Abstract
Here, we provide gain-of-function, loss-of function, and molecular evidence supporting genetic interactions between metastasis associated protein 1 (MTA1) and Six3 and between Six3 and rhodopsin. We discovered that MTA1 physically interacts with the Six3 chromatin in a histone deacetylase-dependent manner, leading to transcriptional suppression of the Six3 gene. MTA1 is also a Six3-interacting corepressor that contributes to a self-negative regulation of Six3 transcription by Six3. In contrast, deletion of the MTA1 alleles in murine embryonic fibroblasts or its knockdown in rat retinal ganglion cells stimulates Six3 expression. MTA1 inactivation in the MTA1-null mice results in an elevated Six3 level and proliferation of the retina cells with no obvious abnormities in eye formation. However, unexpectedly, we discovered an enhanced recruitment of Six3 to the rhodopsin chromatin in retina from the MTA1-null mice; Six3's homeodomain interacts with specific DNA elements in the rhodopsin promoter to stimulate its transcription, resulting in increased rhodopsin expression. Further, in holoprosencephaly patients, Six3 protein with a naturally occurring deletion mutation in the helix 3 of the homeodomain does not bind to rhodopsin DNA or stimulate rhodopsin transcription, implying a potential defective rhodopsin pathway in the affected holoprosencephaly patients. Further Six3 cooperates with Crx or NRL in stimulating transcription from the rhodopsin-luc. These findings reveal a previously unrecognized role for the MTA1 as an upstream modifier of Six3 and indicate that Six3 is a direct stimulator of rhodopsin expression, thus revealing a putative role for the MTA1/Six3/rhodopsin pathway in vertebrate eye.
Keywords: MTA1, transcription repressor, histone deacetylase
It is increasingly accepted that the outcome of gene modifying pathways is mechanistically influenced by epigenetic and heritable modifications of target gene chromatin. Furthermore, perturbation in the expression and activity of chromatin modifiers may contribute to pathologic alterations in their physiologic functions. One such chromatin modifier is metastatic tumor antigen 1 (MTA1), the founding member of the MTA family of coregulators and a component of nucleosome remodeling and histone deacetylase complexes. MTA1 interacts directly with histone deacetylase (HDAC) 1 and 2 and represses transcription by recruiting HDAC corepressor complexes to the target genes (1–3). MTA1 is highly conserved through evolution and ubiquitously expressed (4–7). Although MTA1 levels are increased in cancer cells (8–11), its contribution in the regulation of essential genes in normal tissues, including retina, have as yet not been delineated.
Six3 is a transcription corepressor with a role in the proliferation of retinal precursor cells and contains a homeobox domain and a Six domain (12, 13). Mutations in the homeodomain of the human SIX3 gene have been linked to holoprosencephaly, a complex and multiloci disorder characterized by eye malfunction, severe malformation of the brain, and vision defects in addition to characteristic brain malformations (14). Despite a large body of work in support of an essential role of Six3 in vertebrates, the nature of its upstream regulator(s) remains elusive (12–14). Here, we investigated a potential regulatory role of MTA1 in controlling the Six3 expression and consequently the resulting target gene expression.
Results and Discussion
To explore the role of MTA1 in the Six3 pathway, we took advantage of recently generated stable pooled clones of T7-MTA1 in the HC11 murine epithelial cell line. We unexpectedly found a significant reduction of Six3 mRNA and protein in cells with elevated levels of MTA1 [supporting information (SI) Fig. 6A], raising the possibility that MTA1 might be an upstream regulator of the Six3 pathway. MTA1 also repressed the transcription driven by a Six3 promoter-luc in HeLa cells (SI Fig. 6B). To validate these findings, we studied Six3 expression in murine embryonic fibroblasts from WT, heterozygous, and homozygous MTA1 (KO) mice, recently generated in the R.K. laboratory. We found that the loss of MTA1 alleles in the murine embryonic fibroblasts caused up-regulation of Six3 protein (Fig. 1A).
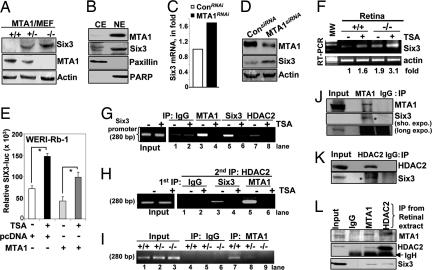
Fig. 1.
MTA1 regulates Six3 expression. (A) Western blot analysis of Six3, MTA1, and actin in the murine embryonic fibroblasts from the MTA1-KO mice. (B) Status of MTA1 and Six3 in the nuclear (NE) and cytoplasmic (CE) fractions from RGC5 cells. Paxillin and PARP were used to demonstrate the purity of CE and NE fractions. (C) Effect of MTA1 siRNA on the levels of Six3 protein in RGC5 cells by qPCR. (D) Effect of MTA1 siRNA on the levels of Six3 protein in the RGC5 cells. (E) Effect of TSA (100 ng/ml) and MTA1 expression on Six3 luc activity in the WERI-Rb-1 cells. (F) RT-PCR analysis of Six3 mRNA in retina's of MTA1-KO and WT mice. (G) ChIP analysis showing recruitment of Six3, MTA1, and HDAC2 onto the Six3 promoter in RGC5 cells. (H) Double ChIP analysis of Six3/HDAC2 or MTA1/HDAC2 complex onto Six3 promoter in RGC5 cells. (I) MTA1 ChIP analysis onto Six3 promoter in the retina's of WT mice but not in KO mice. (J–L) Association of endogenous Six3 with MTA1 (J) and HDAC2 in RGC5 cells (K) and in retinas of the WT mice (L).
Because the published literature suggests a paramount role of Six3 in the development of the eye (12), we next wished to extend these findings to a physiologically relevant retinal ganglion cell line 5 (RGC5) (15), which expresses both MTA1 and Six3 (Fig. 1B), and in which depletion of MTA1 by specific siRNA further up-regulates the expression of Six3 mRNA (Fig. 1C) and protein (Fig. 1D). Further, MTA1 expression in WERI-Rb-1 retinoblastoma cells repressed Six3-luc activity in a TSA-sensitive manner (Fig. 1E). We also noticed a higher level of Six3 mRNA in the cultured retina's from the MTA1-KO mice as compared with the WT mice (Fig. 1F). Because Six3 interacts with groucho repressors, which associate with HDACs in Drosophila (16), HDAC inhibitor trichostatin A (TSA) enhanced the expression of Six3 in both retinas (Fig. 1F, lanes 2 and 4). To confirm that the Six3 gene chromatin is a direct target of MTA1, we performed ChIP assays, using primers encompassing 280 bp of the Six3 promoter region. MTA1 was recruited to Six3 promoter in a TSA-sensitive manner in RGC cells (Fig. 1G), suggesting the role of MTA1/nucleosome remodeling and histone deacetylase complex in the noted MTA1 repression of Six3 transcription. Because MTA1 engages HDAC complexes to repress transcription (17), we found that HDAC2 is also recruited to the Six3 promoter in a TSA-sensitive manner (Fig. 1G). We used Six3 recruitment to its own promoter as a positive control. These findings identify MTA1 as a new upstream regulator of Six3 transcription factor.
To demonstrate the physical interaction of the MTA1/nucleosome remodeling and histone deacetylase complex with the Six3 chromatin, we performed a double ChIP in the RGC5 cells; the initial ChIP was done with anti-MTA1 Ab to immunoprecipitate the MTA1-bound DNA sequences, and the second ChIP was done with the anti-HDAC2 Ab. We found simultaneous coassociation of MTA1 and HDAC2 with the Six3 chromatin and its derecruitment by TSA (Fig. 1H). Interestingly, MTA1 recruitment to the Six3 promoter was 3–5 times more robust than that of Six3-recruitment to its own promoter (Fig. 1H; compare lane 5 with lane 3). MTA1 was bound to the Six3 promoter in the retina from the WT but not KO mice (Fig. 1I). These findings suggested that MTA1 represses Six3 expression in an HDAC-dependent manner in retinal cells, providing an explanation for the repression of Six3 expression at the molecular level.
Because Six3 autorepresses its own expression by a consensus ATTA core motif in its promoter (18), and because this interaction involves MTA1/HDAC (this study), we hypothesized that MTA1 might also contribute to the corepressor activity of Six3. We showed that between the Flag-Six3 and endogenous MTA1 (SI Fig. 7), between the endogenous MTA1 and Six3 (Fig. 1J) and between Six3 and HDAC2 (Fig. 1K) in the RGC5 cells. Endogenous Six3 also associates with MTA1 and HDAC2 in the retina of the WT mice (Fig. 1L). Recombinant MTA1 interacts with amino acids 184–333 of Six3, whereas HDAC2 interacts with the amino acids 120–333 of Six3 (SI Fig. 8). In brief, MTA1 interacts with Six3 in vitro and in physiologic settings.
To test whether the MTA1/Six3 complex participates in the recruitment of Six3 to Six3 gene chromatin, we silenced MTA1 in the RGC5 cells and found a reduced association of Six3 with the Six3 promoter as compared with control siRNA treated cells (Fig. 2A). These results suggested an inherent role of MTA1/Six3 interaction and of MTA1 status in influencing the ability of Six3 to interact with its promoter. To demonstrate a direct or indirect binding of MTA1 to the Six3 promoter, we used the Six3-binding ATTA core-motif sequence in EMSA. We found that recombinant Six3 but not MTA1 binds to the DNA, whereas inclusion of Six3 antibody resulted in the partial loss of Six3/DNA complex with a faint shifted complex (Fig. 2B, lane 3). Interestingly, coincubation of MTA1 enabled the formation of higher Six3/MTA1/DNA complexes (Fig. 2B; compare lanes 3 and 7 with lane 5). These observations suggested that MTA1/Six3 interaction facilitates the binding of Six3 to the core DNA motif, which in turn may lead to the repression of Six3 transcription.
Fig. 2.
Six3 associates with MTA1 in retinal ganglion cells. (A) ChIP, showing Six3 derecruitment from its own promoter in RGC5 cells under MTA1 silencing. (B) EMSA, showing Six3 binding to a synthetic oligo containing ATTA core (lane 3) and a shift with Six3 antibody (lane 4, bracket) and higher-molecular-weight complexes with MTA1 and Six3 (lane 5). Higher protein/DNA complexes are seen in the presence of Six3, MTA1, and Six3 Ab (lane 6). Lane 7 shows no interaction of MTA1 with ATTA DNA oligo.
Because Six3 plays an important role in eye development (12), we next determined the expression characteristics of MTA1 and the status of Six3 in the murine eye from the WT and MTA1-KO mice. We found that MTA1 is expressed in lens epithelial cells, retina at embryonic day (E)14.5 and E17.5, and maintained in the fully differentiated eye and is located in cells of the inner nuclear layer and in scattered retinal ganglion cells in the adult WT eye (Fig. 3 A and B). As expected, there was no detectable expression of MTA1 in eyes from the MTA1-KO mice, as expected (Fig. 3 A and B). MTA1 is also expressed in the cells of the inner nuclear layer and ganglion cell layer in the adult human retina (SI Fig. 9).
Fig. 3.
Six3 expression is up-regulated in retinas from the MTA1-null mice. (A) MTA1 and Six3 IF staining in WT and MTA1-KO mice retinas at different developmental stages. (Scale bar, 100 μm.) (B) Expression of MTA1, rhodopsin, Six3 in retina from WT and MTA1-KO mice. os, outer segment; onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer. (Scale bar, 20 μm.) (C) MTA1 level in retinas from the adult WT and MTA1-KO mice. (D–G) RT-PCR (D), quantitative PCR (E), Northern blot (≈2.3-kb mRNA) (F), and Western blot (G) analyses of Six3 expression in retinas from the adult WT and MTA1-KO mice. (H) EMSAs show Six3 binding to oligo containing ATTA core, using retinal lysates from the WT and MTA1-KO mice. P, probe alone; FP, free probe.
Next, we determined the effect of genetic ablation of MTA1 on the expression pattern and levels of Six3 in embryonic and adult eye (Fig. 3 A and B). We discovered that Six3 colocalizes with the MTA1 during the early E14.5 stage in WT eye, gradually reduces in the E17.5 stage eye, and continues to be expressed in the adult retina (Fig. 3 A and B, green). In contrast, the levels of Six3 were distinctly up-regulated in both embryonic and adult eyes from the MTA1-null mice: during embryonic stages the Six3 expression was present in the whole retina area from the MTA1-KO mice as compared with the limited Six3 expression in the inner area of the WT mice. There was a modest but statistically significant increased proliferation of BrdU-positive cells in E14.5 retina from the KO mice as compared with the WT eyes (SI Fig. 10). In adult mice, the Six3 expression strikingly localized in the outer nuclear layer and was also present in cells in the ganglion cell layer (Fig. 3B). Consistent with the above findings, we found an increased expression of Six3 mRNA and Six3 protein in retina from the MTA1-KO mice as evaluated by RT-PCR, quantitative RT-PCR, northern hybridization, and Western blot analysis (Fig. 3 C–G). Increased Six3 in the retina from MTA1-KO mice was also confirmed by increased ability of retina lysate to form specific Six3/DNA (ATTA core) complex in EMSA (Fig. 3H).
Because there were no obvious abnormalities in the eyes of the MTA1-KO mice (SI Fig. 11), we next compared the expression of a series of cell type-specific markers in the eyes from the WT and KO mice. There was no significant change in the expression characteristics of MAP2, calretinin, calbindin, peripherin-2, GFAP, beta-tubulin-3 (data not shown), anti-cone arrestin, and S and M opsins (SI Fig. 12). However, we discovered increased rhodopsin fluorescence intensity in the outer retinal layer from the MTA1-KO mice as compared with the eyes from age-matched WT mice (Fig. 4A), raising the exciting possibility of a previously unknown relationship between an increased Six3 level and rhodopsin expression. To confirm this hypothesis, we next determined the levels of rhodopsin mRNA and protein in the retina from the WT and MTA1-KO mice. We discovered that retina from the MTA1-KO mice contained increased level of rhodopsin mRNA 3.5 kb transcript (Fig. 4B) as well as of rhodopsin protein (Fig. 4C). Because retina from the MTA1-KO mice also contained an increased level of Six3, and because Six3 could activate or repress the target genes (19, 20), we next explored a coactivator role of Six3 on rhodopsin expression. To directly evaluate this hypothesis, we determined the effect of Six3 or MTA1 on bovine rhodopsin proximal promoter [−130 to + 70 bp, bRho130-luc (21)]. More importantly, a careful analysis of rhodopsin promoter discovered the presence of a core ATTA site (−103 to −106 bp). Results from transient transfection studies in CV1 and 293T cells indicated that Six3 but not MTA1 is a potent activator of rhodopsin-driven transcription (Fig. 4D), revealing a coactivator function of Six3 upon rhodopsin expression.
Fig. 4.
Six3 regulation of rhodopsin expression. (A) Rhodopsin IF in retinas from adult WT and MTA1-KO mice. (Inset) Shown are enlargements of stained rhodopsin region. (Right) Quantification of fluorescence density. (B) Northern blot analysis of rhodopsin mRNA (3.5 kb) in retinas from adult WT and MTA1-KO mice. (C) Six3 protein in retinas from adult WT and MTA1-KO mice. (D) Effect of Six3 on the bRho-130 promoter-luc activity in the CV-1 and 293T cells. (E) ChIP of Six3 recruitment onto indicated regions of the murine rhodopsin promoter, using retinas from adult old MTA1-WT (+/+), -heterozygous (+/−), and -homozygous (−/−) mice.
To establish that rhodopsin gene chromatin is a direct target of Six3 in retina, we next examined the recruitment of Six3 to the proximal rhodopsin promoter encompassing −180 to + 10 bp from the transcription initiation site. We found that Six3 is recruited to the rhodopsin promoter in retinas from the MTA1-KO mice over a readily detectable level of Six3 recruitment in the retina from the WT mice (Fig. 4E). These findings suggest that, in principle, Six3 can bind to the rhodopsin gene chromatin in vivo and may be responsible for the noted up-regulation of rhodopsin in the MTA1-null mice.
To determine whether there was an effect of increased rhodopsin expression on visual function, we next compared the dark-adapted electroretinograms (ERG) of littermate WT and MTA1-KO mice at 2.5 month of age. ERGs were measured in response to brief flashes over a range of increasing stimulus energies. For the high energy stimuli (e.g., 3.0 log sc td s at the top; SI Fig. 13), the ERG consisted of a negative going a-wave, reflecting the mass response of the rod photoreceptors, followed by a positive going b-wave, primarily reflecting rod bipolar cell function. The responses were very similar in wave form and time course for WT and MTA1-KO mice, with responses in the KO at least as large (SI Fig. 14) as responses in the WT. ERGs of WT and KO animals were similar over the entire range of stimulus energies, with the smallest measurable responses at the bottom of the plot (note different amplitude calibration), the negative-going scotopic threshold responses from inner retina, occurring around −5.4 log sc td in both groups. The plots in SI Fig. 14 show amplitudes of a- and b-waves measured at fixed times after the brief flash near the peaks of the a- and b-waves for seven KO and five littermate WT mice. The a-wave amplitudes were slightly larger in the KO animals, but results for the two groups were not significantly different.
Although there was increased rhodopsin in the MTA1-KO mice, ERGs were not significantly larger or more sensitive than in WT littermates. This may be because the increase in rod opsin was not accompanied by an increase in the chromophore, 11-cis retinal. A study of mice in which the rod opsin was overexpressed found that the 11-cis retinal rose only by ≈30% when the quantity of opsin doubled (22). Even in normal mice, only limited supplies of retinoid in excess of the bound chromophore, enough for an additional 25–30% are available (23). This means that photoisomerization rates could hardly increase, limiting potential changes in sensitivity and amplitude of the ERG. Because there was no such loss of photoreceptors in the MTA1-KO mice as oppose to the previous study, our findings point to important inherent regulatory differences between the forced overexpression of exogenous opsin complementary DNA versus natural increase in the levels of opsin expression because of transcription stimulation of opsin promoter chromatin by the currently discovered endogenous regulatory pathway, i.e., MTA1-Six3.
Because the rhodopsin promoter region that interacted with Six3 contained a core ATTA consensus motif (ATTA, −103 to −106), two ATTA like sequences (GTTA, −97 to −100; ATGA, −91 to −94), we next wished to identify the Six3-interacting DNA sequences in the rhodopsin promoter (SI Fig. 15). We designed an oligonucleotide encompassing 26 bp (−90 to −112) of the rhodopsin promoter and performed EMSA, using recombinant Six3 generated by an in vitro translation reaction (either 32P-labeled or unlabeled Six3 protein). We found that Six3 forms a specific complex with the rhodopsin DNA, which could be effectively competed by the cold oligo probe and also supershifted by a specific anti-Six3 antibody (Fig. 5A), suggesting that Six3 could directly bind to the rhodopsin DNA sequence. Similarly, nuclear extracts from the RGC5 cells (SI Fig. 16A) or recombinant Six3 but not Pax5 (SI Fig. 16B) also formed a specific Six3-DNA complex. To determine the specificity of the Six3-interacting DNA sequence, we first mutated the core ATTA sequence to AGCA in the oligo probe and found no major inhibitory effect on Six3 binding (SI Fig. 17, lanes 4–6). Next, we generated six additional oligo probes with mutations in the ATTA and ATTA-like motifs (ATTA, GTTA, or ATGA to GCGC alone or combinations) (SI Figs. 15 and 17), and incubated with the recombinant Six3 in EMSA. This analysis identified both ATTA and ATTA-like motifs as Six3-interacting motif in the rhodopsin promoter, as the oligo probe containing substitution of ATTA or GTTA or ATGA with GCGC failed to form complex with Six3 protein (SI Fig. 17, lanes 13–15).
Fig. 5.
Rhodopsin is a direct transcriptional target of Six3. (A) EMSA showing specific binding of in vitro translated Six3 (lane 2) and a band shift in the presence of Six3 antibody (lane 3) to oligo-containing ATTA core of rhodopsin promoter. P, probe alone; FP, free probe. (B) Effect of Six3 on the WT or mutated-rhodopsin luc activity in 293T cells. (C) EMSA analysis showing Six3 binding onto the WT rhodopsin oligo probe, using the nuclear extracts from the WT and MTA1-KO mice retina. Lack of binding with mutant rhodopsin MT4 oligo (lanes 10–12). (D) Effect of naturally occurring Six3 mutations on Six3's binding ability to the rhodopsin oligo by EMSA. (E) Effect of naturally occurring Six3 mutations on rhodopsin-luc activity in 293T cells. (F) Cooperative coactivator activity of Six3 with NRL or CRX1 in stimulating rhodospin-luc activity in 293T cells. Increasing amounts of either Six3 or CRX1 or NRL (0.25 μg, 0.5 μg, 1 μg) were transfected in lanes 2–10; increasing amounts of CRX1 or NRL (0.25 μg, 0.5 μg, 1 μg) and 0.5 μg of Six3 were transfected in lanes 11–16; and 0.5 μg of Six3, NRL, and Crx1 were transfected in lane 17. (G) A working model summarizes that MTA1 is a negative regulator of Six3 transcription, whereas Six3 is a positive regulator of rhodopsin transcription.
To demonstrate the functionality of the Six3-binding DNA sequence in the rhodopsin, we showed that a corresponding mutation in the bRho130-luc reporter abolishes the ability of Six3 to stimulate transcription of the reporter (Fig. 5B). Having identified the Six3-interacting DNA sequence in the rhodopsin proximal promoter, we next assayed the DNA-binding activity of the nuclear extracts from retinas from the WT and MTA1-KO mice. We found an increased level of Six3/rhodopsin DNA complex by the nuclear extracts from MTA1-KO retinas (Fig. 5C, lanes 4–9; compare with lanes 2 and 3). The identity of the Six3 in the DNA complex was established by showing the lack of binding to the Six3 mutant MT4 probe (Fig. 5C, lanes 10–12). EMSA analysis using retinal nuclear extracts, immunodepleted of Six3 by Six3 Ab or rabbit IgG, also resulted in a significant loss of Six3 binding to rhodopsin DNA oligo (SI Fig. 18). These results are consistent with an increased expression of Six3, with ability to bind to rhodopsin promoter, in the retina from the MTA1-KO mice.
Because mutations in the homeodomain of the human SIX3 gene has been linked to holoprosencephaly, a complex and multiloci disorder characterized by eye malfunction in addition to characteristic brain malformations (14), and because of our data showing rhodopsin expression is under the control of Six3, these observations raised an exciting possibility of a defective Six3/rhodopsin interaction and resulting vision defects in holoprosencephaly patients. We next determined the influence of the four naturally occurring Six3 mutations on the protein's ability to bind to and stimulate the rhodopsin promoter. Surprisingly, we discovered that the deletion of 9-bp (Six3, nucleotides 694–702, AACCCCAGC), which results in the loss of asparagine, proline, and serine at the boarder and beginning of the helix 3 in the homeodomain results in a distinct suppression of Six3-binding to rhodopsin promoter (Fig. 5D, lane 6). However, there were no significant inhibitory effects of the three natural missense mutations (L226V, R257P, and V250A) on the ability of Six3 to bind to the rhodopsin promoter. Because all of the three tested missense Six3 mutants behaved in a similar manner in EMSA, we next examined the effect of most effective Six3.ΔNPS mutant for its ability to induce rhodopsin luciferase as compared with the wild Six3. In this assay, we used Six3.L226V as an additional negative control. Surprisingly, we found that NPS-deletion mutant of Six3 was unable to stimulate rhodopsin luciferase activity (Fig. 5E), and thus consistent with the observed reduced binding in the EMSA assay (Fig. 5E, lane 6). These results suggested that naturally occurring NPS-deletion mutation impair the ability of Six3 to stimulate rhodopsin transcription.
Because we now recognized unusual coactivator functions of Six3 upon rhodopsin transcription, we next investigated whether Six3 cooperates with previously characterized coactivators of rhodopsin promoter, such as NRL (24) and CRX1 (25). We found that Six3 is as effective as NRL or CRX1 in inducing transcription from the rhod-promoter reporter (Fig. 5F, compare lanes 2–4 with lanes 5–10). Interestingly, we found that coexpression of Six3 and NRL or CRX1 further enhances rhodopsin-luc activity compared with the levels achieved by individual coregulators (Fig. 5F, lanes 11–16), whereas combined expression of all three proteins was accompanied by a significant stimulation of rhodopsin transcription as compared with coexpression of two proteins (Fig. 5F, lane 17). Such a transcriptional cooperatively may occur through protein–protein interactions and may result from efficient DNA binding. Because both NRL and CRX1 are widely mutated in the human retinal degeneration, and because Six3, a newly recognized coactivator of rhodopsin, also cooperates with NRL or CRX1, these findings raise possibility of the potential mutations of Six3 and perhaps MTA1, in retinal degeneration diseases. Overall, these observations imply that naturally occurring deletion mutation in the homeodomain of the Six3 in holoprosencephaly patients and perhaps in other human retinal disorders. In summary, findings presented here reveal an unrecognized role for MTA1 as an upstream modifier of Six3, a direct stimulator of rhodopsin expression (Fig. 5G). Thus, our work has revealed a potential regulatory role of MTA1/Six3/rhodopsin pathway in affecting rhodopsin expression in vertebrate eye. Future studies are warranted to reveal the significance of these molecular interactions in the physiology of eye in relevant model systems with varied genetic background.
Methods
Cell Lines.
HEK293T, HeLa and CV1 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA), and RGC5 (15) was maintained in DMEM and F12 medium (1:1) supplemented with 10% FCS.
EMSAs.
Six3 binding to either synthetic ologo's contain ATTA sequence, or WT or mutant rhodopsin promoter oligo (26 nulceotide) using EMSA were done as described in ref. 18.
ChIP, Reporter, and Biochemical Assays.
Chromatin immunoprecipitation assays were done by using either Six3 Ab, MTA1 Ab, or HDAC2 Ab, as described in ref. 2. The primers used for ChIP are listed in SI Fig. 19. Six3-luc or bovine rhodopsin-luc assays were performed. Western blot analyses, immunoprecipitation, and GST-pulldown assays were performed as described in ref. 2.
Mice.
MTA1−/− mice were crossed in C57/BL6 and 129sv mixed background.
RNA Interference, RT-PCR, Quantitative RT-PCR, Northern Blot Analyses, Histology, Immunohistochemistry, Immunofluorescence, and Antibodies.
These assays were performed as described in SI Methods. Oligos used in this study are listed in SI Fig. 19.
Supplementary Material
Acknowledgments
We thank William H. Klein for productive discussions, Ales Cveki (Opthalmology and Visual Sciences, Albert Einstein College of Medicine, Bronx, NY) for providing Flag-Six3, Labelle Yves (Human and Molecular Genetics Research Unit, Pavillion Saint-François d'Assise, Centre Hospitalier Universitaire de Québec City, QC, Canada) for T7-Six3, Guillermo Oliver (Department of Genetics and Tumor Cell Biology, St. Jude Children's Hospital, Memphis, TN) for Six3-Luc, Donald Zack (Departments Opthalmology and Neuroscience, Johns Hopkins School of Medicine, Baltimore, MD) for bovine Rhodopsin-luc, Connie Cepko (Department of Genetics and Howard Hughes Medical Institute, Harvard Medical School, Boston, MA) for CRX1 plasmid, Ross Molday (Department of Biochemistry and Molecular Biology, Centre for Macular Research, University of British Columbia, Vancouver, BC, Canada) for the Rho4D2 mAb, and Cheryl M. Craft and Doheny Eye Research Mary D. Allen laboratory (Los Angeles, CA) for the opsin antibodies. This study was supported in part by The Norman Brinkler Award for Research Excellence and National Institutes of Health Grant CA098823 (to R.K.).
Abbreviations
- En
embryonic day n
- ERG
electroretinograms
- HDAC
histone deacetylase
- KO
knockout.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705878104/DC1.
References
- 1.Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, Vadlamudi RK, Kumar R. Nat Cell Biol. 2001;3:30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Wang RA, Mazumdar A, Talukder AH, Mandal M, Yang Z, Bagheri-Yarmand R, Sahin A, Hortobagyi G, et al. Nature. 2002;418:654–657. doi: 10.1038/nature00889. [DOI] [PubMed] [Google Scholar]
- 3.Toh Y, Kuninaka S, Endo K, Oshiro T, Ikeda Y, Nakashima H, Baba H, Kohnoe S, Okamura T, Nicolson GL, et al. J Exp Clin Cancer Res. 2000;19:105–111. [PubMed] [Google Scholar]
- 4.Chen Z, Han M. Development (Cambridge, UK) 2001;128:4911–4921. doi: 10.1242/dev.128.23.4911. [DOI] [PubMed] [Google Scholar]
- 5.Herman MA, Ch'ng Q, Hettenbach SM, Ratliff TM, Kenyon C, Herman RK. Development (Cambridge, UK) 1999;126:1055–1064. doi: 10.1242/dev.126.5.1055. [DOI] [PubMed] [Google Scholar]
- 6.Simpson A, Uitto J, Rodeck U, Mahoney MG. Gene. 2001;273:29–39. doi: 10.1016/s0378-1119(01)00563-7. [DOI] [PubMed] [Google Scholar]
- 7.Solari F, Bateman A, Ahringer J. Development (Cambridge, UK) 1999;126:2483–2494. doi: 10.1242/dev.126.11.2483. [DOI] [PubMed] [Google Scholar]
- 8.Manauathi B, Kumar R. J Biol Chem. 2007;282:529–533. [Google Scholar]
- 9.Kumar R, Wang RA, Bagheri-Yarmand R. Semin Oncol. 2003;30:30–37. doi: 10.1053/j.seminoncol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki H, Moriyama S, Nakashima Y, Kobayashi Y, Yukiue H, Kaji M, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y. Lung Cancer. 2002;35:149–154. doi: 10.1016/s0169-5002(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 11.Balasenthil S, Kumar R. In: DNA Methylation, Epigenetics and Metastasis. Esteller M, editor. Dordrech, The Netherlands: Springer; 2005. pp. 215–230. [Google Scholar]
- 12.Carl M, Loosli F, Wittbrodt J. Development (Cambridge, UK) 2002;129:4057–4063. doi: 10.1242/dev.129.17.4057. [DOI] [PubMed] [Google Scholar]
- 13.Del Bene F, Tessmar-Raible K, Wittbrodt J. Nature. 2004;427:745–749. doi: 10.1038/nature02292. [DOI] [PubMed] [Google Scholar]
- 14.Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J, Muenke M. Nat Genet. 1999;22:196–198. doi: 10.1038/9718. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamoorthy RR, Agarwal P, Prasanna G, Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ, Yorio T, et al. Brain Res Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Fernandez J, Mische S, Courey AJ. Development (Cambridge, UK) 1999;13:2218–2230. doi: 10.1101/gad.13.17.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh RR, Barnes CJ, Talukder AH, Fuqua SA, Kumar R. Cancer Res. 2005;65:10594–10601. doi: 10.1158/0008-5472.CAN-05-2268. [DOI] [PubMed] [Google Scholar]
- 18.Zhu CC, Dyer MA, Uchikawa M, Kondoh H, Lagutin OV, Oliver G. Development (Cambridge, UK) 2002;129:2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]
- 19.Frassetto LJ, Schlieve CR, Lieven CJ, Utter AA, Jones MV, Agarwal N, Levin LA. Invest Ophthalmol Vis Sci. 2006;47:427–438. doi: 10.1167/iovs.05-0340. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Nishikawa K, Suzuki T, Yamamoto M. Dev Biol. 2001;232:315–326. doi: 10.1006/dbio.2001.0185. [DOI] [PubMed] [Google Scholar]
- 21.Laflamme C, Filion C, Bridge JA, Ladanyi M, Goldring MB, Labelle Y. Cancer Res. 2003;63:449–454. [PubMed] [Google Scholar]
- 22.Otteson DC, Lai H, Liu Y, Zack DJ. BMC Mol Biol. 2005;6:15. doi: 10.1186/1471-2199-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan E, Wang Q, Quiambao AB, Xu X, Qtaishat NM, Peachey NS, Lem J, Fliesler SJ, Pepperberg DR, Naash MI, et al. Invest Ophthalmol Vis Sci. 2001;42:589–600. [PubMed] [Google Scholar]
- 24.Qtaishat NM, Redmond TM, Pepperberg DR. Invest Ophthalmol Vis Sci. 2003;44:1435–1446. doi: 10.1167/iovs.02-0679. [DOI] [PubMed] [Google Scholar]
- 25.Mitton KP, Swain PK, Chen S, Xu S, Zack DJ, Swaroop A. J Biol Chem. 2000;275:29794–29799. doi: 10.1074/jbc.M003658200. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.