Abstract
A previously unidentified class of compounds has been isolated from the regurgitant of the grasshopper species Schistocerca americana. These compounds (named here “caeliferins”) are composed of saturated and monounsaturated sulfated α-hydroxy fatty acids in which the ω-carbon is functionalized with either a sulfated hydroxyl or a carboxyl conjugated to glycine via an amide bond. The regurgitant contains a series of these compounds with fatty acid chains of 15–20 carbons and in varying proportions. Of these, the 16-carbon analogs are predominant and are also most active in inducing release of volatile organic compounds when applied to damaged leaves of corn seedlings. Caeliferins are nonlepidopteran elicitors identified in insect herbivores. This adds a category of insect herbivore-produced elicitors of plant responses, providing further evidence of the ability of plants to detect and respond to a broad range of insect herbivore-produced compounds.
Keywords: American grasshopper, insect herbivory, regurgitant, caeliferin
Insect herbivory provokes direct defenses that impair herbivore performance and induces plants to release volatile organic compounds (VOC) that attract natural enemies of the herbivores (1, 2). The composition of insect-induced VOC varies with different species and even different varieties of plants (3, 4). They are composed of components synthesized by several different biosynthetic pathways, including the lipoxygenase, shikimate, and isoprenoid pathways (5). Induced VOC may be stored in glands, as in cotton, and be released by herbivore damage to the glands (5, 6). However, many plants, such as corn, release VOC soon after herbivore-induced de novo biosynthesis. For some plants, such as lima beans, mechanical damage is sufficient to induce the release of substantial quantities of VOC (7, 8). In most described cases, plants damaged by insect herbivores or treated with regurgitant release greater quantities and/or different proportions of VOC than plants receiving only mechanical damage (5, 9–17). Furthermore, specialist parasitoids can distinguish volatiles induced by host larvae from volatiles induced by nonhost larvae (18, 19). Thus, herbivore-produced substances play a critical role in induction of plant defenses and herbivore-specific volatile emissions.
The first identified nonenzymatic insect herbivore-produced elicitor of plant volatiles was volicitin, N-(17-hydroxylinolenoyl)-l-glutamine, isolated from the regurgitant of beet armyworm, (20, 21). Subsequently, other analogous fatty acid–amino acid conjugates have been identified from regurgitant of larvae of several species of Lepidoptera (22–24). This class of elicitors typically consists of linolenic, linoleic, and oleic acids obtained from the diet or plant on which the larvae feed (25) or from their 17-hydroxy analogs conjugated with glutamine or glutamic acid. Thus far, volicitin, N-linolenoyl-l-glutamine, and N-linolenoyl-l-glutamate have been demonstrated to have significant activity in inducing plants to produce and release VOC (21, 22). However, not all plants respond to these elicitors (8, 26). For example, cow pea releases VOC when fed on by fall armyworm larvae, but not when treated with fatty acid–amino acid elicitors. This phenomenon led to the identification of a previously unidentified type of peptide elicitor in fall armyworm regurgitant, derived by proteolysis from the chloroplastic ATP synthase of the plant on which the herbivore feeds (26). Thus, Spodoptera larvae produce at least two different types of elicitors, both derived in part from the plant, that share similar activity, i.e., induced release of volatiles, but on different host plants of the herbivores. Salivary enzymes of insect herbivores also may influence plant defense responses (27). Therefore, complex interactions between herbivore-produced enzymes and elicitors are likely responsible for the specificity in the blend of VOC released by plants attacked by Lepidoptera herbivores.
Corn seedlings fed on by the grasshopper Schistocerca Americana or treated with its regurgitant were previously found to release VOC similar to that induced by caterpillar feeding or regurgitant treatment (28). Here we describe the isolation, identification, and synthesis of a previously unidentified class of sulfated fatty acids found in regurgitant of S. americana and their biological activity as elicitors of induced VOC in corn seedlings.
Results
Corn seedlings treated with 5 μl of S. americana regurgitant in an excised plant bioassay emitted amounts of VOC equivalent to emissions by seedlings treated with 100 pmol of volicitin or seedlings exposed to grasshopper feeding for 2 h the previous day. The VOC were composed of indole, monoterpenes, sesquiterpenes, and their 11- and 16-carbon homologs. However, no N-acyl fatty acid-type elicitors could be detected in the grasshopper regurgitant by HPLC or liquid chromatography (LC)/MS methods developed for analyses of Lepidoptera larvae regurgitant (21) (data not shown).
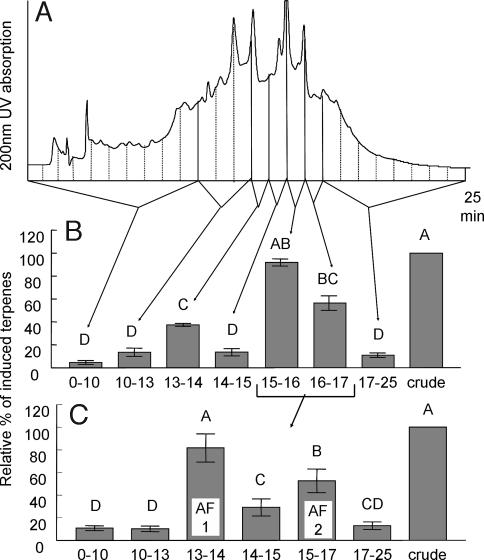
No loss of regurgitant activity was found after protein precipitation. Activity was measured as the combined amount of four induced terpenoids relative to the amount induced by crude regurgitant. The most active regurgitant fraction eluted from a reverse-phase (C18) solid-phase extraction column with 50% acetonitrile in water (water fraction, 4.6 ± 1.4%; 50% acetonitrile, 101.8 ± 20.3%, 100% acetonitrile, 15.1 ± 5.4%; n = 8). Further fractionation of the 50% acetonitrile solid-phase eluant by reverse-phase C18 HPLC with an acidic mobile phase (Fig. 1A) resulted in partial activity in the 13- to 14-min fraction and strong activity in the 15- to 17-min fractions (Fig. 1B). No obvious peaks corresponding to the activity could be seen in the UV trace at 200 nm or at higher wavelengths. The main active fraction (15–17 min) was further separated on the same column by using neutral buffer and a modified gradient. This fraction now separated into two active fractions (Fig. 1C), designated AF1 (13–14 min) and AF2 (15–16 min). Reanalyzing AF1 on HPLC showed a peak with what appeared to be a very low response factor at 200-nm or lower wavelengths, whereas AF2 produced no detectable UV response at any wavelength.
Fig. 1.
HPLC separation and bioassay results of collected fractions. (A) Chromatographic trace (using 200-nm UV detection and pH 4.5 solvent) and 1-ml fraction collection of protein-precipitated and solid-phase extraction (SPE)-purified S. Americana regurgitant. (B) Bioassay results (n = 20) of combined fractions showing strong activity in the 15- to 17-min region and some activity in the 13- to 14-min fraction. (C) Bioassay result (n = 12) of the combined fractions 15–17 min from B after repeated HPLC. Separation was achieved using a neutral (pH 7) solvent and a slow gradient. The active 13- to 14-min fraction was assigned active fraction 1 (AF1) and the 15- to 16-min fraction was assigned AF2. The excised plant assay was used for all bioassays, and the induced volatile release was normalized and analyzed statistically (P < 0.01) as described in Methods.
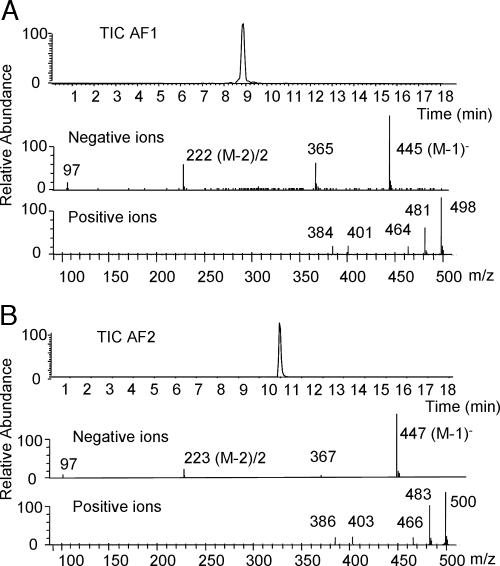
The isolated compounds were analyzed using negative and positive ion electrospray LC/MS. In negative ion mode, both AF1 and AF2 gave very strong M-1 ions at m/z 445 and 447, respectively (Fig. 2). They also gave strong double-charged ions at m/z 222 and 223 (M-2)/2. Repeated daughter ion analyses [see Analysis 1 in supporting information (SI) Text and Fig. 5] showed that both compounds readily lost two 80 atomic mass units (amu)-neutral fragments to give stable ions at m/z 285 and 287, respectively. In positive ion mode, both compounds showed three prominent ions 17 mass units apart (Fig. 2), indicating ammonium salts of three acidic sites and confirming molecular weights of 446 amu and 448 amu.
Fig. 2.
Electro-spray LC/MS total negative ion trace of assigned active fraction 1 (AF1) (A) and AF2 (B) (in Fig. 1C). Negative ion analyses gave a strong (M-1)− ion at m/z 445 (MW 446 amu) for AF1 and (M-1)− ion at m/z 447 (MW 448 amu) for AF2. Both compounds gave strong double-charged ions (M-2)2− at m/z 222 and m/z 223, respectively. Positive ion analyses gave three characteristic ions at MZ 464, 481, and 498 for AF1 and at MZ 466, 483, and 499 for AF2, indicating compounds with a MW of 446 and 448 amu containing three acidic sites forming ammonium salts by the solvent buffer.
Chemical ionization GC/MS analyses (Analysis 2 in SI Text and SI Fig. 6) of methanolysed AF1 gave (M + 1)+ ions at m/z 301 and m/z 303 for AF2. This finding suggested methyl esters of the same fragments as the (M-1)− ions at m/z 285 and 287 in the LC/MS analyses (and the loss of two 80-amu groups during methanolysis). An indicative fragmentation pattern in the electron impact (EI) spectra was the loss of −59, −18, and −18 amu at m/z 241, 223, and 205, respectively, for AF1 (Analysis 3 in SI Text and SI Fig. 7) and 243, 225, and 207, respectively, for AF2, which indicated two alcohols and a carboxylic acid methylester with the first alcohol in the position α to the acid. Cleavage α to the COOCH3 gave the characteristic M-59 fragment. The strong M-30 ion for AF2 at m/z 272 (and a weaker ion at m/z 270 for AF1) indicated a long-range proton transfer to the carbonyl followed by the loss of CH2O. Consequently, the second alcohol was located on the ω-carbon. On the basis of this information and NMR analyses of intact AF1 and AF2, these compounds appeared to be 2,16-dihydroxy C16 fatty acids with the addition of two unknown 80-amu groups. The only difference between the two compounds was the presence of a double bond in AF1, explaining its weak UV absorption. To test the one double-bond hypothesis, the AF1 methyl ester was subjected to hydrogenation, which, as expected, gave a GC/MS peak identical to that of the AF2 methyl ester. The presence of two alcohols was confirmed by acetylation that resulted in the expected increase in molecular weight of 2 × 42 amu for both compounds (Analysis 2 in SI Text and SI Fig. 6). Finally, the presence of only one carboxylic acid was confirmed by ethanolysis and chemical ionization (CI) GC/MS analyses that, for both compounds, gave an ethyl ester with M + 1 ions 14 amu higher than for the corresponding methyl esters.
CI GC/MS analyses of ozonized acetylated AF1-methyl ester gave 10-acetoxydecanal with an M + 1 ion at m/z 215 and a compound with an M + 1 ion at m/z 203 containing a 6-carbon chain with aldehyde and carboxyl methyl ester functions on the opposite ends of the chain and an acetoxy α to the methyl ester. Consequently, the double bond was located between carbon 6 and 7 in the 16-carbon chain.
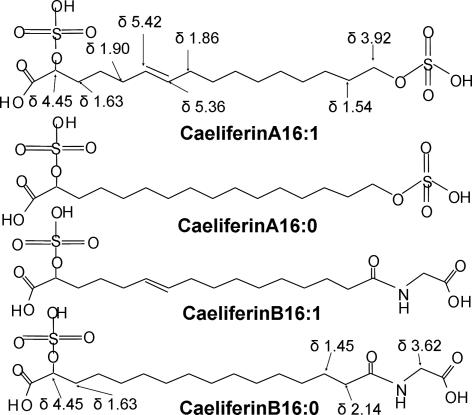
The AF1 methyl ester was also analyzed by GC/FTIR. Two weak absorptions at 3,643 and 3,568 cm−1 for AF1 confirmed two nonidentical alcohols. Furthermore, absorption at 959 cm−1 indicated the presence of a trans double bond. NMR analysis of the original intact AF1 (not subjected to methanolysis) gave 1H at δ = 4.45 ppm (broad), indicative of a proton on an alcohol-substituted carbon α to a carboxylic acid, and 2H at δ = 3.92 ppm, suggesting two protons on an alcohol-substituted ω-carbon. Two protons at δ = 5.42 and 5.36 ppm, J15 Hz, indicated a trans double bond for AF1. However, comparison with spectra of commercial α-hydroxy and ω-hydroxy palmitic acid indicated the α- and ω-protons did not have chemical shifts identical to the comparable protons of AF1 and AF2, confirming that the two substituents on AF1 could not be simple alcohols. Furthermore, the NMR analyses did not indicate the presence of any other organic structure than a di-O(H) substituted C16 fatty acid. There was also no indication of phosphate. However, sulfate esters would explain the consecutive loss of 80 amu in LC/MS analyses [loss of two SO3; molecular weight (MW), 80 amu] as well as the presence of three acidic sites (two sulfates, one carboxylic acid) and the water solubility of the natural products. The most likely candidates for AF1 and AF2 were therefore 2,16-disulfooxy-E6-hexadecenoic acid and 2,16-disulfooxyhexadecanoic acid shown in Fig. 3, which we named caeliferin A16:1 and caeliferin A16:0, respectively.
Fig. 3.
The structures of caeliferin A16:1, and A16:0 and caeliferin B16:1 and B16:0. The H-NMR chemical shifts were obtained for isolated natural compounds dissolved in D2O. Caeliferin A16:1 = (E)-2,16 disulfooxy-6-hexadecenoic acid; caeliferin A16:0 = 2,16 disulfooxyhexadecanoic acid; caeliferin B16:1 = N-[(E)-15-sulfooxy,15-carboxy-10-pentadecenoyl] glycine; and caeliferin B16:0 = N-(15-sulfooxy,15-carboxy pentadecanoyl) glycine.
Both proposed (racemic forms of) dihydroxy acids were synthesized and transformed to disulfate esters (Analysis 4 in SI Text and SI Fig. 8). The synthetic versions of caeliferin A16:1 and A16:0 were analyzed by HPLC, LC/MS, NMR, and, after derivatizing, EI and CI GC/MS and shown to be identical to the natural products. Synthetic and natural products were purified on HPLC and bioassayed (n = 16) at their respective regurgitant concentration (4 nmol/μl for A16:0 and 100 pmol/μl for A16:1) by using the intact plant assay, which is a better mimic of natural damage than the excised plant assay (29). It also required less material per bioassay [0.5-μl regurgitant equivalents (MRE) per seedling]. The bioassay showed none of the treatments to be significantly different from the crude regurgitant treatment. Natural and synthetic caeliferin A16:1 gave a relative response of 82 ± 14% versus 87 ± 14%, and caeliferin A 16:0 gave 86 ± 17% and 100 ± 19%, respectively. However, all treatments were significantly different (P < 0.01) from the buffer control with a relative response of 23 ± 2%. Although it appears chirality is not critical for the biological activity, this will still be addressed in an improved synthesis. Preliminary data indicated that the activity is reduced as the number of sulfates on synthetic caeliferin A16:0 is reduced. However, this could also have been the result of a diminishing solubility in water.
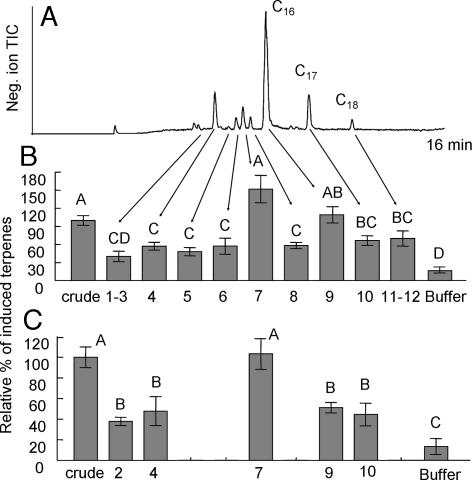
The initial bioassays during isolation and purification indicated that there might be related, but less-active compounds present in the regurgitant (Fig. 1). This was confirmed by LC/MS analyses of the supernatant after precipitation of protein from crude regurgitant (Fig. 4A). Both C17 and C18 (as well as traces of C15, C19, and C20) versions of caeliferin A16:0 were found, with C17 predominant over C18, although there appeared to be only the C16 version of caeliferin A16:1 at detectable amounts. There was also a set of related but different analogs. Of those, peak 4 in Fig. 4A, with a molecular weight of 439 amu, was the most likely compound responsible for the weak activity in fraction 13–14 in Fig. 1B. Sequential daughter ion analyses of this peak revealed only one sulfate to be present. GC/MS analyses of the methyl ester gave a MW of 330 amu, whereas the ethyl ester gave a MW of 358 amu (330 + 2 × 14), indicating a diethyl ester. The acetylated dimethyl ester had a MW of 372 amu (330 + 42), showing the addition of one acetate as expected. EI spectra of the dimethyl ester gave a clear M-59 ion at m/z 271 (M-COOCH3). Thus, peak 4 appeared to be α-sulfooxy hexadecanedioic acid with a calculated molecular weight of 382 amu. However, that is 57 mass units less than the MW given by the LC/MS analyses and indicated the presence of nitrogen. Compound 4 was therefore subjected to a mild methyl ester/acetate procedure especially suitable for compounds containing amino acids (20, 30). GC/MS analysis with on-column injection of the product resulted in three peaks; the mono-acetylated dimethyl ester of the complete compound (minus the sulfate) with a MW of 429, the acetylated methyl ester seen before with a MW of 372 and finally the acetylated methyl ester of glycine (MW 131), giving the final structure of caeliferin B16:0 in Fig. 3. Also, homologs with C17 and C18 fatty acid chains (with traces of C15 and C19) were found in analogy with caeliferin A16:0. There were also traces of an unsaturated version of caeliferin B16:0 (peak 1–3 in Fig. 4A), consequently named caeliferin B16:1 (Fig. 3). Proton NMR analysis of fraction 4 showed the same single proton at a substituted carbon α to a carboxylic acid (δ = 4.45 ppm) (broad) but, as expected, with no indication that an alcohol-substituted ω-carbon was present. However, the two protons at δ = 2.14 ppm confirmed the amide, and finally a singlet with 2 protons at δ = 3.62 ppm confirmed the presence of glycine.
Fig. 4.
Negative ion LC/MS separation and bioassay results of collected fractions. (A) Total ion trace of regurgitant from laboratory-reared S. Americana. The numbers indicate fractions that were collected for intact plant bioassays. Fraction 2, caeliferin B16:1; fraction 4, caeliferin B16:0; fraction 7, caeliferin A16:1; fraction 9, caeliferin A16:0; fraction 10, caeliferin A17:0; and fraction 11, caeliferin A18:0. (B) Normalized result of intact seedling bioassay at natural relative concentrations (0.5-μl regurgitant equivalents), n = 12. (C) Bioassay of selected fractions with all of the concentrations adjusted to that of caeliferin A16:1 (100 pmol in 0.5-μl regurgitant equivalents). Different letters indicate significant differences (P < 0.01).
LC/MS made it possible to collect and bioassay the identified caeliferins and several of the related peaks in Fig. 4A. However, the LC/MS trace could not be used for quantitative estimates due to different response factors for caeliferin A and B. Therefore the absolute amount of the collected compounds in each fraction was established by derivatization and GC analysis. In the first intact plant bioassay, we used the same regurgitant equivalents (0.5 μl) for each fraction tested, maintaining their natural relative concentrations (as in Fig. 1). The results (Fig. 4B) were also very comparable to the earlier results. Caeliferin A16:1 is naturally only a minor component (≈200 pmol/μl) compared with caeliferin A16:0 (≈4 nmol/μl) and B16:0 (≈500 pmol/μl). In a second assay (Fig. 4C) we bioassayed the major compounds at the same concentration (100 pmol per seedling). In this experiment caeliferin A16:1 was significantly more active than any other component, including caeliferin A16:0. The latter was now approximately as active as its C17 and C18 analogs and caeliferin B16:0.
Discussion
We report a new class of insect herbivore-produced plant volatile elicitors with strikingly different chemical structures than those previously described. Yet, the basic moiety of these structures is a fatty acid, such as the fatty acid–amino acid conjugates of the Lepidopteran elicitors, although the most active, as well as the most abundant, compounds from S. americana have 16- rather than 18-carbon chains. Preliminary analyses of regurgitant collected from several other species of Orthoptera indicates that the caeliferins may be present in most, if not all grasshoppers, members of the suborder Caelifera, but not in crickets or katydids in suborder Ensifera. This was the basis for naming these new compounds caeliferins. Interestingly, regurgitant of at least some crickets (31) and katydids (unpublished data) contain fatty acid amides, although we have not been able to find even traces of these in grasshopper regurgitant.
In the Lepidoptera, the fatty acid moiety of the elicitors is derived from the diet on which the larvae feed (25), and variation in proportions of dietary fatty acids results in similar changes in proportions of the corresponding elicitors (32). The composition of the regurgitant of laboratory-reared S. americana is very consistent and does not change with diet or maturing of the insect (data not shown). However, regurgitant from field-collected insects can contain up to 5-fold more of caeliferin A16:1, as well as more of the glycine-containing caeliferin B16:1 and B16:0 than laboratory-reared insects. Furthermore, the regurgitant composition in wild grasshoppers will change into laboratory-reared proportions within a week of caging in the laboratory, independent of what diet the insects are fed. Whether this is diet-related or due to other factors, such as crowding, is not yet known. The origin, or precursors of the S. americana elicitors is not known. On the basis of the natural preference for even carbon chain lengths, we would have expected C16 and C18 to dominate over C17 and, if the origin was plant lipids, then we would expect more C18 than C16 and also double bonds to be cis. However, in S. americana regurgitant, the relative abundance of caeliferin A16:0, A17:0, and A18:0 is ≈30:5:1, and the double bond in caeliferin A16:1 is trans. We have not found hydroxylation at any but the 2 and 16 positions. The 2-hydroxylation might explain the unusual lengths in carbon chains as the first step in a one-carbon chain-shortening sequence. Furthermore, the ω-hydroxyl might be the result of enzymatic reduction of an amide precursor (caeliferin B). However, that still leaves us with a unique and unknown precursor. It appears that all caeliferins in all species investigated are totally sulfated in the pH 5 regurgitant, whereas alkaline or stronger acid conditions result in partially or completely desulfated compounds.
The range of biological activity and function of caeliferins in grasshoppers is not fully understood. Sulfated compounds, for example, glycosaminoglycans, and proteins, have been found in insect ovaries, eggs, and fat bodies (33–35). The hydrophilic sulfate groups make caeliferins especially useful in bridging lipids and water, and as such they may function in digestion. Additionally, they may also be an important part of chemical defense, because grasshoppers regurgitate copious amounts of frothy secretions as a defense against attack. Indeed, investigations (36–38) have shown that both compounds from host plants and insect produced water-soluble components of the regurgitant have deterrent activity (36). Although the deterrent effect of caeliferins remains to be tested, it is probable that the emulsifying properties are important in aiding the water-based regurgitant to carry lipophillic toxic compounds.
It has been well demonstrated that induced plant volatiles play a role in attracting natural enemies of Lepidopteran herbivores, but there is no behavioral evidence that we are aware of that indicates this occurs for grasshoppers. Jasmonic acid signaling is a key player in induced plant response to wounding and feeding damage. Recently it has been shown that a sulfotransferase in Arabidopsis thaliana inactivates 12-hydroxyjasmonate by transforming it to its corresponding sulfate (39). Caeliferins might interact with this system, but at this stage we don't know the full range of activity of caeliferins on plants and signal cascades.
It has been reported that S. americana avoids diet with added monoterpenoids (40). We know, from electroantennogram experiments, that S. americana detects most of the volatile compounds released by induced corn seedlings. It is therefore likely that, under natural conditions, the solitary S. americana will stop feeding on and even avoid induced plants. Thus, the plants will directly benefit from the release of VOC in response to grasshopper feeding and caeliferins. Most of the time the American grasshopper lives a solitary life, but occasionally they transform into a gregarious stage, for example, when crowded. At that stage, plant volatiles could aid in aggregation. Caging will transform wild, solitary, nymphal as well as adult S. americana from solitary to gregarious stage, with the typical change in coloration occurring within a few days. This has so far made it difficult to study changes in behavior, including their response to induced plant volatiles. This physical change occurs in parallel with the change in caeliferin composition, mentioned earlier.
S. americana feeds on a broad range of plants (41). There is now evidence for dramatic variation in the response, or lack of response, to volicitin- and inceptin-types of elicitors from Lepidopteran larvae among plant species (26). It is an intriguing possibility that analogs of caeliferin A and B have different levels of activity on the wide variety of host plants of S. americana or even on different varieties of the same species of plants.
Induced release of volatiles is just one of many levels of defense against phytophagous insects, and it would be surprising if the known elicitors do not also trigger nonvolatile defenses. An investigation of the activity of these elicitors on several plant species in inducing volatile emission, as well as increased levels of jasmonic acid and other plant hormones. The discovery of caeliferins provides new biological tools and directions to explore the physiological ecology and interactions of insects and plants.
Materials and Methods
Insect Rearing.
S. americana were either obtained from a colony maintained at the Department of Entomology and Nematology at the University of Florida or field-collected in Gainesville, FL. The insects were fed lettuce, wheat, or corn leaves and kept in cages in a temperature-controlled greenhouse (25°C during the day/20°C during the night) with natural light, supported by a mixture of high-pressure sodium and metal halide lamps (400W Lucalox; GE, Piscataway, NJ) to provide 14 h of day/10 h of night. To facilitate reproduction and egg laying, cups with moist vermiculite were kept in the cages.
Plants.
All plants were grown in 1-gallon containers (Miracle Grow no. 92695 potting soil fertilized with 1 teaspoon of Osmocote 14-14-14; Scotts-Sierra Horticulture, Marysville, OH). Feed corn variety Delprim (Delley Seeds and Plants, Delley, Switzerland) was planted 10 seeds per pot and maintained on a 12-h dark and 12-h light cycle. One week after germination, the number of seedlings per pot were either reduced to six of uniform size for the cut stem bioassay or transferred to hydroponic chambers (18). The light intensity was 305 μmol·m−2·S−1 of photosynthetically active radiation supplied by a mixture of high-pressure sodium and metal halide lamps (400W GE Lucalox) at the top of the plant canopy with day/night temperatures at 25°C/20°C, respectively. The relative humidity ranged from 60% to 70%. For both bioassays, 10- to 12-day-old seedlings that contained three to four leaves were used for treatments and volatile collections.
Plant Bioassay.
For both bioassays, plants were treated ≈2 h into the scotophase and incubated for ≈16–17 h on the same day/night schedule under which they were grown but with reduced daytime light (2,000 lux) before volatile collection.
Excised Plant.
At the time of treatment, the corn seedlings were cut off near the base of the stem with a razor blade, and the cut end was immediately immersed in 600 μl of 50 mM Na2HPO4 buffer (pH 8) (control) or a test solution in 600 μl of the same buffer, contained in a 1.5-ml vial. Five-microliter regurgitant equivalents (MRE) of the crude regurgitant or subsequent fractions thereof were used to treat each seedling in all bioassays. After incubation, the stem of each plant was recut, wrapped in wet cotton, and placed in a glass volatile collection chamber, and volatiles were collected as described below.
Intact Plant.
At the time of treatment the two oldest leaves of individual plants each received two superficial damage sites by scratching the surface (2 mm × 10 mm) with a razor blade approximately equidistant between the base and tip of the leaf (29). Immediately after damage, 5 μl of the same buffer solution as above containing 0.5-μl regurgitant equivalents (MRE) was applied evenly to the four damage sites. The plants remained in their hydroponic solution during incubation and then were removed from their container, their roots were gently wrapped in soft tissue and dipped in hydroponic solution, the seedlings were placed in the volatile collection chambers, and volatiles were collected as described below.
Volatile Collections.
Volatile chambers (30 cm long × 4 cm i.d.) were placed under the same type and intensity of light as used in rearing and volatiles, were collected on Super Q 80/100 (catalog no. 2735; Alltech Associates, Deerfield, IL) as described in refs. 22 and 28, and were analyzed by gas chromatography (Analysis 5 in SI Text and SI Fig. 9). Volatiles were collected for 2 h for the cut-stem assays and for 1 h for the intact-plant assays. The response by the plant to each test sample was measured as the combined amount of four induced terpenoids: E-β-caryophyllene, E-α-bergamotene, α-humulene, and E-β-farnesene. Although several more volatile components are released by Delprim cultivar in response to insect herbivory (5), these four components show the strongest induced response. All bioassays also included positive controls (0.5 or 5 μl of crude regurgitant, depending on bioassay) and a negative control (buffer only). All samples were replicated three to four times within each bioassay and each bioassay was repeated three to four times (giving a minimum total of n = 9 per treatment). To reduce the variation in response between bioassays, a correlation factor was calculated for each bioassay to give the mean release of induced volatiles for the regurgitant treatments a response of 100. The correlation factor was then used for all samples within that bioassay. Data were analyzed using a Tukey pairwise multiple comparison test.
Collection and Initial Fractionation of Regurgitant.
Regurgitant was collected from both wild and laboratory-reared nymphs and adults as described earlier for Lepidoptera caterpillars (22). The regurgitant was diluted 50:50 with acetonitrile, centrifuged at 16,000 × g for 15 min to remove precipitated proteins, and then filtered through 0.45- and 0.22-μm sterilizing membranes. The supernatant was concentrated to original volume by a gentle stream of N2 and stored at −70°C until used. A C18 solid-phase extraction column (mega bond elute; Waters, Milford, MA) was activated by eluting with 40 ml of acetonitrile and then with 40 ml of water. Two milliliters of concentrated supernatant was added to the top of the column and separated into three fractions by eluting with 20 ml of water, followed by 20 ml of 50:50 water:acetonitrile and then by 20 ml of acetonitrile with gravity flow. Each fraction was concentrated to the original volume before bioassaying.
HPLC Purification.
The active 50% acetonitrile-water fraction was further fractionated by HPLC under acidic conditions (AS3500 Autosampler, Constametric 4100 Pump, and Spectromonitor 3200 variable wavelength detector; Thermo Separation Products, Riviera Beach, FL), while monitoring UV absorption at 220 nm. With the temperature maintained at 60°C, a C18 reverse-phase column (ODS-AMQ, S-5 μm, 200 A, 250 × 4.6 mm i.d.; YMC, Kyoto, Japan) was eluted with a solvent gradient (1.0 ml/min) of 10–70% acetonitrile in water for 20 min followed by 70% acetonitrile for 10 min., with both solvents containing 1 mM NH4Ac:AcOH buffer, pH 4.5 [acetic acid (Aldrich, Milwaukee, WI), acetonitrile UV (B & J Brand High Purity Solvent, Muskegon, MI), and water (Milli-Q UV Plus System; Millipore, Bedford, MA)]. Combined fractions from several repeated separations were evaporated under vacuum to near dryness and desalted on activated C18 solid-phase extraction columns (SPE C18; J.T. Baker, Phillipsburg, NJ) that were rinsed with 2 ml of water, followed by 2 ml of 50% acetonitrile in water. The latter fraction was reduced to dryness and redissolved in bioassay buffer (50 mM phosphate buffer, pH 8) before bioassaying.
The most active fractions from the first HPLC separation were combined and further fractionated by a modified solvent gradient and neutral conditions by using the same column and system as above with UV absorption monitored at 210 nm. The column was eluted with a solvent gradient (1.0 ml/min) of 15–60% acetonitrile in water in 20 min followed by 60% acetonitrile for 10 min, with both solvents containing 5 mM NH4Ac buffer, pH 7.0. Two-minute fractions were collected and desalted as described above before bioassaying.
LC/MS Conditions.
A Thermo Finnigan LCQ Deca XP Max was used with electro spray ionization (sheet gas, 30 arbitrary units; sweep gas, 5 arbitrary units; spray voltage, 5.00 kV; capillary temperature 275°C; and capillary voltage, 3.0 V). The Thermo Separations spectra HPLC system consisted of a quaternary pump P4000, autosampler AS 3000, and diode array detector UV6000. The tertiary solvents consisted of (i) methanol with 0.05% formic acid, (ii) water with 10 mM ammonium formate, and (iii) 90% acetonitrile/10% water with 10 mM ammonium formate. With the column temperature maintained at 60°C and a solvent flow of 1.0 ml/min, the C18 column (ODS-AMQ, S-5 μm, 200 A, 250 × 4.6 mm i.d.; YMC) was eluted with a solvent composition starting with 4:90:6 (i:ii:iii) for 1 min, followed by a gradient to 4:50:30 in 13 min and a fast ramping to 4:0:96 in 2 min and was then kept at that composition for 5 min. UV absorption was monitored at 190–400 nm, and the solvent flow between the UV detector and MS electro spray interface was split 9:1 with a low-volume micro needle valve splitter P450 (Upchurch Scientific, Oak Harbor, WA) making it possible to obtain spectra of eluted compounds and simultaneously collect 90% of the injected material for bioassaying.
GC/MS Conditions.
Derivatized samples were analyzed by GC/MS (6,890/5,973 GC/MS; Agilent, Palo Alto, CA) in both EI and isobutane CI mode. Samples were injected in the splitless mode at 220°C or with cold on-column. The methyl silicone column, (HP1, 30 m × 0.25 mm i.d. × 0.1-μm film thickness; Agilent) was kept at 40°C for 1 min and was then temperature programmed at 10°C/min to 260°C. The He carrier gas flow rate was 30 cm/sec (constant flow), and the transfer line temperature was 250°C. The ion source temperature was 220°C in EI mode and 250°C in CI mode.
Derivatizations for GC/MS.
For methanolysis and ethanolysis 10 μl of regurgitant supernatant after protein precipitation, or the same amount of regurgitant equivalents of HPLC fraction, was added to a vial and evaporated to dryness with N2. Two hundred microliters of dry methanol/HCl (10:1) or ethanol/HCl was added, and the sealed vial was heated to 100°C for 30 min. The mixture was allowed to cool to room temperature, 500 μl of methylene chloride was added, and the solution was extracted twice with 500 μl of water. The organic phase was evaporated to dryness with N2, and 500 μl of fresh methylene chloride was added for GC/MS analyses.
For acetylation, 20 μl of acetyl chloride was added to the above solution, which then was heated to 90°C for 30 min. The solution was then extracted three times with water before GC/MS analyses.
HPLC fractions derivatized for GC/MS analyses (acetylated methanolysis products) were ozonized as described in refs. 20 and 42 and analyzed by GC/MS by using on-column injection.
Double bonds were reduced by gently drying methylene chloride solutions of acetylated methylesters of pure HPLC fractions with N2 and redissolving in ethyl acetate. A few grains of Pd on carbon were added, H2 was gently bubbled through the solution for 1 h, and the solution analyzed on GC/MS.
NMR Analyses.
1H NMR spectra were obtained with Varian (Palo Alto, CA) Unity 600 and Bruker (Billerica, MA) Avance 500 instruments by using a Wilmad (Buena, NY) 520 1A microbore tube. Samples were dissolved in D2O, and 1H NMR chemical shifts are reported with respect to internal tetramethylsilane.
Supplementary Material
Acknowledgments
We thank Peggy Brennan, Mary Searle, Spencer Walse (all at the Center for Medical, Agricultural, and Veterinary Entomology at the United States Department of Agriculture) and Jim Rocca (University of Florida, Gainesville, FL) for technical assistance; and Paul Pare (Texas Tech University, Lubbock, TX), Naoki Mori (Kyoto University, Kyoto, Japan), Ted Turlings (University of Neuchatel, Neuchatel, Switzerland), and John Pickett (Rothamsted Experimental Station, Harpenden, U.K.) for helpful comments.
Abbreviations
- amu
atomic mass unit
- CI
chemical ionization
- EI
electron impact
- LC
liquid chromatography
- MW
molecular weight
- VOC
volatile organic compounds.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705947104/DC1.
References
- 1.Turlings TCJ, Tumlinson JH, Lewis WJ. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 2.Kessler A, Baldwin IT. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 3.Loughrin JH, Manukian A, Heath RR, Tumlinson JH. J Chem Ecol. 1995:1217–1227. doi: 10.1007/BF02228321. [DOI] [PubMed] [Google Scholar]
- 4.Gouinguene S, Degen T, Turlings TCJ. Chemoecol. 2001;11:9–16. [Google Scholar]
- 5.Paré PW, Tumlinson JH. Nature. 1997;385:30–31. [Google Scholar]
- 6.Rose USR, Manukian A, Heath RR, Tumlinson JH. Plant Physiol. 1996;111:487–495. doi: 10.1104/pp.111.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mithofer A, Wanner G, Boland W. Plant Physiol. 2005;137:1160–1168. doi: 10.1104/pp.104.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiteller D, Pohnert G, Boland W. Tetrahedron Lett. 2001;42:1483–1485. [Google Scholar]
- 9.Arimura G, Huber DPW, Bohlman J. Plant J. 2004;37:603–616. doi: 10.1111/j.1365-313x.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- 10.Degenhardt DC, Lincoln DE. J Chem Ecol. 2006;32:725–743. doi: 10.1007/s10886-006-9030-2. [DOI] [PubMed] [Google Scholar]
- 11.De Moraes CM, Mescher MC, Tumlinson JH. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 12.Litvak ME, Monson RK. Oecologia. 1998;114:531–540. doi: 10.1007/s004420050477. [DOI] [PubMed] [Google Scholar]
- 13.Roese USR, Tumlinson JH. Planta. 2005;222:327–335. doi: 10.1007/s00425-005-1528-2. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues-Saona C, Crafts-Brandner SJ, Williams L, Pare PW. J Chem Ecol. 2002;28:1733–1747. doi: 10.1023/a:1020552932566. [DOI] [PubMed] [Google Scholar]
- 15.Turlings TCJ, Bernasconi M, Bertossa R, Bigler F, Caloz G, Dorn S. Biolog Cont. 1998;11:122–129. [Google Scholar]
- 16.Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH. J Chem Ecol. 2000;26:189–202. [Google Scholar]
- 17.Williams L, Rodriguez-Saona C, Pare PW, Crafts-Brandner SJ. Arch Biochem Phys. 2005;58:84–96. doi: 10.1002/arch.20035. [DOI] [PubMed] [Google Scholar]
- 18.De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH. Nature. 1998;393:570–573. [Google Scholar]
- 19.Du YJ, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM. J Chem Ecol. 1998;24:1355–1368. [Google Scholar]
- 20.Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. Science. 1997;276:945–949. [Google Scholar]
- 21.Alborn HT, Jones TH, Stenhagen GS, Tumlinson JH. J Chem Ecol. 2000;26:203–220. [Google Scholar]
- 22.Alborn HT, Brennan MM, Tumlinson JHT. J Chem Ecol. 2003;29:1357–1372. doi: 10.1023/a:1024209302628. [DOI] [PubMed] [Google Scholar]
- 23.Mori N, Alborn HT, Teal EA, Tumlinson JH. J InsectPhysiol. 2001;47:749–757. doi: 10.1016/s0022-1910(00)00171-2. [DOI] [PubMed] [Google Scholar]
- 24.Pohnert G, Jung V, Haukioja E, Lempa K, Boland W. Tetrahedron. 1999;55:11275–11280. [Google Scholar]
- 25.Paré PW, Alborn HT, Tumlinson JH. Proc Natl Acad Sci USA. 1998;95:13971–13975. doi: 10.1073/pnas.95.23.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, Chourey PS, Alborn HT, Teal PEA. Proc Natl Acad Sci USA. 2006;103:8894–8899. doi: 10.1073/pnas.0602328103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW. Nature. 2002;416:599–600. doi: 10.1038/416599a. [DOI] [PubMed] [Google Scholar]
- 28.Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH. J Chem Ecol. 1993;19:411–425. doi: 10.1007/BF00994314. [DOI] [PubMed] [Google Scholar]
- 29.Schmelz EA, Alborn HT, Banchio E, Tumlinson JH. Planta. 2003b;216:665–673. doi: 10.1007/s00425-002-0898-y. [DOI] [PubMed] [Google Scholar]
- 30.Mee JML, Korth J, Halpern B. Biomed Mass Spectrom. 1977;4:178–181. doi: 10.1002/bms.1200040310. [DOI] [PubMed] [Google Scholar]
- 31.Yoshinaga N, Aboshi T, Ishikawa C, Fukui M, Shimoda M, Nishida R, Lait CG, Tumlinson JHT, Mori N. J Chem Ecol. 2007;33:1376–1381. doi: 10.1007/s10886-007-9321-2. [DOI] [PubMed] [Google Scholar]
- 32.De Moraes CM, Mescher MC. Proc Natl Acad Sci USA. 2004;101:8993–8997. doi: 10.1073/pnas.0403248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa A, Werneck CC, Nasciutti LE, Masuda H, Atella GC, Silva LCF. Insect Biochem Mol Biol. 2001;31:31–40. doi: 10.1016/s0965-1748(00)00101-6. [DOI] [PubMed] [Google Scholar]
- 34.Giorgi F, Cecchettini A, Locci MT, Masetti M, Bradley JT. Develop Biol. 1995;167:379–387. doi: 10.1006/dbio.1995.1032. [DOI] [PubMed] [Google Scholar]
- 35.Giorgi F, Falleni A, Cecchettini A, Gremigni V. Biol Cell. 1998;90:183–197. doi: 10.1016/s0248-4900(98)80339-0. [DOI] [PubMed] [Google Scholar]
- 36.Ortego F, Evans PH, Bowers WS. J Chem Ecol. 1997;23:1941–1950. [Google Scholar]
- 37.Sword GA. Oecologia. 2001;128:416–421. doi: 10.1007/s004420100666. [DOI] [PubMed] [Google Scholar]
- 38.Calcagno MP, Avila JL, Rudman I, Otero LD, Alonso-Amelot ME. Physiol Entomol. 2004;29:123–128. [Google Scholar]
- 39.Gidda SK, Miersch O, Levitin J, Wasternack C, Varin L. J Biol Chem. 2003;278:17895–17900. doi: 10.1074/jbc.M211943200. [DOI] [PubMed] [Google Scholar]
- 40.Bernays EA, Chapman RF. Ecol Entomol. 1977;2:1–18. [Google Scholar]
- 41.Capinera JH. Pop Ecol. 1993;22:127–133. [Google Scholar]
- 42.Beroza, Bierl Anal Chem. 1966;38:1976–1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.