Abstract
We used electron microscopy to determine the relative numbers of the three synaptic terminal types, RL, RS, and F, found in several representative thalamic nuclei in cats chosen as representative examples of first and higher order thalamic nuclei, where the first order nuclei relay subcortical information mainly to primary sensory cortex, and the higher order nuclei largely relay information from one cortical area to another. The nuclei sampled were the first order ventral posterior nucleus (somatosensory) and the ventral portion of the medial geniculate nucleus (auditory), and the higher order posterior nucleus (somatosensory) and the medial portion of the medial geniculate nucleus (auditory). We found that the relative percentage of synapses from RL terminals varied significantly among these nuclei, these value being higher for first order nuclei (12.6% for the ventral posterior nucleus and 8.2% for the ventral portion of the medial geniculate nucleus) than for the higher order nuclei (5.4% for the posterior nucleus, and 3.5% for the medial portion of the medial geniculate nucleus). This is consistent with a similar analysis of first and higher order nuclei for visual the system (the lateral geniculate nucleus and pulvinar, respectively). Since synapses from RL terminals represent the main information to be relayed, whereas synapses from F and RS terminals are modulatory in function, we conclude that there is more modulation of the thalamic relay in the cortico-thalamo-cortical higher order pathway than in first order relays.
INTRODUCTION
Sherman and Guillery (1998,2006) first made the point that inputs to thalamic relay cells can be effectively divided into drivers and modulators. A number of features identify driver input, including having powerful, depressing synapses that activate only ionotropic receptors, being the major determinant of receptive field properties of the relay cell, and having singularly large terminals identified with the electron microscope as “RL” (for Round vesicle, Large terminal, and they form symmetric synapses; for details, see Sherman and Guillery, 1998, 2006; Sherman, 2005). A key for this study is that inputs to thalamus terminating in RL, or very large, terminals represent driver inputs. Modulators are associated with weaker, often facilitating synapses that activate ionotropic and metabotropic receptors, and they produce small synaptic terminals. Drivers represent the main information-bearing input to be relayed to cortex and define what is being relayed. For instance, the retinal input is the driver for relay cells of the lateral geniculate nucleus, because it is the retinal input, rather than brainstem or layer 6 cortical input, that represents the main information relayed through the lateral geniculate nucleus.
Based on the source of driver input, subcortical or cortical, there are two distinct types of thalamic relay nuclei (reviewed in Sherman and Guillery, 1996,2006). One, called first order, relays subcortical driver input to cortex and represents the first relay of such information. Examples are the lateral geniculate nucleus that relays retinal input for vision, the ventral posterior nucleus that relays medial lemniscal input for somesthesis, and the ventral portion of the medial geniculate nucleus that relays inferior collicular input for hearing. The other, called higher order, relays information from one cortical area to another, and represents further cortical processing of information already in cortex via first order thalamic nuclei. Higher order relays receive feed forward input from layer 5 of one cortical area, and this driver input is distinguished from layer 6 input, which is a feedback modulatory input that innervates all thalamic relays (Sherman and Guillery, 1996, 2006 ; Sherman, 2005). Examples of these higher order relays are the pulvinar for vision, the posterior nucleus for somesthesia, and the magnocellular portion of the medial geniculate nucleus for hearing (Sherman and Guillery, 1996,2006; Sherman, 2005). Central to this view is evidence showing that synaptic properties of corticothalamic input from layer 5 to higher order nuclei share the same driver properties measured anatomically, physiologically, and pharmacologically as do retinogeniculate input and medial lemniscal input to the ventral posterior nucleus (Schwatrz et al., 1991; Hoogland et al., 1991; Deschênes, et al., 1994; Ojima, 1994; Bourassa, et al., 1995;Bourassa and Deschênes, 1995; Rockland, 1996;Rouiller and Welker, 2000; Bartlett et al., 2000; Guillery, et al., 2001; Li, et al., 2003; Reichova and Sherman, 2004). These higher order relays have only been recently recognized, and they seem to occupy the majority of thalamus (Sherman and Guillery, 2006)1.
However, subtle differences mostly associated with modulatory inputs exist between first and higher order thalamic nuclei. GABAergic inputs from the zona incerta and pretectum plus dopaminergic inputs from an unspecified source selectively target higher order nuclei (Cucchiaro, et al., 1991, 1993; Wang, et al., 2002b; Bokor, et al., 2005; Sanchez-Gonzalez, et al., 2005). Also, cholinergic inputs from the parabrachial region hyperpolarize many higher order relay cells, whereas these inputs appear to depolarize all first order relay cells (Mooney, et al., 2004; Varela and Sherman, 2004). Finally, higher order relay cells exhibit considerably more bursting (Ramcharan, et al., 2005), and Li et al. (2003) have described other subtle differences in the temporal response pattern between some first and higher order relay cells.
The electron microscopic analysis of the higher order, pulvinar complex of the cat by Wang et al. (2002a) suggests another difference. They reported driving (RL) synapses there to be only 3.5% of the total, which is significantly smaller than the 11.7% total for such (retinal) synapses in the first order lateral geniculate nucleus (Van Horn, et al., 2000). The purpose of the present study was to test the hypothesis that this represents a general difference between first and higher order thalamic nuclei by doing this sort of electron microscope analysis in the first and higher order somatosensory and auditory thalamic relays to see if the same difference exists.
EXPERIMENTAL PROCEDURES
Strict sterile surgical procedures were performed in accordance with NIH guidelines for the care and use of laboratory animals, and the protocols used were approved by the IACUC at Stony Brook University and the University of Louisville. Two adult cats were deeply anaesthetized with barbiturate and perfused transcardially with either 4% paraformaldehyde and 0.5% EM grade glutaraldehyde in 0.1M phosphate buffered saline (0.9%, pH 7.4) or 2% paraformaldehyde and 2% glutaraldehyde in 0.1M phosphate buffer (pH 7.4). Our techniques for tissue preparation and analysis have been described previously (Erişir, et al., 1997, 1998; Van Horn, et al., 2000) and are only briefly described here. Areas of interest were blocked and postfixed overnight in the perfusate and sectioned coronally on a Leica VT 100S vibratome at 50μm. Tissue from one case was used from a previous biotinylated dextran amine injection study into area 17 (and so no labeled profiles were seen in the somatosensory and auditory thalamic nuclei studied here); it was thus reacted with an avidin-biotin complex (1:100) followed by a glucoseoxidase, diaminobenzidine intensified reaction. Light level images were taken with a Zeiss Axiocam digital camera. For electron microscopy, the 50μm vibratomed sections, were osmicated, dehydrated, and flat embedded in Durcupan resin.
Four regions of interest were sampled: the ventral posterior nucleus, the medial and lateral sectors of the posterior nucleus (heretofore referred to simply as the posterior nucleus), the ventral portion of the medial geniculate nucleus, and the magnocellular portion of the medial geniculate nucleus. We chose each region of interest from the 50μm thick sections embedded in Durcupan as described above. Each was clearly recognized based on previous cytoarchitectonic maps of thalamus (Snider and Neimer, 1961; Jones, 1985).
Ultra-thin sections of 80nm were cut on a Reichart Ultracut E ultramicrotome and picked up on formvar coated nickel slot grids. Ultra-thin sections used for GABA immunocytochemistry were incubated in GABA antibody at a dilution of 1:750 or 1:1000, washed, and incubated in a secondary IgG antibody conjugated to 15nm gold particles at a dilution of 1:25. Sections were counterstained with uranyl acetate and lead citrate and viewed with a JEOL 1200EXII and a FEI Tecanai 12BioTwin electron microscope. Digital images were taken with an AMT camera and compiled using Adobe Photoshop. Tissue sampling was done by traversing back and forth across the ultrathin section, capturing or photographing images when a clear synaptic contact was evident, and in this way every terminal in a field making a clear synaptic contact was photographed and counted.
Terminal identification was based on Guillery’s classification (Guillery, 1969) modified slightly as described previously (Van Horn, et al., 2000) into one of three types: RL (Round vesicles, Large terminal and asymmetric postsynaptic density; the driver input), RS (Round vesicles, Small terminal and asymmetric postsynaptic density; the modulator input from cortical layer 6 or brainstem) and F (Flattened or pleomorphic vesicles, symmetrical post-synaptic density; the GABAergic modulator input from local interneurons or cells of the thalamic reticular nucleus). F terminals were confirmed by positive GABA immunoreactivity. F terminals can be further subdivided into two types: F1 terminals derive from axons mainly of interneurons, reticular cells, or some sources extrinsic to the thalamus such as the zona incerta or pretectum (Barthó, et al., 2002; Bokor, et al., 2005), and F2 terminals derive from the dendrites of interneurons; we did not distinguish between these F terminals in the present study and they are not considered further here, but additional details can be found elsewhere (Sherman and Guillery, 1996,2006).
RESULTS
Our data includes 947 synapses, each assigned to a specific terminal type: RL, RS or F (see EXPERIMENTAL PROCEDURES). These include 453 from the first order thalamic nuclei (222 from the ventral posterior nucleus and 231 from the ventral portion of the medial geniculate nucleus) and 494 from the higher order thalamic nuclei (295 from the posterior nucleus and 199 from the magnocellular portion of the medial geniculate nucleus). Figure 1 shows the zones sampled from these thalamic nuclei. These thus represent data from first and higher order nuclei for somatosensory and auditory information. Figures 2-4 show representative electron micrographs of each of the terminal types from each of the nuclei sampled. Table 1 summarizes the data collected and also includes for comparison similar data previously published for the lateral geniculate nucleus and pulvinar (i.e., the first and higher order thalamic visual nuclei).
Figure 1.
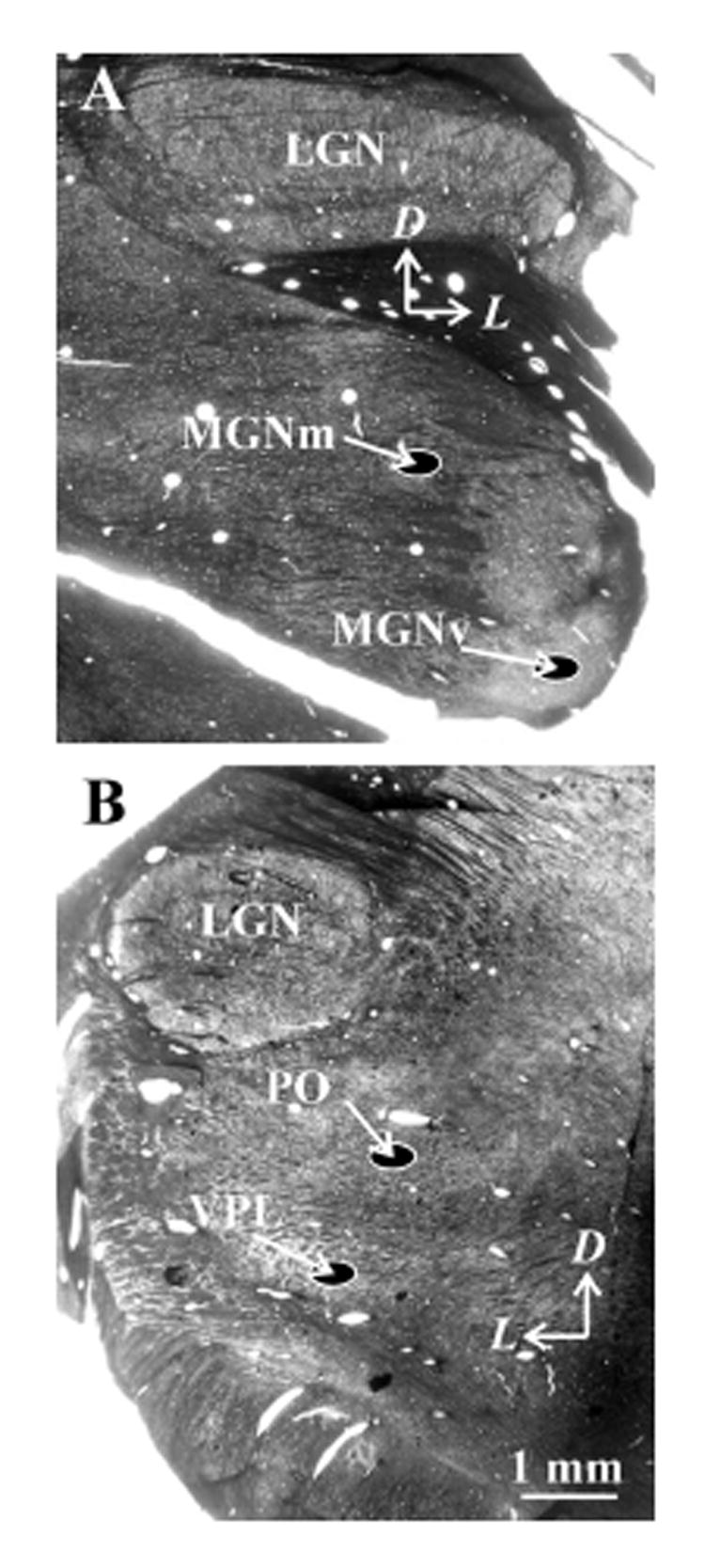
Light level images of embedded 50 μm sections, cut coronally showing areas sampled at the electron microscopic level. Arrows indicate dorsal (D) and lateral (L) directions. A: Areas for auditory nuclei. B: Areas for somatosensory nuclei. Abbreviations: LGN, lateral geniculate nucleus, MGNm, magnocellular portion of the medial geniculate nucleus; MGNv, ventral portion of the medial geniculate nucleus; PO, the posterior nucleus; Pul, pulvinar, VP, ventral posterior nucleus LGN, lateral geniculate nucleus; MGNm, medial geniculate nucleus.
Figure 2.
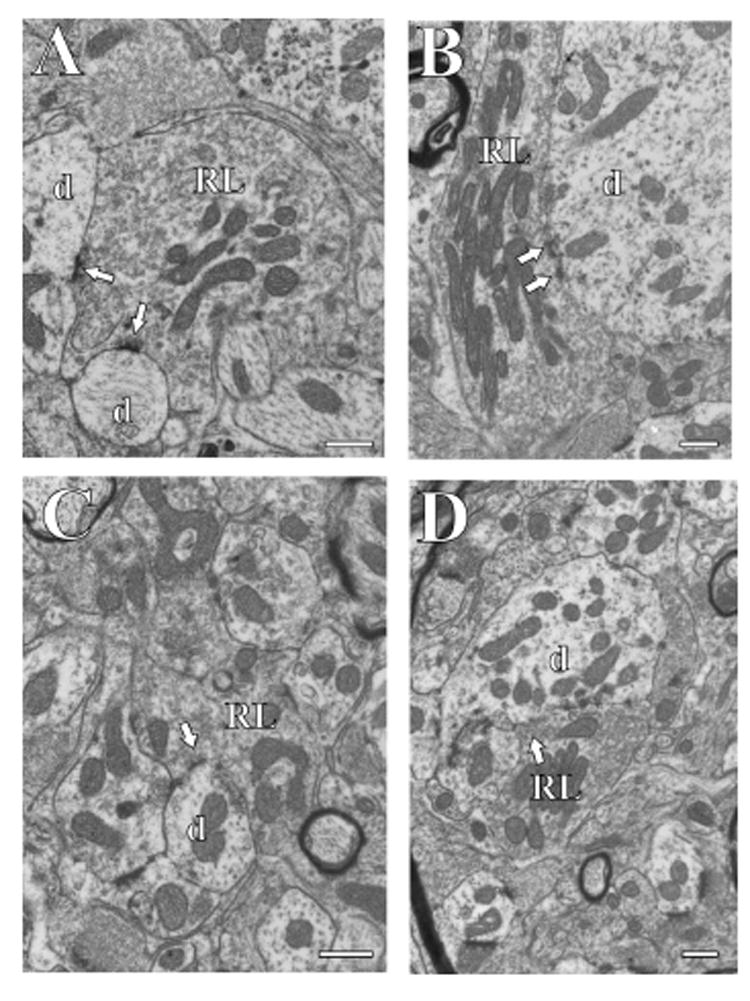
Electron micrographs of RL terminals (RL) in different thalamic nuclei. The arrows point to synapses, and postsynaptic dendrites (d) are also shown. The scale bar in each panel is 0.5 μm. A: Posterior nucleus. B: Ventral posterior nucleus. C: Ventral portion of the medial geniculate nucleus. D: Medial portion of the medial geniculate nucleus.
Figure 4.
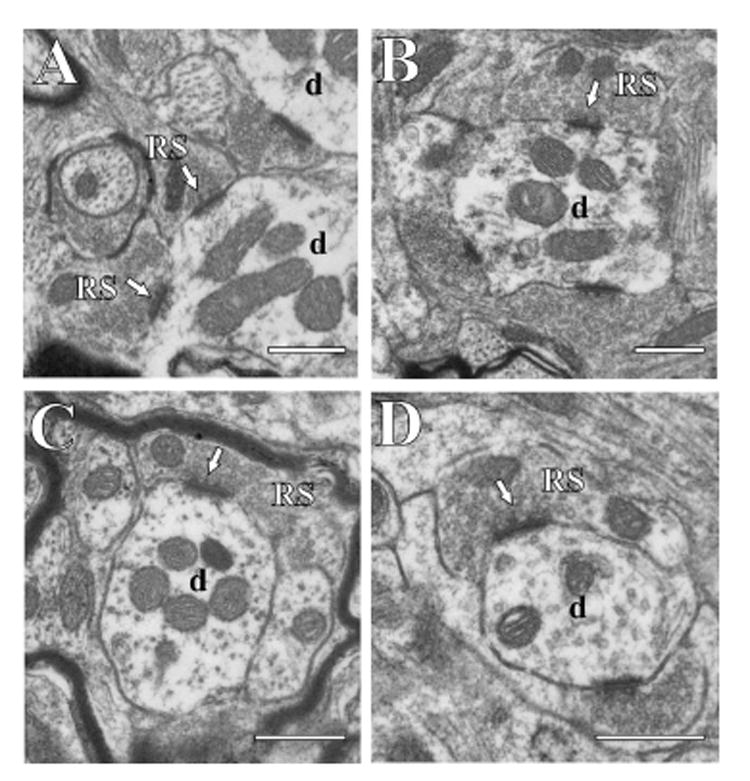
Electron micrographs of RS terminals (RS) in different thalamic nuclei. The arrows point to synapses, and postsynaptic dendrites (d) are also shown. The scale bar in each panel is 0.5 μm. sample areas A: Posterior nucleus. B: Ventral posterior nucleus. C: Ventral portion of the medial geniculate nucleus. D: Medial portion of the medial geniculate nucleus.
Table 1.
Relative Number of Synapses
| RL | RS | F | Total | |
|---|---|---|---|---|
| Ventral Posterior Nucleus | 28 | 146 | 48 | 222 |
| Posterior Medial Nucleus | 16 | 211 | 68 | 295 |
| Medial Geniculate Nucleus (ventral) | 19 | 159 | 53 | 231 |
| Medial Geniculate Nucleus (magnocellular) | 7 | 166 | 26 | 199 |
| Lateral Geniculate Nucleus* | 207 | 613 | 339 | 1159 |
| Pulvinar§ | 93 | 1333 | 257 | 1683 |
Data from Van Horn et al. (10)
Data from Wang et al. (9)
Relative number of RL Synapses
Of particular interest is the relative number of RL synapses among the various thalamic nuclei, because these have been identified as driver synapses (see DISCUSSION). As can be seen from Table 1 and Figure 5, these are the minority in all thalamic nuclei. In our sample, RL synapses represent 12.6% of synapses in the ventral posterior nucleus, 8.2% in the ventral portion of the medial geniculate nucleus, 3.5% in the magnocellular portion of the medial geniculate nucleus, and 5.4% in the posterior nucleus. This is consistent with previously published data for the visual thalamic nuclei, since RL synapses are 17.9% in the lateral geniculate nucleus (Van Horn, et al., 2000) and 5.5% for the pulvinar (Wang, et al., 2002a).
Figure 5.
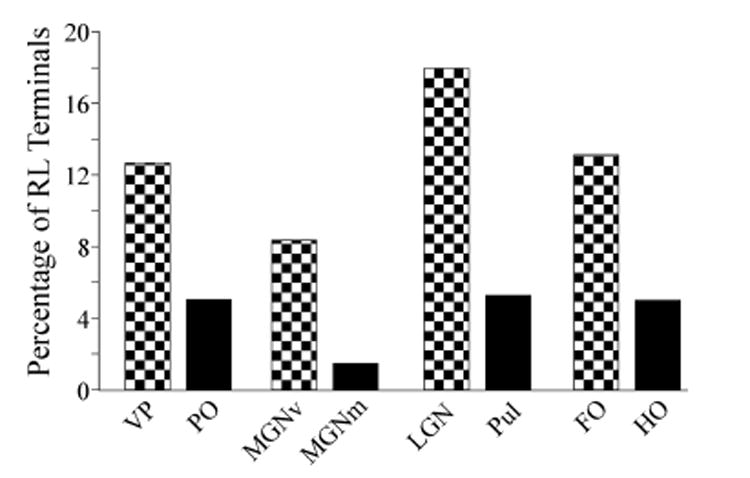
Relative percentage of synapses from RL terminals in various thalamic nuclei. In each case, the first order nuclei are indicated by hatched bars, and the higher order nuclei by solid bars. data for the left 4 thalamic nuclei are from the present paper; data from the LGN are from Van Horn et al. (2000) and from the Pul are from Wang et al. (2002a). The averages for the right hand pair of bars were computed by averaging the RL percentages from each of the other thalamic nuclei so that different sample sizes between nuclei does not distort the average. Abbreviations: FO, first order thalamic nucleus; LGN, lateral geniculate nucleus, HO, higher order thalamic nucleus; MGNm, magnocellular portion of the medial geniculate nucleus; MGNv, ventral portion of the medial geniculate nucleus; PO, the posterior nucleus; Pul, pulvinar, VP, ventral posterior nucleus.
Note that these data actually over-represent RL synapses. That is, because they are the largest synapses in the tissue, they will be sampled more frequently unless certain controls for synapse size are instituted. This has been done for the visual nuclei, so that, when corrected for sampling error, the number of RL synapses in the lateral geniculate nucleus drops from 17.9% to 11.7% (Van Horn, et al., 2000), and in the pulvinar, from 5.5% to 3.5% (Wang, et al., 2002a). However, since we in this study are interested in differences in the relative distribution of RL terminals among thalamic nuclei, we did not apply this correction for sample size. This assumes that sampling errors for RL terminals are roughly the same among thalamic nuclei, and since this seems to be the case for the lateral geniculate nucleus and pulvinar (Van Horn, et al., 2000; Wang, et al., 2002a), we consider this to be a reasonable assumption. Nonetheless, this assumption has not yet been verified experimentally.
Although RL synapses were the minority in all thalamic nuclei studied (indeed, the fewest of any synaptic type; see Table 1), their relative distribution was nonetheless larger in first order versus higher order nuclei. (All statistical comparisons below are based on the χ2-test.) Thus RL synapses were greater in number in the ventral posterior nucleus compared to the posterior nucleus (12.6% versus 5.4%; p< 0.005), and they were greater in the ventral portion of the medial geniculate nucleus compared to the magnocellular portion (8.2% versus 3.5%; p<0.05). When we combine the data from the first order (the ventral posterior nucleus and the ventral portion of the medial geniculate nucleus) and higher order (the magnocellular portion of the medial geniculate nucleus and the posterior nucleus), the differences are more striking (p<0.001). We get a similar result if we just compare the RL-to-RS ratio: it is greater the ventral posterior nucleus compared to the posterior nucleus (0.12 versus 0.4; p< 0.02), and it is greater in the ventral portion of the medial geniculate nucleus compared to the magnocellular portion (0.19 versus 0.07; p<0.005)
In contrast to these differences between first and higher order nuclei, we found no differences in comparisons between the same type of nucleus. That is, the percentages of RL synapses were not significantly different in the ventral posterior nucleus versus the ventral portion of the medial geniculate nucleus (first order; 12.6% versus 8.2%; p>0.1), nor were these percentages different in the posterior nucleus versus the magnocellular portion of the medial geniculate nucleus (higher order; 5.4% versus 3.5%; p>0.1).
Relative number of F versus RS synapses
While the above analysis indicates that RL synapses are more common in first order versus higher order thalamic nuclei with no differences in relative numbers between first order or higher order nuclei, there are some curious difference in the distribution of the other terminal types. For the somatosensory nuclei, there are no significant differences in the relative number of RS and F synapses (p>0.1). However, in the auditory nuclei, there is a lower RS to F terminal ratio in the ventral portion of the medial geniculate nucleus (p<0.005). A comparison within relay type shows that this variability is due to differences in higher order nuclei, because the relative numbers of RS and F terminals is the same for the ventral posterior nucleus and the ventral portion of the medial geniculate nucleus (p>0.1), but there are relatively more RS synapses in the magnocellular portion of the medial geniculate nucleus than in the posterior nucleus (p<0.005).
Comparison with Visual Thalamic Nuclei
Our data from the somatosensory and auditory thalamic nuclei indicate that RL synapses are relatively more common in first order compared to higher order nuclei. This is consistent with previously published data using the same techniques from the visual thalamic nuclei (Van Horn, et al., 2000; Wang, et al., 2002a). This is summarized in Table 1 and Figure 5. We can thus conclude that for these major sensory pathways, RL terminals are more commonly found in first order than in higher order nuclei. If we average across all of these thalamic nuclei (pooling the averages from each nucleus rather than raw numbers of synapses to account for the different sample sizes across thalamic nuclei), we find that RL synapses comprise 12.9% of all synapses sampled in first order nuclei but only 4.8% in higher order nuclei (p<0.001).
DISCUSSION
The main conclusion that we can draw from the present results is that, of the three main types of synapse—RL, RS, and F—found in the major sensory pathways of thalamus, the ratio of RL to RS plus F synapses is consistently and significantly lower in the higher order nuclei than in the first order nuclei. Thus our data for the somatosensory and auditory thalamic nuclei, when added to the earlier studies of the visual nuclei (Van Horn, et al., 2000; Wang, et al., 2002a) extend this difference between first and higher order thalamic nuclei. Other differences also exist among these thalamic nuclei, as suggested by the observation that relative numbers of F and RS synapses vary in ways that so far defy systematics. The relative number of RL synapses described here is the one difference seen so far in synapse numbers that follows a consistent pattern.
Functional Significance of Main Observations
We suggest that the main significance of the difference in relative RL synaptic numbers between first and higher order thalamic nuclei can appreciated by understanding what is meant by RL synapses and first and higher order thalamic nuclei.
Drivers versus modulators
As noted in INTRODUCTION, inputs to thalamic relay cells can be effectively divided into drivers and modulators and, based on the source of the driver input—subcortical or cortical layer 5—thalamic relays could be characterized, respectively, as first and higher order. We have associated RL terminals in higher order thalamic nuclei as emanating from layer 5 of cortex. Evidence that this occurs is available from many examples (reviewed in Sherman, 2005; Sherman and Guillery, 2006): from visual cortex to the pulvinar (Bourassa and Deschênes, 1995; Rockland, 1998;Rouiller and Welker, 2000), from somatosensory cortex to the posterior nucleus (Hoogland et al., 1991; Bourassa et al., 1995); and from the auditory cortex to the magnocellular part of the medial geniculate nucleus (Ojima, 1994; Bartlett et al., 2000). Evidence for this also exists for the medial dorsal nucleus (Schwatrz et al., 1991)
Nonetheless, as noted in the first footnote, some of the RL terminals in higher order nuclei may be subcortical in origin. Indeed, Kelly et al. (2003) have provided clear evidence that there are RL terminals in parts of the pulvinar that are tectal in origin, indicating that the pulvinar complex may include both first and higher order relays. There are also inferior collicular inputs to the medial division of the medial geniculate nucleus and spinal inputs to the posterior nucleus, raising the possibility that these, too, represent mixed first and higher order relays, but in these cases, it is not yet known if the inferior collicular or spinal inputs to these respective nuclei produce RL terminals. This possibility that these thalamic nuclei putatively identified as higher order may also contain first order relays, as seems the case for the pulvinar (Kelly, et al., 2003), does not affect the main conclusion that synapses from driving (RL) terminals are relatively rare in these thalamic nuclei. It also seems reasonable to conclude that this also applies to the subset of these synapses that derive from layer 5 of cortex.
All other inputs that are not drivers to thalamic nuclei are considered to be modulators, meaning that their role, rather than to provide basic information to be relayed, is to modulate the relay of driver input (Sherman and Guillery, 1998, 2006). For instance, layer 6 input from cortex is not responsible for the basic receptive field properties of lateral geniculate relay cells, but instead imparts a variety of subtle effects on their target cells’ response properties, including the transition between burst and tonic firing (Godwin et al., 1996; Wang et al., 2006). Furthermore, local GABAergic inputs, by inhibiting relay cells, can affect the overall responsiveness of relay cells and, by altering membrane voltage, play a role in controlling their voltage dependent properties. Among these modulators, synapses from RS terminals derive mainly from glutamatergic layer 6 cells of cortex or from cholinergic cells of the brainstem parabrachial region; synapses from F terminals represent GABAergic inputs, mostly from local interneurons or cells of the thalamic reticular nucleus (Sherman and Guillery, 1996, 2006).
First order versus higher order thalamic nuclei
The other point to be made in interpreting the results summarized here is the distinction between first and higher order thalamic nuclei (for details, see Sherman and Guillery, 1996, 2006). Simply put, first order nuclei represent the initial relay of a particular type of information from a subcortical source, like the retina or medial lemniscus, to cortex; higher order nuclei represent the relay of information from one cortical area to another in a cortico-thalamo-cortical route. Important to this concept is the point that all of these thalamic nuclei receive a modulatory input from cortical layer 6 that is mostly feedback in nature, while the higher order nuclei in addition receive a driver input from cortical layer 5 that is effectively feedforward. Thus with regard to the functional circuitry of these nuclei, a subcortical driver input in first order nuclei is replaced by a layer 5 driver input in higher order nuclei.
When the above implications for RL terminals and first versus higher order thalamic nuclei are considered, the conclusion follows that the driver/modulator synaptic ratio is significantly lower for higher order than for first order nuclei. Strictly speaking, this conclusion should be limited to the sensory systems for which data are available, namely the visual, somatosensory, and auditory systems, but it is plausible that this distinction be extrapolated throughout thalamus (for a fuller account of first and higher order thalamic nuclei, see Sherman and Guillery, 2006).
Other Differences Between First Order and Higher Order Thalamic Nuclei
Other differences between first and higher order thalamic nuclei exist, and our observations should be seen in this context. There are several subcortical, presumably modulatory, sources that tend to innervate higher order rather than first order nuclei. These include GABAergic inputs from the anterior pretectal nucleus and zona incerta (Barthó, et al., 2002; Bokor, et al., Acsády, 2005) and dopaminergic inputs from an as yet undefined source (Sanchez-Gonzalez, et al., 2005). There is also evidence for a difference in the postsynaptic effects of cholinergic inputs to thalamic relay cells (Mooney, et al., 2004; Varela and Sherman, 2004): in first order nuclei, these are depolarizing, but in higher order nuclei, these are hyperpolarizing for a substantial minority of relay cells. Finally, and perhaps related to the these differences in innervation patterns, recordings from behaving monkeys show that cells in higher order thalamic nuclei tend to be in burst mode much more commonly than are those in first order nuclei (Ramcharan, et al., 2005).
Conclusions
The main conclusion here is that the driver/modulator ratio is higher for first than for higher order thalamic nuclei. These observations, when put in the context of the extra GABAergic and dopaminergic modulatory inputs noted in INTRODUCTION (Barthó, et al., 2002; Bokor, et al., 2005; Sanchez-Gonzalez, et al., 2005), suggest that higher order nuclei may have modulatory effects not observed in first order nuclei. This, in turn, suggest that modulation of corticocortical processing through thalamus (i.e., involving cortico-thalamo-cortical pathways) differs from and may be more extensive than that seen in first order thalamic nuclei. It would be of obvious value to extend these studies to other first and higher order thalamic nuclei as they are identified.
Figure 3.
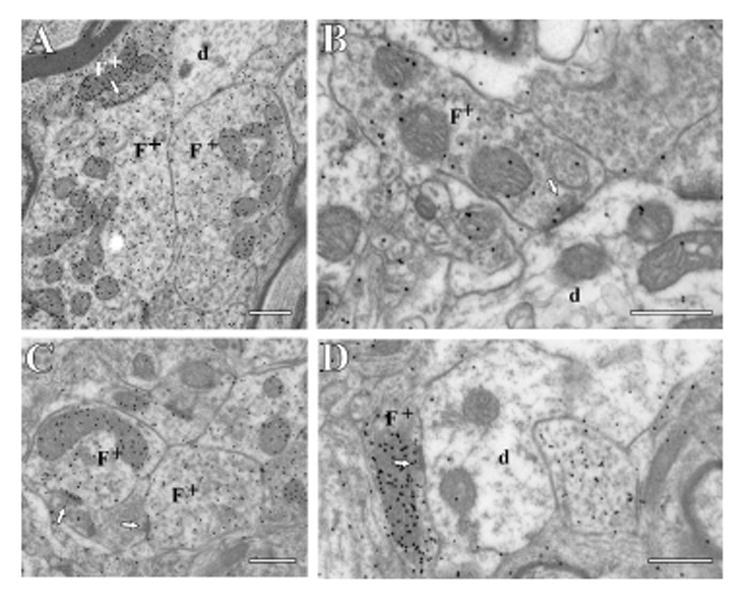
Electron micrographs of F terminals (F) with GABA postembedding immunolabeling in different thalamic nuclei. All of the F terminals are GABA-positive (as shown by immunogold particles) and are thus indicated as F+. terminals The arrows point to synapses, and postsynaptic dendrites (d) are also shown. The scale bar in each panel is 0.5 μm. sample areas A: Posterior nucleus. B: Ventral posterior nucleus. C: Ventral portion of the medial geniculate nucleus. D: Medial portion of the medial geniculate nucleus.
Acknowledgments
We would like to thank Dr. Martha E. Bickford for generously supplying some of the tissue used in this study.
Supported by USPHS Grant EY03038
Footnotes
For simplicity, we shall refer to these higher order relays by their cytoarchitectonic designations, but many or all of these may also include limited regions of first order relay (see ref. (Sherman and Guillery, 2006). For example, part of the posterior nucleus may receive spinothalamic input that is relayed to cortex in a first order fashion.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bartlett EL, Stark JM, Guillery RW, Smith PH. Comparison of the fine structureof cortical and collicular terminals in the rat medial geniculate body. Neurosci. 2000;100:811–828. doi: 10.1016/s0306-4522(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Barthó P, Freund TF, Acsády L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Bokor H, Frere SGA, Eyre MD, Slezia A, Ulbert I, Luthi A, Acsády L. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45:929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Deschênes M. Corticothalamic projections from the primary visual cortex in rats: A single fiber study using biocytin as an anterograde tracer. Neurosci. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: A single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Cucchiaro JB, Bickford ME, Sherman SM. A GABAergic projection from the pretectum to the dorsal lateral geniculate nucleus in the cat. Neurosci. 1991;41:213–226. doi: 10.1016/0306-4522(91)90211-6. [DOI] [PubMed] [Google Scholar]
- Cucchiaro JB, Uhlrich DJ, Sherman SM. Ultrastructure of synapses from the pretectum in the A-laminae of the cat’s lateral geniculate nucleus. J Comp Neurol. 1993;334:618–630. doi: 10.1002/cne.903340409. [DOI] [PubMed] [Google Scholar]
- Deschênes M, Bourassa J, Pinault D. Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 1994;664:215–219. doi: 10.1016/0006-8993(94)91974-7. [DOI] [PubMed] [Google Scholar]
- Erişir A, Van Horn SC, Sherman SM. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1517–1520. doi: 10.1073/pnas.94.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erişir A, Van Horn SC, Sherman SM. Distribution of synapses in the lateral geniculate nucleus of the cat: Differences between laminae A and A1 and between relay cells and interneurons. J Comp Neurol. 1998;390:247–255. [PubMed] [Google Scholar]
- Godwin DW, Vaughan JW, Sherman SM. Metabotropic glutamate receptors switch visual response mode of lateral geniculate nucleus cells from burst to tonic. J Neurophysiol. 1996;76:1800–1816. doi: 10.1152/jn.1996.76.3.1800. [DOI] [PubMed] [Google Scholar]
- Guillery RW. The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Z Zellforsch. 1969;96:1–38. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Van Lieshout DP. Connections of higher order visual relays in the thalamus: A study of corticothalamic pathways in cats. J Comp Neurol. 2001;438:66–85. doi: 10.1002/cne.1302. [DOI] [PubMed] [Google Scholar]
- Hoogland PV, Wouterlood FG, Welker E, van der Loos H. Ultrastructure of giant and small thalamic terminals of cortical origin: a study of the projections from the barrel cortex in mice using Phaseolus vulgaris leuco-agglutinin (PHA-L) Exp Brain Res. 1991;87:159–172. doi: 10.1007/BF00228517. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York: Plenum Press; 1985. [Google Scholar]
- Kelly LR, Li J, Carden WB, Bickford ME. Ultrastructure and synaptic targets of tectothalamic terminals in the cat lateral posterior nucleus. J Comp Neurol. 2003;464:472–486. doi: 10.1002/cne.10800. [DOI] [PubMed] [Google Scholar]
- Li JL, Bickford ME, Guido W. Distinct firing properties of higher order thalamic relay neurons. J Neurophysiol. 2003;90:291–299. doi: 10.1152/jn.01163.2002. [DOI] [PubMed] [Google Scholar]
- Li JL, Guido W, Bickford ME. Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. J Neurophysiol. 2003;90:3429–3440. doi: 10.1152/jn.00456.2003. [DOI] [PubMed] [Google Scholar]
- Mooney DM, Zhang L, Basile C, Senatorov VV, Ngsee J, Omar A, Hu B. Distinct forms of cholinergic modulation in parallel thalamic sensory pathways. Proc Natl Acad Sci U S A. 2004;101:320–324. doi: 10.1073/pnas.0304445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. cc. 1994;4:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12236–12241. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: Distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Rockland KS. Two types of corticopulvinar terminations: Round (type 2) and elongate (type 1) J Comp Neurol. 1996;368:57–87. doi: 10.1002/(SICI)1096-9861(19960422)368:1<57::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rockland KS. Convergence and branching patterns of round, type 2 corticopulvinar axons. J Comp Neurol. 1998;390:515–536. doi: 10.1002/(sici)1096-9861(19980126)390:4<515::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Res Bull. 2000;53:727–741. doi: 10.1016/s0361-9230(00)00364-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ML, Dekker JJ, Goldman-Rakic PS. Dual mode of corticothalamic synaptic termination in the mediodorsal nucleus of the rhesus monkey. J Comp Neurol. 1991;309:289–304. doi: 10.1002/cne.903090302. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: Distinguishing “drivers” from “modulators”. Proc Natl Acad Sci USA. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- Snider RS, Neimer WT. A Stereotaxix Atlas of the Cat Brain. Chicago, IL: University of Chicago Press; 1961. [Google Scholar]
- Van Horn SC, Erişir A, Sherman SM. The relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. J Comp Neurol. 2000;416:509–520. [PubMed] [Google Scholar]
- Varela C, Sherman SM. A further difference between first and higher order thalamic relays in response to cholinergic input. Society for Neuroscience. 2004 Society for Neuroscience Abstracts Program No 528.16. [Google Scholar]
- Wang S, Eisenback MA, Bickford ME. Relative distribution of synapses in the pulvinar nucleus of the cat: Implications regarding the “driver/modulator” theory of thalamic function. J Comp Neurol. 2002a;454:482–494. doi: 10.1002/cne.10453. [DOI] [PubMed] [Google Scholar]
- Wang ST, Eisenback M, Datskovskaia A, Boyce M, Bickford ME. GABAergic pretectal terminals contact GABAergic interneurons in the cat dorsal lateral geniculate nucleus. Neurosci Lett. 2002b;323:141–145. doi: 10.1016/s0304-3940(01)02533-2. [DOI] [PubMed] [Google Scholar]
- Wang W, Jones HE, Andolina IM, Salt TE, Sillito AM. Functional alignment of feedback effects from visual cortex to thalamus. Nat Neurosci. 2006;9:1330–1336. doi: 10.1038/nn1768. [DOI] [PubMed] [Google Scholar]


