Abstract
Nelarabine (506U78) is a soluble pro-drug of 9-β-d-arabinofuranosylguanine (ara-G), a deoxyguanosine derivative. We treated 26 patients with T-cell acute lymphoblastic leukemia (T-ALL) and 13 with T-cell lymphoblastic lymphoma (T-LBL) with nelarabine. All patients were refractory to at least one multiagent regimen or had relapsed after achieving a complete remission. Nelarabine was administered on an alternate day schedule (days 1, 3, and 5) at 1.5 g/m2/day. Cycles were repeated every 22 days. The median age was 34 years (range, 16-66 years); 32 (82%) patients were male. The rate of complete remission was 31% (95% confidence interval [CI], 17%, 48%) and the overall response rate was 41% (95% CI, 26%, 58%). The principal toxicity was grade 3 or 4 neutropenia and thrombocytopenia, occurring in 37% and 26% of patients, respectively. There was only one grade 4 adverse event of the nervous system, which was a reversible depressed level of consciousness. The median disease-free survival (DFS) was 20 weeks (95% CI, 11, 56), and the median overall survival was 20 weeks (95% CI, 13, 36). The 1-year overall survival was 28% (95% CI, 15%, 43%). Nelarabine is well tolerated and has significant antitumor activity in relapsed or refractory T-ALL and T-LBL.
Introduction
Although current treatment results in complete remission (CR) in 80% to 90% of adults with newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL) or lymphoblastic lymphoma (T-LBL), approximately half of these patients relapse within the first 2 years.1 In addition, patients who fail to achieve a CR have an extremely poor prognosis. A response rate of 50% is achieved among patients with relapsed or refractory T-ALL/LBL who receive high- or intermediate-dose cytarabine-based combination chemotherapy, but remissions are short.2–5 After salvage chemotherapy, allogeneic hematopoietic stem cell transplantation (SCT) remains the only treatment offering long-term survival, and those patients undergoing SCT while in remission have the best chance for prolonged survival.
Nelarabine (compound 506U78; Arranon) is a pro-drug that is demethylated by adenosine deaminase to 9-β-d-arabinofuranosylguanine (ara-G), a deoxyguanosine analog.6 Previous studies demonstrated that immature T lymphocytes and T lymphoblasts are sensitive to the cytotoxic effects of deoxyguanosine.6–12 The accumulation of deoxyguanosine triphosphate with subsequent inhibition of ribonucleotide reductase, inhibition of DNA synthesis, and resultant cell death accounts for T-cell toxicity.8–12 However, the clinical use of deoxyguanosine is limited because of its rapid degradation by the purine nucleoside phosphorylase (PNP) present in red blood cells. In 1983, Cohen et al7 reported that ara-G was resistant to degradation by PNP and was also toxic to T lymphoblasts at micromolar concentrations.
Laboratory studies demonstrated that conversion of ara-G to its 5′-triphosphate, ara-GTP, is required for lymphocytotoxicity mediated by the inhibition of DNA synthesis.10–12 Ara-G is phosphorylated via deoxycytidine kinase and mitochondrial deoxyguanosine kinase.9–13 The increased activity of ara-G against T cells compared with B cells was initially thought to be due to decreased catabolism of ara-GTP in T cells relative to B cells. However, recent studies suggest that the rate of ara-GTP catabolism is similar in both T cells and B cells and that initial ara-G concentrations are higher in T cells for a given dose. Thus, T cells have a greater intracellular exposure to ara-GTP.11
Ara-G is difficult to synthesize using traditional techniques and it is poorly water soluble.14 For these reasons, it has not been studied in the clinic. Use of advanced enzyme technology has allowed synthesis of nelarabine, a compound that is 10 times more water soluble than ara-G.15 Nelarabine is rapidly demethylated in blood by adenosine deaminase to ara-G. Thus, many of the obstacles to the use of ara-G have been circumvented by the development of nelarabine.
A phase 1 study explored the administration of nelarabine as a 1-hour intravenous infusion daily for 5 consecutive days.16 Ninety-three adult and pediatric patients with refractory hematologic malignancies were enrolled in this multi-institutional trial. The half-life of nelarabine was determined to be approximately 20 minutes because of rapid conversion of the drug to ara-G. The half-life of ara-G is approximately 3 hours, but the intracellular half-life of the active ara-GTP is more than 24 hours.17,18 As predicted by preclinical in vitro studies, the highest response rates were observed in 39 evaluable patients with T-cell ALL (CR, 23%; partial remission [PR], 31%), 7 patients with T-CLL (PR 29%), and 15 patients with T-cell lymphoma (PR 13%). Responses were seen at all dose levels tested. The maximum tolerated dose (MTD) was 40 mg/kg/d in adults and 60 mg/kg/d in children when given for 5 days. Neurotoxicity, consisting of somnolence, encephalopathy, seizures, mental status changes, obtundation, and ascending paralysis, was dose-limiting.16 In an effort to minimize neurologic toxicity, a dose of 1.8 g/m2/d was tested in adults on an alternate-day schedule (days 1, 3, and 5). This regimen was well tolerated. Subsequent phase I studies evaluated further dose escalation using this schedule and reported an MTD of 2.2 g/m2/day.
A phase 2 open-label multicenter clinical trial was initiated to evaluate the efficacy and safety of single agent nelarabine in adult subjects with refractory or relapsed T-lineage ALL/LBL. Although a dose of 2.2 g/m2/d was initially chosen, the study was amended after 3 patients received therapy to a dose of 1.5 g/m2/d on days 1, 3, and 5 to decrease the potential risk of neurologic toxicities, because most patients with ALL would have previously received cranial irradiation and neurotoxins such as vincristine or intrathecal chemotherapy.
Patients, materials, and methods
Patient population
The Institutional Review Boards at each participating institution approved the study protocol. All patients gave written informed consent before study participation in accordance with the Declaration of Helsinki. Patients older than 16 years of age who had T-cell ALL or LBL that was either refractory to at least one induction regimen or in first or later relapse were eligible. For patients with ALL, relapsed or refractory disease was defined by the presence of more than 10% lymphoblasts in the bone marrow or more than 1000 lymphoblasts/μL in the blood. Prior therapy for relapsed disease was permissible. Leukemia or lymphoma cells were required to express at least 2 of the following cell surface antigens: CD1a, CD2, CD3 (surface or cytoplasmic), CD4, CD5, CD7, and CD8. Leukemia cells were also required to be negative for myeloperoxidase or Sudan Black B histochemical stains. If the only T cell markers present were CD4 and CD7, the leukemia cells must also have lacked the myeloid markers CD33 and/or CD13. Patients with more than 25% lymphoblasts in the bone marrow either at initial diagnosis or at study entry were considered to have ALL rather than LBL.
Patients were not allowed to have evidence of central nervous system (CNS) leukemia or lymphoma that would require intrathecal or craniospinal radiation therapy. A lumbar puncture (LP) was not required in asymptomatic patients. Subjects with a seizure disorder, grade 3 or greater neurologic toxicity during prior treatment, or pre-existing neuropathy of grade 2 or greater at the time of registration regardless of causality were not eligible. Within 48 hours before the first administration of nelarabine, a serum creatinine level was obtained in all patients, and a calculated creatinine clearance of 50 mL/min or greater, using the Cockcroft equation, was required.
Treatment
Nelarabine was administered as an intravenous infusion over 2 hours initially at a dose of 2.2 g/m2/d on days 1, 3, and 5, but this dose was amended to 1.5 g/m2/d on days 1, 3, and 5. Although no neurotoxicity was seen in the 3 patients treated at the 2.2 g/m2 dose, safety data from other studies began to accumulate, and neurologic toxicity was seen in these other studies. To assure patient safety, the nelarabine dose was reduced from 2.2 to 1.5 g/m2/d. Three subjects received the 2.2 g/m2 dose and 36 of the 39 subjects received the 1.5 g/m2 dose. The data from all treated subjects were included in all analyses. Nelarabine was administered as a 5 mg/mL solution without further dilution. Antiemetic prophylaxis with steroids was not allowed. Hematopoietic growth factors were not used prophylactically. Patients were assessed for disease response on day 22; if residual leukemia or lymphoma was present, a second course of nelarabine was administered as above. However, if the day 22 marrow was hypocellular (cellularity ≤ 15%), then a repeat bone marrow biopsy was obtained on day 29 to assess response. If residual leukemia/lymphoma was documented at the point of marrow recovery (cellularity > 15%), then a second course of nelarabine was administered on the same schedule as above.
Patients achieving a CR were eligible to receive 2 additional courses of nelarabine as consolidation therapy at the same dose and schedule as outlined above. Additional courses of nelarabine could be given after discussion with the study chair. A calculated creatinine clearance of 50 mL/min or greater using the Cockcroft equation was documented before drug administration for each cycle. Each course of chemotherapy was administered only after adequate recovery of peripheral blood counts (eg, absolute neutrophil count > 1500/μL and platelets > 100 000/μL). If residual or recurrent leukemia/lymphoma or extramedullary disease was documented after 2 courses of nelarabine, the patient was removed from protocol therapy. If clinically indicated, a LP was performed to rule out CNS leukemia/lymphoma before each consolidation course. Patients who achieved a response and were candidates for an allogeneic SCT were removed from the protocol. All patients were followed for relapse and survival.
Response assessment
The criteria used for treatment efficacy in T-ALL were adapted from Cheson et al.19 CR was defined as an absolute neutrophil count greater than 1500/μL, no circulating blasts, platelets greater than 100 000/μL, bone marrow cellularity over 20% with trilineage hematopoiesis, and fewer than 5% marrow blast cells, none of which could appear neoplastic. If the morphologic result was ambiguous, a second bone marrow examination was performed 1 week later. All previous extramedullary manifestations of disease must have been absent (eg, lymphadenopathy, splenomegaly, skin or gum infiltration, testicular masses, or CNS involvement). CR with incomplete hematopoietic recovery (CRi) was defined as bone marrow blast counts less than 5% and no other evidence of disease but with neutrophils more than 1500/μL or platelets more than 100 000/μL. A partial remission (PR) required that all of the CR criteria were met with the exception of the bone marrow, which was defined by a reduction of 50% or more leukemia blast cells as long as the absolute blast count was between 5% and 25%. Relapsed disease was defined as the reappearance of unequivocal leukemia blast cells in the blood or the bone marrow (> 5%), in the CNS (positive cytospin examination of cerebrospinal fluid) or in any other extramedullary site after a CR or progression to over 25% leukemia blasts cells in the marrow after a PR.
For patients with T-LBL, the criteria for CR were similar and required the disappearance of all measurable disease, signs, symptoms, and biochemical changes related to the tumor. PR was defined as a reduction of 50% or more in the sum of the products of the perpendicular diameters of all measurable lesions compared with pretreatment measurements. No new lesions could appear, and no existing lesions could enlarge. Stable disease was defined as a less-than 50% reduction and no more than a 25% increase in the sum of the products of 2 perpendicular diameters of all measured lesions, and the appearance of no new lesions.
The assessment of response to treatment was based on the results of complete blood cell counts (CBC), platelet count, differential count, bone marrow aspirate, bone marrow biopsy, and physical examination. Extramedullary sites involved with leukemia before treatment (eg, mediastinal lymphadenopathy) were also re-examined if clinically indicated. Results of immunophenotyping, cytochemistry, and cytogenetic analyses were to be used as supportive data but were not required for clinical assessment. The protocol specified that a validated response required maintenance for at least 1 month. Subjects who proceeded with additional nelarabine treatment were to have no evidence of disease recurrence during this 1-month interval.
Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. CALGB statisticians performed statistical analyses. As part of the quality assurance program of the CALGB, members of the Data Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 19 patients (47.5%) of the 40 patients enrolled on this study.
Results
Patient characteristics
Forty subjects were enrolled in the study from August 15, 1998, to September 12, 2001, and 39 received at least one dose of study drug. One patient withdrew consent before treatment in this study because of insurance issues. Thus, 39 treated subjects were included in the analyses (Table 1). Most subjects were male (82%) and most were white (69%); 23% were African American. The median age at study entry was 34 (range, 16-66) years. Eighteen patients (46%) were between 16 and 30 years old, and only 5 patients (13%) were under age 21.
Table 1.
Patient baseline characteristics (n=39)
| Characteristic | Data |
|---|---|
| Age, y, no. (%) | |
| 16–20 | 5 (13) |
| 21–30 | 13 (33) |
| 31–50 | 15 (39) |
| 51–64 | 4 (10) |
| Over 65 | 2 (5) |
| Median age (range), y | 34 (16-66) |
| Sex, no. (%) | |
| Male | 32 (82) |
| Female | 7 (18) |
| Race or ethnic group, no. (%) | |
| White | 27 (69) |
| African American | 9 (23) |
| Native American | 1 (3) |
| Hispanic | 1 (3) |
| Oriental | 1 (3) |
| Median WBC count (range), × 109/L | 6.8 (0.0006-526.3) |
| Extramedullary disease, no. (%) | |
| Yes | 26 (67) |
| No | 12 (31) |
| Unknown | 1 (3) |
| History of CNS leukemia, no. (%) | |
| None | 35 (90) |
| One occurrence | 3 (8) |
| More than 1 occurrence | 1 (3) |
| ECOG performance status, no. (%) | |
| 0 (fully active) | 11 (28) |
| 1 (ambulatory, capable of light work) | 17 (44) |
| 2 (in bed less than 50% of time) | 6 (15) |
| 3 (in bed more than 50% of time) | 5 (13) |
| Median time since initial diagnosis (range), mo. | 14.1 (0.6-200.9) |
WBC indicates white blood cells; CNS, central nervous system, ECOG, Eastern College Oncology Group.
Twenty-six patients (67%) had T-ALL at study entry, and 13 (33%) had T-LBL (Table 2). Eleven patients (28%) had received one prior multiagent induction regimen, and 28 (72%) had received 2 or more prior regimens (Table 2). Moreover, of the 28 patients with ≥ 2 prior inductions, 17 (61%) had not achieved a CR with the most recent induction attempt. Nineteen patients (49%) had disease that was refractory to their most recent therapy. Twenty-six patients (67%) had extramedullary disease, which was either lymphadenopathy or splenomegaly. A history of prior CNS leukemia was reported in 4 patients (10%), and only one patient (3%) had more than one occurrence of CNS leukemia. The majority of patients (72%) had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1 at baseline.
Table 2.
Prestudy patient treatment histories
| Characteristic | Data |
|---|---|
| Disease classification at study entry, no. (%) | |
| T-ALL | 26 (67) |
| T-LBL | 13 (33) |
| No. of prior courses of induction therapy (%) | |
| 1 prior course | 11 (28) |
| More than 1 prior course | 28 (72) |
| Response to most recent prior therapy, n (%) | |
| Relapsed after CR | 20 (51) |
| Refractory | 19 (49) |
| Number of prior multiagent regimens excluding surgery | |
| Median (range) | 2 (1-5) |
| 1, no. (%) | 11 (28) |
| 2, no. (%) | 10 (26) |
| 3, no. (%) | 9 (23) |
| 4, no. (%) | 7 (18) |
| 5, no. (%) | 2 (5) |
| Prior high-dose methotrexate, no. (%) | 9 (23) |
| Prior high-dose cytarabine, no. (%) | 25 (64) |
| Prior irradiation, no. (%) | 19 (49) |
| Prior stem cell transplant, no. (%) | 5 (13) |
The most common prior chemotherapy agents were vincristine, cytarabine, cyclophosphamide, and methotrexate. In addition, most patients (82%) had received prior corticosteroid treatment. Doxorubicin, daunorubicin, or idarubicin had been included in the chemotherapy regimens of 62%, 46%, and 8% of patients, respectively; all together 85% (33 of 39) of the patients had received an anthracycline before enrollment on this study.
Response
The study was initially opened at a dose of 2.2 g/m2 but was amended to a dose of 1.5 g/m2 to decrease the potential risk of neurologic toxicity. Three subjects received the 2.2 g/m2 dose, and data from these subjects were included in all analyses. Thirty-six of the 39 subjects received 1.5 g/m2 on days 1, 3, and 5. All 39 subjects completed at least one course; 14 received only one course; 17 received 2 courses; 5 received 3 courses; 2 received 5 courses, and one patient received 6 courses. The range of cumulative doses of nelarabine for all subjects was 4.3 to 26.9 g/m2. Overall, subjects spent an average of 68 days on therapy, with a range of 10 to 451 days.
Sixteen patients (41%) achieved a CR (n = 12; 31%) or PR (n = 4) (Table 3). Of the 12 CR patients, 10 (26%) achieved a CR with full hematologic recovery. Two of the 12 patients did not have documented evidence of full hematologic recovery and were thus classified as having had a CRi. One of these patients withdrew from protocol treatment without demonstrating platelet and absolute neutrophil count (ANC) recovery to receive a cord blood SCT. The second CRi patient received 2 induction courses of nelarabine and eventually achieved full hematologic recovery but then relapsed 17 days later. The response in this subject was not classified as a CR because it was not maintained for 1 month.
Table 3.
Summary of efficacy endpoints
| Endpoints | No. (%) | [95% CI] or (range) |
|---|---|---|
| Overall CR + CRi + PR | 16 (41) | [26%, 58%] |
| CR+CRi | 12 (31) | [17%, 48%] |
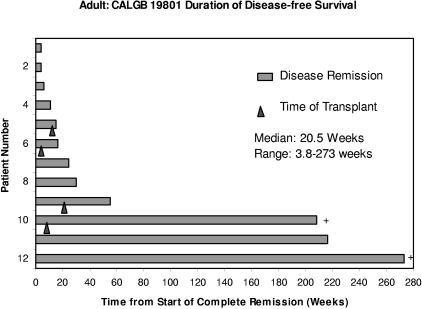
| Median duration of CR+CRi, wk | 20.5 | (3.8-273.3) |
| CR | 10 (26) | [13%, 42%] |
| Median duration of CR, wk | 27 | (4.1-273.3) |
| CRi | 2 (5) | — |
| PR in T-ALL patients* | 4 (10) | |
| CR + CRi + PR | 11 (42) | [23%, 63%] |
| CR+CRi | 8 (31) | [14%, 52%] |
| PR in T-LBL patients† | ||
| CR + CRi + PR | 5 (38) | [14%, 68%] |
| CR | 4 (31) | [9%, 61%] |
| DFS, median wks | 20 | (11-56) |
| DFS at 1 year | 7 (25) | [6%, 50%] |
| OS, median wks | 20 | (13-36) |
| Survival at 1 year | 7 (28) | [15%, 43%] |
CR indicates complete remission; CRi, incomplete remission; CR+CRi, complete remission with or without hematologic recovery, PR, partial remission; DFS, disease-free survival; OS, overall survival.
N = 26.
N = 13.
There were no differences in response rates between T-ALL and T-LBL. Eleven of 26 patients (42%) with T-ALL and 5 of 13 patients (38%) with T-LBL had a response of any kind (CR, CRi, or PR). Of the 26 patients with T-ALL, 8 (31%) had a CR with or without hematopoietic recovery (CRi). Four of the 13 patients (31%) with T-LBL had CR that was confirmed 1 month later (Table 3).
Of the 28 patients who had previously received at least 2 prior induction regimens, 10 (36%) experienced a CR, CRi, or PR (Table 4). In comparison, 6 of 11 (55%) patients who had received only one prior induction regimen demonstrated a CR, CRi, or PR to nelarabine. Nineteen patients had previously failed to respond to their most recent prior induction attempt (Table 5). Of those 19 patients, 4 (21%) experienced a CR after nelarabine, and all of these CRs were confirmed 1 month later. All 4 of these patients had received 2 or more prior induction regimens.
Table 4.
Summary of response rates by number of prior multiagent regimens
| Overall* |
CR + CRi |
PR |
Less than PR | OS |
Survival at 1 y |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | No. wks, median | 95% CI | % | 95% CI | ||
| 1 prior induction | 6 (55) | 23-82 | 4 (36) | 11-69 | 2 (18) | 2-52 | 5 (45) | 19.8 | 12.0-219.4 | 36 | 11-63 |
| More than 1 prior induction | 10 (36) | 19-56 | 8 (29) | 13-49 | 2 (7) | 1-24 | 18 (64) | 20.3 | 10.4-36.4 | 25 | 11-42 |
| Total | 16 (41) | 26-58 | 12 (31) | 17-48 | 4 (10) | 3-24 | 23 (59) | 19.8 | 13.0-36.4 | 28 | 15-43 |
Abbreviations are as in Table 3.
CR + CRi + PR.
Table 5.
Summary of responses in patients with disease refractory to most recent therapy
| N (%) | 95% CI | |
|---|---|---|
| Number of patients | 19 (100) | — |
| Overall* | 7 (37) | 16, 62 |
| CR+CRi | 4 (21) | 6, 46 |
| Partial response | 3 (16) | — |
| Less than a partial response | 12 (63) | — |
| Survival at 1 year | 7 (37) | 17, 57 |
Two patients remained in remission at the date of last contact. One patient proceeded directly to high-dose therapy with SCT while still in a nelarabine-induced remission and has remained in a CR for longer than 4 years. A second patient has remained in CR for longer than 5.25 years without subsequent anticancer therapy after treatment with 3 cycles of nelarabine (Figure 1). Before enrollment, this patient with T-LBL had relapsed after 2 multiagent induction regimens, one of which included autologous SCT.
Figure 1.
Duration of disease-free survival in weeks. ▴ corresponds to the time of stem cell transplantation; + represents patients who remain in continuous remission.
Survival
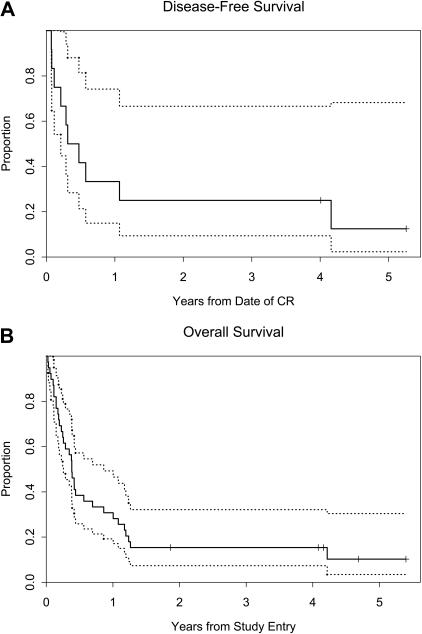
The median survival for all treated patients was 20 weeks, with a 95% confidence interval (CI) of 13 to 36 weeks. The Kaplan-Meier estimate for survival is shown in Figure 2. The median survival for those patients with one prior induction regimen was 20 weeks with a 95% CI of 12 to 219 weeks (Table 4). For patients who had received 2 or more prior inductions, the median survival was 20 weeks with a 95% CI of 10 to 36 weeks. The 1-year survival rate for all treated patients was 28% (95% CI, 15% to 43%) (Table 3 and Figure 2). Of the 28 patients with 2 or more prior inductions, the 1-year survival rate was 25% (95% CI, 11% to 42%). The 1-year survival rate for patients whose disease failed to respond to their most recent prior induction was 37% (95% CI, 17% to 57%) (Table 5).
Figure 2.
Kaplan-Meier curves. (A) Disease-free survival (DFS) and (B) overall survival (OS). The dotted curves represent the 95% confidence limits.
Stem-cell transplantation
Successful engraftment after SCT was not an end point in the original study design, but these data were collected retrospectively. Seven subjects who were treated with nelarabine subsequently received a SCT. Four patients who achieved a CR and one patient who was classified as a CRi underwent SCT. Two subjects who did not respond to nelarabine subsequently received SCT. Engraftment data for 5 of these 7 patients were collected.
Full hematopoietic engraftment was documented on days 7, 11, and 18 after transplant in 3 of the 7 patients who underwent a SCT. One patient died shortly after transplant before engrafting, and engraftment data for another patient are unknown. This latter patient was removed from the study treatment after cycle 1 to go directly to SCT without additional anticancer therapy and was still alive at last contact 4 years later. Myeloid engraftment was not reported in 2 additional patients, but both were still alive almost 3 years after SCT, suggesting that engraftment had in fact occurred.
Toxicity
Toxicity data were available on 38 of 39 treated patients. Nine subjects (24%) experienced a grade 3 adverse event (AE), and 16 subjects (42%) experienced a grade 4 event judged by the investigator to be possibly or probably related to nelarabine (Table 6). The most frequent grade 3 or higher nonhematologic events were fatigue (n = 7, 18%) and muscle weakness (n = 4, 11%). Although gastrointestinal symptoms (nausea, diarrhea, stomatitis, or vomiting) were reported in 51% of subjects, only one of these events was severe (diarrhea grade 3).
Table 6.
Summary of adverse events (all treated subjects with available toxicity data, n=38)
| Adverse event | Events, no. (%) |
||
|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | |
| Blood/bone marrow | |||
| Neutropenia | 5 (13) | 11 (29) | 0 |
| Thrombocytopenia | 7 (18) | 6 (16) | 0 |
| Anemia | 6 (16) | 2 (5) | 0 |
| Cardiovascular | |||
| Hypertension | 1 (3) | 0 | 0 |
| Hepatic | |||
| Aspartate AT | 1 (3) | 1 (3) | 0 |
| Alanine AT | 1 (3) | 1 (3) | 0 |
| Bilirubin | 2 (5) | 0 | 0 |
| Infection (ANC greater than 500) | 1 (3) | 1 (3) | 0 |
| Febrile neutropenia | 3 (8) | 0 | 0 |
| Neurologic | |||
| Aphasia | 1 (3) | 0 | 0 |
| Hallucinations | 1 (3) | 0 | 0 |
| Depressed consciousness | 0 | 1 (3) | 0 |
| Depression | 1 (3) | 0 | 0 |
| Confusion | 1 (3) | 0 | 0 |
| Neuropathy, peripheral | 1 (3) | 0 | 0 |
| Seizure | 1 (3) | 0 | 0 |
| Constitutional/other | |||
| Diarrhea | 1 (3) | 0 | 0 |
| Fatigue | 6 (16) | 1 (3) | 0 |
| Muscle weakness | 4 (11) | 0 | 0 |
| Myalgia | 1 (3) | 0 | 0 |
AT indicates aminotransferase; ANC, absolute neutrophil count per microliter.
The most frequent reasons for withdrawal from the study were for progressive or relapsed disease (n = 14, 36%) and no response to therapy (n = 14, 36%). Three subjects were withdrawn because of toxicity. One patient withdrew after 3 cycles as a result of proteinuria in the nephrotic range that was thought to be unrelated to nelarabine. Two patients withdrew from protocol therapy because of peripheral sensory neuropathy grade 2 after 3 and 5 cycles, respectively. Three additional patients withdrew to receive other therapies, and 2 patients died, one from a motor vehicle accident while in long-term remission.
Peripheral sensory neuropathy was reported in 14 patients (37%); all events were either grade 1 or 2. Peripheral motor neuropathy was reported in 8 patients (21%); only one was grade 3. In addition, one patient had a grade 3 seizure after receiving a cumulative dose of 4.5 g/m2 of nelarabine; this was thought to be more likely to be due to imipenem and renal dysfunction. Another patient experienced grade 3 aphasia after receiving 4.5 g/m2 of nelarabine. There was only one grade 4 AE of the nervous system, which was a reversible depressed level of consciousness (Table 6).
At the time of this report, 34 patients (87%) have died. Of the 34 deaths, 31 were disease-related and 3 were unrelated to the disease or to the protocol treatment. No deaths were related to nelarabine. Deaths thought to be unrelated to underlying disease were attributed to pneumocystic pneumonia, an automobile accident, and graft-versus-host disease. Two patients died from disease progression while receiving therapy. Three patients died while on study after receiving only one cycle of nelarabine. One died of respiratory failure secondary to progressive disease. Another died from multiorgan failure reported as hypotension. The cause of death was considered by the investigator to be unrelated to the study drug. The third patient died from a pleural effusion and pneumothorax related to disease progression.
Discussion
This study was an open-label multicenter phase II study performed to assess the efficacy and safety of single agent nelarabine in adult subjects with refractory or relapsed T-lineage ALL/LBL. The patients treated on this study were representative of the general population of patients with relapsed T-ALL/LBL in terms of their age, gender, functional status, and the amount of previous therapy they had received. The study population was balanced between subjects whose disease failed to respond after their most recent prior therapy and those who had achieved a CR after their most recent induction attempt (49% vs 51%). Most subjects (67%) had extramedullary disease at the time of enrollment. All of the patients had received at least one prior induction chemotherapy regimen, and 72% had received 2 or more prior attempts at induction. Five patients (13%) had also undergone a prior SCT. Thus, this was a heavily pretreated group of patients with poor prognoses.
It is noteworthy that 4 of the 19 subjects (21%) who had just failed to respond after their most recent induction attempt exhibited a CR or CRi after treatment with nelarabine. All of these patients had received 2 or more prior induction regimens before responding to nelarabine. This suggests that the mechanism of action of nelarabine differs from that of the other standard agents that these patients had previously received.
The median survival for subjects with 2 or more prior inductions was 20 weeks, and 25% of this group of patients was still alive at 1 year. Historical survival data for patients in this population are not known, but the results observed here are similar to those reported for children and adults treated with multiagent regimens after failure of one prior regimen.2–5 For example, Thomas et al5 reported 24% 1-year survival in 57 patients with relapsed or refractory T-ALL treated with various multiagent regimens.
Berg et al recently reported results from a trial conducted by the Children's Oncology Group (COG) in children with refractory T-cell malignancies treated with nelarabine for 5 consecutive days.20 An overall response (OR; CR plus PR) rate of 55% was seen in patients with T-ALL in first relapse and an OR rate of 27% was seen with T-ALL in second relapse. Grade 3 or 4 neurologic adverse events were seen in 18% of the patients, including peripheral neuropathy, hallucinations, seizures, and one episode of a Guillain-Barré—like syndrome.
Neurotoxicity from nelarabine has been an ongoing concern since the early phase I studies. The majority of the neurologic events reported in the current trial were reversible grade 1 or 2. However, there were 7 severe (grade 3 or 4) neurologic AEs in 4 patients among 38 treated patients whose toxicity data were available. In 2 of these cases, the events had other likely causes and were thought to be unrelated to nelarabine. Thus, although neurologic AEs were reported, the number (n = 2) of severe neurologic AEs likely related to nelarabine was low.
The neurologic AEs that occurred in this study may have been related, at least in part, to prior chemotherapy with other neurotoxic agents, such as vincristine, methotrexate, or cytarabine, or to intrathecal (IT) therapy. Because patients with active CNS disease were excluded, patients did not receive concurrent IT chemotherapy on this study. The relatively low rate of neurologic events observed on this study was possibly due to the lack of concurrent intrathecal treatment.
The treatment goal for patients with relapsed or refractory ALL/LBL is most often to prepare the patient for allogeneic SCT. Therefore, the response must be of sufficient duration to allow for a transplant evaluation. The durations of responses (CR + CRi) observed on this study were all in excess of 4 weeks. When time to response is added to the duration of response, an adequate period of disease control was obtained. Thus, all patients with CRs on this study had their disease controlled for at least 7 weeks, which in most cases should provide sufficient time to arrange for a SCT. Goekbuget et al21 reported results from a similar phase II study with nelarabine by the German Multicenter ALL Group wherein 47% of patients with relapsed T-ALL or T-LBL achieved a CR. Impressively, 76% of the patients achieving a CR were able to proceed to a SCT. Within the current study, 7 patients underwent a SCT, and myeloid engraftment was established in 5 patients. Although preliminary, these data suggest that SCT after nelarabine administration is safe without an increase in transplant-related toxicities.
In summary, nelarabine demonstrated clinically significant antineoplastic activity with acceptable toxicity in adult patients with relapsed/refractory T-ALL/LBL. Neurotoxicity was mostly mild and reversible in this limited study population. These data led to the approval of nelarabine for this indication in the United States by the Food and Drug Administration. Nelarabine has the potential to provide an alternative chemotherapeutic approach to adult patients with T-ALL/LBL and should be evaluated in frontline regimens in sequence with other standard chemotherapy agents in an attempt to increase the cure rate.
Supplementary Material
Acknowledgments
This work was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, Chairman). D.J.D. was supported by CA32291, D.Y. and S.E.C. were supported by CA33601, A.T.S. supported by CA13612, J.P.G. was supported by supported by CA47577, B.S.M. was supported by CA47559, F.R.A was supported by CA20319, and R.A.L. was supported by CA41287.
We acknowledge Kelly Sullivan for assistance with the manuscript and Ilene Galinksy for technical assistance.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
D.J.D. was the overall principal investigator, performed research, analyzed data, and wrote the manuscript. D.Y. was the principal statistician, analyzed data, and edited the manuscript. J.L.J. was the staff statistician, analyzed data, and edited the manuscript. S.E.C. performed research, and edited the manuscript. R.M.S., A.T.S., and J.P.G. performed research and edited the manuscript. B.S.M. was the coprincipal investigator, performed research, and edited the manuscript. F.R.A. performed research, and edited the manuscript. R.A.L. performed research, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: R.A.L. gave expert testimony to FDA; the remaining authors declare no competing financial interests.
Correspondence: Daniel J. DeAngelo, Dana-Farber Cancer Institute, 44 Binney Street, Boston, MA 02115; e-mail: ddeangelo@partners.org.
References
- 1.Laport GF, Larson RA. Treatment of adult acute lymphoblastic leukemia. Semin Oncol. 1997;24:70–82. [PubMed] [Google Scholar]
- 2.Giona F, Testi AM, Rondelli R, et al. ALL R-87 protocol in the treatment of children with acute lymphoblastic leukaemia in early bone marrow relapse. Br J Haematol. 1997;99:671–677. doi: 10.1046/j.1365-2141.1997.4413253.x. [DOI] [PubMed] [Google Scholar]
- 3.Giona F, Annino L, Rondelli R, et al. Treatment of adults with acute lymphoblastic leukaemia in first bone marrow relapse: results of the ALL R-87 protocol. Br J Haematol. 1997;97:896–903. doi: 10.1046/j.1365-2141.1997.1102926.x. [DOI] [PubMed] [Google Scholar]
- 4.Testi AM, Del Giudice I, Arcese W, et al. A single high dose of idarubicin combined with high-dose ARA-C for treatment of first relapse in childhood 'high-risk' acute lymphoblastic leukaemia: a study of the AIEOP group. Br J Haematol. 2002;118:741–747. doi: 10.1046/j.1365-2141.2002.03706.x. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Lambe CU, Averett DR, Paff MT, Reardon JE, Wilson JG, Krenitsky TA. 2-Amino-6-methoxypurine arabinoside: an agent for T-cell malignancies. Cancer Res. 1995;55:3352–3356. [PubMed] [Google Scholar]
- 7.Cohen A, Lee JW, Gelfand EW. Selective toxicity of deoxyguanosine and arabinosyl guanine for T-leukemic cells. Blood. 1983;61:660–666. [PubMed] [Google Scholar]
- 8.Gelfand EW, Lee JW, Cohen A. A sensitivity of T-leukemic cells to deoxyguanosine and arabinosyl guanine. Adv Exp Med Biol. 1984;165:309–314. doi: 10.1007/978-1-4757-0390-0_59. [DOI] [PubMed] [Google Scholar]
- 9.Shewach DS, Mitchell BS. Differential metabolism of 9-beta-d-arabinofuranosylguanine in human leukemic cells. Cancer Res. 1989;49:6498–6502. [PubMed] [Google Scholar]
- 10.Shewach DS, Daddona PE, Ashcraft E, Mitchell BS. Metabolism and selective cytotoxicity of 9-beta-d-arabinofuranosylguanine in human lymphoblasts. Cancer Res. 1985;45:1008–1014. [PubMed] [Google Scholar]
- 11.Verhoef V, Fridland A. Metabolic basis of arabinonucleoside selectivity for human leukemic T- and B-lymphoblasts. Cancer Res. 1985;45:3646–3650. [PubMed] [Google Scholar]
- 12.Ullman B, Martin DW., Jr Specific cytotoxicity of arabinosylguanine toward cultured T lymphoblasts. J Clin Invest. 1984;74:951–955. doi: 10.1172/JCI111514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert ME, Greenberg ML, Chaffee S, et al. Pharmacologic purging of malignant T cells from human bone marrow using 9-beta-d-arabinofuranosylguanine. Transplantation. 1991;52:634–640. doi: 10.1097/00007890-199110000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Reist EJ, Goodman L. Synthesis of 9-beta-d-arabinofuranosylguanine. biochemistry. 1964;3:15–18. doi: 10.1021/bi00889a004. [DOI] [PubMed] [Google Scholar]
- 15.Krenitsky TA, Koszalka GW, Tuttle JV. Purine nucleoside synthesis, an efficient method employing nucleoside phosphorylases. Biochemistry. 1981;20:3615–3621. doi: 10.1021/bi00515a048. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–3403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 17.Kisor DF, Plunkett W, Kurtzberg J, et al. Pharmacokinetics of nelarabine and 9-beta-d-arabinofuranosyl guanine in pediatric and adult patients during a phase I study of nelarabine for the treatment of refractory hematologic malignancies. J Clin Oncol. 2000;18:995–1003. doi: 10.1200/JCO.2000.18.5.995. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi V, Plunkett W, Rodriguez CO, Jr, et al. Compound GW506U78 in refractory hematologic malignancies: relationship between cellular pharmacokinetics and clinical response. J Clin Oncol. 1998;16:3607–3615. doi: 10.1200/JCO.1998.16.11.3607. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 20.Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children's Oncology Group. J Clin Oncol. 2005;23:3376–3382. doi: 10.1200/JCO.2005.03.426. [DOI] [PubMed] [Google Scholar]
- 21.Goekbuget N, Arnold R, Atta J, et al. Compound GW506U78 has high single-drug activity and good feasibility in heavily pretreated relapsed T-lymphoblastic leukemias (T-ALL) and T-lymphoblastic lymphoma (T-LBL) and offers the option for cure with stem cell transplantation (SCT). Blood. 2005;106:47a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.