Abstract
Study objectives
Disseminated strongyloides is a rarely reported phenomenon and occurs in immuno-suppressed patients with chronic Strongyloides stercoralis infection. Typically, patients present with pulmonary symptoms but subsequently acquire Gram-negative sepsis. Several cases have been noted in Minnesota, and their presentation, diagnostic evaluation, and clinical outcomes were reviewed.
Design
A retrospective chart review was conducted of complicated strongyloides infections from 1993 to 2002 in Minneapolis and St. Paul, MN. Cases were identified by reviewing hospital microbiology databases.
Setting
Metropolitan hospitals with large immigrant populations.
Results
Nine patients, all of Southeast Asian heritage, were identified. Eight patients immigrated to the United States ≥ 3 years prior to acute presentation. All patients were receiving antecedent corticosteroids; in five patients, therapy was for presumed asthma. Absolute eosinophil counts > 500/μL occurred in only two patients prior to steroid initiation. Eight patients presented with respiratory distress, and Gram-negative sepsis developed in four patients. Four patients had evidence of right-heart strain on ECG or echocardiography at the time of presentation. Three patients died; all had eosinophil counts of < 400/μL.
Conclusions
Serious complications, including death, may occur in patients with chronic strongyloides infection treated with corticosteroids. Strongyloides hyperinfection usually presents as acute respiratory failure and may initially mimic an asthma exacerbation or pulmonary embolism. Southeast Asian patients presenting with new-onset “asthma,” acute respiratory distress, and/or Gram-negative sepsis should undergo evaluation to exclude strongyloides infection.
Keywords: acute respiratory failure, disseminated strongyloides, Gram-negative sepsis, Strongyloides stercoralis
Strongyloides stercoralis infection is a common cause of morbidity and mortality throughout the world, particularly in developing countries, where more than a hundred million are estimated to have chronic infection.1,2 Death from strongyloides is primarily due to hyperinfection or disseminated disease.1,3 Hyperinfection occurs when the parasite load increases and rhabditiform larvae penetrate the bowel mucosa. Hyperinfection implies confinement of the strongyloides larvae to the organs normally involved in the pulmonary autoinfection cycle (ie, GI tract, lungs, and peritoneum). Strongyloides dissemination is defined as larvae migrating to end organs not usually involved in the normal cycle of the parasite, such as brain and skin.4 Gram-negative sepsis and meningitis can occur because strongyloides larvae facilitate the invasion of enteric bacteria through the bowel mucosa into the host’s circulation. Although this is classically viewed as an infectious disease issue, these patients will frequently present with severe respiratory distress or Gram-negative sepsis to a pulmonology or intensive care specialist in the ICU.
Strongyloidiasis hyperinfection may occur years after exposure as a result of the unique ability of strongyloides to autoinfect the host without an obligatory external portion of the life cycle.5,6 Two cases of hyperinfection/dissemination occurring > 50 years after last known host exposure in an endemic area were recently reported.6,7 Although antecedent treatment with corticosteroids accounts for a majority of reported cases,6,8,9 there have been numerous case reports10,11 of strongyloides hyperinfection as a result of chemotherapeutic agents used in the treatment of cancer8 and HIV.
Originally reported in 1966, the first large case series12 of the syndrome of disseminated S stercoralis was published in 1978 from Thailand. The mortality rate of 61 to 85%1,12 may be an overestimate, as it was derived from the accumulation of single published case reports. We report nine cases of strongyloides hyperinfection or dissemination presenting to two county hospitals in Minneapolis and St. Paul, MN.
Materials and Methods
Cases were identified by reviewing microbiology laboratory records from 1992 to 2002 for S stercoralis at HealthPartners’/Regions Hospital and Hennepin County Medical Center. Regions Hospital is a 427-bed urban hospital with an associated Center for International Health serving primarily residents of St. Paul, MN. Hennepin County Medical Center is a 360-bed urban hospital serving primarily Minneapolis, MN. Medical records were reviewed by experts in tropical medicine for inclusion in the case series. Analysis is descriptive.
Results
S stercoralis larvae were identified in nine patients with a clinical syndrome consistent either with hyperinfection syndrome or dissemination. Larvae were found in the stool (n = 9), sputum (n = 7), and skin (n = 1) [Fig 1, 2]. Demographics of the patients with strongyloides hyperinfection/dissemination are presented in Table 1. Adult-onset asthma was diagnosed in six patients 6 months to 10 years prior to presentation.
Figure 1.
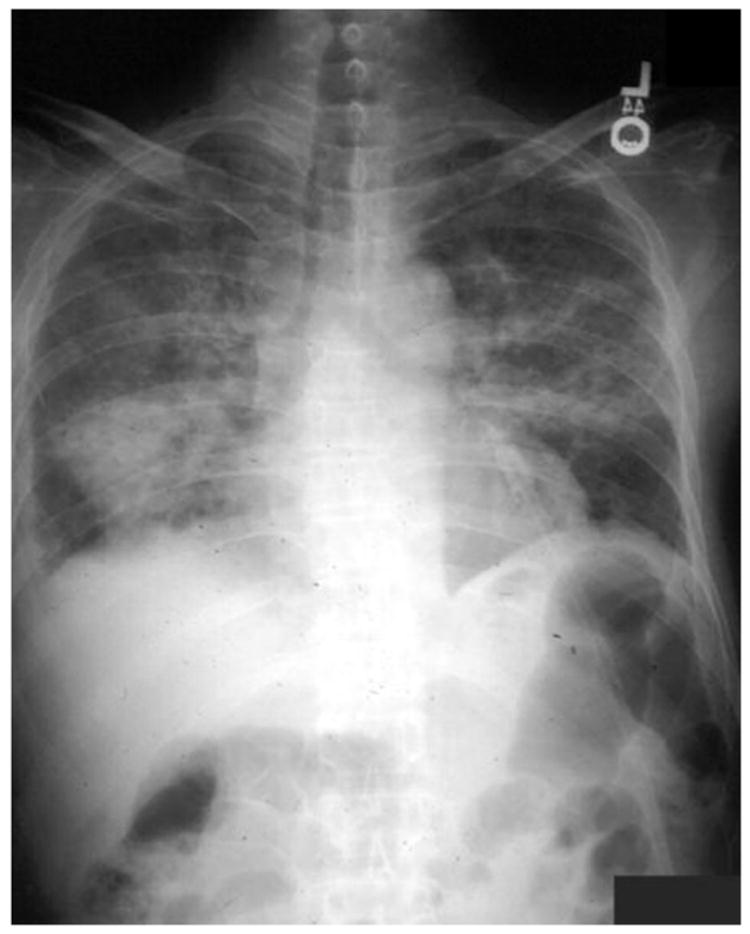
Chest radiograph of a Vietnamese man in the United States for 8 years, with fever, rash, and pneumonia after receiving steroids for uveitis.
Figure 2.
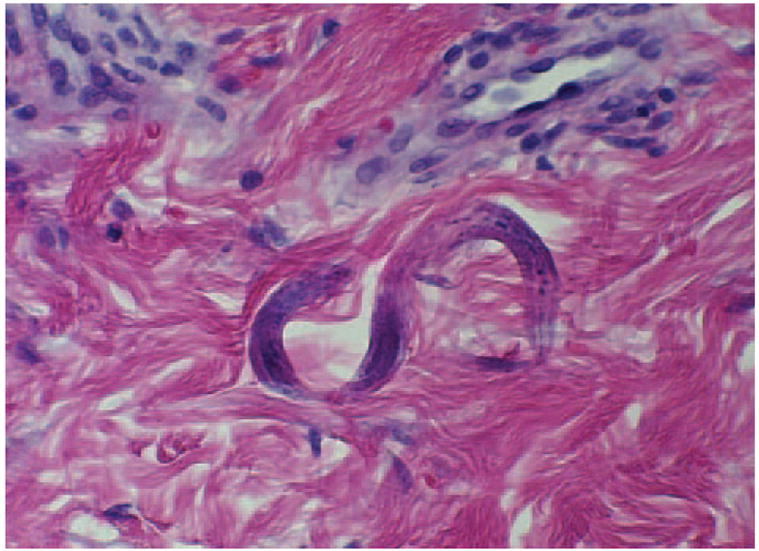
Dermal biopsy sample showing filariform larva of S stercoralis.
Table 1.
Demographic Information and Medical History of Case Patients*
| Age, yr | Gender | Ethnicity | Duration of US Residence | Comorbidities | Respiratory Symptoms at Presentation | Asthma Diagnosis | Outcome and Sequelae |
|---|---|---|---|---|---|---|---|
| 42 | Female | Cambodian | 6 mo | Asthma; hepatitis B | Productive cough | 3 yr prior | Complete recovery; recurrent rash despite repeated treatment |
| 24 | Male | Hmong | 3 yr | Asthma | Wheezing; pleuritic chest pain; shortness of breath | 1 mo prior | Complete recovery; follow-up unknown |
| 34 | Male | Hmong | > 5 yr | Hepatitis B | Productive cough; wheezing; shortness of breath | None | Survived acute event but had severe pulmonary hypertension |
| 52 | Male | Vietnamese | > 5 yr | Uveitis; hepatitis C | Wheezing; shortness of breath | None | Complete recovery with severe malnutrition |
| 46 | Female | Hmong | 8 yr | Asthma; hypertension; chronic renal insufficiency | Wheezing; shortness of breath | 10 yr prior | Survived acute event; died of cerebrovascular accident shortly after |
| 69 | Male | Hmong | 4 yr | COPD; hypothyroidism | Productive cough; wheezing; shortness of breath; pleuritic chest pain | None | Death on day 8 |
| 72 | Male | Laotian | 7 yr | Asthma; hypertension | Shortness of breath; pleuritic chest pain | 1.5 mo prior | Perforated bowel and death |
| 76 | Male | Vietnamese | > 5 yr | Diabetes mellitus, hypertension; aspergillosis | None | 1 mo prior | Complete recovery; 48 h in ICU; follow-up unknown |
| 34 | Female | Hmong | 4 yr | Asthma; hepatitis B | Cough | 6 mo prior | Death on day 12 |
All patients were receiving corticosteroids ranging from 7 days to 4 years.
Eight patients presented with acute pulmonary symptoms, including wheezing, shortness of breath, and pleuritic chest pain. One patient was admitted with an FEV1/FVC ratio of 80% that changed to 100% 10 months after treatment. Another patient had an initial peak flow of 200 L/min on hospital admission; this increased to 325 L/min at the time of discharge. All patients had normal chest radiographic findings on presentation, but five patients acquired bilateral, patchy infiltrates during hospitalization. One patient acquired bilateral pleural effusions. All five patients with abnormal chest radiographic findings required intubation and mechanical ventilation. Eight patients had associated GI symptoms, including abdominal pain, nausea and vomiting. Skin rashes ranging from urticarial to an abdominal macular rash were present in five of the nine cases. The eosinophil count was < 500/μL in seven patients and < 400/μL in four patients. In seven patients, sputum microscopy revealed S stercoralis larvae. In two of the seven patients, S stercoralis larvae were also identified on BAL. Evidence of right-heart strain, either on ECG or echocardiography, was present in four patients. This finding can be misleading on presentation. In fact, two patients underwent evaluation for acute pulmonary embolism prior to the diagnosis of strongyloides hyperinfection.
Three patients died (33% mortality). In all three deaths, no eosinophilia was present (cells < 400/μL). Thiabendazole treatment was administered to six patients, while two patients received ivermectin. One patient had no treatment documented. The duration of treatment ranged from 3 to 18 days. Complicating infections occurred in four patients and included bacteremia and Escherichia coli meningitis, Klebsiella pneumonia, Proteus mirabilis bacteremia, and Enterobacter cloacae pneumonia.
Discussion
Strongyloides hyperinfection may mimic new-onset asthma or an exacerbation of asthma, COPD, or pulmonary embolism. In fact, four patients in this series had ECG or echocardiographic findings suggestive of acute pulmonary embolism, and two patients had this diagnosis investigated at initial presentation; this has not been reported previously. In adults from endemic areas presenting with acute-onset asthma or other acute pulmonary symptoms, strongyloides should be considered as a potential etiology.
Although eosinophilia is a common finding in patients with chronic strongyloides infection,1,10 it is an unreliable predictor of hyperinfection. The lack of eosinophilia (> 400/μL) while receiving immuno-suppressant therapy cannot reliably exclude underlying chronic strongyloides infection.13
Minneapolis/St. Paul has a large refugee and immigrant populations from many endemic areas for strongyloides (East and West Africa, Eastern Europe, countries of the former Soviet Union, and Latin America), but two other large case series have documented disseminated strongyloidiasis: one series6 described seven patients from Thailand, and the other study,12 in a nonendemic setting, found that 30% of patients were of Southeast Asian heritage while another 60% had immigrated from the Caribbean. All patients in this case series were of Southeast Asian descent, predominantly of Laotian (Hmong) heritage. Endemic areas also exist in the United States, most notably in West Virginia, and this diagnosis must be considered in any patient presenting with consistent symptoms who resided in endemic areas.
Conclusion
Given the serious complications of strongyloides hyperinfection and dissemination, clinicians must maintain a high level of suspicion for patients from endemic areas presenting with new-onset wheezing, acute respiratory distress, and/or Gram-negative sepsis. Diagnosis may be established through sputum or stool examination for S stercoralis larvae. Elevated eosinophil counts may fail to identify those at highest risk of mortality. In the acute setting, particularly in a patient of Southeast Asian origin with acute respiratory distress complicated by Gram-negative infection, patients should be tested and treated for strongyloides regardless of peripheral eosinophil count or duration of residence outside an endemic area. Serologic studies, although sensitive,14 may delay diagnosis and should be used only as confirmation in a severely ill patient.
Acknowledgments
We thank Jason Sanchez, MD, for his contribution of a case report, and Charles Cartwright, PhD, for the photograph.
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 2.Genta RM. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11:755–767. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- 3.Muennig P, Pallin D, Sell RL, et al. The cost-effectiveness of strategies for the treatment of intestinal parasites in immigrants. N Engl J Med. 1999;340:773–779. doi: 10.1056/NEJM199903113401006. [DOI] [PubMed] [Google Scholar]
- 4.Nutman TB, Keiser PB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemos LB, Qu Z, Laucirica R, et al. Hyperinfection syndrome in strongyloidiasis: report of 2 cases. Ann Diagnost Pathol. 2003;7:87–94. doi: 10.1053/adpa.2003.50019. [DOI] [PubMed] [Google Scholar]
- 6.Lim S, Katz K, Krajden S, et al. Complicated and fatal strongyloides infection in Canadians: risk factors, diagnosis and management. Can Med Assoc J. 2004;171:479–484. doi: 10.1503/cmaj.1031698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill GV, Beeching NJ, Khoo S, et al. A British Second World War veteran with disseminated strongyloidiasis. Trans R Soc of Trop Med Hyg. 2004;98:382–386. doi: 10.1016/j.trstmh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Genta RM, Miles P, Fields K. Opportunistic Strongyloides stercoralis infection in lymphoma patients: report of a case and review of the literature. Cancer. 1989;63:1407–1411. doi: 10.1002/1097-0142(19890401)63:7<1407::aid-cncr2820630729>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Cruz T, Reboucas G, Rocha H. Fatal strongyloidiasis in patients receiving corticosteroids. N Engl J Med. 1966;275:1093–1096. doi: 10.1056/NEJM196611172752003. [DOI] [PubMed] [Google Scholar]
- 10.Lessnau KD, Can S, Talavera W. Disseminated. Strongyloides stercoralis in human immunodeficiency virus-infected patients: treatment failure and a review of the literature. Chest. 1993;104:119–122. doi: 10.1378/chest.104.1.119. [DOI] [PubMed] [Google Scholar]
- 11.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 12.Leelarasamee A, Nimmannit S, Na Nakorn S, et al. Disseminated strongyloidiasis: report of seven cases. Southeast Asian J Trop Med Public Health. 1978;9:539–542. [PubMed] [Google Scholar]
- 13.Potter A, Stephens D, De Keulenaer B. Strongyloides hyper-infection: a case for awareness. Ann Trop Med Parasitol. 2003;97:855–860. doi: 10.1179/000349803225002453. [DOI] [PubMed] [Google Scholar]
- 14.Loutfy MR, Wilson M, Keystone JS, et al. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66:749–752. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]


