Abstract
Keratocytes of the corneal stroma secrete a specialized extracellular matrix essential for vision. These quiescent cells exhibit limited capacity for self-renewal and after cell division become fibroblastic, secreting nontransparent tissue. This study sought to identify progenitor cells for human keratocytes. Near the corneal limbus, stromal cells expressed ABCG2, a protein present in many adult stem cells. The ABCG2-expressing cell population was isolated as a side population (SP) by cell sorting after exposure to Hoechst 33342 dye. The SP cells exhibited clonal growth and continued to express ABCG2 and also PAX6, product of a homeobox gene not expressed in adult keratocytes. Cloned SP cells cultured in medium with fibroblast growth factor-2 lost ABCG2 and PAX6 expression and upregulated several molecular markers of keratocytes, including keratocan, aldehyde dehydrogenase 3A1, and keratan sulfate. Cloned corneal SP cells under chondrogenic conditions produced matrix staining with toluidine blue and expressed cartilage-specific markers: collagen II, cartilage oligomatrix protein, and aggrecan. Exposure of cloned SP cells to neurogenic culture medium upregulated mRNA and protein for glial fibrillary acidic protein, neuro filament protein, and beta-tubulin II. These results demonstrate the presence of a population of cells in the human corneal stroma expressing stem cell markers and exhibiting multipotent differentiation potential. These appear to be the first human cells identified with keratocyte progenitor potential. Further analysis of these cells will aid elucidation of molecular mechanisms of corneal development, differentiation, and wound healing. These cells may be a resource for bioengineering of corneal stroma and for cell-based therapeutics.
Keywords: Cornea, Keratocyte, Keratocan, Keratan sulfate, ABCG2, PAX6, Side population, Adult stem cells, Progenitor cells, Chondrogenesis
Introduction
As the outermost layer of the eye, the cornea both serves as a barrier and provides an optical function, transmitting and focusing incident light on the retina. The corneal stroma is a connective tissue making up 90% of the corneal thickness, with physical properties that provide the cornea its essential character. The stroma is composed of collagenous lamellae consisting of tightly packed collagen fibrils embedded in a hydrated matrix of glycoproteins and proteoglycans. The keratocytes are a population of quiescent, neural crest–derived cells, sandwiched between the lamellae, responsible for secretion of the unique stromal extracellular matrix. In response to acute injury, keratocytes become mitotic, adopt a fibroblastic phenotype, and move to the injured area [1, 2]. This fibroblastic activation results in deposition of new extracellular matrix [3] with a composition and light transmission properties different from that of the normal, uninjured stroma. Stromal scarring can also result from a number of chronic conditions in addition to acute wound healing. Scar tissue is long-lasting and can cause a permanent disruption of vision.
Keratoplasty is currently the only effective method providing recovery of vision after corneal blindness. Although donated corneal tissue currently meets the needs of most recipients in the U.S., worldwide 8 to 10 million individuals suffer from corneal blindness without access to therapy. Additionally, numerous individuals reject allogeneic corneal tissue, and the supply of donated corneas may soon be reduced by the increasing number of refractive surgeries. Because of these problems, there is significant interest in development of artificial and bioengineered corneas. Griffith et al. [4] have demonstrated that corneal equivalents generated from the three corneal cell layers mimic human corneas in key physical and physiological functions. These studies used immortalized cell lines transformed with retrovirus, consequently not suitable for transplantation. Focus has therefore turned to stem cells as a source of tissues for use in cell-based therapy and corneal tissue engineering.
Stem cells have self-renewal ability as well as the capability for multilineage differentiation and in vivo functional reconstruction of several tissues [5]. Embryonic stem cells derived from the inner cell mass of the blastocyst are pluripotent cells that can be propagated indefinitely in an undifferentiated state. Stem cells exist in many adult tissues as well. Compared with embryonic stem cells, tissue-specific stem cells have less self-renewal ability. However, recent studies suggest that tissue-specific stem cells can differentiate into lineages other than the tissue of origin [6]. Adult stem cells occur in small numbers compared with the somatic cells of the tissue, and isolation can be difficult. Adult stem cells have the ability to efflux fluorescent dye Hoechst 33342, leading to reduced red and blue fluorescence in fluorescence-activated cell sorting (FACS) [7]. These cells are referred to as “side population” (SP) cells. The SP cells are lost after treatment with verapamil, a drug that blocks action of the ATP-binding cassette transporter G family member known as ABCG2. This transporter protein has been identified as the Hoechst efflux pump [8–10] and as a specific marker for many kinds of stem cells such as hematopoietic [8, 9, 11], mesenchymal [8], muscle [8], neural [12, 13], cardiac [14], islet [15], and keratinocyte [16, 17] stem cells. Recently stem cells in the corneal epithelium have been shown to express ABCG2 and to separate as an SP [18, 19]; however, no studies have identified SP cells or other forms of stems cells from the human corneal stroma, nor has generation of differentiated human keratocytes from stem cells been demonstrated.
In this study, we identify an SP of cells from the human corneal stroma which exhibits many aspects of stem cells, including clonal growth in vitro, extended lifespan, and the ability to differentiate into several different cells types, including keratocytes of the cornea.
Materials and Methods
Materials
Antibodies used included anti-keratocan (made in our lab) [20], ABCG2 (a gift from Dr. Bates [21]; National Cancer Institute, Bethesda, MD, http://www.cancer.gov) for immunostaining and ABCG2 (Clone BXP-21; Chemicon International, Temecula, CA, http://www.chemicon.com) for immunostaining and immunoprecipitation, Pax6 (Covance, Princeton, NJ, http://www.covance.com), ALDH (aldehyde dehydrogenase; a gift from Dr. R. Lindahl) [22], J36 to keratan sulfate (made in our lab) [23], beta-tubulin III, glial fibrillary acidic protein (GFAP) (Chemicon International), NF200 against neurofilament protein (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com), and collagen II (Abcam, Inc., Cambridge, MA, http://www.abcam.com). Secondary antibodies for Western blotting, peroxidase-labeled anti-mouse and anti-rabbit IgG, were from Santa Cruz Biotechnology (Santa Cruz, CA, http://www.scbt.com). For fluorescent staining, anti-mouse Alexa 488, anti-rabbit Alexa 488, anti-rabbit Alexa 647, nuclear dye TO-PRO-3 iodide, and Vybrant DiD were obtained from Molecular Probes, Inc. (Eugene, OR, http://probes.invitrogen.com).
Cell Culture
Human corneas were obtained from the Center for Organ Recovery & Education (Pittsburgh, http://www.core.org). Donor human corneas not usable for transplantation were rinsed and incubated in 2.4 U/ml Dispase II (Roche Diagnostics, Pleasanton, CA, http://www.roche-diagnostics.com) overnight at 4°C. Epithelial and endothelial cells were removed by dissection and debridement, and the stroma was minced into 2-mm cubes. Stroma was digested up to 3 hours at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) containing 1 mg/ml collagenase type L (Sigma-Aldrich) and 0.2 mg/ml testicular hyaluronidase (Sigma-Aldrich). Primary stromal cells were cultured at 1 × 104 per cm2 in a humidified atmosphere containing 5% CO2 in a medium (stem cell growth medium [SCGM]) modified from Jiang et al. [6] containing DMEM/MCDB-201 (Sigma-Aldrich) with 2% fetal bovine serum (FBS) (HyClone, Logan, UT, http://www.hyclone.com), 10 ng/ml epidermal growth factor (Invitrogen Corporation, Carlsbad, CA, http://www.invitrogen.com), 10 ng/ml platelet-derived growth factor (PDGF-BB) (Sigma-Aldrich), 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenous acid (ITS) (Invitrogen), 1,000 units per ml leukemia inhibitory factor (LIF) (Chemicon International), ×1 linoleic acid–bovine serum albumin (LA-BSA), 0.1 mM ascorbic acid-2-phosphate, 10−8 M dexamethasone, 100 IU/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, and 1.25 μg/ml amphotericin B (Sigma-Aldrich). When 80%–90% confluent, cells were trypsinized and subcultured.
Cell Sorting
At passage four, trypsinized cells were incubated at 1.0 × 106 cells per ml in DMEM with 2% FBS and 5 μg/ml Hoechst 33342 dye (Molecular Probes, Inc.) for 90 minutes at 37°C. To inhibit ABCG2-mediated efflux of Hoechst dye, cells were preincubated for 20 minutes with 50 μg/ml verapamil (an inhibitor of multidrug resistance proteins) (Sigma-Aldrich) before Hoechst 33342 incubation. After staining, the cells were washed twice in Hanks’ balanced salt solution (HBSS) with 2% FBS and then stored in cold HBSS with 2% FBS on ice. Immediately before sorting, 2 μg/ml propidium iodide (Sigma-Aldrich) was added to identify nonviable cells for flow cytometric analysis. Cells were analyzed on a MoFlo (DakoCytomation, Fort Collins, CO, http://www.dakocytomation.us) high-speed cell sorter, using 350-nm excitation [24]. Cells showing reduced fluorescence of both blue (670 nm) and red (450 nm), a “side population,” were collected [7]. Dead cells stained with propidium iodide were omitted from the population.
SP Cell Culture, Cloning, and Differentiation
After sorting, SP cells were cultured in SCGM. At 80%–90% confluence, these cells were cloned by limiting dilution and subcultured at a density of 1 × 104 cells per cm2. Cloned cells were used in all subsequent experiments. To determine differentiation potential, cloned passage-18 SP cells were incubated 2 weeks in Advanced D-MEM (Invitrogen Corporation) supplemented with fibroblast growth factor 2 (FGF2) 10 ng/ml (keratocyte differentiation medium [KDM]). Neural differentiation medium (NDM) contained Advanced D-MEM with 10 ng/ml epithelial growth factor, 10 ng/ml FGF2 and 1 μM all-trans retinoic acid. Retinoic acid was added every 3 days, and the cells were kept 2–3 weeks to induce neurogenesis. For chondrogenesis, 2 × 105 cells per tube were used in a pellet culture [25]. Chondrocyte differentiation medium (CDM) contained DMEM/MCDB-201, 2% FBS, 0.1 mM ascorbic acid-2-phosphate, 10−7M dexamethasone, 10 ng/ml TGFβ1 (transforming growth factor β1), and 100 μg/ml sodium pyruvate. The medium was changed every 3 days. Pellets were cultured for 2–3 weeks. Fibroblastic differentiation was induced in DMEM/F12 with 10% FBS. After three passages, fibroblasts were subcultured in different media as described above to assess the potential for reversion.
Reverse Transcription–Polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR
Qualitative RT-PCR was carried out as previously described [20]. PCR products were separated on 6% acrylamide gel and detected by SYBR Gold (Molecular Probes, Inc.). Amplification of 18S rRNA was carried out for each cDNA as a qualitative external control. Primers used are shown in Table 1. Quantitative RT-PCR was performed using SYBR Green RT-PCR Reagents (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) according to the manufacturer’s instructions. The reaction was carried out for 40 cycles of 15 seconds at 95°C and 1 minute at 60°C after initial incubation at 95°C for 10 minutes. Reaction volume was 50 μl, containing ×1 SYBR Green PCR buffer, 3 mM Mg2+, 200 μM dATP, dCTP, dGTP and 400 μM dUTP, 0.025 units per ml AmpliTaq Gold polymerase, 5 μl cDNA, and forward and reverse primers at optimized concentrations. A dissociation curve for each reaction was generated on the Gene-Amp ABI Prism 7700 Sequence Detection System (Applied Bio-systems) to confirm the absence of nonspecific amplification. Amplification of 18S rRNA was performed for each cDNA (in triplicate) for normalization of RNA content. Threshold cycle number (Ct) of amplification in each sample was determined by ABI Prism Sequence Detection System software (Applied Bio-systems). Relative mRNA abundance was calculated as the Ct for amplification of a gene-specific cDNA minus the average Ct for 18S expressed as a power of 2, that is, 2−Delta;Ct. Three individual gene-specific values thus calculated were averaged to obtain mean ± SD. One-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests were used to evaluate the different gene expression of cells in different media.
Table 1.
Primers for reverse transcription–polymerase chain reaction
| Gene | DNA sequence |
|---|---|
| Keratocan | Forward: ATCTGCAGCACCTTCACCTT
Reverse: CATTGGAATTGGTGGTTTGA |
| ABCG2 | Forward: TGCAACATGTACTGGCGAAGA
Reverse: TCTTCCACAAGCCCCAGG |
| Pax6 | Forward: CAATCAAAACGTGTCCAACG
Reverse: TAGCCAGGTTGCGAAGAACT |
| Keratin 12 | Forward: CTACCTGGATAAGGTGCGAGCT
Reverse: TCTCGCATTGTCAATCTGCA |
| Neurofilament | Forward: GAGGAACACCAAGTGGGAGA
Reverse: CTCCTCCTCTTTGGCCTCTT |
| GFAP | Forward: ACTACATCGCCCTCCACATC
Reverse: CAAAGGCACAGTTCCCAGAT |
| Aggrecan | Forward: TGAGGAGGGCTGGAACAAGTACC
Reverse: GGAGGTGGTAATTGCAGGGAACA |
| COMP | Forward: ACAATGACGGAGTCCCTGAC
Reverse: AAGCTGGAGCTGTCCTGGTA |
| Collagen II | Forward: CCGGGCAGAGGGCAATAGCAGGTT
Reverse: CAATGATGGGGAGGCGTGAG |
Immunodetection
ABCG2 was detected by immune precipitation after cell surface biotinylation with Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL, http://www.piercenet.com). Initially, Protein G magnetic beads (Dynal Biotech LLC, Brown Deer, WI, http://www.dynalbiotech.com) were used to clear the cell extract. ABCG2 antibody (clone BXP-21; Chemicon International) was preincubated with Protein G Beads and then the loaded beads were incubated with samples overnight. The eluted proteins were separated on 4%–20% SDS-PAGE gel, transferred to polyvinylidene difluoride (PVDF) membranes, and streptavidin-HRP (BD Biosciences Pharmingen, San Jose, CA, http://www.bdbiosciences.com/pharmingen) was used to detect biotinylated proteins using luminescent substrate [20].
Proteoglycans were recovered from culture media by ion exchange chromatography on SPEC-NH2 microcolumns (Ansys Diagnostics) as described previously [20]. Proteoglycans were digested with keratanase II and endo-beta-galactosidase [26]. Digested and undigested samples, normalized for cell number, were run on a 4%–20% SDS-PAGE gel, transferred to PVDF membrane, and subjected to immunoblotting with antibody against keratocan [20] and antibody J36 against keratan sulfate [23]. For cell associated proteins, cells were lysed by M-PER® (Pierce) with 1% v/v protease inhibitor cocktail (Sigma-Aldrich). Equal amounts of lysate protein were separated on SDS-PAGE gels as described above.
Histology
Cells cultured in plastic plates were rinsed briefly in phosphate-buffered saline (PBS), fixed for 12–15 minutes in 3% paraformaldehyde in PBS at room temperature, rinsed in PBS, and stored at 4°C in 50% glycerol and 50% PBS (v/v) until staining. Cells cultured in pellets were rinsed and fixed as described above and then embedded in OCT compound [25] and cut into 8 μ sections. Human corneas were washed and embedded in OCT at −80°C. Before staining, they were cut into 10 μ sections and fixed in acetone for 10 minutes at −20°C. Nonspecific binding was blocked with 10% heat-inactivated goat serum. Sections were incubated for 1 hour at room temperature with primary antibodies. After two washes, secondary antibodies were added followed by incubation for 1 hour at room temperature. The samples were photographed using a confocal microscope with a ×40 oil objective (Bio-Rad Laboratories, Hercules, CA, http://www.bio-rad.com). Pellet culture sections were stained for 1 minute in 0.1% toluidine blue solution in 0.1 M acetic acid.
Results
ABCG2-Expressing Cells in the Corneal Stroma
The ATP-binding cassette transporter ABCG2 has been identified as a specific marker for stem cells in a number of adult tissues [8, 12–17]. We hypothesized that similar to other adult tissues, stem cells in the corneal stroma would express the ABCG2 protein. Immunofluorescent staining of frozen human corneal sections, using a polyclonal antibody to extracellular domains of the ABCG2 protein, identified positive cells in human corneal stroma. These cells were rare but most often observed in the peripheral region of the stroma posterior to the limbal feature known as the Palisades of Vogt, in which the epithelium exhibits extensive folding (Figs. 1A, 1B). These results confirmed recent reports [18, 19] that epithelial cells in this region express ABCG2 protein (Fig. 1B, triangles). A number of cells in the anterior stroma in this region also expressed ABCG2 (Fig. 1B, arrows). A second monoclonal antibody against ABCG2, Clone BXP21, identified a similar population of cells in the anterior stroma (Fig. 1C, arrows). This antibody also stained rare cells in more central regions of the stroma in which epithelial staining was absent (Fig. 1D).
Figure 1.
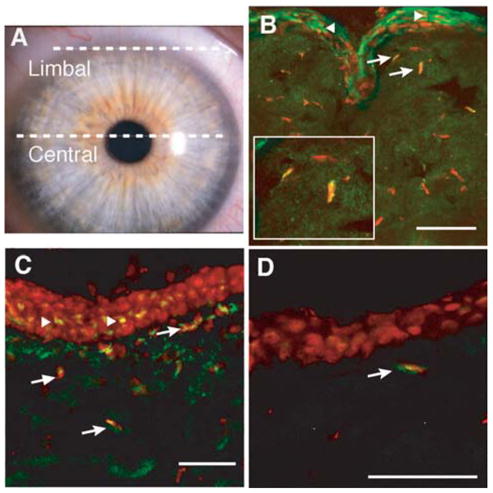
ABCG2 protein localization in human cornea. (A): Transverse frozen sections were obtained from the limbal and central regions of unfixed human corneas as indicated by white lines in the photograph. (B): ABCG2 protein (green) was detected in epithelial and stromal cells of limbal sections, using the antibody described by Litman et al. [21]. Cells were counterstained with To-Pro-3 (red). Arrows show stained stromal cells and triangles indicate stained epithelial cells. Inset shows magnification of two cells designated by arrows. (C): ABCG2 protein could also be also demonstrated in limbal sections, using monoclonal antibody BXP21 (green). (D): ABCG2 was detected in occasional stromal cells in central cornea (arrow), using antibody BXP21 as in (C). Solid white bars = 50 μM.
The ABCG2 transporter protein acts to remove Hoechst dye from cells, thus allowing isolation of cells in which ABCG2 is active by use of FACS [8–10]. To get enough human stromal cells for FACS analysis, primary cultures were expanded in a culture medium (SCGM) modified from Jiang et al. [6] in which stem cells can proliferate without loss of differentiation potential. In passage four, stromal cells stained with Hoechst 33342 contained an SP of 1.3% of the cells in which both red and blue Hoechst fluorescence were reduced (Fig. 2A, arrow). Preincubation with verapamil, an inhibitor of ABCG2 function (Fig. 2B), virtually eliminated the SP cells. The isolated SP cells were cultured in SCGM and then were subcultured and cloned.
Figure 2.
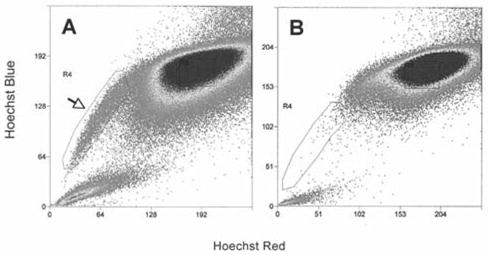
Flow cytometric isolation of side population from cultured human corneal stromal cells. (A): Passage-four stromal cells stained with Hoechst 33342 were analyzed using 350-nm excitation with blue (635 nm) and red (488 nm) emission. Cells showing reduction of both blue and red fluorescence (side population cells) were collected as defined by the box outlined on the left (arrow). (B): An analysis similar to (A) but with a preincubation in 50 μM verapamil before incubation with Hoechst 33342.
At passage 18, the cloned SP cells continued to express mRNA for ABCG2, as did noncultured primary stromal cells. SP cells transferred into a variety of media in which they exhibited differentiated characteristics showed a significant (p < .001) reduction of ABCG2 mRNA (Fig. 3A). SP cells passaged three times as fibroblasts in 10% FBS and then returned to SCGM did not regain ABCG2 expression (Fig. 3A). ABCG2 protein could be detected in the SP cells by direct immunostaining (Fig. 3B) or by immunoprecipitation of biotin-labeled cell-surface proteins using antibody to the ABCG2 protein (Fig. 3B, inset). SP cells incubated 1 week in KDM lost this expression of ABCG2 protein (Fig. 3C).
Figure 3.
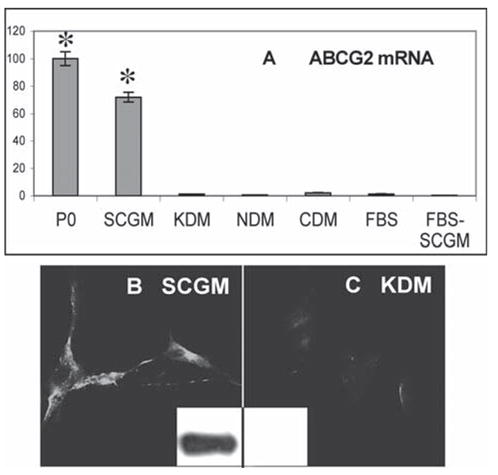
Expression of ABCG2 in cultured side population cells. (A): mRNA for ABCG2 was detected in stromal cells by quantitative reverse transcription–polymerase chain reaction. Side population cells, selected as in Figure 2 and maintained in culture for 18 serial passages in SCGM, were compared with freshly isolated uncultured stromal cells (P0) and with side population cells after conditioning 1–3 weeks with KDM, NDM, CDM, and 10% FBS. After passaging four times in FBS, cells were transferred back to SCGM (FBS-SCGM). (B): ABCG2 protein expression in SCGM was detected by immunostaining cultured cells and by immunoblotting (inset). (C): ABCG2 detected in KDM as in (B). Error bars show SD of triplicate analyses. Asterisks indicate significant difference (p < .001) from cells in differentiation media and FBS. Abbreviations: CDM, chondrocyte differentiation medium; FBS, fetal bovine serum; KDM, keratocyte differentiation medium; NDM, neural differentiation medium; SCGM, stem cell growth medium.
PAX6 Expression in ABCG2-Positive Stromal Cells
PAX6 is a homeobox transcription factor expressed in embryonic ocular precursor cells and epithelial cells but absent in adult keratocytes [27]. Immunostaining for PAX6 showed rare stromal cells expressing this protein (Figs. 4A, 4B). These PAX6-positive stromal cells also stained for ABCG2 (Fig. 4C). We found that the cultured SP cells maintained a strong nuclear staining for PAX6 (Fig. 4D). Transcripts for PAX6 could be amplified from RNA from corneal epithelial cells and from noncultured stromal cells (Fig. 4E) and, as expected, PAX6 mRNA was present in SCGM-cultured SP cells. PAX6, however, was absent in SP cells cultured in KDM or in fibroblastic cells (Fig. 4E). To control for epithelial cell contamination in the stromal and SP cell preparations, we examined the same RNA samples for the presence of keratin 12, an epithelial-specific gene product. As shown in Figure 4F, keratin 12 mRNA was detected only in epithelial cells but not in the unfractionated stromal cells or in any of the SP cell populations. These experiments show the SP cells to have a specific phenotype, stable through subculture, cloning, and more than 20 serial passages. The absence of keratin 12 marker in the unfractionated cells from which the SP cells are derived demonstrates this population to have been isolated without contamination by epithelial cells.
Figure 4.
PAX6expressioninstromalcells.(A):PAX6protein(green) was detected in nuclei of corneal epithelial cells but rarely in stromal cells. Counterstain was DiD. (B): Magnification of the inset of (A) showing a single PAX6-positive stromal cell (arrow). (C): PAX6 (green) colocalized with ABCG2 staining (red) in stromal cells. (D): SP cells in SCGM were stained for PAX6 protein (green) and counterstained with cytoplasmic myosin (red). (E): Reverse transcription–polymerase chain reaction showed PAX6 mRNA present in freshly isolated stromal cells (P0) and epithelial cells (Epi) as well as SP cells cultured in SCGM, but not in SP cells cultured in KDM or FBS. (F): mRNA for keratin 12 was detected in freshly isolated epithelial cells but not in freshly isolated stromal cells (P0) or SP cells under any test conditions. Bars = 30 μM. Abbreviations: FBS, fetal bovine serum; KDM, keratocyte differentiation medium; NDM, neural differentiation medium; SCGM, stem cell growth medium; SP, side population.
Keratocyte Differentiation
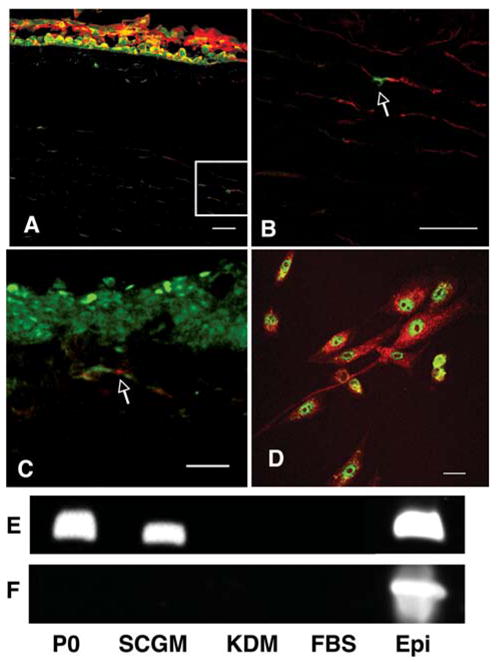
In acute wound healing, keratocytes rapidly become fibroblastic. Similarly, keratocytes in vitro irreversibly lose their characteristic phenotype. We found that the human stromal SP cells maintained the ability to assume several well-identified aspects of the keratocyte phenotype. When transferred to a serum-free medium containing FGF2 (KDM) for 1 week or more, cloned SP cells upregulated expression of mRNA for keratocan, a proteoglycan core protein that in adults has expression uniquely localized in the corneal stroma (Fig. 5A). Uncultured stromal cells, primarily keratocytes, express this gene but SP cells in SCGM do not. SP cells differentiated to fibroblasts in 10% FBS did not express keratocan either. The expression of keratocan protein was readily detected by immunostaining of the SP cells after incubation in KDM (Fig. 5C). Keratocan could also be isolated from culture media by ion exchange chromatography as a proteoglycan. The keratocan protein was detected in this proteoglycan fraction after treatment with keratanase and Western blotting (Fig. 5D). Keratan sulfate, a glycosaminoglycan unique in abundance in the corneal stroma, was also detected in the culture media of the SP cells in KDM using immunoblotting (Fig. 5D) as was an increased abundance of ALDH, a protein present in elevated amounts in keratocytes in vivo.
Figure 5.
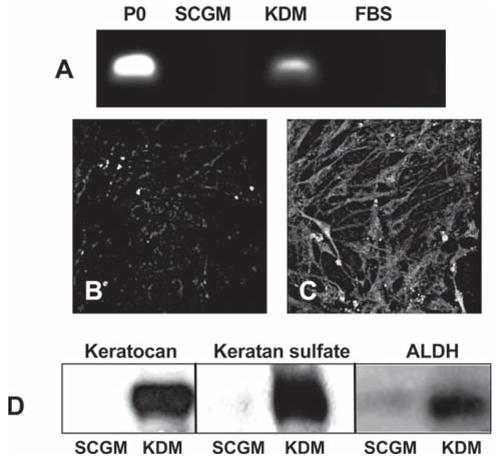
Expression of keratocyte-specific genes by SP cells. (A): Keratocan mRNA was amplified by reverse transcription–polymerase chain reaction in uncultured primary stromal cells (P0) and passage-18 SP cells in SCGM, KDM, or FBS medium as described in Materials and Methods. (B, C): Keratocan protein was detected by immunostaining in SP cells cultured in SCGM (B) or after transfer to KDM (C). (D): Keratocan protein, keratan sulfate, and ALDH were detected by immunoblotting of extracts of passage-18 SP cells cultured in SCGM or after transfer to KDM. Abbreviations: ALDH, aldehyde dehydrogenase; FBS, fetal bovine serum; KDM, keratocyte differentiation medium; NDM, neural differentiation medium; SCGM, stem cell growth medium; SP, side population.
Differentiation to Nonocular Cells
One well-documented aspect of adult stem cells is the ability to differentiate into a number of different cell types. We examined this property in the cloned SP cells by culturing them as a pellet in medium found to induce chondrogenesis in adult stem cells [25, 28]. Under these conditions, mRNA and protein for several cartilage-specific extracellular matrix molecules could be detected (Fig. 6). mRNAs for collagen II, aggrecan, and cartilage oligomatrix protein (COMP) were detected in the chondrogenic conditions but not in the SP precursor, keratocyte, or fibroblastic cells (Fig. 6A). Collagen II and COMP protein expression was confirmed using immunoblotting (Fig. 6B). After several weeks, a significant amount of extracellular matrix was deposited in the pellet cultures. The matrix deposited under chondrogenic conditions stained strongly with toluidine blue, a characteristic resulting from proteoglycan accumulation typical of cartilage (Figs. 7B, 7D). Pellet cultures in SCGM failed to deposit a similar matrix (Figs. 7A, 7C).
Figure 6.
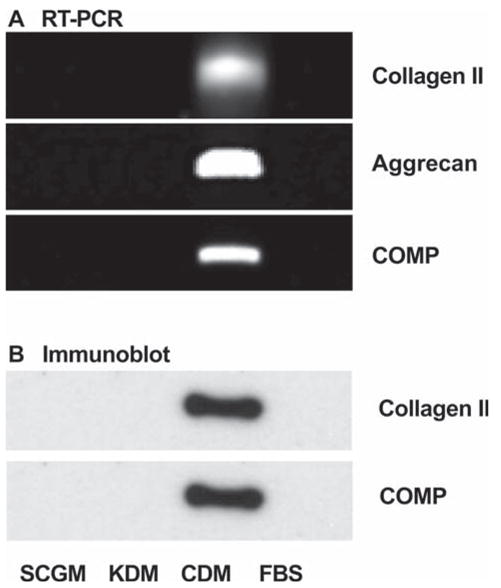
Induction of chondrocyte-specific genes in side population cells. (A): mRNAs for collagen type II, COMP, and aggrecan were detected by RT-PCR in side population cells under culture conditions described in Figure 3. (B): Protein expression of collagen II and COMP were demonstrated by immunoblotting under the same culture conditions. Abbreviations: CDM, chondrocyte differentiation medium; COMP, cartilage oligomatrix protein; FBS, fetal bovine serum; KDM, keratocyte differentiation medium; RT-PCR, reverse transcription–polymerase chain reaction.
Figure 7.
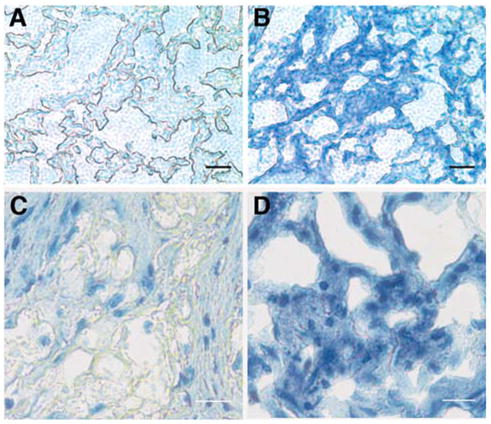
Inductionofcartilagematrixinpelletculture. Sidepopulation cells (2 × 105) were cultured as pellets in (A, C) SCGM or (B, C) CDM for 3 weeks as described in Materials and Methods. Sections of the pellets were stained using toluidine blue to detect proteoglycan staining typical of cartilage. In addition, (C, D) were counterstained with hematoxylin to visualize cell nuclei. Two magnifications are shown. Bars = 100 μ (A, B), 50 μ (C, D). Abbreviations: CDM, chondrocyte differentiation medium; SCGM, stem cell growth medium.
When the cloned SP cells were incubated with high levels of FGF2 and retinoic acid in a medium reported to induce neurogenesis, mRNA upregulation of both GFAP and neurofilament protein was observed (Fig. 8A). ANOVA analysis found a statistically significant (p < .01) 2.5-fold increase in expression of GFAP and neurofilament protein mRNAs in NDM compared with the other conditions. Increases in GFAP and neurofilament proteins were detected by Western blot (Figs. 8B, 8C). By immunofluorescent staining, the cells positive for neurofilament (Fig. 8D), GFAP (Fig. 8E), and beta-tubulin III (Fig. 8F) were present among cells cultured in NDM. SP cells passaged three times in 10% FBS (fibroblasts) and then cultured in NDM did not produce these neural marker proteins (Figs. 8G–8I). This demonstrates that clonal lines of SP cells retain a potential for neural differentiation in addition to the ability to form keratocytes and chondrocytes.
Figure 8.
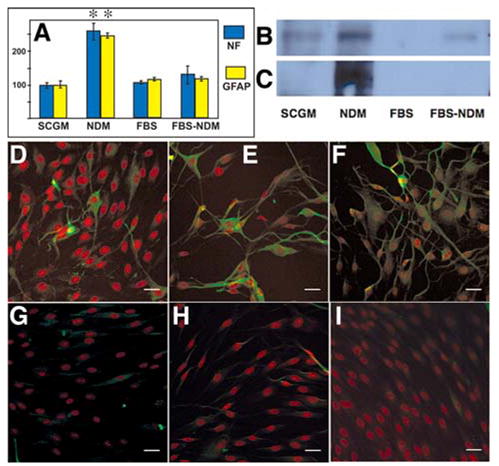
Induction of neural gene expression in SP cells. (A): mRNA pools for NF and GFAP were quantified by reverse transcription–polymerase chain reaction in SP cells exposed to NDM, SCGM, FBS, or NDM after culture in FBS (FBS-NDM) as described in Materials and Methods. Error bars show SD of triplicate analyses. Asterisks indicate significant (p < .05) increase compared with all other samples. Extracts of cells cultured in same media were subject to immunoblotting, using antibodies to neurofilament 200 protein (B) or to GFAP (C). SP cells in NDM (D–F) or FBS-NDM (G–I) were immunostained for neurofilament protein 200 (D, G), GFAP (E, H), and beta-tubulin III (F, I) (green). Counterstain (red) was DiD. Bars = 30 μM. Abbreviations: FBS, fetal bovine serum; GFAP, glial fibrillary acidic protein; KDM, keratocyte differentiation medium; NDM, neural differentiation medium; NF, neurofilament protein; SCGM, stem cell growth medium; SP, side population.
Discussion
We report here a population of cells in human corneal stroma expressing the ABCG2 transporter protein which can be isolated by FACS as SP after labeling with Hoechst dye. These cells are clonogenic and can be expanded in vitro to 100 or more population doublings. These cells do not exhibit aspects of differentiated corneal cells or of fibroblasts but show stable expression of ABCG2 and PAX6 genes. SP cells from a single clone can be induced to express differentiation markers unique to corneal keratocytes and, additionally, markers of chondrogenesis and neural cells. Once the cells are differentiated, they do not reassume aspects of the SP cells. Together, all of these observations demonstrate that these cells represent a resident population of adult stem cells in the corneal stroma. Demonstration of cells with the capability of generating human keratocytes in vitro is novel and has significant implications for corneal cell–based therapy and tissue engineering.
This report appears to be the first to demonstrate the presence of ABCG2-expressing stem cells in the corneal stroma. Previously, we observed clonogenic cells in bovine stroma which could be expanded and can also give rise to keratocytes [29]. The current work confirms the presence of keratocyte precursor cells in human, as well as bovine, corneas. It also expands on our previous study by demonstrating isolation of these cells using FACS and documentation of the multipotent differentiation of the precursor cells. ABCG2 expression and the formation of an SP are now widely reported to be characteristic of adult stem cells [8, 12–17]. Corneal epithelium, a self-renewing tissue long known to harbor a stem cell population, also contains an ABCG2 SP thought to represent precursors for the differentiated corneal epithelial cells [19].
ABCG2-expressing SP cells have frequently been reported to display the ability to express differentiated markers of multiple tissues in response to different environmental stimuli [5, 6, 9, 10, 12,15,24,28,30,31]. Although the biological implications of such multipotency are not yet fully understood, this unusual property has become a useful means of confirming the stem-like nature of these cells. In the case of cells from the corneal stroma, several genes and proteins never expressed in the adult cornea, such as collagen type II, aggrecan, and neurofilament protein, could be induced in clonal lines of cells of stromal origin. Our current data are not sufficient to demonstrate that the stromal SP cells can differentiate into fully functional chondrocytes or neurons, but they do serve to show that clonal isolates of these cells are not fully committed to a single developmental lineage. Thus, according to current conventions, these cells should be considered multipotent adult stem cells as opposed to committed progenitor cells
The stem-like character of these cells is also reinforced by their expressionofPAX6, a homeobox gene expressed in early development by ocular precursor cells [32]. Absence of PAX6 or mutation of the gene leads to complete loss of eye formation or formation of small or underdeveloped eyes [33]. During formation of the anterior segment, periocular precursor cells for both keratocytes and epithelial cells express PAX6 protein [34]. The expression continues in adult corneal epithelium but not in stroma [35]. Thus, the expression of PAX6 in SP cells in vitro and its downregulation as the cells express markers of keratocyte differentiation recapitulate the developmental pathway of embryonic stromal cells. A further demonstration of their stem-like characteristic is the ability of the cells to grow clonally and to divide well beyond the Hayflick limit of approximately 50 to 70 population doublings characteristic of adult somatic human cells [36, 37].
Stem cells are thought to reside in a specific niche in which external signals control quiescence and/or differentiation into daughter cells [38]. Our observations (Fig. 1) showed ABCG2-positive cells to be more abundant in peripheral cornea compared with that in central; however, no clear morphological niche was identified in the current study. Stem cells in the corneal epithelium also appear to reside in a limbal niche, and a recent study indicated PAX6 expression to be essential for correct function of epithelial stem cells [39]. In the current study, care was taken to remove epithelial and endothelial cells before isolation of the stromal cells. The fact that the stromal cells used for isolation of the SP cells contained no keratin 12 mRNA(Fig. 4) indicated that epithelial cells were excluded during the isolation of these cells. Although they lack an obvious niche, stromal ABCG2-positive cells are most abundant subjacent to ABCG2-expressing cells in the limbal epithelium (Fig. 1), suggesting a relationship between the populations. Because limbal ABCG2-positive cells lack keratin 12 expression [40], distinguishing differences between these populations will require further analysis.
Clearly, the most important contribution of this study is the finding that a biological source of differentiated keratocytes persists in human corneas. Stromal connective tissue exhibits a number of unique biophysical properties essential for vision. Secretion of this tissue, however, is a volatile feature of the keratocyte phenotype. It has long been observed that human stromal cells rapidly become fibroblastic in vitro. Recent studies showed that keratocytes maintained some aspects of their differentiated phenotype if cultured on amniotic membrane, but lost even that after several population doublings [41]. These in vitro findings are consistent with the long-term persistence of fibrotic tissue in human cornea [20]. Presumably, the fibroblastic cells that generate such tissue are not replaced with keratocytes, even after extended periods of time. Access to cells in which the keratocyte phenotype can be induced will allow gene profiling and subsequent elucidation of the molecular events that control this differentiation pathway. The ability to expand stromal stem cells in vitro may provide material for cell-based therapy for stromal scarring. Accessibility to an essentially unlimited supply of differentiated keratocytes also opens the possibility of producing stromal tissue for use in bioprosthetic corneas.
The practical applicability of stem cell–based therapies and bioengineered tissues may be less complicated in the corneal stroma than in many of the other applications for which stem cells are being developed. The cornea enjoys an immune privileged status that results in a rejection rate of only 10% of unmatched allografts [42]. Rejection appears to be the result of antigen-presenting cells in the donor tissue, not the result of a rejection of the stromal keratocytes [42, 43]. It has recently been shown that bone marrow–derived cells are normal components of corneal stroma in most or all corneas, even in the absence of inflammation [44]. Thus, the ability to generate bioengineered tissues composed of pure keratocytes, free of these antigen-presenting cells, may provide bioprosthetic stromal material that is tolerated as well or better than the whole-tissue allografts now in use.
Acknowledgments
The authors would like to thank Dr. Hongmei Shen for her help in cell sorting and Cindy Stone for her help in the histology. This work was supported by National Institutes of Health grants EY13806, EY09368, and P30-EY08098, Research to Prevent Blindness, and Eye and Ear Foundation of PGH. J.L.F. is a Jules and Doris Stein Research to Prevent Blindness Professor.
Footnotes
Disclosures
The authors indicate no potential conflicts of interest.
References
- 1.Carlson EC, Wang IJ, Liu CY, et al. Altered KSPG expression by keratocytes following corneal injury. Mol Vis. 2003;9:615–623. [PubMed] [Google Scholar]
- 2.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 3.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 4.Griffith M, Osborne R, Munger R, et al. Functional human corneal equivalents constructed from cell lines. Science. 1999;286:2169–2172. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- 5.Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002;12:502–508. doi: 10.1016/s0962-8924(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 7.Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 9.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Turnquist H, Jackson J, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 11.Abbott BL. ABCG2 (BCRP) expression in normal and malignant hematopoietic cells. Hematol Oncol. 2003;21:115–130. doi: 10.1002/hon.714. [DOI] [PubMed] [Google Scholar]
- 12.Cai J, Cheng A, Luo Y, et al. Membrane properties of rat embryonic multipotent neural stem cells. J Neurochem. 2004;88:212–226. doi: 10.1046/j.1471-4159.2003.02184.x. [DOI] [PubMed] [Google Scholar]
- 13.Jang YK, Park JJ, Lee MC, et al. Retinoic acid-mediated induction of neurons and glial cells from human umbilical cord-derived hematopoietic stem cells. J Neurosci Res. 2004;75:573–584. doi: 10.1002/jnr.10789. [DOI] [PubMed] [Google Scholar]
- 14.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Lechner A, Leech CA, Abraham EJ, et al. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293:670–674. doi: 10.1016/S0006-291X(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 16.Triel C, Vestergaard ME, Bolund L, et al. Side population cells in human and mouse epidermis lack stem cell characteristics. Exp Cell Res. 2004;295:79–90. doi: 10.1016/j.yexcr.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Terunuma A, Jackson KL, Kapoor V, et al. Side population keratinocytes resembling bone marrow side population stem cells are distinct from label-retaining keratinocyte stem cells. J Invest Dermatol. 2003;121:1095–1103. doi: 10.1046/j.1523-1747.2003.12531.x. [DOI] [PubMed] [Google Scholar]
- 18.de Paiva CS, Chen Z, Corrales RM, et al. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe K, Nishida K, Yamato M, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 20.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litman T, Jensen U, Hansen A, et al. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ ABCG2. Biochim Biophys Acta. 2002;1565:6–16. doi: 10.1016/s0005-2736(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 22.Boesch JS, Lee C, Lindahl RG. Constitutive expression of class 3 aldehyde dehydrogenase in cultured rat corneal epithelium. J Biol Chem. 1996;271:5150–5157. doi: 10.1074/jbc.271.9.5150. [DOI] [PubMed] [Google Scholar]
- 23.Edward DP, Yue BY, Sugar J, et al. Heterogeneity in macular corneal dystrophy. Arch Ophthalmol. 1988;106:1579–1583. doi: 10.1001/archopht.1988.01060140747049. [DOI] [PubMed] [Google Scholar]
- 24.Giangreco A, Shen H, Reynolds SD, et al. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L624–L630. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- 25.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 26.Funderburgh JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 27.Sivak JM, Mohan R, Rinehart WB, et al. Pax-6 expression and activity are induced in the reepithelializing cornea and control activity of the transcriptional promoter for matrix metalloproteinase gelatinase B. Dev Biol. 2000;222:41–54. doi: 10.1006/dbio.2000.9694. [DOI] [PubMed] [Google Scholar]
- 28.Suva D, Garavaglia G, Menetrey J, et al. Non-hematopoietic human bone marrow contains long-lasting, pluripotential mesenchymal stem cells. J Cell Physiol. 2004;198:110–118. doi: 10.1002/jcp.10396. [DOI] [PubMed] [Google Scholar]
- 29.Funderburgh ML, Du Y, Mann MM, et al. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;10:1371–1373. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hierlihy AM, Seale P, Lobe CG, et al. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 31.Asakura A, Seale P, Girgis-Gabardo A, et al. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramaesh T, Collinson JM, Ramaesh K, et al. Corneal abnormalities in Pax6+/− small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci. 2003;44:1871–1878. doi: 10.1167/iovs.02-0576. [DOI] [PubMed] [Google Scholar]
- 34.Baulmann DC, Ohlmann A, Flugel-Koch C, et al. Pax6 heterozygous eyes show defects in chamber angle differentiation that are associated with a wide spectrum of other anterior eye segment abnormalities. Mech Dev. 2002;118:3–17. doi: 10.1016/s0925-4773(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 35.Sivak JM, West-Mays JA, Yee A, et al. Transcription factors Pax6 and AP-2alpha interact to coordinate corneal epithelial repair by controlling expression of matrix metalloproteinase gelatinase B. Mol Cell Biol. 2004;24:245–257. doi: 10.1128/MCB.24.1.245-257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayflick L. The cell biology of aging. J Invest Dermatol. 1979;73:8–14. doi: 10.1111/1523-1747.ep12532752. [DOI] [PubMed] [Google Scholar]
- 37.Rubin H. The disparity between human cell senescence in vitro and lifelong replication in vivo. Nat Biotechnol. 2002;20:675–681. doi: 10.1038/nbt0702-675. [DOI] [PubMed] [Google Scholar]
- 38.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 39.Collinson JM, Chanas SA, Hill RE, et al. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6(+/−) mouse. Invest Ophthalmol Vis Sci. 2004;45:1101–1108. doi: 10.1167/iovs.03-1118. [DOI] [PubMed] [Google Scholar]
- 40.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Espana EM, He H, Kawakita T, et al. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci. 2003;44:5136–5141. doi: 10.1167/iovs.03-0484. [DOI] [PubMed] [Google Scholar]
- 42.Streilein JW. New thoughts on the immunology of corneal transplantation. Eye. 2003;17:943–948. doi: 10.1038/sj.eye.6700615. [DOI] [PubMed] [Google Scholar]
- 43.Hamrah P, Huq SO, Liu Y, et al. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 44.Wilson SE, Mohan RR, Netto M, et al. RANK, RANKL, OPG, and M-CSF expression in stromal cells during corneal wound healing. Invest Ophthalmol Vis Sci. 2004;45:2201–2211. doi: 10.1167/iovs.03-1162. [DOI] [PubMed] [Google Scholar]


