Abstract
Clinical studies suggest that treatment with vaccines comprised of idiotype protein may be associated with improved clinical outcome in follicular lymphoma patients. The time-consuming process required to generate patient-specific vaccines is a major limitation, however. Here we report results of a pilot clinical trial with a novel autologous, tumor-derived proteoliposome vaccine formulation that could be rapidly produced within a single day. Vaccination was safe, induced autologous tumor-specific type 1 cytokine responses in 5 out of 10 follicular lymphoma patients, and was associated with induction of a sustained complete response in one patient. Other patients had large tumor burdens and progressed after a median duration of 8 months. These results suggest that further testing of this vaccine formulation, particularly in the setting of minimal disease, is warranted. Furthermore, the proteoliposome formulation may provide a model for vaccine development for other human cancers, for which tumor-associated antigens need not be defined.
Introduction
Therapeutic vaccination with the unique determinants of the variable regions of the clonal tumor immunoglobulin molecule, termed idiotype (Id), induces humoral and cellular immune responses and is associated with prolonged progression-free survival in patients with follicular lymphoma.1–6 The production of Id protein by hybridoma or recombinant DNA technology is expensive and labor-intensive, however, requiring up to 6 months to manufacture the vaccine for each individual patient.7 We therefore developed a novel vaccine formulation where membrane proteins were directly extracted from autologous tumor cells and incorporated into liposomes along with IL-2 to produce membrane-patched proteoliposomes. Reports in the literature indicate that the antigen encapsulated in liposomes is delivered into both the endosomal and cytosolic processing pathways of antigen presenting cells, thereby generating both CD4+ and CD8+ T cell responses.8,9 IL-2 was chosen as a vaccine component due to its ability to expand activated T cells. Furthermore, we have previously demonstrated that IL-2 has a specific interaction with small unilamellar lipid vesicles leading to the formation of multilamellar coalescent vesicles used for vaccines.10 Testing in a mouse lymphoma model showed this formulation to be at least as potent as the prototype Id protein vaccine in inducing tumor protection (see accompanying Brief Report, Popescu MC et al). Here we report the results of our pilot clinical trial to evaluate the safety, feasibility, and immunogenicity of this novel vaccine formulation in patients with Stage III and IV follicular lymphoma.
Patients and methods
Patients
After obtaining signed informed consent, eleven previously untreated or treated patients with Stage III or IV follicular lymphoma grade 1 or grade 2 were enrolled in this National Cancer Institute institutional review board-approved Phase I clinical trial (Table 1). All patients underwent a lymph node biopsy prior to starting treatment to obtain tissue for vaccine production. Clinical responses were assessed by physical examination, computerized tomography (CT) scans, and bilateral bone marrow biopsies according to the non-Hodgkin lymphoma International Workshop Criteria.11
Table 1.
Patient characteristics and clinical outcome
| UPN | Age/Sex | Clinical Stage | Histology/Grade | Prior Chemotherapy/Response* | Time to vaccination (months)** | Prevaccine Status | Postvaccine Status (months)*** |
|---|---|---|---|---|---|---|---|
| 1 | 32/M | IVA | FL/2 | PACE/PR | 9 | PR | SD (22)# |
| 2 | 56/F | IVA | FL/2 | PACE/PR | 10 | PR | PD (15) |
| 3 | 33/M | IVA | FL/1 | PACE/PR | 8 | PR | PD (17) |
| 4 | 42/M | IVA | FL/1 | PACE/CR | 83 | PD## | PD (10) |
| 5 | 53/M | IVA | FL/1 | PACE/CR | 20 | PD## | CR (30+)### |
| 6 | 53/F | IVA | FL/1 | PACE/CR | 12 | PD## | PD (12) |
| 7 | 20/F | IVA | FL/1 | PACE/PR | 8 | PD## | PD (3) |
| 8 | 60/M | IVA | FL/1 | PACE/CR | 15 | PD## | PD (8) |
| 9 | 48/M | IIIA | FL/2 | None | n/a | PD## | PD (5) |
| 10 | 44/M | IIIA | FL/1 | None | n/a | PD## | PD (3) |
| 11 | 52/M | IVA | FL/2 | None | n/a | PD## | PD (2) |
UPN indicates unique patient number; FL, follicular lymphoma; PACE, prednisone, doxorubicin, cyclophosphamide, and etoposide; PR, partial response; SD, stable disease; PD, progressive disease; CR, complete response.
Best response to chemotherapy
Time to vaccination from last chemotherapy
Months after starting vaccination.
Lost to follow-up
The duration of progressive disease varied from 6 to 12 months prior to vaccine administration
Remains in complete remission 44 months after completion of the vaccination.
Vaccine formulation and administration
Proteins from whole cell membranes were directly extracted from 2 × 109 lymph node biopsy cells with detergent. The membrane proteins were incorporated into liposomes along with IL-2 to produce membrane-patched proteoliposome (Oncoquest-L) vaccine. Each vaccine was formulated on a per milliliter basis with membrane proteins obtained from approximately 1.6 × 108 biopsy cells, 4 × 106 IU of IL-2, and 80 mg of dimyristoylphosphatidylcholine (DMPC), which was used to generate liposomes. Idiotype and total protein dose were assayed in each vaccine preparation and were observed to be fairly uniform. The mean idiotype concentration in the vaccine was 2.37 μg/mL (standard deviation ± 1.02 μg/mL). The mean total protein concentration in the vaccine was 464 μg/mL (standard deviation ± 89 μg/mL). The vaccine was injected subcutaneously at 2 separate sites, either in arms or legs, at a dose of 0.5 mL per site for a total of 5 doses of the vaccine at months 0, 1, 2, 3, and 4. Two patients (UPN 9 and 11) received half the dose of each vaccine subcutaneously and the other half intratumorally in an enlarged inguinal lymph node. Patients who received prior chemotherapy were immunized at least 6 months after the completion of the chemotherapy, to allow time for immunological recovery. Patients 7, 10, and 11 did not complete the 5 vaccinations due to progression of disease.
Immunological assays
Cytokine induction, interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT), and Granzyme B ELISPOT assays were performed in prevaccine and postvaccine peripheral blood mononuclear cell (PBMC) samples in parallel as previously described.6,12–14 Immune responses could not be assessed in patient 2 due to the unavailability of tumor cells.
A positive response for cytokine induction assay was defined as a response 2 or more times greater than the negative controls, which included postvaccine PBMC alone, tumor cells alone, prevaccine PBMC alone and prevaccine PBMC + tumor cells.6 There was no significant production of cytokines above the detection limit (< 15.6 pg/mL) with tumor cells alone in all 10 patients that were assessed.
A significant difference in the precursor frequency of tumor-reactive T cells between the prevaccine and postvaccine samples was determined by the Student t test for paired mean values.6,12–14
Results and discussion
Autologous tumor-specific T-cell responses were induced by immunization
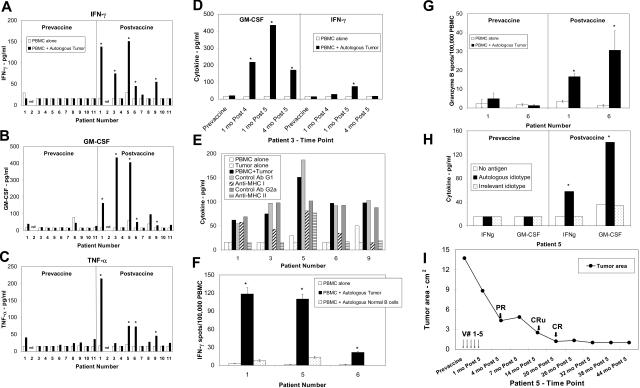
Postvaccine but not prevaccine PBMCs from 5 out of 10 patients responded to autologous tumor cells by producing significant amounts of IFN-γ, GM-CSF, and/or TNF-α compared with PBMC or tumor alone (Figures 1A-C). The PBMC response against the tumor cells was confirmed with samples from multiple postvaccine time points from each patient (Figure 1D) and was partially inhibited by anti-HLA class I and/or class II blocking antibodies, suggesting that both CD4+ and CD8+ T-cells were involved in the anti-tumor immune responses (Figure 1E). The preferential induction of MHC class I or II immune responses in some patients may reflect the presence or absence of the respective T-cell epitopes or the preferential presentation of tumor antigens through the endosomal or cytosolic processing pathways following vaccination.
Figure 1.
Tumor-specific cellular immune responses were induced following vaccination. Cryopreserved prevaccine and postvaccine PBMCs were cultured in either medium alone, or with sCD40Lt activated autologous tumor cells in the presence or absence of HLA class I and class II blocking antibodies or their respective isotype control antibodies or sCD40Lt activated autologous normal B cells or autologous or irrelevant idiotype protein and cytokine production was detected by ELISA or ELISPOT as previously described.6,12–14 Cytokine production in the supernatants for IFN-γ (A, D), GM-CSF (B, D), and TNF-α (C) was measured by ELISA. (D) Representative data on the cytokine production in response to autologous tumor cells from PBMC samples obtained at various time points in patient 3. (E) Tumor-reactive immune responses in postvaccine PBMC were associated with both HLA class I and class II molecules. IFN-γ production is shown for patients 1, 3, 5, and 6, and TNF-α production is shown for patient 9. (F) Significantly increased numbers of IFN-γ spots were detected by ELISPOT in response to autologous tumor cells but not in response to autologous normal B cells in 3 patients (P < .05). (G) Significantly increased numbers of Granzyme B spots were detected by ELISPOT in response to autologous tumor cells in postvaccine but not prevaccine PBMC samples (P < .05). (H) Postvaccine but not prevaccine PBMC from patient 5 produced significant amounts of cytokines in response to autologous idiotype protein compared with control isotype-matched irrelevant idiotype protein. (I) Induction of complete remission following vaccination. Vaccination time points are indicated by arrows. The sum of the product of the perpendicular diameters of 4 separate lymph nodes measured on CT scans is represented on the y-axis. PR - partial response; CRu - complete response, unconfirmed; CR - complete response.
Tumor-reactive T-cell responses were also confirmed by an independent IFN-γ ELISPOT assay in patients 1, 5, and 6 (Figure 1F). The calculated precursor frequency of tumor-reactive T cells in these 3 patients was significantly increased in postvaccine PBMC (range 19-115 IFN-γ spots/105PBMC), compared with prevaccine PBMC (range 2-7 IFN-γ spots/105PBMC) (P value < .05 using the Student t test). Interestingly, these T-cell responses were observed to be specific against autologous tumor cells since there was no significant reactivity against autologous peripheral blood normal B cells in all 3 patients tested (Figure 1F). The specificity of the postvaccine T-cell response was also confirmed for all 3 cytokines, IFN-γ, GM-CSF, and TNF-α by a cytokine induction assay in these 3 patients (data not shown). Postvaccine but not prevaccine PBMCs from patients 1 and 6 also produced significant amounts of Granzyme B in response to autologous tumor cells (Figure 1G).
Idiotype-specific immune responses were evaluated in 4 patients (patients 4, 5, 8, and 11) in whom tumor-derived idiotype protein could be produced by hybridoma technology. Postvaccine PBMC from only one of the 4 patients specifically recognized autologous tumor-derived idiotype protein by producing significant amounts of cytokines. (Figure 1H and data not shown). Anti-idiotypic antibody responses were not detected in any of these patients.
Clinical outcome
The vaccine was well tolerated, and injection site reactions such as erythema and induration lasting up to a week were noted in all patients. There were no grade 3 or 4 adverse events due to the vaccine. No evidence of autoimmunity was noted either by clinical or laboratory parameters in any patient. Laboratory parameters monitored for detection of autoimmunity included antinuclear antibodies, anti–ds DNA antibodies, peripheral blood B-cell numbers, and immunoglobulin levels. Patient 5, who had relapsed from doxorubicin-containing chemotherapy, achieved a complete remission 14 months after the completion of the vaccination and remains in remission 44 months after the completion of vaccination (Figure 1I). Patient 1 remained in stable disease for at least 22 months after completing vaccination. The remaining 9 patients, most of whom had large tumor burdens, progressed after a median duration of 8 months (range 2 to 17 months - Table 1).
These results suggest that this novel membrane-proteoliposome vaccine formulation is safe and could be produced rapidly from primary, autologous follicular lymphoma samples. Despite the use of total membrane proteins as antigenic material in the vaccine formulation, we did not observe any clinical or laboratory evidence of autoimmunity in any patient on this trial. The absence of reactivity against normal B cells (Figure 1F) suggests that the T-cell responses may have been directed against tumor-associated antigens that were uniquely expressed in the lymphoma cells such as the idiotype (Figure 1H). However, it is also possible that antitumor immune responses may have been induced against tumor antigens other than idiotype with this vaccine formulation. The complete response observed in patient 5 and the prolonged stabilization of disease observed in patient 1 are unlikely due to IL-2, due to the fact that both these patients had evidence of induction of a strong anti-tumor T-cell response following vaccination (Figures 1A-C, F), and continued regression of the tumors in patient 5 was observed several months after completion of the vaccination (Figure 1I), suggesting the induction of a sustained anti-tumor T-cell response. These results suggest that additional testing of this formulation may be warranted, particularly in the setting of low tumor burden or minimal residual disease.
Although this novel vaccine formulation requires the generation of a custom-made product for each patient, it offers several advantages over patient-specific idiotype vaccines. First, this formulation can be produced rapidly within a single day, in contrast to the 2 to 6 months required to manufacture idiotype vaccine for each patient.7 Second, in addition to the membrane idiotype protein, this vaccine formulation may induce immune responses against other unrecognized tumor-associated antigens. Finally, this novel formulation may serve as a model for vaccine development against other human malignancies including certain leukemias, lymphomas, and solid tumors where tumor-associated antigens have not been defined.
Acknowledgments
We thank the physicians, pharmacy, and nursing staff of the 13E unit in Buildng 10, NIH Clinical Center, for their patient care. We thank A. Malyguine, S. Strobl, and K. Shafer-Weaver for performing the ELISPOT assays and Amgen for generously providing the sCD40Lt. We also thank the patients for participating in this trial. We thank Jessie Horton and Miriam Ferraro for help with data management and Biomira USA Inc. for manufacturing the vaccine. We thank Alison Woo for editorial assistance in preparation of the manuscript.
This work was supported by cooperative research and development agreement with Biomira USA Inc, Cranbury, NJ. This publication has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-12 400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U. S. Government.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contributions: Designed research (S.S.N., R.J.R., M.C.P., L.W.K.), performed research (S.S.N., B.L.G., L.H., S.T.L., A.R.F., J.H., L.W.K.), analyzed data (S.S.N., B.L.G., S.T.L., A.R.F., J.H., L.W.K.), wrote paper (S.S.N., L.W.K.).
Conflict of interest disclosure: MCP, RJR, and LWK have declared a financial interest in XEME Biopharma, Inc., whose potential products were studied in the present work.
Correspondence: Sattva S. Neelapu, MD, 1515 Holcombe Blvd, Unit 903, M. D. Anderson Cancer Center, Houston, TX 77030; E-mail: sneelapu@mdanderson.org
References
- 1.Kwak LW, Campbell MJ, Czerwinski DK, et al. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 2.Hsu FJ, Caspar CB, Czerwinski D, et al. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma—long-term results of a clinical trial. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- 3.Bendandi M, Gocke CD, Kobrin CB, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 4.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman JM, Czerwinski DK, Davis TA, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 6.Neelapu SS, Baskar S, Gause BL, et al. Human autologous tumor-specific T-cell responses induced by liposomal delivery of a lymphoma antigen. Clin Cancer Res. 2004;10:8309–8317. doi: 10.1158/1078-0432.CCR-04-1071. [DOI] [PubMed] [Google Scholar]
- 7.Hurvitz SA, Timmerman JM. Recombinant, tumour-derived idiotype vaccination for indolent B cell non-Hodgkin's lymphomas: a focus on FavId. Expert Opin Biol Ther. 2005;5:841–852. doi: 10.1517/14712598.5.6.841. [DOI] [PubMed] [Google Scholar]
- 8.Harding CV, Collins DS, Kanagawa O, et al. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991;147:2860–2863. [PubMed] [Google Scholar]
- 9.Rao M, Alving CR. Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen-presenting cells. Adv Drug Deliv Rev. 2000;41:171–188. doi: 10.1016/s0169-409x(99)00064-2. [DOI] [PubMed] [Google Scholar]
- 10.Boni LT, Batenjany MM, Neville ME, et al. Interleukin-2-induced small unilamellar vesicle coalescence. Biochem Biophys Acta. 2001;1514:127–138. doi: 10.1016/s0005-2736(01)00377-7. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphoma. NCI Sponsored International Working Group. J Clin. Oncol. 1999;18:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 12.Malyguine A, Strobl S, Shafer-Weaver K, et al. A modified human ELISPOT assay to detect specific responses to primary tumor cell targets. J Transl Med. 2004;2:9–19. doi: 10.1186/1479-5876-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neelapu SS, Kwak LW, Kobrin CB, et al. Vaccine induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005;11:986–991. doi: 10.1038/nm1290. [DOI] [PubMed] [Google Scholar]
- 14.Shafer-Weaver K, Sayers T, Strobl S, Derby E, Ulderich T, Baseler M, Malyguine A. The Granzyme B ELISPOT assay: an alternative to the 51Cr-release assay for monitoring cell-mediated cytotoxicity. J Transl Med. 2003;1:14. doi: 10.1186/1479-5876-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]