Abstract
Background
The effects of the murine monoclonal anti-PcrV antibody Mab166 on acute lung injury induced by Pseudomonas aeruginosa were analyzed in a rat model.
Methods
Lung injury was induced by the instillation of P. aeruginosa strain PA103 directly into the left lungs of anesthetized rats. One hour after the bacterial instillation, rabbit polyclonal anti-PcrV IgG, murine monoclonal anti-PcrV IgG Mab166 or Mab166 Fab-fragments were administered intratracheally directly into the lungs. The degree of alveolar epithelial injury, amount of lung edema, decrease in oxygenation and extent of lung inflammation by histology were evaluated as independent parameters of acute lung injury.
Results
These parameters improved in rats that had received intratracheal instillation of either rabbit polyclonal anti-PcrV IgG, murine monoclonal anti-PcrV IgG Mab166 or Mab166 Fab-fragments in comparison with the control group.
Conclusion
Mab166 and its Fab fragments have potential as adjuvant therapy for acute lung injury due to P. aeruginosa pneumonia.
Background
Pseudomonas aeruginosa (P. aeruginosa) pneumonia frequently causes bacteremia and sepsis in immunocompromised and mechanically ventilated patients [1-7]. This leads to an increased morbidity and mortality compared with pneumonia caused by other pathogens [1-5]. The rapid systemic dissemination of P. aeruginosa is associated with the fact that some strains of P. aeruginosa cause acute lung epithelial injury by inducing the necrosis of the lung epithelium [8,9]. To protect patients who are at risk for the development of P. aeruginosa pneumonia and sepsis, therapy would have to be given prior to the development of extensive lung injury, as dissemination and multi-system organ failure occur once significant lung epithelial injury is produced [10]. In addition, resistance to antibiotics is a major problem in the therapy of P. aeruginosa infections in critically ill patients. Therefore, a need for non-antibiotic based adjuvant therapies for virulent P. aeruginosa has created more interest in generating antibody reagents against the Pseudomonal virulence factors causing acute lung injury.
The pathogenicity of P. aeruginosa appears to be related to its repertoire of toxins. Type III secretion is a recently identified toxin secretion system found in most pathogenic gram-negative bacteria [11,12]. Requiring intimate contact with eukaryotic cell surfaces, this bacterial secretion system delivers its toxins directly into the cytosol of the eukaryotic cells, thereby modulating the host immune response [13]. The virulence of type III secretory cytotoxins in P. aeruginosa is associated with acute lung epithelial damage and dissemination of inflammatory cytokines and bacteria from the lungs to the circulation [10]. To date, four type III secretory toxins (ExoS, ExoT, ExoU and ExoY) have been identified in P. aeruginosa. Cytotoxic P. aeruginosa possesses the type III secreted cytotoxin ExoU, which is necessary for causing acute necrotic cell death [14-16].
We have documented that clinical isolates of P. aeruginosa expressing the type III secretory proteins was associated with higher morbidity and poorer outcome than that for patients infected with P. aeruginosa strains that did not secrete these proteins [17]. In addition, a correlation between poor prognosis of patients with ventilator-associated pneumonia caused by P. aeruginosa and the bacterial expression of type III secretion was also reported [18]. PcrV is one component of the P. aeruginosa type III secretion system and is homologous to the Yersinia V-antigen (LcrV) [16]. PcrV appears to be an integral component of the translocation apparatus of the type III secretion system mediating the delivery of the type III secretory toxins into target eukaryotic cells [19]. Active and passive immunization against PcrV improved acute lung injury and mortality of mice infected with cytotoxic P. aeruginosa [19]. The major effect of immunization against PcrV was due to the blockade of translocation of the type III secretory toxins into eukaryotic cells [19]. Furthermore, we demonstrated that the therapeutic administration of a polyclonal anti-PcrV IgG prevented septic shock and acute lung injury in a rabbit model of P. aeruginosa pneumonia, and that the effects of the anti-PcrV antibody were independent of the Fc-fragments of IgG [20].
We recently generated a murine monoclonal anti-PcrV antibody, Mab166, that was found to be protective against P. aeruginosa-induced mortality when coinstilled with the bacteria in lungs or intraperitoneally administered to mice before infection [21]. More recently, major advances have been made in the development of antibodies safe for human patients; this has been accomplished by engineering recombinant antibodies to decrease the immunogenicity of murine antibodies (chimeric and humanized antibodies) and by developing transgenic animals that produce human monoclonal antibodies. Mab166 could be humanized if it proves to be effective in protecting animals from virulent P. aeruginosa. Our objective in this study was to test the protective effects of a murine monoclonal antibody, an antibody that could be humanized, in a model of early P. aeruginosa lung infection. If effective in early infection, the monoclonal antibody would be as effective or even more protective when given prior to the development of infection. Therefore, we investigated the protective properties of intratracheally administered Mab166 and its Fab fragments on acute lung injury in a rat model of P. aeruginosa pneumonia.
Methods
Animals
Certified pathogen-free, Sprague Dawley male rats (body weight, 280–380 g) were purchased from Charles River Laboratories (Wilmington, MA). The rats were housed in cages with filter tops in specific pathogen-free conditions. Sterile food and water were provided ad lib. All experiments were done in compliance with Animal Care Committee rules of the University of California at San Francisco, U.S.A., and all protocols were approved prior to the start of the experiments.
P. aeruginosa strain and culture conditions
P. aeruginosa PA103 was used in this study. Bacteria from frozen stocks, stored at -70°C in 10% sterile skim milk solutions, were streaked onto trypticase soy agar plates. Five milliliters of a deferrated dialysate of trypticase soy broth supplemented with 10 mM nitrilotriacetic acid (Sigma Chemical, St. Louis, MO), 1% glycerol, and 100 mM monosodium glutamate was inoculated with a loop of bacteria and grown at 33°C for 13 h under shaking conditions. Cultures were centrifuged at 8,500 × g for 5 min and the media discarded. The bacterial pellet was washed twice in lactated Ringer's (L/R) solution and diluted to the appropriate concentration of CFU/ml in L/R solution, as determined by spectrophotometry. Plating out the known dilutions on sheep blood agar plates confirmed the bacterial concentrations.
Surgical preparation and ventilation
The rat model for P. aeruginosa pneumonia was reported previously [22,23]. Briefly, rats were anesthetized with 100 mg/kg of pentobarbital sodium administered intraperitoneally. An endotracheal tube (PE-240, Clay Adams, Parsippany, NJ) was inserted into the trachea via an open tracheostomy. The rats were ventilated with a constant-volume respirator (Harvard Apparatus, South Natick, MA) with an inspired O2 fraction of 1.0, peak airway pressures of 8–12 cmH2O and a 2 cm positive end expiratory pressure (PEEP). The respiratory rate was adjusted to maintain PaCO2 between 35 and 45 mmHg. The rats remained anesthetized, intubated and ventilated throughout the entire experiment. The right carotid artery was canulated with a polyethylene tube (PE-50, Clay Adams) to monitor systemic arterial pressure, administrate drugs and obtain blood samples.
Bacterial instillate preparation and administration
The instillate consisted of 5% bovine serum albumin (BSA), 2 mg of Evans blue dye, and 1 μCi of 131I-labeled albumin, and P. aeruginosa, at a final concentration of 5 × 107 CFU/ml in L/R solution to a total volume of 1 milliliter; Colloid osmotic pressure of the instillate was adjusted by adding 5% BSA as an established method to quantify liquid clearance of lung epithelial barriers as an index of lung edema [24]. The bacteria were added just before airspace instillation if the experiment was to include bacteria. A sample of the instillate was saved for radioactivity measurement (counts/min/g) in a γ-ray counter (Auto-Gamma, model 5550, Packard, Downers Grove, IL) and quantitative bacterial cultures on sheep blood agar plates to assure accurate inoculations. The instillates were delivered slowly, over a 30 min period using a polyethylene tube (PE-10, Clay Adams) into the left lungs.
Interventions
Mab166 IgG (IgG2bκ) or its Fab fragments were previously prepared in PBS and stored at -70°C [21]. Experimental groups are listed in Table 1. One additional group of rats was used as the sham control group; rats received L/R solution not containing IgG. In three groups of rats, we co-instilled P. aeruginosa PA103 (5 × 107 CFU) with 4 mg/kg of either mouse monoclonal isotype-matched control IgG (IgG2b, clone #20116.11, R&D System, Minneapolis, MN), rabbit anti-PcrV polyclonal IgG, or murine monoclonal Mab166 IgG intratracheally. In another three groups, rats received either PBS or 4 mg/kg of either anti-PcrV polyclonal IgG, Mab166 IgG, or Mab166 Fab fragments one hour after the airspace instillation of P. aeruginosa PA103 (5 × 107 CFU).
Table 1.
Experimental groups.*
| Groups | Infection (P. aeruginosa) | Intervention | n |
| Control | None | 3 | |
| Co-instillation | Antibodies were premixed with PA103 | ||
| Control IgG | PA103 (5 × 107 CFU), IT | Control IgG (IgG2b), 4 mg/ml IT | 3 |
| Rab anti-PcrV | PA103 (5 × 107 CFU), IT | Polyclonal anti-PcrV IgG, 4 mg/ml IT | 3 |
| Mab166 | PA103 (5 × 107 CFU), IT | Monoclonal Mab166, 4 mg/ml IT | 3 |
| Therapeutic | Antibodies were intratracehally instilled 1 h after the instillation of PA103 | ||
| w/o IgG | PA103 (5 × 107 CFU), IT | Phosphate-buffered saline | 5 |
| Rab anti-PcrV | PA103 (5 × 107 CFU), IT | Polyclonal anti-PcrV IgG, 4 mg/ml IT | 3 |
| Mab166 | PA103 (5 × 107 CFU), IT | Monoclonal Mab166, 4 mg/ml IT | 5 |
| Mab166 Fab | PA103 (5 × 107 CFU), IT | Fab fragments of Mab166, 4 mg/ml IT | 3 |
*IT: Intratracheal administration
General experimental protocol
After surgical preparation, blood pressure and gas exchanges were allowed to stabilize. Systemic arterial pressure and airway pressure were continuously monitored using an on-line data logging system (Powerlab, ADInstruments, Mountain View, CA). Blood samples were collected every hour for gas exchange measurement, 131I-albumin radioactivity count and bacterial culture. The rats were kept anesthetized and paralyzed throughout the experiment. Four hours after bacterial instillation, rats were deeply anesthetized and exsanguinated. Pleural fluids were obtained for radioactivity counts. The lungs were removed through a sternotomy; the left and right lobes were weighed and homogenized separately for water to dry weight ratio measurement and radioactivity counts.
Measurement of lung injury
Lung injury was quantified in two different ways, as previously described [22,23]. The first method evaluates the integrity of the lung epithelial barrier by quantifying the efflux of 131I-albumin from the alveolar to the bloodstream. Total 131I-albumin instilled into the lung was determined by measuring duplicate samples of the instillate for total radioactivity (cpm/g) and multiplying this amount by the total volume instilled into the lung. Circulating plasma 131I-albumin was measured from blood samples obtained every hour and at the end of the experiment. The plasma fraction was calculated by multiplying the counts per gram times the plasma volume [body weight × 0.07 (1-hematocrit)]. The second method, the water to dry weight ratio, is a well-accepted index of lung edema. Lung homogenates were placed in pre-weighed aluminum pans and dried to a constant weight in an oven at 80°C for 3 days. The excess water in the experimental lung was calculated with an equation described previously [22,23].
Histology analysis
Lungs were perfused with 10% buffered formalin phosphate for fixation and were embedded in paraffin. Mounted sections were stained with hematoxylin-eosin and observed under light microscopy.
Statistical analysis
Results are presented as mean ± standard errors. The difference between the control IgG-treated group and the Mab166 IgG or Mab166 Fab fragments-treated group was analyzed. Two-way analysis of variance (ANOVA), repeated measure, followed by the Newman-Keuls t-test or unpaired Student's t-test was used for comparisons of data. Significance was accepted at P value of < 0.05.
Results
Coinstillation of Mab166 with P. aeruginosa decreased induced acute lung injury
First, to evaluate the maximal blocking effects of anti-PcrV IgGs on P. aeruginosa-induced acute lung injury, we coinstilled either rabbit-derived polyclonal anti-PcrV IgG, murine monoclonal anti-PcrV IgG Mab166 (IgG2b), or irrelevant monoclonal control IgG (IgG2b) (4 mg/kg, respectively) with P. aeruginosa PA103 (5 × 107 CFU) into the lungs of the anesthetized ventilated rats under artificially controlled ventilation. The antibodies were premixed with P. aeruginosa three min before the instillation. The acute alveolar lung injury was quantified as the efflux of the coinstilled radioactive alveolar protein tracers (131I-albumin) into the circulation every one-hour during the 4-h experimental period.
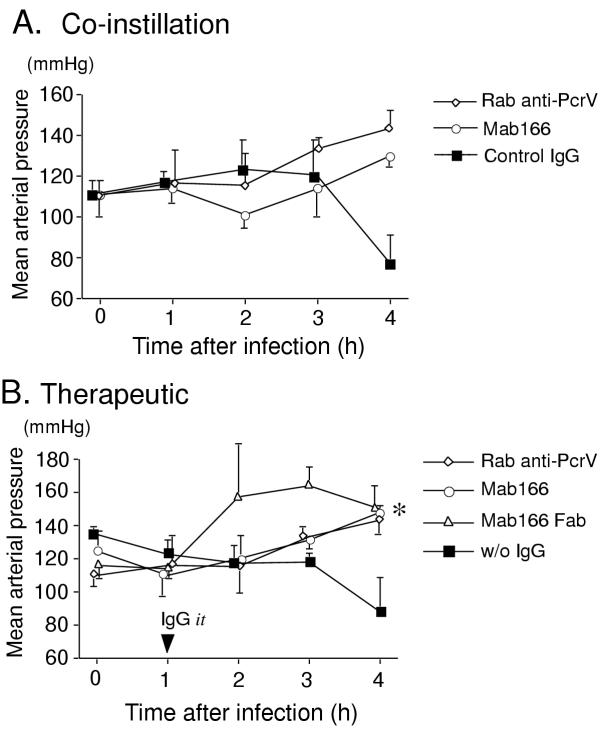
The control rats that received the lactated Ringer's solution supplemented with 5% bovine serum albumin but without bacteria did not show any lung epithelial injury (Fig. 1). Wet to dry weight ratios of the lungs increased to approximately 6 in the control rats (Fig. 2). Note a wet to dry weight ratio of the lung of a normal rat is between 3.5–4.0 (data not shown). The rats that received P. aeruginosa mixed with control irrelevant monoclonal IgG intratracheally developed significant acute epithelial injury in 4 h (Fig. 1). Severe lung edema was observed in this group of rats 4 h after bacterial instillation (Fig. 2). The arterial blood pressure decreased below 80 mmHg after 4 h time point (Fig. 3). Arterial blood oxygenation severely decreased to approx. 100 mmHg immediately after bacterial instillation, never normalized (Fig. 4). Metabolic acidosis gradually developed over the 4 h in this group of rats (Fig. 5).
Figure 1.
Quantification of acute lung epithelial injury. The efflux of alveolar protein tracer (131I-albumin) from lungs to the circulation was calculated in 4-h experiments of rats as an index of acute lung epithelial injury. In the control group (Control, no bacteria), only lactated Ringer's solution was instilled into the airspace of the rats and no therapeutic intervention was taken. Three sets of rats were co-instilled P. aeruginosa PA103 with 4 mg/kg of either irrelevant monoclonal IgG (Control IgG), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV), or murine monoclonal anti-PcrV IgG (Mab166). Four sets of rats were intratracheally administered either PBS, rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV)(4 mg/kg), murine monoclonal anti-PcrV IgG (Mab166)(4 mg/kg), or Mab166 Fab (4 mg/kg). Data are shown as means+standard errors. The numbers of rats are listed in Table 1. +P < 0.05 to the control group (no bacteria) and *P < 0.05 to the control IgG group in co-instillation and to the group without IgG (PBS) in therapeutic administration by two way-ANOVA, repeated measure, followed by the Newman-Keuls t-test.
Figure 2.
Quantification of lung edema. Water-to-wet weight ratios of the lungs were measured at 4-h time points in the rats infected with P. aeruginosa. as an index of acute lung edema. In the control group (Control, no bacteria), only lactated Ringer's solution was instilled into the airspace of the rats and no therapeutic intervention was taken. Three sets of rats were co-instilled P. aeruginosa PA103 with 4 mg/kg of either irrelevant monoclonal IgG (Control IgG), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV), or murine monoclonal anti-PcrV IgG (Mab166). Four sets of rats were intratracheally administered either phosphate-buffered saline (PBS), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV)(4 mg/kg), murine monoclonal anti-PcrV IgG (Mab166)(4 mg/kg), or Mab166 Fab (4 mg/kg). Data are shown as means+standard errors. The numbers of animals are listed in Table 1. +P < 0.05 to the control group (no bacteria) and *P < 0.05 to the control IgG group in co-instillation and to the group without IgG (PBS) in therapeutic administration by two way-ANOVA, followed by the Newman-Keuls t-test.
Figure 3.
Mean arterial blood pressure. The mean arterial blood pressure was measured for 4 h in the rats. A. Either irrelevant monoclonal IgG (Control IgG, filled squares), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV, open diamonds), or murine monoclonal anti-PcrV IgG (Mab166, open circles) (4 mg/kg, respectively) was co-instilled with P. aeruginosa PA103 (5 × 107 CUF) in the airspaces of the rats. B. Either PBS without IgG (w/o IgG, filled squares), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV, open diamonds), murine monoclonal anti-PcrV IgG (Mab166, open circles), or Fab fragments Mab166 (Mab166 Fab, open triangles) (4 mg/kg, respectively) was intratracheally instilled one hour after the airspace instillation of P. aeruginosa PA103 (5 × 107 CUF). Data are shown as means ± standard errors. The numbers of animals are listed in Table 1. *P < 0.05 in the Mab166 group to the control group (w/o IgG) at the 4 h time point by unpaired t-test.
Figure 4.
The oxygenation of arterial blood. The oxygen pressure of the arterial blood was measured for 4 h in the rats. A. Either irrelevant monoclonal IgG (Control IgG, filled squares), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV, open diamonds), or murine monoclonal anti-PcrV IgG (Mab166, open circles) (4 mg/kg, respectively) was co-instilled with P. aeruginosa PA103 (5 × 107 CUF) in the airspaces of the rats. B. Either PBS without IgG (w/o IgG, filled squares), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV, open diamonds), murine monoclonal anti-PcrV IgG (Mab166, open circles), or Fab fragments Mab166 (Mab166 Fab, open triangles) (4 mg/kg, respectively) was intratracheally instilled one hour after the airspace instillation of P. aeruginosa PA103 (5 × 107 CUF). Data are shown as means ± standard errors. The numbers of animals are listed in Table 1.
Figure 5.
Metabolic acidosis. Base excess was measured for 4 h in the rats as an index of metabolic acidosis. A. Either irrelevant monoclonal IgG (Control IgG, filled squares), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV, open diamonds), or murine monoclonal anti-PcrV IgG (Mab166, open circles) (4 mg/kg, respectively) was co-instilled with P. aeruginosa PA103 (5 × 107 CUF) in the airspaces of the rats. B. Either PBS with out IgG (w/o IgG, filled squares), rabbit polyclonal anti-PcrV IgG (Rab anti-PcrV, open diamonds), murine monoclonal anti-PcrV IgG (Mab166, open circles), or Fab fragments Mab166 (Mab166 Fab, open triangles) (4 mg/kg, respectively) was intratracheally instilled one hour after the airspace instillation of P. aeruginosa PA103 (5 × 107 CFU). Data are shown as means ± standard errors. The numbers of animals are listed in Table 1. *P < 0.05 in the Mab166 group to the control group (w/o IgG) at the 4 h time point by unpaired t-test.
The rats that received P. aeruginosa premixed with rabbit polyclonal anti-PcrV IgG intratracheally developed significantly lower levels of lung injury. Alveolar epithelial injury was significantly lower than that of rats that had received control IgG (Fig. 1), and lung edema was less, although not significantly (Fig. 2). Blood pressure was normal for the 4 h (Fig. 3), arterial blood oxygenation recovered by the 4 h time point (Fig. 4), and acidosis did not developed (Fig. 5). Finally, in the rats which received P. aeruginosa premixed with murine monoclonal anti-PcrV IgG Mab166, the lung epithelial injury and lung edema were significantly less than in the other groups (Fig. 1 and 2). Arterial blood pressures and acid-base status of these rats were normal for 4 h (Fig. 3 and 5), and the arterial blood oxygenation was the best among the three groups (Fig. 4). Thus, co-instillation of Mab166 with P. aeruginosa was the most protective.
Therapeutic administration of Mab166 intratracheally protects against P. aeruginosa-induced acute lung injury
Next, we evaluated the therapeutic administration of anti-PcrV IgG in our rat model. In this series of the experiments, we administered either rabbit polyclonal anti-PcrV IgG, murine monoclonal anti-PcrV IgG Mab166, Fab fragments of Mab166 (4 mg/kg, respectively), or PBS alone without IgG 1 h after the instillation of P. aeruginosa (5 × 107 CFU) into the lungs of the anesthetized ventilated rats. The rats that received PBS alone 1 h after bacterial instillation showed a significant increase in lung epithelial injury and lung edema after 4 h. The arterial blood pressure gradually decreased to 80 mmHg over the experimental periods. The arterial blood oxygenation remained significantly decreased (Fig. 4). Severe metabolic acidosis developed over the 4 h (Fig. 5).
The rats that had received either rabbit polyclonal anti-PcrV IgG, murine monoclonal anti-PcrV IgG Mab166, or Fab fragments of Mab166 (4 mg/kg) intratracheally showed significant improvement of alveolar epithelial injury and lung edema 4 h after bacterial instillation (Fig. 1). The protective effect of Mab166 Fab fragments on lung epithelial injury was the most significant among the three antibodies, while rabbit polyclonal and murine monoclonal anti-PcrV IgGs were better in improving lung edema than Mab166 Fab fragments (Fig. 2). Hypotension did not develop in the three groups of rats that received any anti-PcrV antibodies (Fig. 3). The arterial oxygenation in the three treated groups of rats was significantly improved compared to the untreated rats (Fig. 4). Although mild metabolic acidosis did develop in the rats that had received either rabbit polyclonal anti-PcrV IgG or Mab166 Fab, the rats that had received Mab166 did not become acidotic (Fig. 5).
We compared the lung histology between the rats treated with Mab166 and the rats treated with control IgG (Fig. 6). While the rats that received control IgG one hour after bacterial instillation showed severe neutrophil recruitment and destruction of alveolar structures (Fig. 6A), the rats that received Mab166 had almost no neutrophils in their airspaces and had preservation of normal alveolar structures (Fig. 6B). As a result, therapeutic administration of Mab166 showed comparable effects to rabbit polyclonal anti-PcrV IgG in preventing acute lung injury and subsequently occurring systemic distress. The therapeutic administration of Mab166 Fab fragments also had the same or better effects than the administration of Mab166 IgG.
Figure 6.
Lung histology. Four hours after the intratracheal instillation of P. aeruginosa PA103 (5 × 107 CFU), the rats were euthanized and their lungs were perfused with 10% buffered formalin phosphate for fixation and were embedded in paraffin. Mounted sections were stained with hematoxylin-eosin and observed in light microscopy. A. The rat received irrelevant control IgG (4 mg/kg) intratracheally one hour after bacterial instillation. B. The rat received Mab166 (4 mg/kg) intratracheally one hour after bacterial instillation. Magnification of objective lens 20× (left figures) and 40× (right figures).
Discussion
The widespread use of antibiotics has generated multiple antibiotic-resistant microorganisms, and there is a new need for non-antibiotic based adjuvant therapies for microbial infections. Antibody-based immunotherapy is one of the adjuvant therapies that can help treat antibiotic-resistant bacterial infections. In this investigation, we showed that intratracheal administration of murine monoclonal anti-PcrV IgG Mab166 improved acute lung injury in infected animals. An important consideration in the comparisons of effectiveness of various treatments of lung infections in experimental animal models is the ability to produce a consistent quantity of bacterial induced lung injury. In our rat model, the administration of P. aeruginosa (5 × 107 CFU) for an interval (4 h) consistently leads to modest quantities of lung injury. Using independent measurement of lung epithelial injury and of lung edema, we have been able to evaluate the therapeutic effects of various antibodies on acute lung injury [22]. The effects of Mab166 were comparable to the administration of rabbit polyclonal anti-PcrV IgG. The intratracheal administration of Mab166 (4 mg/kg) significantly improved the lung epithelial injury caused by cytotoxic P. aeruginosa. Lung edema, measured as wet/dry ratios of the lungs, decreased significantly in the rats treated with intratracheal Mab166. The lung wet/dry ratios of the rats instilled with bacteria and treated with any of anti-PcrV IgGs were lower that those of the control rats (no bacteria) probably due to the ability of Pseudomonal exotoxin A to increase lung liquid clearance. Note P. aeruginosa strain PA103 used in this study is a high producer of type II secretory exotoxin A and P. aeruginosa treated with anti-PcrV IgG would still secrete exotoxin A which has been shown to increase the lung liquid clearance (and decrease lung edema) [25] although exotoxin A itself does not cause neither lung epithelial injury nor lung edema [26]. Hemodynamics, oxygenation, and metabolic acidosis were improved by the treatment with intratracheal Mab166. Lung histology in the rat treated with Mab166 showed significant improvement and preservation of normal structures. We previously showed that F(ab')2 fragments of rabbit polyclonal anti-PcrV IgG prevented sepsis and allowed survival in a rabbit model of P. aeruginosa infection [20]. Similarly, the Fab fragments of the murine monoclonal anti-PcrV IgG (Mab166 Fab) had comparable therapeutic effects to the whole IgG molecules of Mab166 in preventing P. aeruginosa-induced acute lung injury. Because, Fab portions had the same therapeutic effects as whole IgG in P. aeruginosa-induced lung injury, the Fc-dependent opsonization of the bacteria does not seem critical for the efficacy of the anti-PcrV antibodies.
Intratracheal administration of Fab is attractive for the following reasons: 1) Direct delivery of therapeutic agents in the site of infection is advantageous pharmacokinetically. Only limited amounts of systemically administered IgGs (intravenously, or intramuscularly) reach the airspaces of the lung. 2) The administration of the whole IgG may cause some inflammatory side effects, because the Fc-portion of IgG may induce unfavourable inflammatory responses such as complement fixation, activation of macrophages. In our study, Fab fragment had the same therapeutic potency as the whole IgG and the therapeutic administration of Fab fragments may overcome the disadvantages of the intratracheal administration of whole IgG.
Since the discovery of the production of monoclonal antibodies by Kohler and Milstein in 1975, only a handful of antibodies had been used in human therapy [27]. The main difficulty with monoclonal antibodies is that mouse antibodies are seen by the human immune system as foreign, and the patient mounts an immune response against them, producing "human anti-mouse antibodies (HAMA)". These not only cause the therapeutic antibodies to be eliminated from the host, but also cause the formation of immune complexes that damage the kidneys. Therefore, technology has focused on methodology that produces less immunogenic monoclonal antibodies. More recently, the techniques to engineer recombinant chimera and humanized antibodies have been developed to decrease the immunogenicity of murine antibodies [28]. Due to the multiple antibiotic resistance mechanisms that P. aeruginosa possesses, the need for adjunctive therapies is becoming more important. Therefore, anti-PcrV antibody-based immunotherapies are potential therapeutic options for immunocompromised patients infected with P. aeruginosa.
Conclusions
Intratracheal administration of the murine monoclonal anti-PcrV antibody Mab166 and its Fab fragments protected rats infected with Pseudomonas aeruginosa from acute lung injury. Mab166 and its Fab fragments are potential useful adjuvant therapies for acute lung injury secondary to P. aeruginosa pneumonia.
Authors' contributions
K. Fuare carried out animal studies, and drafted the manuscript. J. Fujimoto, D. W. Shimabukuro, N. Shime and K. Moriyama participated in the animal studies. T. Ajayi edited the manuscript. E. G. Spack contributed to the production and purification of antibodies. J. P. Wiener-Kronish and T. Sawa conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Abbreviations
P. aeruginosa:Pseudomonas aeruginosa, IT: Intratracheal administration
Acknowledgments
Acknowledgements
This research was supported by NIH grant HL067600 and American Lung Association Research Grant RG-004-N to T. Sawa, NIH grants RO1 HL59239 & AI44101, and a grant sponsored by InterMune, Inc. (Brisbane, California, U.S.A.) to J. P. Wiener-Kronish.
Contributor Information
Karine Faure, Email: karine-faure@invivo.edu.
Junichi Fujimoto, Email: junfuji@med.yokohama-cu.ac.jp.
David W Shimabukuro, Email: shimabud@anesthesia.ucsf.edu.
Temitayo Ajayi, Email: tajayi@itsa.ucsf.edu.
Nobuaki Shime, Email: shime@koto.kpu-m.ac.jp.
Kiyoshi Moriyama, Email: kmor7200@itsa.ucsf.edu.
Edward G Spack, Email: tspack@intermune.com.
Jeanine P Wiener-Kronish, Email: wienerkj@anesthesia.ucsf.edu.
Teiji Sawa, Email: teiji@itsa.ucsf.edu.
References
- Almirall J, Mesalles E, Klamburg J, Parra O, Agudo A. Prognostic factors of pneumonia requiring admission to the intensive care unit. Chest. 1995;107:511–516. doi: 10.1378/chest.107.2.511. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Regnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. doi: 10.1001/jama.274.12.968. [DOI] [PubMed] [Google Scholar]
- Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- Vidal F, Mensa J, Almela M, Martinez JA, Marco F, Casals C, Gatell JM, Soriano E, Jimenez de Anta MT. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med. 1996;156:2121–2126. doi: 10.1001/archinte.156.18.2121. [DOI] [PubMed] [Google Scholar]
- Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gilbert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- Parrillo JE, Parker MM, Nathanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans Advances in the understanding of pathogenesis, cardiovascular dysfunction and therapy. Ann Intern Med. 1990;113:227–237. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Taylor GD, Buchanan-Chell M, Kirkland T, McKenzie M, Wiens R. Bacteremic nosocomial pneumonia. A 7-year experience in one institution. Chest. 1995;108:786–788. doi: 10.1378/chest.108.3.786. [DOI] [PubMed] [Google Scholar]
- Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest. 1991;88:864–875. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener-Kronish JP, Sakuma T, Kudoh I, Pittet JF, Frank D, Dobbs L, Vasil ML, Matthay M. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol. 1993;75:1661–1669. doi: 10.1152/jappl.1993.75.4.1661. [DOI] [PubMed] [Google Scholar]
- Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104:743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener-Kronish JP, Frank DW, Sawa T. Mechanisms of lung epithelial cell Injury by acute by Pseudomonas aeruginosa. In: Clark RSG, Carcillo JA, editor. In Molecular biology of acute lung injury. Boston: Kluwer Academic Publishers; 2001. pp. 149–161. [Google Scholar]
- Galan JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- Frank DW. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr TL, Mende-Mueller LM, Friese MB, Frank DW. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infection. J Infect Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Sawa T, Yahr TL, Ohara M, Kurahashi K, Gropper MA, Wiener-Kronish JP, Frank DW. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- Shime N, Sawa T, Fujimoto J, Faure K, Allmond LR, Karaca T, Swanson BL, Spack EG, Wiener-Kronish JP. Therapeutic administration of anti-PcrV F(ab') 2 in sepsis associated with Pseudomonas aeruginosa. J Immunol. 2001;167:5880–5886. doi: 10.4049/jimmunol.167.10.5880. [DOI] [PubMed] [Google Scholar]
- Frank DW, Vallis A, Wiener-Kronish JP, Roy-Burman A, Spack EG, Mullaney BP, Megdoud M, Marks JD, Fritz R, Sawa T. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J Infect Dis. 2002;186:64–73. doi: 10.1086/341069. [DOI] [PubMed] [Google Scholar]
- Ernst EJ, Hashimoto S, Guglielmo J, Sawa T, Pittet JF, Kropp H, Jackson JJ, Wiener-Kronish JP. Effects of antibiotic therapy on Pseudomonas aeruginosa-induced lung injury in a rat model. Antimicrob Agents Chemother. 1999;43:2389–2394. doi: 10.1128/aac.43.10.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa T, Kurahashi K, Ohara M, Gropper MA, Doshi V, Larrick JW, Wiener-Kronish JP. Evaluation of antimicrobial and lipopolysaccharide-neutralizing effects of a aynthetic CAP18 fragment against Pseudomonas aeruginosa in a mouse model. Antimicrob Agents Chemother. 1998;42:3269–3275. doi: 10.1128/aac.42.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayr C, Garat C, Meignan M, Pittet JF, Zelter M, Matthay MA. Alveolar liquid and protein clearance in anesthetized ventilated rats. J Appl Physiol. 1994;76:2636–2642. doi: 10.1063/1.357560. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Hashimoto S, Pian M, McElroy MC, Nitenberg G, Wiener-Kronish JP. Exotoxin A stimulates fluid reabsorption from distal airspaces of lung in anesthetized rats. Am J Physiol. 1996;270:L232–L241. doi: 10.1152/ajplung.1996.270.2.L232. [DOI] [PubMed] [Google Scholar]
- Kudoh I, Wiener-Kronish JP, Hashimoto S, Pittet JF, Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol. 1994;267:L551–L556. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Gavilondo JV, Larrick JW. Antibody engineering at the millennium. Biotechniques. 2000;29:128–138. doi: 10.2144/00291ov01. [DOI] [PubMed] [Google Scholar]