiological temperatures and suggest the enclosed active site structure is important for maintaining the structural integrity of the enzyme.
Results
3Dpol-NTP complex structures
Electron density maps from the 3Dpol-NTP crystals reveal excellent density for GTP, moderate density for both CTP and UTP, and poor density for ATP (Figure 2). We solved the structures in the presence of either Mg2+ or Mn2+, but definitive electron density was only observed for the larger manganese ions whose positions were confirmed by calculating anomalous difference electron density maps (Figure 3). The electron density for ATP was quite weak with both metal ions and only the Mg2+ structure has been deposited in the PDB. All four NTPs bind polio 3Dpol at the same site that is near the catalytic center of the enzyme, but NTPs are not yet in a catalytically competent position because the triphosphate group is not yet positioned over the active site aspartic acid residues, as has been seen in other polymerases (see Discussion). The overall structures of the polymerase does not change significantly as a result of NTP binding and structural changes are limited to small movements of the ring finger whose basic residues are involved in the ligand interactions (Table 1, Figure 2(f)).
Figure 2.

Structures of 3Dpol complexed with ribonucleotides in the presence of Mg2+. The NTPs make bridging interactions between the fingers and palm domains. The bases are stacked on Arg174 from the ring finger, the ribose interacts with Arg174 and Asp238 from the palm, and the triphosphate moiety interacts with Arg163 and Lys167 from the ring finger and the backbone amide of residue 236 from the palm. (a) 2.35 Å resolution 2Fo-Fc electron density map contoured at 1.5σ around UTP. (b) 2.25 Å resolution 2Fo-Fc electron density map contoured at 1.5σ around CTP. (c) 2.6 Å resolution 2Fo-Fc electron density map contoured at 1.5σ around ATP and (d) 2.6 Å resolution Fo-Fc electron density map (blue) contoured at 1.8σ around ATP. (e) 2.35 Å resolution 2Fo-Fc electron density map contoured at 1.5σ around GTP. (f) Comparison of the apo 3Dpol structure (red) with all the 3Dpol-NTP structures (grey) with showing the minor shift in the ring finger position as result of interactions with bound NTPs.
Figure 3.

Structures of 3Dpol complexed with ribonucleotides in the presence of Mn2+ with anomalous difference maps to identify the positions of the chelated Mn2+ cations. (a) 2.6 Å resolution 2Fo-Fc map (green) contoured at 1.5σ around UTP and a 2.6 Å resolution anomalous map contoured at 3.5σ Mn2+ (blue; λ = 1.54 Å). (b) 2.5 Å resolution 2Fo-Fc map (green) contoured at 1.5σ around CTP and a 2.5 Å resolution anomalous map (blue; λ = 1.54 Å) at 3.9σ around Mn2+. The * marks the location of weak third peak in the anomalous difference maps. (c) 2.5 Å resolution 2Fo-Fc map (green) contoured at 1.5σ around GTP and a 2.2 Å resolution anomalous map contoured at 10.0σ around Mn2+ (blue; λ = 1.9 Å). (d) Comparison of divalent cation positions in poliovirus and other polymerases. The 3Dpol-GTP-Mn2+ structure from panel (c) is shown with its Mn2+ ions depicted as large stippled spheres and inside these spheres are smaller spheres corresponding to Mn2+ ions from panels a,b,c (blue), Mn2+ ions (orange) from hepatitis C virus 8; 10 and rabbit hemorrhagic disease virus5, Mg2+ (yellow) from bacteriophage φ6 13 and Sm2+ (green) from human rhinovirus serotype 14 3. The polymerase structures were superimposed using Cα atoms from the active site GDD motif and three residues on either side of it.
Table 1.
Summary of crystallographic data
| Complex | 3Dpol+UTP+Mg2+ | 3Dpol+CTP+Mg2+ | 3Dpol+GTP+Mg2+ | 3Dpol+ATP+Mg2+ | 3Dpol+UTP+Mn2+ | 3Dpol+CTP+Mn2+ | 3Dpol+GTP+Mn2+ |
|---|---|---|---|---|---|---|---|
| PDB Code | 2IM2 | 2IM0 | 1RA71 | 2ILY | 2IM3 | 2IM1 | 2ILZ |
| Space Group | P65 | P65 | P65 | P65 | P65 | P65 | P65 |
| Unit Cell (Å) | a=b=129.0 c=113.1 | a=b=127.7 c=113.0 | a=b= 128.0 c= 113.3 | a=b=128.5 c=112.9 | a=b=128.1 c=112.8 | a=b=128.9 c=113.0 | a=b=128.2 c=112.6 |
| X-Ray Source | Cu-Kα 1.54 Å | ALS 4.4.2 0.98 Å | Cu-Kα 1.54 Å | Cu-Kα 1.54 Å | Cu-Kα 1.54 Å | Cu-Kα 1.54 Å | Cu-Kα 1.54 Å |
| Resolution Limits (Å)2 | 30 - 2.35 (2.43-2.45) | 30 - 2.25 (2.33-2.25) | 30 - 2.35 (2.43-2.35) | 30 - 2.6 (2.69-2.60) | 30 - 2.6 (2.69-2.60) | 30 - 2.5 (2.59-2.50) | 30 - 2.5 (2.59-2.50) |
| C α RMSD vs. apo | 0.26 Å | 0.18 Å | 0.23 Å | 0.23 Å | 0.26 Å | 0.28 Å | 0.27 Å |
| Reflections | |||||||
| Total Collected | 313203 | 372805 | 215638 | 168973 | 276952 | 302975 | 136298 |
| Unique | 43793 | 49540 | 43126 | 31801 | 32149 | 36470 | 36244 |
| Redundancy | 7.2 (7.1) | 7.5 (6.8) | 5 (3.9) | 5.31 (5.02) | 8.6 (8.5) | 8.3 (8.3) | 3.8 (3.7) |
| I/σ | 16.6 (4.6) | 13.1 (3.6) | 16.2 (2.1) | 11.9 (3.7) | 15.6 (4.7) | 15.5 (4.7) | 9.9 (2.2) |
| Completeness (%) | 98.5 (97.7) | 99.7 (99.5) | 98.6 (96.2) | 97.6 (96.3) | 99.3 (98.8) | 98.8 (98.5) | 99.8 (100) |
| Rmerge (%) | 7.7 (43.9) | 8.5 (46.2) | 8.2 (55.7) | 11.5 (45.8) | 9.0 (45.8) | 8.2 (44.6) | 8.1 (47.6) |
| Refinement | |||||||
| Resolution Range (Å) | 30 - 2.35 | 30 - 2.25 | 30 - 2.35 | 30 - 2.6 | 30 - 2.6 | 30 - 2.5 | 30 - 2.5 |
| R | 22.9 | 23.7 | 22.4 | 22.6 | 23.0 | 23.1 | 23.0 |
| R-free3 | 26.6 | 26.0 | 26.2 | 25.4 | 26.3 | 26.3 | 25.5 |
| Ramachandran statistics4 | |||||||
| Favored | 378 | 373 | 379 | 368 | 363 | 360 | 366 |
| Allowed | 30 | 36 | 30 | 39 | 44 | 48 | 42 |
| Generous | 2 | 1 | 1 | 3 | 4 | 2 | 2 |
| Disfavored | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
Previously published (reference 1) and shown for comparison.
Data in parentheses are for the highest resolution shell.
R-free was computed using 10% of the data assigned randomly.
Ramachandran statistics do not include glycine and proline residues.
The structures show that a number of hydrogen bond and electrostatic interactions are responsible for binding and positioning the NTPs. The nucleotides are all bound with their 2′ OH group forming a hydrogen bond with Asp238 that is crucial for activity and we have previously shown1 that Asp238 is positioned by the buried N-terminus of the proteolytically processed 3Dpol. If this hydrogen bond is eliminated either by mutating Asp238 to alanine or by soaking native crystals in 2′ deoxy GTP then there is no electron density to indicate occupancy of the nucleotide binding site (data not shown). The bound nucleosides are positioned such that the bases interact with the guanidinium group of Arg174 from the ring finger in a cation-π stacking interaction and the Arg174 guanidinium group is further locked in place by a charge interaction with Glu161 from the other end of the ring finger. This also positions Arg174 within hydrogen bonding distance of the ribose ring oxygen. The triphosphate moiety is involved in ionic interactions with Lys167 and Arg163 of the ring finger that drop down from the roof of the NTP entry tunnel, and the β-phosphate is hydrogen bonded to the backbone amide of residue 236 in the palm domain.
The observed sugar pucker in all the 3Dpol-NTP complex structures is of the C3′-endo type where the C3′ shows the largest deviation from the sugar plane towards the C4′-C5′ bond and the C2′ deviates away from this bond in the exo direction. The C3′-endo pucker is likely dictated by the pair of hydrogen bonds between the 2′ hydroxyl and Asp238, and between the 4′ oxygen and Arg174. GTP is clearly bound in the syn conformation with the base swung over the ribose while ATP, UTP, and CTP are all bound in the lower energy anti conformation with the base swung away from the sugar. Although the electron density for UTP and CTP is not as good as that for GTP, refinement of these nucleosides in the syn conformation yielded significant negative Fo-Fc difference electron density around their exocyclic O2 atoms. ATP clearly has the weakest density of the four NTPs and we interpret it to be predominantly in the anti conformation based on the Fo-Fc difference density maps calculated without ATP in the model (Figure 2(d)). There is one hydrogen bonding interaction between the protein and the base moiety whereby the residue 175 backbone carbonyl interacts with the N1 nitrogen of GTP or the exocyclic amino groups of CTP and ATP. Last, the ribose moieties of CTP, UTP, and ATP are highly superimposable while the GTP ribose is slightly tilted down toward the palm domain in order to accommodate the base in the syn conformation (Figure 2(f)).
NTP mediated thermal stabilization
We used 2, 6-ANS fluorescence to examine the thermal denaturation of 3Dpol in the presence of NTPs because the high absorbance from millimolar nucleoside concentrations hindered our ability to directly monitor protein secondary structure by CD spectroscopy. ANS is an amphipathic dye commonly used to investigate protein folding intermediates due to the dramatic increase in its quantum yield upon insertion into the hydrophobic environment of a protein during the unfolding process22. The ANS detected melting profile of 3Dpol in the absence of NTPs shows an initial pre-transition linear decrease in the fluorescence signal, the main melting transition that is observed as a rapid decrease in ANS fluorescence, and then a linear post-transition increase in the signal with increasing temperature (Figure 4(a)). Immediately before the melting transition we observe a slight plateau in the ANS fluorescence signal, indicating there is some structural change taking place prior to complete protein denaturation. As shown in Figure 4, the addition of nucleotides results in two pronounced NTP concentration dependent changes in the 3Dpol melting profile; 1) a significant increase in the ANS signal immediately preceding the melting transition and 2) an increase in the temperature of the main melting transition. In an effort to separate and quantify the signals arising from the pre-transition increase in ANS fluorescence and the thermal denaturation event itself, we turned to fitting the data to a thermal denaturation model (Equation 1, see Methods) that accounts for the linear pre- and post-transition slopes and characterizes the melting transition by its midpoint (Tx, in Kelvin) and a width described by an enthalphy change (ΔH)23. In carrying out this analysis, we would like to note that the thermal denaturation of 3Dpol is not reversible and results in a precipitated protein sample. We therefore do not rigorously interpret the enthalphy change obtained from this curve fit and instead use it to simply model our data and quantify the apparent midpoint of the melting transition in the presence of increasing NTP concentrations.
Figure 4.

ANS fluorescence detected thermal melting profiles of poliovirus 3Dpol in the absence (a) and presence of 10 and 20 mM CTP (b,c). A subset of the data (☉) reflecting the melting transition and linear pre- and post-transition slopes were fit to a thermal denaturation model according to Equation 1, resulting in the curve fit shown by a solid line in (a-c). (d) This fitted curve was then subtracted from all the datapoints to yield a residual curve showing the NTP concentration dependent increase in the pretransition ANS fluorescence signal.
Equation 1 fit the observed melting data quite well if we exclude the data points corresponding to the pre-transition fluorescence increase (solid lines in Figure 4). This allowed us to fit the pre- and post-transition slopes and determine the apparent mid-point (Tx) of the underlying melting transition with reasonable precision. As shown in Figure 5, this analysis shows that all four NTPs significantly stabilize the protein, increasing the apparent melting point by ∼6°C from 42° C to 48°C in a saturatable concentration dependent manner where maximal increase in melting temperature requires ∼10 mM NTP. With this mathematical model of the melting transition data, we could subtract the calculated curve corresponding to the melting transition from the complete dataset and visualize the residual curve corresponding to the pretransition increase that was excluded from the curve fit (Figure 4(d)). This analysis showed that the pre-transition event begins at ∼32°C, has a reasonably symmetric profile centered at ∼38-39 °C, and has an amplitude that increases with increasing NTP concentration. Similar data were observed for all four NTPs and no single NTP stood out as being particularly special in the extent to which it elicited the pre-melting transition increase in ANS fluorescence. In the absence of NTPs the apo form of 3Dpol also undergoes this transition, but with a much lower amplitude that accounts for the observed leveling out of the pre-transition slope just before the main melting transition (Figure 4(a)).
Figure 5.

NTP mediated stabilization of 3Dpol thermal melting. The apparent melting point of the protein, determined by fitting to Equation 1 as in Figure 4, is plotted against NTP concentration. All four NTPs increase the melting point of the protein from 42°C to 47-48°C in a concentration dependent manner. Error bars reflect the 95% confidence intervals of the apparent melting point value obtained from the curve fit.
Apo and mutant 3Dpol thermal denaturation
Combined with our structures of the 3Dpol-NTP complexes, the observed NTP dependent stabilization of 3Dpol suggests that bridging interactions between the fingers and palm domains provided by the bound NTPs act to stabilize the protein. We therefore hypothesized that a similar stabilizing role may be played by the interaction between the index finger and the thumb domain, the structure that creates the NTP entry tunnel and enclosed active site motif that is common to RNA-dependent RNA polymerases. We set out to characterize the importance of this structural interaction in 3Dpol by making a series of mutations predicted to destabilize the protein by weakening this interaction. These experiments were done in the absence of NTPs and we were therefore able to directly observe the conformation of the protein by using circular dichroism spectroscopy at 222 nm to monitor secondary structure during the melting transition.
In these experiments the apo form of 3Dpol was observed to have a melting temperature of ∼39°C with a very sharp transition that ended in protein precipitation at temperatures above 45°C (Figure 6(a)). The melting transitions we observed by CD spectroscopy were asymmetric or biphasic (see below) and we could not adequately model the data using Equation 1. As a result, we instead characterized the apparent melting temperatures (Tx) by simply determining the inflection point in a plot of the first derivative of the CD data, i.e. the difference between adjacent θ222 data points, versus temperature (Figure 6(b), Table 2). The melting temperatures observed by this analysis were consistently 2-3 °C lower than those obtained in the ANS fluorescence experiments. This difference is likely due to differences in the thermal calibration of the two instruments and the two analysis methods (curve fit vs. slope analysis) we used. It is also known that ANS binding can stabilize protein structure and lead to an increase in the apparent melting temperature of the protein22; 24. Importantly, for the interpretations we make in this work, we do not rely on directly comparing the apparent melting points obtained by the two techniques; the effects of NTP binding were determined by ANS fluorescence while the effects of mutations on the stability of the apo protein were determined by CD spectroscopy.
Figure 6.

Thermal denaturation of full-length and N-terminally truncated versions of poliovirus 3Dpol. (a) Denaturation profiles of native 3Dpol (●), 3Dpol-Δ22 (×), and 3Dpol-Δ68 (□) obtained by CD spectroscopy at a wavelength of 222 nm. (b) Slope analysis plot (Δθ vs. T) of data in panel (a) where the maxima indicate the thermal transition temperatures of the various proteins (Tx - summarized in Table 2) (c) Far UV CD spectra of full-length 3Dpol(○) obtained at 10°C and the 3Dpol-Δ68 mutant at 10°C (●), 50°C (◆), 60°C (□), and 90°C (+).
Table 2.
Effects of Mutations on Polymerase function and stability
| Mutation | RNA Binding | Activity | Tx1 | Tx2 |
|---|---|---|---|---|
| μM Kd | % | °C | °C | |
| 3Dpol | 1.8 ± 0.1 | 100 ± 4.0 | 39 | na |
| 3Dpol-Δ22 | 3.4 ± 0.2 | −0.3 ± 0.7 | 37 | 50 |
| 3Dpol-Δ68 | 6.5 ± 0.4 | 0.0 ± 0.2 | 34 | 76 |
| 3Dpol-F30A | − | 4.3 ± 2.1 | − | − |
| 3Dpol-F34A | 1.7 ± 0.1 | 4.3 ± 1.5 | − | − |
| 3Dpol-F30A-F34A | 2.2 ± 0.1 | −0.3 ± 0.7 | 38 | na |
| 3Dpol-F30D-F34D | 3.0 ± 0.1 | 2.0 ± 1.0 | 35 | 57 |
| 3Dpol-403A | 1.7 ± 0.1 | 2.0 ± 0.2 | 33.5 | 39.5 |
| 3Dpol-403D | 2.3 ± 0.2 | 1.3 ± 0.6 | 34.5 | 44.5 |
| 3Dpol-69-380 | − | −0.3 ± 1.2 | 37 | 75 |
- = not determined, na = not applicable
When the first 68 residues of the protein were deleted (3Dpol-Δ68), the melting profile showed two distinct melting transitions at ∼33.5°C and ∼76°C (Figure 6(a)). At temperatures above 75°C this truncation mutant also precipitated with large white flakes in the sample, but at the plateau between 35°C and 65°C the mutant appeared only slightly turbid. Deletion of the first 22 residues from the N-terminus (3Dpol-Δ22) results in an enzyme with two-thirds of the index finger, thereby preserving the possibility of interactions with the thumb domain and a majority of the interactions with the ring finger (Figure 1(b)). Like 3Dpol-Δ68, the 3Dpol-Δ22 protein exhibited a biphasic melting transition (Figure 6(a)). The first transition temperature is approximately ∼37°C, which is lower than that of the full-length protein but higher than that of the 3Dpol-Δ68 construct, showing that residues 23-68 of the index finger make a positive contribution to structural stability.
The biphasic melting curves of the Δ22 and Δ68 N-terminally truncated proteins were suggestive of a multi-state unfolding transition that could hypothetically correspond to the selective unfolding of different polymerase domains. To further investigate this possibility, we collected CD spectra of the proteins in the native conformation at 10°C, in the intermediate plateau region at 50°C and 60°C, and in the fully denatured state at 90°C. As shown in Figure 6(c), the CD spectra obtained from the 3Dpol-Δ68 melting intermediates are quite different from those of the full length and 3Dpol-Δ68 proteins in their native conformation at 10°C. The native spectra are indicative of a mixture of α-helix and β-strand content that is consistent with the structure of 3Dpol. The melting intermediate form, however, appears to be almost pure β-strand conformation and we cannot reconcile the CD spectrum of the intermediate with that expected from the selective unfolding of any single polymerase domain, such as the thumb or fingers. The change in secondary structure is also not reversible by cooling the protein back down, and as a result we attribute this β-strand rich intermediate species to some misfolded and aggregated form of the protein whose structure has little or no relationship to that of native 3Dpol. While we did not pursue this further, we find it noteworthy that the presence of a biphasic melting curve correlates with the length and hydrophobic content of the index finger sequence; proteins with large N-terminal truncations (3Dpol-Δ68) have significant plateaus, proteins with smaller truncations (3Dpol-Δ22) have smaller plateaus, and point mutations that replace hydrophobic residues with polar ones also develop plateaus (see below). This suggests that the presence of the intermediate is dependent on solubility following some initial partial unfolding event that likely involves the index finger sequence; the native protein rapidly aggregates following this initial unfolding event while the less hydrophobic mutants remain somewhat soluble, allowing the intermediate to be observed.
Fingers-Thumb Interface
To more specifically investigate the relationship between the thermal stability of 3Dpol and the enclosed active site structure, we examined the melting profile of full-length polymerases with point mutations located at the tip of the index finger that disrupt the index finger-thumb interaction (Figure 7(a)). We mutated the hydrophobic phenylalanines that insert into the thumb to alanines and aspartic acids. The individual point mutations of phenylalanines 30 and 34 to alanines yielded polymerases with thermal melting profiles almost identical to that of the native polymerase, while the double mutant (3Dpol-F30A-F34A) displayed a single transition with a melting temperature approximately one degree lower than that of the native polymerase (Figure 7(c), Table 2). Mutation of these phenylalanines to negatively charged aspartic acid residues, which would disfavor insertion into the top of the thumb, result in a biphasic melting profile with transition temperatures of ∼35°C and ∼57°C (Figure 7(c)). We also altered the fingers-thumb interface by mutating tryptophan 403, which is located at the top of the thumb and plays an important role in the native structure where it serves as a “hook” for the loop at the tip of the index finger. Structural comparison of the complete full-length structure1 with the 3Dpol-Δ68 structure1 reveals large conformational differences around Trp403, demonstrating there is significant structural flexibility at the top of the thumb domain in the absence of an index finger (Figure 7(a) and (b)). To test the role of this conserved hydrophobic residue in stabilizing the native structure, we mutated Trp403 to both alanine and aspartic acid. The aspartic acid mutation resulted in a ∼4°C drop in the melting temperature, resulting in a slightly biphasic melting profile with transition temperatures centered around ∼35°C and ∼45.5°C (Figure 7(d)). The alanine mutant reduced the melting temperature by 5-6°C and also displayed a slightly biphasic profile (Figure 7(d)).
Figure 7.

Thermal denaturation of 3Dpol mutants in the Fingers-Thumb domain interface. (a) Detailed view of the top of the thumb domain in full-length poliovirus 3Dpol (a) and 3Dpol-Δ68 (b) structures. The loop composed of residues 401-408 adopts different conformations in these structures, highlighting the flexibility of this region. The solvent exposed conformation of Trp403 is stabilized by a crystal packing contact in the 3Dpol-Δ68 structure. (c) Denaturation profiles of 3Dpol-F30A-F34A (Δ) and 3Dpol-F30D-F34D (▼). The data from native 3Dpol protein is depicted with a solid black line. (d) Denaturation profiles of 3Dpol-W403A (◇) and 3Dpol-W403D (☒) with the native 3Dpol data depicted with a solid black line.
RNA Binding and Polymerase Activity
The fluorescence polarization based RNA binding assays showed that all the mutant polymerases retained essentially native RNA binding abilities (Table 2). The largest reductions in affinity came from the N-terminal truncation mutants that showed a ∼3.5-fold decrease for 3Dpol-Δ68 and a ∼2-fold decrease for 3Dpol-Δ22. These differences are fairly small and correspond to ΔΔG values of less that 1 kcal/mol. The index finger and thumb point mutants show only minor decreases in the RNA binding affinity that roughly correlate with their relative effects on the first thermal transition temperature. Despite essentially normal RNA binding of these mutants, we observe drastic effects on catalytic activity in the polyA templated oligo-dT extension assay. All N-terminal truncation mutants were completely dead in elongation assays (Table 2), which is explained by our previously demonstrated requirement for a native N-terminus1. It is more surprising that mutations having small effects on the thermal transition temperatures and RNA binding, such as F30A, F34A, and F30A-F34A, are also inactive in the elongation assay. These data suggest that mutations of the index finger-thumb interface may have more pronounced and destabilizing effects in the context of the 3Dpol elongation complex, whose structure and dynamics we do not yet know much about.
Discussion
In this work we have solved the structures of poliovirus 3Dpol in complex with all four NTPs and shown that they bind in a common site where they interact with both the fingers and palm domains. This binding location suggested that NTP binding, previously known to prevent thermal inactivation of 3Dpol, stabilizes the polymerase structure by providing bridging interactions between the palm and fingers domain. Consistent with this, we show that NTP binding increase the melting point of the protein by ∼6°C while mutations that weaken the fingers-thumb interface reduce the melting point of the protein by 4-6°C. Our results demonstrate that the interaction between the fingers and thumb domains is important for the structural integrity and function of poliovirus 3Dpol.
3Dpol-NTP Complex Structures
The crystal structures of the 3Dpol-NTP complexes presented here show that all four natural nucleotide triphosphates bind to poliovirus polymerase in the same pre-insertion site. The site is structurally analogous to sites seen in RNA polymerases from hepatitis C virus, bacteriophage φ6, and bacteriophage T7. These enzymes all show initial ribonucleotide binding near the catalytic center in a site composed of basic residues from the top of the NTP entry tunnel and divalent cations bound to conserved aspartate residues in the palm domain 10; 13; 25. Although we do not observe the NTPs in a catalytic conformation in poliovirus 3Dpol, structural studies of the bacteriophage φ6 polymerase with bound NTPs show three NTP binding orientations termed the interrogation, catalytic, and priming sites in that protein13. The site seen in our 3Dpol complexes corresponds to the interrogation site, which is thought to be the initial binding position where recognition of the correct cognate base occurs. Similarly, bacteriophage T7 RNA polymerase initially binds NTPs in an interrogation position termed the “preinsertion site” and the triphosphate moiety is then pushed into the active site by a rotation of the O helix that contains several basic residues interacting with the triphosphates and is structurally analogous to our ring finger 20; 26; 27.
NTP Recognition
In the poliovirus 3Dpol-NTP complexes there are three main areas of interactions between the protein and bound ligand that together select for a ribonucleotide triphosphate in the binding site with little base specificity. The triphosphates interact with basic residues from the ring finger that forms the roof of the NTP entry tunnel, the nucleobase participates in a cation-π interaction28 with Arg174 from the ring finger, and the 2′ hydroxyl group forms a very important hydrogen bond with Asp238 in the palm domain. We have previously shown that the positioning of the Asp238 side chain for this hydrogen bond is dependent on the proteolytic processing of the protein to generate its native N-terminus, which is buried in a pocket just behind Asp2381. Consistent with this, if we mutate Asp238 to alanine then we do not observe any electron density for any bound NTP, including GTP that normally shows stellar electron density in 2Fo-Fc maps (Figures 2(e) and 3(c)) and simulated anneal omit maps1. Likewise, soaks with 2′ deoxy NTPs also do not result in any electron density for a bound nucleotide, further illustrating the importance of the 2′ OH-Asp238 hydrogen bond in selecting for an intact ribose group. It is also noteworthy that the relative quality of the electron density maps from the different NTPs rank according to their reported Kd values, which range from 4 μM for GTP to 130 μM for ATP 29 and may reflect the cellular availability of each ribonucleotide 30.
The NTPs are all bound with a C3′-endo sugar pucker and, with the exception of GTP, they are in the anti conformation. There is one direct contact between the bases and the protein whereby the residue 175 backbone carbonyl forms a hydrogen bond with the NH proton of GTP and the exocyclic amino group of CTP and ATP. The positions of the divalent metal ions are consistent with those observed in other polymerase structures (Figure 3(d)) and the two-metal mechanism for catalysis involving the binding of two divalent cations that have distinct roles in catalysis 31; 32; 33. In our 3Dpol-NTP structures these metal ions are coordinated by the three conserved aspartate residues from the catalytic site (Asp328, Asp329, Asp233) and the triphosphate moiety of the NTP (Figure 3). Occasionally we observed a diffuse additional anomalous difference peak that may be from a third Mn2+ ion with partial occupancy at a site that is above the triphosphate moiety (Figure 3(b)). While the purpose and functional relevance of this cation binding site is unclear, a similarly placed third metal ion has also been observed in hepatitis C virus polymerase complexed with UTP-Mn2+ 25 and the bacteriophage φ polymerase complexed with template-primer and Mg2+/Mn2+ 13.
Protein stability
Prior biochemical experiments have shown that 3Dpol is readily inactivated by incubation at temperatures of 37-42°C and this inactivation can be prevented by the addition of NTPs 17; 18. The structures of the 3Dpol-NTP complexes suggest the NTPs may be stabilizing the structure against thermal denaturation by providing bridging interactions between the fingers and palm domains. In the second part of this study we examined this in further detail by characterizing the stability of various 3Dpol-NTP complexes and 3Dpol mutants using ANS fluorescence and circular dichroism spectroscopy. The resulting data show that 3Dpol has a melting point of only ∼40°C, indicating that in its apo form the protein is only marginally stable at physiological temperatures. NTP binding increases the melting point of the protein by 5-6°C, providing an explanation for the retention of enzymatic activity observed when the protein is incubated at elevated temperature in the presence of NTPs. Conversely, mutations that disrupt the fingers-thumb interface decrease the melting point of the protein. A minor ∼1°C reduction was observed when the two phenylalanine residues (Phe30, Phe34) that insert into the top of the thumb domain were mutated to alanine, while a much larger ∼4°C reduction in the transition temperature was observed when they were mutated to aspartic acids. Mutation of Trp403, the residue at the top of the thumb that forms a “hook” for the index finger loop (Figure 7(a)), to either alanine or aspartic acid drastically reduces the melting transition by ∼6°C. Our conclusion from these data is that the index finger reaching across the active site and interacting with the thumb acts as a key structural interaction that locks down and stabilizes the conformation of the entire protein.
Further insight into the stability of 3Dpol was obtained from ANS fluorescence based melting data that suggests 3Dpol exists in a molten globule state at physiological temperatures. Molten globules are often found in proteins that must undergo significant dynamics in order to carry out their function and therefore have stable secondary structure elements with loosely packed hydrophobic cores19. They exhibit increased ANS binding due to the exposure of hydrophobic patches that can bind this dye and it is not unusual to observe molten globule intermediates during thermal denaturation experiments. Our data suggests the apo form of 3Dpol is in a molten globule state from ∼32 to 40°C, the region where the ANS detected melting profile of the apo protein levels out just before the main melting transition (Figure 4(a) and (d)). As expected for a molten globule, the θ222nm CD signal remains largely unchanged through this transition region (Figure 6(a)), indicating that the secondary structure of the protein is not changing significantly prior to the main unfolding event. The low melting point of the apo protein means that the molten globule state does not become significantly populated before the protein unfolds and aggregates and we therefore observe only a fairly small residual ANS fluorescence signal (Figure 4(d)). In the presence of NTPs, however, the structure is stabilized and we observe a significant increase in the ANS signal before the protein unfolds at temperatures approaching 45-48°C (Figure 4). Our interpretation of these data is that NTP binding causes an increase in the temperature of the unfolding transition that leads to aggregation, but it does not prevent parts of the protein from entering the molten globule state prior to this point. NTP binding thus increases the population of the molten globule state by allowing it to exist at temperatures where the apo form of the protein has already unfolded.
We do not know exactly which parts of the protein become molten globules, but strongly suspect it is the fingers domain based on its relatively loosely packed structure and the observation that it can unfold independently of the remainder of the protein. The crystal structures show that the atomic B-factors are significantly higher in the fingers domain than the palm and thumb domains (62 Å2 versus 42 Å2 and 43 Å2, respectively). In addition, the previously solved structure of the 3Dpol-Δ68 mutant1 showed that the entire index finger sequence can be deleted from the protein without affecting the expression and folding of the palm and thumb domains. Finally, the original partial 3Dpol structure14 and those from three other crystal forms34 showed that the fingers domain could be selectively disordered by what was later realized to be crystal packing clashes in the pinky finger region1. We have attempted to directly show that the fingers domain is responsible for the pre-transition increase in ANS fluorescence, but these experiments have proven difficult because the fingers domain is composed of three distinct regions of primary sequence (1-68, 96-190, 269-285), precluding a simple deletion of the domain. An expression construct lacking the C-terminal thumb domain did not yield any protein, consistent with the idea that the protein is not stable if the index fingertip cannot be buried in the thumb. We were able to get low protein expression from a residue 69-380 construct that lacks both the index finger and the thumb. This protein showed biphasic melting behavior similar to that of the Δ22 and Δ68 deletions, indicating that the thumb domain is not the part of the protein responsible for the biphasic melting profile.
3Dpol Structural Integrity
Combining our structural, mutational and thermal stability data, we propose that the enclosed active site motif of 3Dpol plays a major role in holding an inherently unstable fingers domain structure together. The data suggest that the index finger traversing across the active site and interacting with the thumb domain is an important structural support that stabilizes the fingers domain by trapping the ring finger underneath it, and this in turn stabilizes the rest of the fingers domain because the ring finger is linked to the pinky finger (Figure 1(b)). The insertion of Phe30 and Phe34 from the index finger into the groove at the top of the thumb and the burial of Trp403 are important and this is likely the interaction that melts at ∼40°C, leading to an unfolding of the entire fingers domain and immediate aggregation of the protein. Mutations that weaken or eliminate interactions in this interface reduce the melting temperature of the protein. Conversely, the binding of NTPs stabilizes the folded conformation by providing a set of bridging interactions between the ring finger and the palm domains that further stabilize the ring finger conformation (Figure 2(f)) and raising the trigger point for the unfolding of the fingers domain by 5-6 °C. While the addition of NTPs increases the melting temperature of the event that triggers protein aggregation, it does not prevent the remainder of the molecule from undergoing significant conformational dynamics in a molten globule state as the temperature is raised, giving rise to the large observed increase in ANS fluorescence.
Implications for catalytic cycle
The notion that the fingers domain is a highly dynamic part of the polymerase is consistent with its function. This is the domain that interacts with both the template RNA and incoming NTPs and we expect that it must be able to readily interconvert between multiple conformations during the catalytic cycle. In the case of poliovirus 3Dpol, the Cameron laboratory has shown that enzyme undergoes conformational changes after binding RNA and after incorporation of the first nucleotide that both increase the half-life of the 3Dpol-RNA complex18, indicating that the polymerase can exist in multiple stable conformations. Although we do not yet have a structure of a picornaviral RdRP complex in the catalytic closed conformation, we can draw parallels between these polymerases and the multiple structures of bacteriophage T7 RNA polymerase at different points in the catalytic cycle 20; 26; 27; 35. In T7 RNAP, the NTP initially binds in a preinsertion site where it makes several ionic interactions with residues from the O helix in the fingers domain. During the catalytic cycle this helix undergoes a significant swinging motion that pushes the NTP into the active site in order to properly position the triphosphate moiety for catalysis 20; 27. In our structures of poliovirus 3Dpol we also observe NTP binding in a preinsertion site where it has several interactions with the ring finger that appear to be structurally analogous to the O-helix-NTP interactions in T7 RNA polymerase. By analogy, we expect that the 3Dpol fingers domain, and the ring finger in particular, will have to ratchet back and forth as NTPs are continually bound and pushed into the catalytic site during elongation. These motions are likely correlated with conformational changes in the pinky finger that interacts with the template RNA because the ring finger sequence is actually an insertion in the pinky finger, providing an efficient way of coupling NTP repositioning by the ring finger with RNA translocation. This model for the 3Dpol catalytic cycle requires significant structural flexibility in the fingers domain, which is consistent with the observed molten globule behavior of the protein at physiological temperatures.
The index finger-thumb interaction is a conserved feature of all RdRP structures solved thus far, yet there is significant diversity in the apparent strength of this inter-domain linkage. The picornaviral polymerases have very similar conformations that are characterized by an index finger loop lying on top of the thumb domain surface, wrapping around a hydrophobic “hook” residue (e.g. Trp403), and inserting a few residues into a hydrophobic cleft at the top of the thumb (Figure 1(c)). In contrast, the closely related caliciviral polymerases display much more extensive interactions in this region of the structure (Figure 8(a)). In the rabbit hemorrhagic disease virus polymerase there is a large loop at the top of the thumb that folds over the index finger and back onto the hydrophobic hook residue, forming an enclosed tunnel through which the index finger passes. The Norwalk virus polymerase further augments this extended loop motif by having the thumb loop ensnare a second tryptophan residue from the portion of the index finger that passes through the tunnel. Finally, the hepatitis C virus polymerase has a fully exposed index finger loop like that of the picornaviral polymerases (Figure 8(b)). Similar to our finding that F30A and F34A mutations in poliovirus 3Dpol abolish activity, point mutations of Leu30 to serine or arginine in HCV polymerase have also been shown to abolish enzymatic activity 16. Interestingly, the top of the HCV polymerase thumb is also the interaction site for a recently discovered class of non-nucleoside benzimidazole-based inhibitors that function by disrupting interaction with the λ1 loop that is equivalent to the index finger tip 36. This suggests the fingers-thumb interaction is dynamic enough to allow the inhibitors access to the binding pocket on the thumb, resulting in displacement of the index fingertip (Figure 8(b)).
Figure 8.

Comparison of Fingers-Thumb connection in different RdRP structures. (a) Two orthogonal views of the interactions at the top of the thumb in poliovirus (PV1), rabbit hemorrhagic disease virus (RHDV5), and Norwalk virus (NWV6) polymerases. The index finger is colored green, the ring finger is colored yellow, and the thumb is shown as a grey surface with its tryptophan “hook” residue colored magenta. Note how a loop on the thumbs of the RHDV and NWV polymerases forms a flap over the top of the index finger that interacts with the hook residue, forming a small tunnel through which the index finger passes in a β-strand conformation. In Norwalk virus polymerase this loop also wraps around Trp29 from the index finger (cyan), further stabilizing the interaction. (b) Structures of the native fingers-thumb interaction in hepatitis C virus polymerase8 and a benzimidazole-based inhibitor complex36 showing how this compound binds the top of the thumb where the index fingertip (λ1-loop) normally interacts.
Conclusions
In closing, we have shown that poliovirus polymerase has a melting point of only ∼40°C and is only marginally stable at physiological temperatures where it exhibits molten globule type behavior. The structures of 3Dpol complexes with all four NTPs show that these ligands bind in a pre-insertion site where they provide bridging interactions between the palm and fingers domains that stabilize the structure and increase its melting point. Conversely, mutations that disrupt the interactions between the fingers and thumb domains decrease the melting point of the protein. The data implicate the structural linkage between the fingers and thumb domains as being important for the structural integrity of the enzyme while at the same time allowing for significant conformational dynamics within the fingers domain during the catalytic cycle. Elucidating the specific molecular details of these conformations will require solving the structures of picornaviral elongation complexes at various stages of the catalytic cycle.
Materials and Methods
Protein Purification and Crystallization
The 3Dpol construct used for crystallization studies contains the L446D and R455D mutations on the thumb domain and were expressed in and purified from E. coli BL-21 (DE3) pLysS cells using inducible T7 based expression plasmids as previously described1. The 3Dpol construct used for the ANS binding studies contained the L446D mutation and a C-terminal hexa-histidine tag and was purified similarly except the first chromatography column was replaced with a Ni-chelating column and the protein was eluted with a linear gradient from 10 mM to 500 mM imidazole in 300 mM NaCl, 5% glycerol (v/v), 50 mM TRIS (pH 8.0), 0.02% NaN3 (w/v). Protein concentrations were determined by absorbance at 280 using extinction coefficients obtained from the Expasy web site (http://www.expasy.org/tools/protparam.html).
Crystals were grown by hanging drop vapor diffusion at 16°C using ∼12 mg/ml protein. 3Dpol-L446D-R455D crystals grew in 4 days with a precipitant/well solution containing 2 M sodium acetate, 0.1 M cacodylic acid (pH 7.1), 2 mM DTT and 0.02% (w/v) sodium azide. Crystals were soaked for 5 to 10 hours at 4°C in a solution containing either 10 mM ATP, CTP, GTP, or UTP and either 10 mM MnCl2 or MgCl2 in the crystallization mixture listed above. All crystals were transferred into corresponding precipitant/ligand solutions containing 30% (v/v) glycerol prior to flash freezing. Structures of 3Dpol in complexes with all four NTPs could also be obtained by cocrystallization of the polymerase with 10 mM NTPs.
Structure determination
Diffraction data were collected at the MBC beamline 4.2.2 at the Advanced Light Source (Berkeley, CA) and by a R-AXIS IV imaging plate detector with CuKα radiation. Reflections were integrated, merged, and scaled using d*TREK 37. The initial structure solutions were obtained by molecular replacement with CNS 38 using the complete polymerase structure (PDB code 1RA6) as the search model. The new Rfree reflection datasets for each crystal were uncoupled from the remainder of the data with a 1500K simulated annealing step. Manual model rebuilding was performed using O 39 and refined with the CNS package using the maximum likelihood intensity (MLI) target. NTP parameter sets were obtained from the Dundee PRODRG2 Server (http://davapc1.bioch.dundee.ac.uk/programs/prodrg) 40. Crystallographic details are listed in Table 1 and structure figures were generated with Pymol Molecular Graphics System (www.pymol.org) 41.
ANS Binding Studies
ANS fluorescence data were obtained on an Aviv Instruments model ATF-105 spectrofluorometer using a 1 cm pathlength quartz cell containing 1.6 mL of 2 ╭M 3Dpol, 25 mM Tris (pH 7.5), 25 mM NaCl, 200 ╭M 2-anilinonaphthalene-6-sulfonate, and varying concentrations of NTPs. An emission wavelength of 445 nm (8 nm bandwidth) was monitored with an excitation wavelength of 360 nm (2.5 nm bandwidth) as a function of temperature. Thermal denaturation experiments were run from 5° to 60°C in one-degree steps with a 2°C per minute rate of increase, one minute equilibration time, and 20 second data averaging time. Thermal transition temperatures (Tx) were obtained using a least squares nonlinear regression analysis of fluorescence signal vs. T plots to the following equation23:
| Equation 1 |
where y is the observed fluorescence signal, yf and yu are the intercepts, mf and mu are the slopes of the pre- and post-transition baselines, Tx is the midpoint of the transition curve, T is the temperature (in Kelvin), and R is the gas constant.
Polymerase Activity Assays
20 μL PolyA/oligo(dT) polymerase extensions reactions containing 0.01 μg/μL polyA template with an average length of 300 nt, 0.005 μg/μL oligo dT15, 50 mM HEPES (pH 7.5), 25 μM UTP, 0.5 mM each GTP, CTP, ATP, 4 mM DTT, 1.5 mM Mg acetate, 60 μM ZnCl2, 0.1 % NP40, 0.01 Ci/μL [α-32P]UTP, and 300 nM 3Dpol were assembled on ice in a 96-well microtiter plate. The entire plate was then transferred onto a 30°C heatblock for the elongation reaction that was quenched at various times between 10 and 30 minutes. Replicate samples for each protein occupy an entire column (8-wells) or half-row (6-wells) of the plate so that multiple time points can easily be quenched by addition of 30 μL of 0.5 mM EDTA using a multichannel pipettor. Incorporation of 32P-uracil radiolabel was evaluated by filtering ∼35 μL of the quenched reactions through Hybond-N+ nylon membrane supported on Whatman paper in a Schleicher & Schuell 96-well dotblot filter apparatus. The membrane is washed both before and after addition of the reaction with 25 mM MES (pH 6.5), 2.5 mM Mg acetate, 40 μM ZnCl2, 10% glycerol (v/v), 2 mM DTT. The amount of radiolabeled RNA bound to the membrane was quantified using a phosphorimager and the activities were determined by linear regression of the radiolabel signal vs. reaction time. Comparison of the slopes of these 32P incorporation curves yielded activities of the mutant proteins relative to wild-type 3Dpol controls that were always performed at the same time using the same reagents.
RNA binding assays
RNA binding affinities were determined by fluorescence polarization using a RNA homo-duplex labeled at its 5′ end with fluorescein. The sequence of this RNA, CAGUGGCCGGCC, is similar to sym/sub used extensively in the Cameron lab as a substrate for 3Dpol elongation18 but with a 2 bp longer duplex region. Fluorescence polarization measurements were conducted using black 384-well flat-bottom polystyrene plates in a Perkin Elmer Victor V Model 1420 Multilabel Counter using 480 nm excitation and 535 nm emission bandpass filters. The 50 μL reactions contained 50 mM HEPES (pH 7.0), 4 mM DTT, 1.5 mM Mg acatate, 60 μM ZnCl2 0.1 % NP40, 50 mM NaCl, 10 nM annealed 5′ fluorescein end-labeled RNA, and polymerase at various concentrations from 1 nM to 20 μM. To ensure the protein was soluble, the polymerase was serially diluted 1.2-1.5 fold in a 200 mM NaCl buffer to generate a series of samples containing 3Dpol at 10X the final concentration used in the assay. Small volumes of these protein dilutions (5 μL) were first transferred to the microtiter plate followed by larger volumes (45 μL) of a mixture containing the remaining components of the reaction adjusted so as to obtain a uniform final NaCl concentration of 50 mM. Reactions were assembled at 4°C and allowed to equilibrate for 30 minutes prior to reading the FP signals. Equilibrium dissociation constants were determined by directly fitting the polarization data to a single site binding curve using Kaleidagraph (Synergy Software). The Kd values obtained were >100-fold higher than the 10 nM RNA concentration and consequently the free 3Dpol concentration of was taken to equal the total 3Dpol concentration when fitting the data.
Coordinates
The coordinates and structure factors have been deposited at the Protein Databank with ID codes as listed in Table 1.
Acknowledgements
We thank Robert Woody for helpful discussions, Alan Kennan and the entire Kennan lab for assistance with the CD experiments, Mike Maloney for preliminary work with the 3Dpol-Δ68 protein, and Jay Nix of the Molecular Biology Consortium synchrotron beamline 4.2.2 at the Advanced Light Source for assistance with X-ray data collection. This work was supported by grant R01-AI059130 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson AA, Peersen OB. Structural basis for proteolysis-dependent activation of the poliovirus RNA-dependent RNA polymerase. Embo J. 2004;23:3462–71. doi: 10.1038/sj.emboj.7600357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrer-Orta C, Arias A, Escarmis C, Verdaguer N. A comparison of viral RNA-dependent RNA polymerases. Curr Opin Struct Biol. 2006;16:27–34. doi: 10.1016/j.sbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Love RA, Maegley KA, Yu X, Ferre RA, Lingardo LK, Diehl W, Parge HE, Dragovich PS, Fuhrman SA. The crystal structure of the RNA-dependent RNA polymerase from human rhinovirus: a dual function target for common cold antiviral therapy. Structure. 2004;12:1533–44. doi: 10.1016/j.str.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer-Orta C, Arias A, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J Biol Chem. 2004;279:47212–21. doi: 10.1074/jbc.M405465200. [DOI] [PubMed] [Google Scholar]
- 5.Ng KK, Cherney MM, Vazquez AL, Machin A, Alonso JM, Parra F, James MN. Crystal structures of active and inactive conformations of a caliciviral RNA-dependent RNA polymerase. J Biol Chem. 2002;277:1381–7. doi: 10.1074/jbc.M109261200. [DOI] [PubMed] [Google Scholar]
- 6.Ng KK, Pendas-Franco N, Rojo J, Boga JA, Machin A, Alonso JM, Parra F. Crystal structure of norwalk virus polymerase reveals the carboxyl terminus in the active site cleft. J Biol Chem. 2004;279:16638–45. doi: 10.1074/jbc.M400584200. [DOI] [PubMed] [Google Scholar]
- 7.Ago H, Adachi T, Yoshida A, Yamamoto M, Habuka N, Yatsunami K, Miyano M. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Structure Fold Des. 1999;7:1417–26. doi: 10.1016/s0969-2126(00)80031-3. [DOI] [PubMed] [Google Scholar]
- 8.Bressanelli S, Tomei L, Roussel A, Incitti I, Vitale RL, Mathieu M, De Francesco R, Rey FA. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc Natl Acad Sci U S A. 1999;96:13034–9. doi: 10.1073/pnas.96.23.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesburg CA, Cable MB, Ferrari E, Hong Z, Mannarino AF, Weber PC. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat Struct Biol. 1999;6:937–43. doi: 10.1038/13305. [DOI] [PubMed] [Google Scholar]
- 10.O′Farrell D, Trowbridge R, Rowlands D, Jager J. Substrate complexes of hepatitis C virus RNA polymerase (HC-J4): structural evidence for nucleotide import and de-novo initiation. J Mol Biol. 2003;326:1025–35. doi: 10.1016/s0022-2836(02)01439-0. [DOI] [PubMed] [Google Scholar]
- 11.Choi KH, Groarke JM, Young DC, Kuhn RJ, Smith JL, Pevear DC, Rossmann MG. The structure of the RNA-dependent RNA polymerase from bovine viral diarrhea virus establishes the role of GTP in de novo initiation. Proc Natl Acad Sci U S A. 2004;101:4425–30. doi: 10.1073/pnas.0400660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao Y, Farsetta DL, Nibert ML, Harrison SC. RNA synthesis in a cage--structural studies of reovirus polymerase lambda3. Cell. 2002;111:733–45. doi: 10.1016/s0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- 13.Butcher SJ, Grimes JM, Makeyev EV, Bamford DH, Stuart DI. A mechanism for initiating RNA-dependent RNA polymerization. Nature. 2001;410:235–40. doi: 10.1038/35065653. [DOI] [PubMed] [Google Scholar]
- 14.Hansen JL, Long AM, Schultz SC. Structure of the RNA-dependent RNA polymerase of poliovirus. Structure. 1997;5:1109–22. doi: 10.1016/s0969-2126(97)00261-x. [DOI] [PubMed] [Google Scholar]
- 15.Hobson SD, Rosenblum ES, Richards OC, Richmond K, Kirkegaard K, Schultz SC. Oligomeric structures of poliovirus polymerase are important for function. Embo J. 2001;20:1153–63. doi: 10.1093/emboj/20.5.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labonte P, Axelrod V, Agarwal A, Aulabaugh A, Amin A, Mak P. Modulation of hepatitis C virus RNA-dependent RNA polymerase activity by structure-based site-directed mutagenesis. J Biol Chem. 2002;277:38838–46. doi: 10.1074/jbc.M204657200. [DOI] [PubMed] [Google Scholar]
- 17.Richards OC, Yu P, Neufeld KL, Ehrenfeld E. Nucleotide binding by the poliovirus RNA polymerase. J Biol Chem. 1992;267:17141–6. [PubMed] [Google Scholar]
- 18.Arnold JJ, Cameron CE. Poliovirus RNA-dependent RNA polymerase (3D(pol)). Assembly of stable, elongation-competent complexes by using a symmetrical primer-template substrate (sym/sub) J Biol Chem. 2000;275:5329–36. doi: 10.1074/jbc.275.8.5329. [DOI] [PubMed] [Google Scholar]
- 19.Bychkova VE, Ptitsyn OB. The Molten Globule in Virto and In Vivo. CHEMTRACTS-Bichemistry and Molecular Biology. 1993;4:133–163. [Google Scholar]
- 20.Temiakov D, Patlan V, Anikin M, McAllister WT, Yokoyama S, Vassylyev DG. Structural basis for substrate selection by t7 RNA polymerase. Cell. 2004;116:381–91. doi: 10.1016/s0092-8674(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SJ, Taylor JS, Beese LS. Processive DNA synthesis observed in a polymerase crystal suggests a mechanism for the prevention of frameshift mutations. Proc Natl Acad Sci U S A. 2003;100:3895–900. doi: 10.1073/pnas.0630532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matulis D, Baumann CG, Bloomfield VA, Lovrien RE. 1-anilino-8-naphthalene sulfonate as a protein conformational tightening agent. Biopolymers. 1999;49:451–8. doi: 10.1002/(SICI)1097-0282(199905)49:6<451::AID-BIP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Pace CN, Hebert EJ, Shaw KL, Schell D, Both V, Krajcikova D, Sevcik J, Wilson KS, Dauter Z, Hartley RW, Grimsley GR. Conformational stability and thermodynamics of folding of ribonucleases Sa, Sa2 and Sa3. J Mol Biol. 1998;279:271–86. doi: 10.1006/jmbi.1998.1760. [DOI] [PubMed] [Google Scholar]
- 24.Celej MS, Montich GG, Fidelio GD. Protein stability induced by ligand binding correlates with changes in protein flexibility. Protein Sci. 2003;12:1496–506. doi: 10.1110/ps.0240003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bressanelli S, Tomei L, Rey FA, De Francesco R. Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides. J Virol. 2002;76:3482–92. doi: 10.1128/JVI.76.7.3482-3492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin YW, Steitz TA. Structural basis for the transition from initiation to elongation transcription in T7 RNA polymerase. Science. 2002;298:1387–95. doi: 10.1126/science.1077464. [DOI] [PubMed] [Google Scholar]
- 27.Yin YW, Steitz TA. The structural mechanism of translocation and helicase activity in T7 RNA polymerase. Cell. 2004;116:393–404. doi: 10.1016/s0092-8674(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 28.Mao L, Wang Y, Liu Y, Hu X. Molecular determinants for ATP-binding in proteins: a data mining and quantum chemical analysis. J Mol Biol. 2004;336:787–807. doi: 10.1016/j.jmb.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 29.Arnold JJ, Cameron CE. Poliovirus RNA-dependent RNA polymerase (3Dpol): presteady-state kinetic analysis of ribonucleotide incorporation in the presence of Mg2+ Biochemistry. 2004;43:5126–37. doi: 10.1021/bi035212y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 31.Sosunov V, Sosunova E, Mustaev A, Bass I, Nikiforov V, Goldfarb A. Unified two-metal mechanism of RNA synthesis and degradation by RNA polymerase. Embo J. 2003;22:2234–44. doi: 10.1093/emboj/cdg193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sosunov V, Zorov S, Sosunova E, Nikolaev A, Zakeyeva I, Bass I, Goldfarb A, Nikiforov V, Severinov K, Mustaev A. The involvement of the aspartate triad of the active center in all catalytic activities of multisubunit RNA polymerase. Nucleic Acids Res. 2005;33:4202–11. doi: 10.1093/nar/gki688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steitz TA. A mechanism for all polymerases. Nature. 1998;391:231–2. doi: 10.1038/34542. [DOI] [PubMed] [Google Scholar]
- 34.Hobson S. Crystallographic and Biochemical Studies of Higher Order Poliovirus Polymerase Structures. Ph.D., University of Colorado; 2000. [Google Scholar]
- 35.Steitz TA. Visualizing polynucleotide polymerase machines at work. Embo J. 2006;25:3458–68. doi: 10.1038/sj.emboj.7601211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Marco S, Volpari C, Tomei L, Altamura S, Harper S, Narjes F, Koch U, Rowley M, De Francesco R, Migliaccio G, Carfi A. Interdomain communication in hepatitis C virus polymerase abolished by small molecule inhibitors bound to a novel allosteric site. J Biol Chem. 2005;280:29765–70. doi: 10.1074/jbc.M505423200. [DOI] [PubMed] [Google Scholar]
- 37.Pflugrath JW. The finer things in X-ray diffraction data collection. Acta Crystallogr D Biol Crystallogr. 1999;55(Pt 10):1718–25. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- 38.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 5):905–21. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 39.Jones TA, Zou JY, Cowan SW, Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47(Pt 2):110–9. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 40.Schuttelkopf AW, van Aalten DM. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr. 2004;60:1355–63. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 41.DeLano WL. The PYMOL Molecular Graphics System. DeLano Scientific, San Carlos; CA USA: 2002. [Google Scholar]


