Abstract
BACKGROUND AND OBJECTIVE
To describe the changes revealed by multifocal electroretinography (ERG) in patients taking hydroxychloroquine.
PATIENTS AND METHODS
Six patients being treated for various inflammatory conditions with hydroxychloroquine for periods ranging from 8 months to 7 years were consecutively evaluated. Each examination included measurement of Snellen visual acuities, Amsler grid assessment, and automated visual field testing. In some cases, funduscopic examinations were complimented by photography and fluorescein angiography. Multifocal ERG was performed for all patients.
RESULTS
Three patients (six eyes) were found to have distinctive abnormalities on multifocal ERG consisting of pericentral depression of ERG signals. The abnormalities on multifocal ERG corresponded with the patients’ subjective descriptions and the visual field depiction of their pericentral scotomas. All affected patients had been taking hydroxychloroquine for at least 7 years. One patient with generalized depression on multifocal ERG had possible hydroxychloroquine retinopathy. Two patients (three eyes) had relatively normal results on multifocal ERG.
CONCLUSION
Multifocal ERG objectively demonstrates depression of signals in the perifoveal region in visually symptomatic patients with long-term hydroxychloroquine use. Even patients with normal visual acuity and no fundus abnormalities can have abnormal results. Although we have not yet identified patients with abnormalities on multifocal ERG before the onset of symptoms, multifocal ERG may be useful in monitoring patients at risk and may provide an earlier opportunity to identify maculopathy.
INTRODUCTION
Originally used as antimalarial agents, chloroquine and hydroxychloroquine are now being used as effective treatment for various connective tissue diseases, especially systemic lupus erythematosus and rheumatoid arthritis.1 In 1959, chloroquine retinopathy was first described in three cases with classic bull’s eye retinopathy, which was attributed to lengthy treatment.2 Affected patients present with difficulty reading and blurred distance vision, along with increased granularity or patchy depigmentation in a ring-shaped pattern circumscribing the macula.3,4
Johnson and Vine examined nine patients taking a cumulative dose of hydroxychloroquine of greater than 1,000 g and noted that eight patients taking 400 mg/d had no evidence of retinopathy, whereas one patient taking more than 600 mg/d had retinopathy.5 Bernstein concurred with this study, but noted that patients with chronic renal insufficiency have a higher risk for hydroxychloroquine retinopathy because most of the drug is normally excreted in the urine.6 There have been no reported cases of hydroxychloroquine retinopathy when the dose was no greater than 6.5 mg/kg of body weight per day.6,7
There are approximately 100,000 patients per year in the United States exposed to hydroxychloroquine, yet no universal agreement on screening methods exists. Common detection methods include baseline color fundus photographs,8 clinical examinations, automated perimetry, and home Amsler grid testing.6 Easterbrook has suggested that the presence of corneal chloroquine or hydroxychloroquine deposits should prompt the clinician to carefully assess drug dosage per kilogram of body weight and macular function.9 The British Cardiff group, which has concluded that color vision, Amsler grid testing, and perimetry testing were not helpful in screening, has ceased screening for hydroxychloroquine retinopathy unless the patient becomes symptomatic.10 However, they did not use any electrophysiologic measures of retinal function.
Full-field electroretinography (ERG) has not been demonstrated to be a reliable method for identifying early hydroxychloroquine retinopathy. Results of standard ERG may be normal in early and well-established retinopathy.11 The scotoma needs to occupy a majority of the visual field before full-field ERG results are considered abnormal.12
Multifocal ERG, as described by Sutter and Tran, is a relatively new technique to record the electrical activity of the retina.13 The advantage of multifocal ERG over the older techniques, including full-field and foveal ERG, rests in its ability to record and localize retinal electrical contributions over 103 individual locations that span the central 45° (Fig. 1). Maturi et al. found fundus mottling and otherwise normal full-field ERG results in a patient with hydroxychloroquine retinopathy, but multifocal ERG revealed markedly decreased retinal responses.14 We have been monitoring patients taking hydroxychloroquine with periodic multifocal ERG, and our experience with 6 patients is described in this article.
Figure 1.
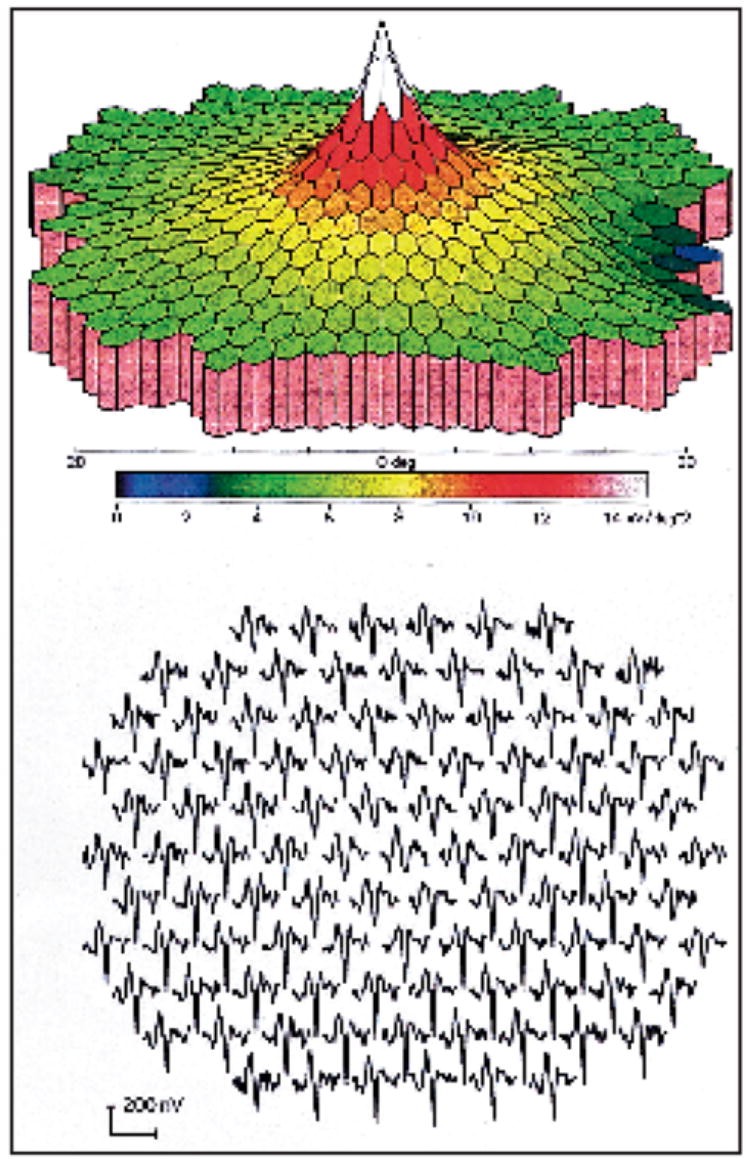
Normal multifocal electroretinogram. (Top) 3D density plot. (Bottom) Wave forms.
PATIENTS AND METHODS
Six patients who were taking hydroxychloroquine for a variety of conditions were examined with multifocal ERG (Table). Both eyes were tested in all but one patient, who had no light perception in one eye from previous vascular occlusion. Patients had been referred to the Neuro-ophthalmology Service of The New England Eye Center from various sources. Three patients complained of loss of vision in their central visual field despite relative preservation of acuity at the time they were examined. The other three patients had vague visual symptoms. Some patients were tested once and others underwent multiple tests.
TABLE.
Summary of Clinical Cases: Hydroxychloroquine Retinopathy
| Patient | Age (Y) | Disease | Dose (mg/d) | Duration | Symptoms | Visual Acuity | Visual Field* | Retina | Cornea | Multifocal ERG |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | Dermatomyositis | 400 | 7 y | “Cloudy areas”, donut shaped | OD = 20/25 OS = 20/25+ | (24-2) OU = paracentral scotomas | OU = normal | Normal | OU = Paracentral depression |
| 2 | 45 | SLE | 500 | 5 y | Glare, brief spells of central visual field obscuration(“like looking through a glass”) | OD = 20/20 OS = 20/20 | (30-2) OU = central scotomas | OU = normal | Normal | OU = paracentral depression |
| 3 | 68 | SLE | 400 | 7 y | Flashes of light | OD = 20/20 OS = 20/20 | (10-2) OU= paracentral depression | Macular pigment changes | Normal | OU = paracentral depression |
| 4 | 83 | Rheumatoid arthritis; OS = AION | 400 | 12 y | Difficulty with dark adaptation | OD = 20/20 OS = NLP | (24-2) OU = normal | OD = normal OS = linear deposits | Normal | OU = general depression |
| 5 | 36 | Rheumatoid arthritis | 400 | 8 mo | Blurred vision, more in the left eye than the right; light sensitivity (“like looking through a screen”) | OD = 20/15 OS = 20/15 | (30-2) OU = normal | OU = normal | Normal | OD = general depression; OS = normal |
| 6 | 28 | SLE | 400 | 2 y | Blurred spots | OD = 20/15 OS = 20/15 | (24-2) OU = normal | OU = normal | Normal | OU = normal |
OD = right eye; OS = left eye; OU = both eyes; SLE = systemic lupus erythematosus; NLP = no light perception; ERG = electroretinography; AION = anterior ischemic optic neuropathy.
Measured by the Humphrey Visual Filed Instrument, Carl Zeiss Meditech, Dublin, CA, at settings of 10-2, 24-2, and 30-2.
All patients underwent an initial complete ophthalmic examination, including testing of Snellen visual acuity, color (AOHRR plates), and visual fields (Humphrey Visual Field Instrument; Carl Zeiss Meditech, Dublin, CA). Retinal findings were documented photographically, and in some cases with fluorescein angiography. Multifocal ERG was performed using the Visual Evoked Response Imaging System (VERIS; EDI, Inc., San Mateo, CA) for the 11 eyes tested.
The fellow eye was patched and a ground electrode was attached to the ipsilateral ear lobe. After a topical anesthetic was administered, a Burian–Allen bipolar contact lens electrode was placed in the test eye. Due to the refractive nature of the lens, the patient underwent refraction with an external spherical lens to at least 20/30. The patient was seated with his or her chin on the rest and forehead against the bar. The VERIS program created a multifocal flicker in combination with periodic global flash stimuli on a monochrome monitor. Recordings lasted less than 12 minutes and relied on the patient to fixate on a central circle. First-order kernel amplitude responses were analyzed. Latencies were not measured. Multifocal ERG was performed by either an optometrist or a certified technician specially trained to use the VERIS.
RESULTS
The eyes of three patients showed distinctive abnormalities on multifocal ERG when compared with those of a normal individual (Fig. 1). In each, this consisted of pericentral depression of signals with relative preservation of the central peak and of signals from 15° outward to the peripheral limits of the testing area at 20° (Table). The appearance of the abnormalities on multifocal ERG was similar in each case (Fig. 2). The area of abnormality in each affected eye corresponded to the areas in which each patient described the visual disturbance on an Amsler grid. Each affected patient had relative preservation of visual acuity ranging from 20/15 to 20/20 and visual field abnormalities detected to varying degrees by the Humphrey Visual Field Instrument, programs 30-2, 24-2, or 10-2 (Table). There was usually a small area of depression within the central 5° to 10° (Fig. 3). Each affected patient had few or no abnormalities in the retina, including on fluorescein angiography (Figs. 4 and 5).
Figure 2.
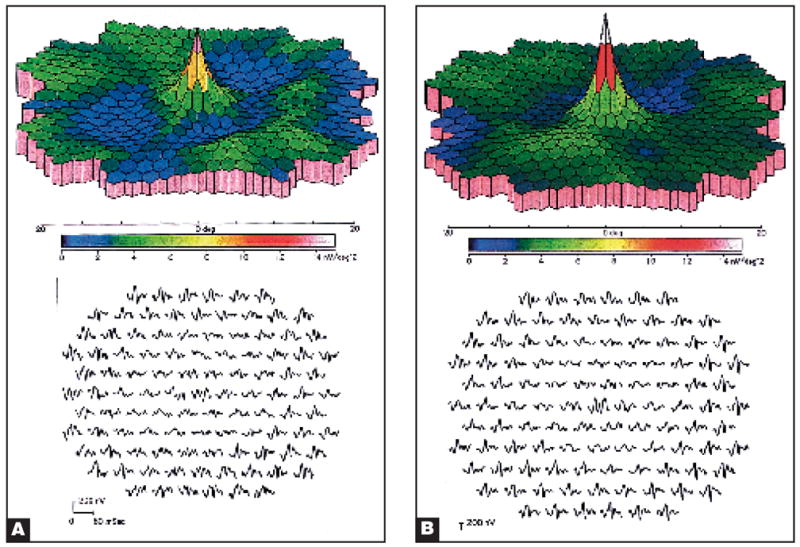
Multifocal electroretinograms of (A) the right eye and (B) the left eye of patient 1 showed depressed 3D density plots and wave forms.
Figure 3.
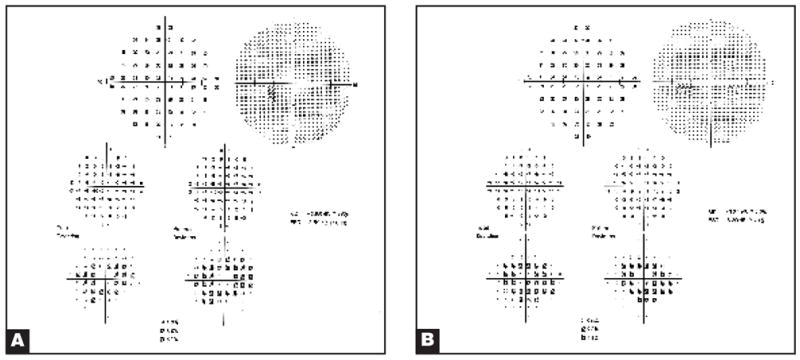
Humphrey visual field test (setting 10-2) of (A) the right eye and (B) the left eye of patient 1 showed pericentral ring defects.
Figure 4.
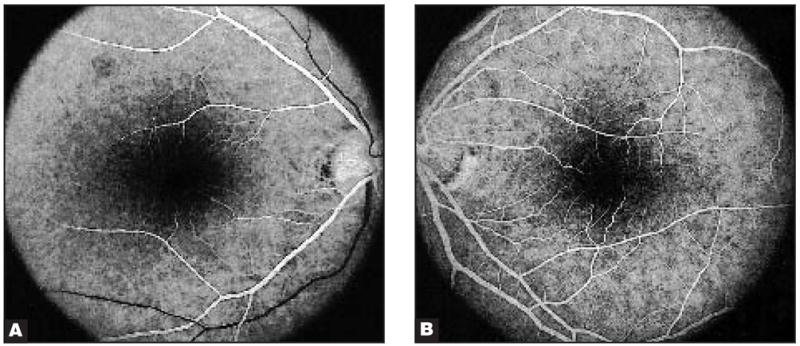
Fundus fluorescein angiography of (A) the right eye and (B) the left eye of patient 1 appeared normal.
Figure 5.
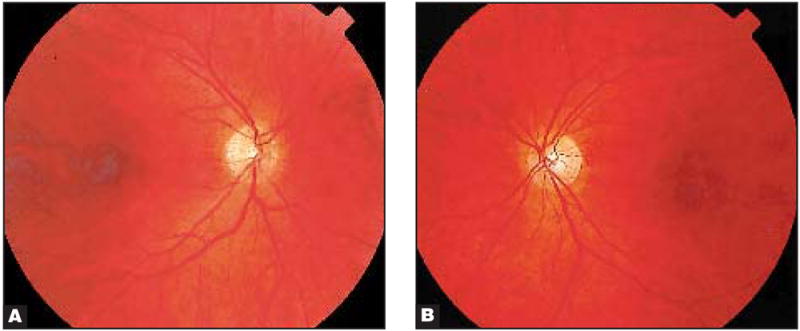
Fundi of (A) the right eye and (B) the left eye of patient 1 appeared normal.
One elderly patient showed generalized depression on multifocal ERG. She had been taking hydroxychloroquine for 12 years. Only one eye was tested because she had ischemic optic neuropathy in the other eye. The other relatively asymptomatic eye, which retained a visual acuity of 20/25 and a normal visual field (Humphrey 24-2). It was believed that she had possible hydroxychloroquine retinopathy.
The other two cases showed no abnormalities on multifocal ERG except case 4, in which there was generalized depression of the signals from the right eye, presumably due to artifact from poor contact between the electrode and the cornea. Repeated multifocal ERG was performed for 2 patients who remained symptomatic after discontinuing the drug. In both cases, the recordings were similar to the initial recordings.
DISCUSSION
The mechanism of retinopathy induced by chloroquine and hydroxychloroquine has not been fully delineated, yet in vitro evidence suggests it affects the photoreceptors indirectly via the retinal pigment epithelial cells. Bernstein and Ginsberg suggested that chloroquine binds to pigment cells containing melanin, most prominently in the retinal pigment epithelium and iris.15 In vitro, chloroquine can inhibit certain enzymes, including diphosphopyridine nucleotide–cytochrome C reductase16 and erythrocyte and serum cholinesterase.17 The inhibition of metabolism of the retinal pigment epithelium, which disrupts the metabolic support for the photoreceptors, may be the mechanism of chloroquine-induced retinopathy.6
Early screening devices such as fundus examinations, color vision testing, automated perimetry, and Amsler grid testing are currently recommended to detect the changes of hydroxychloroquine toxicity.6 In our experience, results of these tests have been normal unless the patient has a visual complaint, at which point the damage appears to be irreversible despite discontinuation of therapy. The early pigment stippling of the macula in an asymptomatic patient is often reversible following discontinuation of the drug.6
Fluorescein angiography does not usually contribute additional information if fundus photographs are normal. It does highlight the macular pigmentary changes seen in established chloroquine and hydroxychloroquine maculopathy, but rarely enhances the accuracy of the diagnosis. Fluorescein angiography may not be as sensitive as color photography in diagnosing chloroquine retinopathy; however, there is utility in fluorescein angiography when considering etiologies other than antimalarial-induced retinopathy.18 Serial electro-oculography was not reliable for routine use in detecting hydroxychloroquine retinopathy.19 Definition of a significant fall in electro-oculography ratio is not well established. Reports of reduced electro-oculography in patients with untreated rheumatoid arthritis further makes this an unreliable screening device.20
In reported cases employing full-field ERG, the recordings have been unreliable for detection of chloroquine or hydroxychloroquine toxicity. Using full-field and foveal ERG on 20 patients, Sassaman et al. suggested only foveal ERG may be of some value in detecting retinal toxicity prior to the onset of clinical signs.21 In Easterbrook’s study of 26 patients with early chloroquine retinopathy, 4 of 6 with an absolute scotoma demonstrated normal findings on full-field ERG. Easterbrook suggested that ERG findings are usually normal in early retinopathy, thereby being of little utility.9 Full-field ERG findings were abnormal only in advanced cases.3,12,15 Abnormal findings on ERG include diminished photopic and scotopic responses.3
The enhanced resolution of the multifocal ERG over 103 locations illustrates the potential for detection of early functional loss of drug-induced maculopathy. Other applications with multifocal ERG include diabetic retinopathy,22 age-related macular degeneration,22 cytomegalovirus retinitis,23 retinitis pigmentosa,24 and central serous chorioretinopathy.25
In a previous case report, Maturi et al.14 found depressed central signals on multifocal ERG in a 45-year-old patient with a 6-year history of hydroxychloroquine use. However, in this case, abnormalities in the retinal pigment epithelium and fluorescein angiographic evidence of bull’s eye maculopathy were present, along with decreased Snellen visual acuities to less than 20/50. In our affected patients, the central peak on multifocal ERG was relatively preserved, consistent with retention of visual acuities of 20/20 in each affected eye. Furthermore, in our 3 affected patients, there was no clinically detectable maculopathy.
We believe that multifocal ERG is a more definitive test for demonstrating retinal dysfunction from hydroxychloroquine toxicity in the setting of a normal-appearing fundus with changes on Amsler grid and perimetry and with relatively normal visual acuity. Multifocal ERG has the potential to be used as an early screening device for patients taking high doses of hydroxychloroquine before they become symptomatic or show clinical signs of toxicity. Because stabilization and perhaps reversibility are more likely if retinopathy is detected early, the development of multifocal ERG for detection of the earliest stages of hydroxychloroquine retinopathy is paramount.15
Future studies of chloroquine and hydroxychloroquine retinal toxicity with multifocal ERG should be directed at the determination of the earliest point at which multifocal ERG detects retinopathy. Additionally, such investigations would also be designed to evaluate whether hydroxychloroquine-induced retinopathy is reversible when the hydroxychloroquine is discontinued.
Acknowledgments
Supported in part by the NIH EY RO1-13178, Bethesda, Maryland (JSS), the Glaucoma Research Foundation, San Francisco, California (JSS), and NIH P 30 Core Grant and a fellowship from the Charlton Student Research Fund, Boston, Massachusetts (SCS).
References
- 1.Weinberg DV. Retina and vitreous: trauma: toxic retinopathies. In: Yanoff M, Duker JS, editors. Ophthalmology. St. Louis, MO: Mosby; 1999. pp. 8.46.1–8.46.2. [Google Scholar]
- 2.Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959;2:478–480. doi: 10.1016/s0140-6736(59)90604-x. [DOI] [PubMed] [Google Scholar]
- 3.Okun E, Gouras P, Bernstein H, Von Sallman L. Chloroquine retinopathy. Arch Ophthalmol. 1963;69:59–71. doi: 10.1001/archopht.1963.00960040065013. [DOI] [PubMed] [Google Scholar]
- 4.Finbloom D, Silver K, Newsome DA, Gunkel R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. 1985;12:692–694. [PubMed] [Google Scholar]
- 5.Johnson MW, Vine AK. Hydroxychloroquine therapy in massive total doses without retinal toxicity. Am J Ophthalmol. 1987;104:139–144. doi: 10.1016/0002-9394(87)90005-5. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein HN. Ocular safety of hydroxychloroquine. Ann Ophthalmol. 1991;23:292–296. [PubMed] [Google Scholar]
- 7.Rynes RI, Krohel G, Falbo A, Reinecke RD, Wolfe B, Bartholomew LE. Ophthalmologic safety of long-term hydroxychloroquine treatment. Arthritis Rheum. 1979;22:832–836. doi: 10.1002/art.1780220805. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz RS, Saatci OA. Chloroquine and hydroxychloroquine retinopathy: how to follow affected patients. Ann Ophthalmol. 1991;23:290–291. [PubMed] [Google Scholar]
- 9.Easterbrook M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol. 1990;25:249–251. [PubMed] [Google Scholar]
- 10.Morsman CDG, Livesey SJ, Richards IM, Jessop JD, Mills PV. Screening for hydroxychloroquine retinal toxicity: is it necessary? Eye. 1990;4:572–576. doi: 10.1038/eye.1990.79. [DOI] [PubMed] [Google Scholar]
- 11.Easterbrook M. Ocular effects and safety of antimalarial agents. Am J Med. 1988;85(suppl 4A):23–29. doi: 10.1016/0002-9343(88)90358-0. [DOI] [PubMed] [Google Scholar]
- 12.Weiner A, Sandberg MA, Gaudio AR, Kini MM, Berson EL. Hydroxychloroquine retinopathy. Am J Ophthalmol. 1991;112:528–534. doi: 10.1016/s0002-9394(14)76853-9. [DOI] [PubMed] [Google Scholar]
- 13.Sutter EE, Tran D. The field topography of ERG components in man: I. The photopic luminance response. Vis Res. 1992;32:433–446. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- 14.Maturi RK, Folk JC, Nichols B, Oetting TT, Kardon RH. Hydroxychloroquine retinopathy. Arch Ophthalmol. 1999;117:1262–1263. doi: 10.1001/archopht.117.9.1262. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein HN, Ginsberg J. The pathology of chloroquine retinopathy. Arch Ophthalmol. 1964;71:238–245. doi: 10.1001/archopht.1964.00970010254019. [DOI] [PubMed] [Google Scholar]
- 16.Mushinski JF, Yielding KL, Munday JS. A comparison of the in vitro effects of steroid hormones and certain antimalarial compounds. Arthritis Rheum. 1962;5:118. [Google Scholar]
- 17.Wright CI, Sabine J. Cholinesterases of human erythrocytes and plasma and their inhibition by anti-malarial drugs. Pharmacology and Experimental Therapeutics. 1948;93:230–239. [PubMed] [Google Scholar]
- 18.Cruess AF, Schachat AP, Nicholl J, Augsburger JJ. Chloroquine retinopathy: is fluorescein angiography necessary? Ophthalmology. 1985;92:1127–1129. [PubMed] [Google Scholar]
- 19.Bishara SA, Matamoros N. Evaluation of several tests in screening for chloroquine maculopathy. Eye. 1989;3:777–782. doi: 10.1038/eye.1989.121. [DOI] [PubMed] [Google Scholar]
- 20.Percival SP, Behrman J. Ophthalmological safety of chloroquine. Br J Ophthalmol. 1969;53:101–109. doi: 10.1136/bjo.53.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sassaman FW, Cassidy JT, Alpern M, Maaseidvaag E. Electroretinography in patients with connective tissue diseases treated with hydroxychloroquine. Am J Ophthalmol. 1970;70:515–523. doi: 10.1016/0002-9394(70)90884-6. [DOI] [PubMed] [Google Scholar]
- 22.Palmowski AM, Sutter EE, Bearse MA, Jr, Fung W. Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Invest Ophthalmol Vis Sci. 1997;38:2586–2596. [PubMed] [Google Scholar]
- 23.Bearse MA, Jr, Sutter EE. Imaging localized retinal dysfunction with the multifocal electroretinogram. J Opt Soc Am A. 1996;13:634–640. doi: 10.1364/josaa.13.000634. [DOI] [PubMed] [Google Scholar]
- 24.Hood DC, Holopigian K, Greenstein V, et al. Assessment of local retinal function in patients with retinitis pigmentosa using the multi-focal ERG technique. Vis Res. 1998;38:163–179. doi: 10.1016/s0042-6989(97)00143-0. [DOI] [PubMed] [Google Scholar]
- 25.Marmor MF, Tan F. Central serous chorioretinopathy: bilateral multifocal electroretinographic abnormalities. Arch Ophthalmol. 1999;117:184–188. doi: 10.1001/archopht.117.2.184. [DOI] [PubMed] [Google Scholar]


