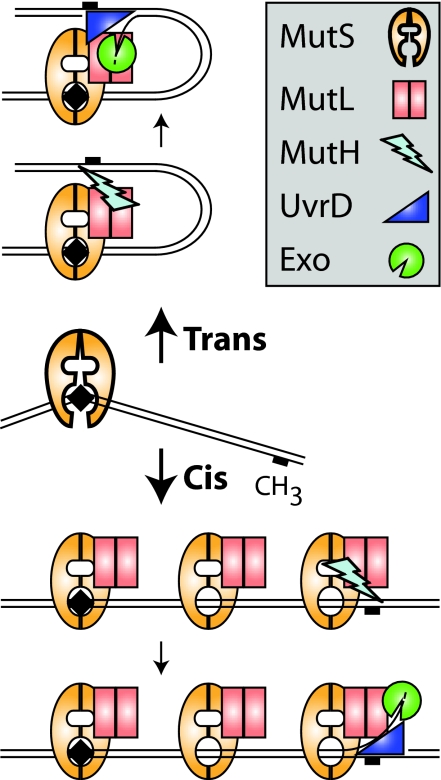
How can the activities of different proteins that act at two distant sites on DNA be coupled? In cis mechanisms, an intact DNA helix is required so that the protein molecules can slide (diffuse), translocate, polymerize, or otherwise communicate along the DNA helix with the second site. Examples include the nucleotide-dependent translocation of type I and III restriction endonucleases (1) and the polymerization of SeqA that blocks bacterial replication origins (2). In trans mechanisms, contacts occur between the two sites, and the intervening DNA is looped out; this also could occur between sites on different DNAs. Trans mechanisms are common in the regulation of gene expression (3). The DNA mismatch repair (MMR) system must recognize mispairs resulting from replication errors, distinguish between newly synthesized and parental DNA strands, and coordinate an excision repair reaction so that the misincorporated base is removed from the newly synthesized strand (4). In Escherichia coli, MutL mediates communication between the mispairs, which are recognized by MutS, and a distant hemimethylated GATC site, which is cleaved by MutH on the newly synthesized strand that is not yet modified by the Dam methylase (Fig. 1). The single-strand break created by MutH can direct excision either 3′ or 5′ to the mispaired base and is the key postinitiation intermediate in MMR that allows excision to proceed by means of the UvrD helicase and one of four single-stranded DNA exonucleases. Efficient MutH cleavage depends on MutS, MutL, and the mispair; however, the mechanism by which this coupling occurs and drives appropriate directionality of excision is poorly understood even though E. coli MMR was first reconstituted with purified proteins almost 20 years ago (4). In a recent issue of PNAS, Pluciennik and Modrich (5) provided welcome insight into the problem; their results support a cis mechanism for MMR.
Fig. 1.
Cis and trans models for mismatch repair.
MMR mechanisms have been the subject of much debate. Much of the controversy over cis and trans mechanisms for both the bacterial and the conserved eukaryotic MMR system is not over experimental results. There is substantial consensus on the biochemical properties of MMR proteins. Instead, the debate focuses on which interpretations and which experimental results describe biologically relevant MMR mechanisms. In an early cis model, it was suggested that MMR proteins might form a nucleoprotein filament on the DNA from the mismatch to the initiation site, although no evidence for such a filament has emerged (6). Other cis models for MMR have been influenced by the fact that MutS and its eukaryotic homologs (MSHs, for MutS homologs) dissociate from mispairs when challenged with ATP. Mispair-bound MutS and MSHs challenged with ATP can be trapped on mispair-containing DNA by DNA end blocks, indicating that this dissociation occurs along the DNA helix (7–10). This result was initially interpreted as active translocation (11) in analogy to type I and type III restriction enzymes (1). However, it is clear that MutS, MutSα (the eukaryotic Msh2–Msh6 heterodimer), and MutSβ (the eukaryotic Msh2–Msh3 heterodimer) do not undergo ATP hydrolysis-driven translocation but rather hydrolyze ATP to produce an ADP-bound form that binds to a mispair. Subsequent ATP binding by mispair-bound MutS, but not hydrolysis [hydrolysis is inhibited in the mispair-bound state to promote a two-ATP-bound state (12, 13)], drives a conformational change that allows sliding on the DNA (7, 8). ATP thus acts as a cofactor that induces specific conformational changes, predicted to yield clamp-like structures based on the ADP-bound MutS structures and structural comparisons to the Rad50 ATPase (14). Because this mechanism allows several MutS dimers to load on the DNA, these MutS dimers could interact through their tetramerization interface to produce DNA loops like those observed (one initial round of ATP hydrolysis would be required, but hydrolysis-driven translocation would not occur). This type of structure could mediate communication along the DNA helix; however, MutS tetramerization is not particularly required for MMR (15), and there are no reports of tetramerization for the eukaryotic MSH complexes.
In contrast, the trans models for MMR emphasize the fact that the ATP-loaded state of MutS is the target of MutL association, and that the MutS/MutL complex has a longer residence time at mispairs than ATP-challenged MutS (8, 9). The first evidence for a trans model came from analysis of MutH activation where a reaction in which the mispair was on one substrate but the hemimethylated GATC was on a second was as efficient as when both sites were on the same substrate (9). In the trans models, sliding of MutS alone could be interpreted as an abortive reaction in the absence of MutL assembly. Moreover, the relative ease that MutS sliding can be blocked is potentially problematic, because naked DNA is not a commonly available substrate. Despite this fact, complexes of MutS/MutL or their eukaryotic equivalents are not refractory to sliding (8, 10). In addition, a dominant MMR-defective mutant MutSα complex Msh2–Msh6-G1142D has a defect in sliding but retains the ability to assemble MutLα (Saccharomyces cerevisiae Mlh1-Pms1) (16), emphasizing the importance of sliding.
Pluciennik and Modrich (5) have directly tested a key aspect that distinguishes the cis and trans models: a requirement for a continuous and unblocked DNA helix. In one experiment, they demonstrate that binding of a catalytically inactive mutant EcoRI to an EcoRI recognition site located between the mispair and the hemimethylated GATC site significantly reduced but did not eliminate mispair-dependent activation of MutH. The failure to completely inhibit the reaction was attributed to the fact that even a tightly binding protein cannot occupy its binding site 100% of the time. In a second experiment, double-strand breaks were used to interrupt the shorter distance between the mispair and the hemimethylated GATC on a plasmid, which completely eliminated MutH activation. The results of these elegantly simple experiments fall strongly on the side of a cis mechanism for MutH activation.
How can we reconcile these results with those showing that MutH can be activated by MutS, MutL, and a mispair in trans (9)? Previous studies compared MutH activation by MutS and MutL on an oligonucleotide duplex substrate containing a GATC site and a mispair 66 bases apart with activation when the mispair and the GATC sites were located on separate oligonucleotide substrates (9). In these experiments, cis and trans MutH activation occurred at the same rate even though relative site concentrations favored the cis reaction. However, the size of the MutS–MutL footprint on a mispair-containing substrate is sufficiently large that the GATC site on the cis substrate was likely occluded (9), leading us to suggest that, even on the cis substrate, MutH activation was likely in trans. In the current study, when the mispair and the GATC site were located 1,000 bp apart on a long DNA substrate, activation of MutH occurred at significantly higher rates than seen on the cis or trans substrates in the trans reactions described above. A possible explanation for the slow, but not zero, rate of trans activation is that the surfaces mediating the interaction of MutS–MutL with MutH-GATC can functionally associate in trans but are greatly facilitated by communication along DNA in cis.
The results of Pluciennik and Modrich (5) also seem at odds with experiments demonstrating that separating a mispair from a 5′ single-strand break by different blocks did not inhibit mismatch-stimulated 5′ excision in human extracts (17). MutSα and a mismatch stimulate this excision, presumably mediated by Exo1 (4), possibly in trans or through looping to bypass the block. This stimulation of excision is a different step than MutH activation (which is specific to bacterial methyl-directed MMR) and could have different mechanistic requirements. Thus, excision might not be the initiating signal in eukaryotes but rather lie downstream of an initiating event, which is currently not understood. Indeed, the observation that the eukaryotic MutL homolog Pms2 has an endonuclease activity implies that the initiation of eukaryotic MMR may be similar to the activation of MutH (18); however, if Mlh1-Pms2 does catalyze the initiating single-strand break, then how it is targeted to the newly synthesized DNA strand is unclear. Furthermore, it will be interesting to examine the effect of DNA blocks on the subsequent steps in the bacterial system such as when the UvrD helicase and the exonuclease are loaded at a single-strand break, especially because UvrD helicase activity is stimulated by MutL (19).
Given the results of Pluciennik and Modrich (5), how might MMR work, and what advantages might a cis mechanism bring to MMR? During mispair recognition, binding of the mispair by MutS promotes ATP binding and reduces the rate of hydrolysis, potentially giving time for MutL to recognize a metastable ATP-bound MutS (12, 13). Sliding of this MutS–MutL complex along the DNA may serve several roles in mismatch repair initiation (nick-formation) and excision. First, this sliding complex could activate prebound MutH at, or potentially both deliver MutH to and activate MutH at, a hemimethylated GATC site to generate a single-strand nick to initiate the repair reaction. Second, the MutS–MutL complex has high affinity for double-strand ends (10), and if it also had a high affinity for single-strand breaks, then sliding MutS–MutL complexes could “label” strand-specific breaks associated with mispairs and target them for excision repair rather than ligation. Additionally, ATP-bound MutL is known to help load and activate the UvrD helicase in E. coli MMR (19), and labeled breaks could directly control directionality of UvrD and subsequent strand excision. Third, sliding of the complex away from the mispair would allow other MutS–MutL complexes to be loaded onto the DNA (10). Multiple MutS–MutL sliding clamps could promote the net processivity of strand excision by directing reloading of the excision enzymes if they dissociate (10). Successful removal of the mispair would prevent further MutS–MutL recruitment and squelch the signals for continued excision. Additionally, there is only a small window of time for MMR to occur before strand specificity is lost. Formation of a chromosomal region with multiple MutS–MutL molecules may help to inhibit activities, such as Dam methylase in E. coli, which eliminates the newly synthesized DNA signal.
Only MutS and MutL are conserved in eukaryotic MMR, and the eukaryotic homologs share all of the key features described above. The signal for newly synthesized strands has not been identified in eukaryotes; however, the conserved biochemical features of MutS and MutL could be consistent with direct recruitment to strand breaks generated during the process of replication itself in eukaryotic MMR (and in bacteria lacking the methyl-directed component of MMR). Remarkably, both bacterial and eukaryotic MMR systems interact with replication processivity factors (the bacterial β-clamp and eukaryotic PCNA) (4, 20). Recruitment to regions of actively replicating DNA would bring MutS and MutL to where new mispairs are generated and signals for newly synthesized DNA still exist, and such coupling between replication proteins and mispairs might occur by a cis communication mechanism.
Footnotes
The authors declare no conflict of interest.
See companion article on page 12709 in issue 31 of volume 104.
References
- 1.Bourniquel AA, Bickle TA. Biochimie. 2002;84:1047–1059. doi: 10.1016/s0300-9084(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 2.Kaguni JM. Annu Rev Microbiol. 2006;60:351–375. doi: 10.1146/annurev.micro.60.080805.142111. [DOI] [PubMed] [Google Scholar]
- 3.Saiz L, Vilar JM. Curr Opin Struct Biol. 2006;16:344–350. doi: 10.1016/j.sbi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Iyer RR, Pluciennik A, Burdett V, Modrich PL. Chem Rev. 2006;106:302–323. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 5.Pluciennik A, Modrich P. Proc Natl Acad Sci USA. 2007;104:12709–12713. doi: 10.1073/pnas.0705129104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modrich P. Annu Rev Biochem. 1987;56:435–466. doi: 10.1146/annurev.bi.56.070187.002251. [DOI] [PubMed] [Google Scholar]
- 7.Gradia S, Subramanian D, Wilson T, Acharya S, Makhov A, Griffith J, Fishel R. Mol Cell. 1999;3:255–261. doi: 10.1016/s1097-2765(00)80316-0. [DOI] [PubMed] [Google Scholar]
- 8.Mendillo ML, Mazur DJ, Kolodner RD. J Biol Chem. 2005;280:22245–22257. doi: 10.1074/jbc.M407545200. [DOI] [PubMed] [Google Scholar]
- 9.Schofield MJ, Nayak S, Scott TH, Du C, Hsieh P. J Biol Chem. 2001;276:28291–28299. doi: 10.1074/jbc.M103148200. [DOI] [PubMed] [Google Scholar]
- 10.Acharya S, Foster PL, Brooks P, Fishel R. Mol Cell. 2003;12:233–246. doi: 10.1016/s1097-2765(03)00219-3. [DOI] [PubMed] [Google Scholar]
- 11.Allen DJ, Makhov A, Grilley M, Taylor J, Thresher R, Modrich P, Griffith JD. EMBO J. 1997;16:4467–4476. doi: 10.1093/emboj/16.14.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazur DJ, Mendillo ML, Kolodner RD. Mol Cell. 2006;22:39–49. doi: 10.1016/j.molcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Antony E, Hingorani MM. Biochemistry. 2003;42:7682–7693. doi: 10.1021/bi034602h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sixma TK. Curr Opin Struct Biol. 2001;11:47–52. doi: 10.1016/s0959-440x(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 15.Mendillo ML, Putnam CD, Kolodner RD. J Biol Chem. 2007;282:16345–16354. doi: 10.1074/jbc.M700858200. [DOI] [PubMed] [Google Scholar]
- 16.Hess MT, Mendillo ML, Mazur DJ, Kolodner RD. Proc Natl Acad Sci USA. 2006;103:558–563. doi: 10.1073/pnas.0510078103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Hays JB. EMBO J. 2004;23:2126–2133. doi: 10.1038/sj.emboj.7600153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Matson SW, Robertson AB. Nucleic Acids Res. 2006;34:4089–4097. doi: 10.1093/nar/gkl450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez de Saro FJ, Marinus MG, Modrich P, O'Donnell M. J Biol Chem. 2006;281:14340–14349. doi: 10.1074/jbc.M601264200. [DOI] [PubMed] [Google Scholar]