Abstract
Endophthalmitis is an infection of the posterior segment of the eye that frequently results in loss of vision. This devastating result occurs despite prompt and often aggressive therapeutic and surgical intervention. Over the past decade, research has centered on determining the bacterial and host factors involved in this potentially blinding disease. The initial focus on the bacterial factors responsible for intraocular virulence has recently expanded into analysis the inflammatory response to infection, including the molecular and cellular interactions between the pathogen and host. This review discusses the epidemiology and therapeutic challenges posed by endophthalmitis, as well as recent findings from the analysis of interactions between the host and pathogen. Based on these findings, a model for the pathogenesis of endophthalmitis is presented. A more comprehensive understanding of the molecular and cellular interactions taking place between pathogen and host during endophthalmitis will expose possible therapeutic targets designed to arrest the infection and prevent vision loss.
Keywords: Endophthalmitis, bacteria, retina, vitreous, infection, therapy
1. Introduction
Bacterial endophthalmitis can occur following the introduction of an infectious agent into the posterior segment of the eye, causing intraocular infection and inflammation. Contamination of the posterior segment with bacteria can occur during a surgical procedure (post-operative), following trauma caused by a penetrating foreign object (post-traumatic), or during metastasis of bacteria into the eye from a distant infection site (endogenous). The clinical presentation of endophthalmitis can vary widely, from a mild and therapeutically responsive inflammation, to complete vision loss or loss of the eye itself, despite the use of aggressive therapy and surgery. The majority of endophthalmitis cases are a complication of intraocular surgery. The isolates involved are usually normal flora of the surface of the eye and surrounding mucosa, such as Staphylococcus epidermidis, which are generally not highly virulent. Therapy for endophthalmitis caused by avirulent pathogens is usually successful, often with retention of full visual acuity. Conversely, post-traumatic endophthalmitis is typically caused by environmental pathogens such as Bacillus cereus, resulting in severe and potentially blinding infections. Cases of endophthalmitis caused by highly virulent pathogens are often nonresponsive to treatment, resulting in significant vision loss.
1.1 Post-operative Endophthalmitis
The rates of post-operative endophthalmitis have been low for many years, but recent reports suggested that this type of ocular infection may be on the rise. Fluctuations in the number of cases appear to correlate with the type of intraocular surgery performed (Busbee, 2006). Post-operative endophthalmitis has been reported as a consequence of nearly every type of ocular surgery, but is most common following cataract surgery. A recent report identified an increase in the number of post-operative endophthalmitis cases, from 0.1% in the 1990s, to 0.2% for the period 2000 to 2003 (West, et al., 2005). Among the types of cataract surgeries performed, phacoemulsification accounted for approximately 48% of the post-operative endophthalmitis cases, while 38.5% and 6.6% of cases followed extracapsular or intracapsular extraction, respectively (Ng, et al., 2005).
Numerous reports have demonstrated that Gram-positive bacteria cause the vast majority of post-operative endophthalmitis cases. Coagulase-negative staphylococcal isolates are the most common, causing 47 to 70% of all post-operative endophthalmitis cases. Other species involved include Staphylococcus aureus, streptococci, enterococci, and Gram-positive rods such as Bacillus. Gram-negative bacteria were isolated from a relatively low number of post-operative endophthalmitis cases (6%). Most intraocular infections resulting from infection with coagulase-negative staphylococci can be treated with antibiotic and anti-inflammatory agents, resulting in restoration of partial or complete vision. However, the more virulent the bacterial strain, the more devastating the visual outcome. Intraocular infections with S. aureus, enterococci, Bacillus, or Gram-negative strains are often intractable, and blindness or loss of the eye itself is not uncommon (Josephburg, 2006; Ng, et al., 2005).
The therapeutic success of treatment of post-operative endophthalmitis depends largely on accurate and prompt diagnosis. Of the Gram-positive endophthalmitis cases cited in the Endophthalmitis Vitrectomy Study (EVS), 84% of cases resulted in at least 20/100 visual acuity, while 50% of these cases resulted in 20/40 visual acuity. Severe cases were caused by more virulent strains. Eyes infected with Gram-negative bacteria, streptococci, and S. aureus were more difficult to treat, and only 30% of these eyes attained 20/100 visual acuity. Of the entire EVS patient group, 11% had 5/200 vision and 5% had no light perception (Josephburg, 2006). Since the early 1980’s, the preferred route of antibiotic administration changed from the use of subconjunctival and intravenous antibiotics to the immediate injection of antibiotics directly into the vitreous (Baum, et al., 1982). However, the visual outcome of patients has not improved significantly as a result of this change (Josephburg, 2006; Ng, et al., 2005).
1.2. Post-traumatic Endophthalmitis
Although there are not as many cases of post-traumatic endophthalmitis as there are following intraocular surgery, infection rates following penetrating injuries are higher. The rates of endophthalmitis following ocular trauma ranged from 3% to 17%, with an increased likelihood for significant vision loss due to the potential virulence of environmental isolates involved (Jonas, et al., 2000; Meredith, 1999; O’Brien and Choi, 1995; Thompson, et al., 1993). Staphylococci are the most common causes of post-traumatic endophthalmitis, with B. cereus ranked as the second most common cause. B. cereus is ten times more likely to be isolated from cases of post-traumatic endophthalmitis than from post-operative endophthalmitis cases (Das, et al., 2005).
During traumatic injury, the penetrating intraocular foreign body (IOFB) may remain lodged in the eye. Ocular injuries with IOFBs accounted for 18 to 40% of all penetrating eye injuries. The involvement of IOFBs in penetrating injuries predisposes a risk of developing endophthalmitis (Brinton, et al., 1984; Thompson, et al., 1993, 1995; Williams, et al., 1988). In such cases, the vast majority of IOFBs were typically found in the posterior segment. Of all penetrating eye injuries, 2% to 16% developed sight-threatening endophthalmitis, the incidence and severity of which were dependent upon whether IOFB removal was immediate or delayed (Essex, et al., 2004; Jonas, et al., 2000; Lieb, et al., 2003; Thompson, et al., 1995).
Post-traumatic endophthalmitis is difficult to treat, not only because of the potential virulence of infecting organisms, but also because of the varying time between injury and treatment, the condition of the injury upon presentation, and the age of the patient. All factors considered, immediate therapeutic intervention is critical in order to target all potential organisms and arrest the potentially destructive inflammatory response.
1.3. Endogenous Endophthalmitis
Endogenous endophthalmitis occurs when the interior of the eye is seeded with bacteria from a distant site of infection. This form of endophthalmitis occurs most often in immunocompromised individuals, those with prolonged indwelling medical devices, and intravenous drug abusers. Endogenous endophthalmitis is relatively rare, accounting for only 2% to 8% of all endophthalmitis cases (Jackson, et al., 2003; Okada, et al., 1994; Romero, et al., 1999), and is associated with a poor visual prognosis. The visual outcome of endogenous endophthalmitis has not improved over the past 55 years, despite the use of improved antibiotics and aggressive surgical intervention. Patients with severe systemic infections may not present with ocular symptoms initially, and therefore the focus on the eye may be limited until a patient complaint of ocular pain or change in vision.
Over 50% of endogenous endophthalmitis cases are caused by fungal organisms, particularly Candida albicans (which causes 75–80% of fungal cases) (Romero, et al., 1999). The bacterial causes of endogenous endophthalmitis vary geographically. Gram-negative bacteria cause 32% to 37% of cases in North America and 70% of cases in East Asia. Among Gram-negative endogenous endophthalmitis cases, Klebsiella spp. is the most common etiologic agent. Bacillus spp. and coagulase-negative staphylococci are the most common causes of Gram-positive endogenous endophthalmitis (Jackson, et al., 2003). Despite aggressive therapeutic and surgical intervention, these patients typically retain only count fingers vision (Jackson, et al., 2003).
Immunocompromise is an important factor in the development of endogenous endophthalmitis. In a recent review, 56% of patients with endogenous bacterial endophthalmitis were also immunocompromised, and diabetes was the most common underlying disease involved (Jackson, et al., 2003). The increased risk of infection to diabetics has been well-documented; however, no correlation has been shown between diabetes and post-operative or post-traumatic endophthalmitis. Links between underlying ocular diseases associated with diabetes (i.e. diabetic retinopathy) have not been established. For endogenous endophthalmitis, Type II diabetes is the most common underlying condition, particulary in patients with secondary Klebsiella liver abscess (Jackson, et al., 2003). Intravenous drug use, which over time can also lead to immunocompromise, is the second most common underlying condition associated with endogenous endophthalmitis. Approximately one-third of endogenous endophthalmitis patients reported were under treatment of an immunosuppressive agent (Jackson, et al., 2003).
2. Therapeutic Challenges
Treatment of bacterial infections and inflammation in the interior of the eye poses a unique dilemma. Anatomic barriers and the delicate nature of the interior of the eye are factors to consider during treatment. Key anatomic barriers that prevent adequate treatment of endophthalmitis are the inner and outer blood-retinal barrier and the blood-aqueous humor barrier, collectively called the blood-ocular barrier (Cunha-Vaz 1997, 2004). The blood-ocular barrier, similar to the blood-brain barrier, consists of tight junctions between the endothelial cells and basement membrane of retinal capillaries and retinal pericytes. It not only protects the interior of the eye from assault by cells, macromolecules, and drugs, but also prevents the entrance and subsequent activity of most systemic antimicrobial and anti-inflammatory drugs. Intraocular barriers can be bypassed by direct injection of drugs into the vitreous. Photoreceptors and other cells of the retina are exquisitely sensitive to insult, and high doses of antimicrobial agents necessary to sterilize the eye may have toxic effects on the retina, potentially disrupting the biochemical pathways necessary for vision. In addition, damage can occur not only from toxic virulence factors produced by the organism in the eye, but also from bystander damage caused by the influx of inflammatory cells into the posterior segment. Overall, clinicians must take into account a myriad of unique challenges posed by the blood-ocular barrier, bystander damage of the immune response, and potential damage caused by drugs in order to protect the vision of patients with bacterial endophthalmitis.
The clinical presentation of endophthalmitis can vary widely. The outcome of infection depends on many factors, including the age and immune status of the patient, condition of the eye upon presentation, the infecting organism’s virulence and antibiotic susceptibility profile, and the time between injury/surgery and therapy. However, regardless of the source of infection, clinicians often do not know the identity of the infecting strain and must treat the eye empirically. Vancomycin, aminoglycosides, and cephalosporins are commonly used treat bacterial endophthalmitis. To be effective, these antibiotics may require direct injection into the vitreous, because the blood-ocular barrier may prevent adequate penetration into the vitreous at levels above the minimal inhibitory concentration for the infecting pathogen when these drugs are administered systemically. Experimental studies have demonstrated that without early treatment with intravitreal antibiotics, vision may be lost (Aguilar, et al., 1996; Forster, 1992).The EVS suggested that systemic antibiotic administration following vitrectomy was not an effective adjunct therapy for endophthalmitis because systemic use in combination with intravitreal antibiotics did not improve visual outcome or vitreal clarity (Endophthalmitis Vitrectomy Study, 1995).
The most commonly utilized therapeutic combinations for intravitreal injections have included vancomycin (1.0 mg) and amikacin (0.4 mg) or ceftazidime (2.2 mg). Vancomycin has been reported to have 100% effectiveness against the most common causative Gram-positive endophthalmitis organisms (Benz, et al., 2004; Recchia, et al., 2005). Amikacin and ceftazidime have approximately the same success rate against Gram-negative organisms (89%) (Han, et al., 1996). However, toxicity to retinal cells has been reported following amikacin use (Campochiaro and Conway, 1991). Ceftazidime has been recommended as the antibiotic of choice for endophthalmitis caused by Gram-negative species (Compichiaro and Lim, 1994; Jackson, et al., 2003).
Fluoroquinolone antibiotics, while used frequently as topical agents for corneal infections, are effective against most intraocular pathogens. Fourth generation fluoroquinolones, such as gatifloxacin and moxifloxacin, have been shown to penetrate the blood-ocular barrier, suggesting their potential effectiveness as intraocular drugs (Busbee 2004). Gatifloxacin administered orally prior to pars plana vitrectomy resulted in aqueous and vitreous concentrations that were above the MIC90 for the most common endophthalmitis pathogens (Hariprasad, et al., 2003). However, topical administration of moxifloxacin and gatifloxacin resulted in vitreous concentrations of each antibiotic that were below the MIC90 for common endophthalmitis pathogens (Costello, et al., 2006). Compared with other quinolones, moxifloxacin has been reported to penetrate more effectively into intraocular tissues following topical administration (Robertson, et al. 2005; Yagci, et al., 2006). Concerns remain regarding possible toxic effects of fluoroquinolones and further evaluation of intraocular toxicity is necessary. Unfortunately, fluoroquinolone resistance among ocular pathogens appears to be on the rise, which may impact future therapeutic regimens for this disease (Miller, et al., 2006).
Inflammation during infection is necessary for the clearance of organisms, but can result in bystander damage to the interior of the eye. Intraocular inflammation can occur following intravitreal injection of bacteria or their components, such as toxins or cell wall constitutents that are shed from the organisms during infection, either during bacterial replication (Callegan, et al., 1999a, 2002b; Fox, et al., 1984; Kufoy, et al., 1990), or following treatment with cell wall-active antbiotics (Callegan, et al., 2002b). To minimize ocular inflammation during endophthalmitis, clinicians often use intravitreal injection of dexamethasone in conjunction with antibiotics. The use of steroids in the treatment of endophthalmitis has been controversial and there remains no clear evidence of their benefit for this disease. Experimental models and clinical studies have reported that concomitant administration of dexamethasone with antibiotics were detrimental (Meredith, et al., 1996), beneficial (Gan, et al., 2005; Liu, et al., 2000; Smith, et al., 1997; Yildirim, et al., 2002) or had no effect (Aguilar, et al., 1996; Ermis, et al., 2005; Pollack, et al., 2004; Shah, et al., 2000). Despite the controversy, dexamethasone is frequently used as an adjunct to antibiotics for the treatment of endophthalmitis.
Vitrectomy is often used to debride and remove the nidus of infection during severe cases of endophthalmitis. The vitreous is removed with a miniature hand-held cutting device and replaced with transparent fluid to maintain intraocular pressure and pH necessary for visual function. The EVS reported that in suspected endophthalmitis cases following intraocular surgery, vitrectomy was an effective adjunct to antibiotic therapy, but only for patient who had lost vision to only light perception (Endophthalmitis Vitrectomy Study, 1995). In cases of post-traumatic endophthalmitis where the eye contains retained IOFBs, immediate vitrectomy and intravitreal antibiotic injection has been recommended (Abu El-Asrar, et al., 1999). For endogenous endophthalmitis, adjunct vitrectomy conferred significant improvements in vision, but any delay in vitrectomy resulted in a comparative loss of visual acuity (Yoon, et al., 2003). Most reports agree that vitrectomy should be performed without delay in severe cases of endophthalmitis, especially those involving IOFBs.
3. The Contribution of Bacterial Virulence Factors to Pathogenicity
3.1. Bacillus
Bacillus spp. are a major cause of rapidly blinding cases of post-traumatic and endogenous endophthalmitis. The majority of patients with Bacillus endophthalmitis lost significant visual function or the eye itself in less than 2 to 3 days (Das, et al., 2001, 2005; David, et al., 1994; Ho, et al., 1982; O’Day, et al., 1981). B. cereus causes the vast majority of Bacillus endophthalmitis cases. However, endophthalmitis can also be caused by Bacillus thuringiensis, a bacterium commonly used for organic gardening and farming that is genetically and phenotypically similar to B. cereus (Callegan, et al., 2006a). For B. cereus and B. thuringiensis, the quorum sensing transcriptional regulator plcR controls the expression of many extracellular virulence factors (Agaisse, et al., 1999). In an experimental rabbit model of endophthalmitis, B. cereus and B. thuringiensis were significantly less virulent when plcR was nonfunctional (Callegan, et al., 2003). Wild type Bacillus caused severe intraocular inflammation by 12 hours postinfection. Severe intraocular inflammation did not occur until 30 hours postinfection in eyes infected with plcR-deficient Bacillus. Wild type Bacillus also caused nearly complete loss of retinal function by 18 hours, while plcR-deficient mutants required 36 hours to reach >90% reduction in retinal function. Reduced virulence was likely due to reduced toxin expression by plcR-deficient strains. The ability of B. cereus toxins to induce the type of damage seen in endophthalmitis has been demonstrated in a model of sterile endophthalmitis, in which bacterial supernatants from wild type and plcR-deficient B. cereus were examined in mice (Figure 1). Supernatant from wild type B. cereus caused rapid retinal function loss and more severe inflammation than did supernatant from plcR-deficient B. cereus (R. T. Ramadan, B. D. Novosad, and M. C. Callegan, Abstr. Molec. Pathogen. Infectious Inflammatory Eye Res. Conf., 2005). In terms of individual toxins, those tested to date (hemolysin BL, phosphatidylinositol-specific phospholipase C, and phosphatidylcholine-specific phospholipase C) contributed little to the overall pathogenesis of experimental B. cereus endophthalmitis (Callegan, et al., 1999b, 2002a). Taken together, these data highlight the importance of quorum sensing to the pathogenicity of Bacillus endophthalmitis and its role as a potential therapeutic target.
Figure 1. Retinal function loss following intravitreal injection of wild type and quorum sensing-defective Bacillus cereus and supernatants.
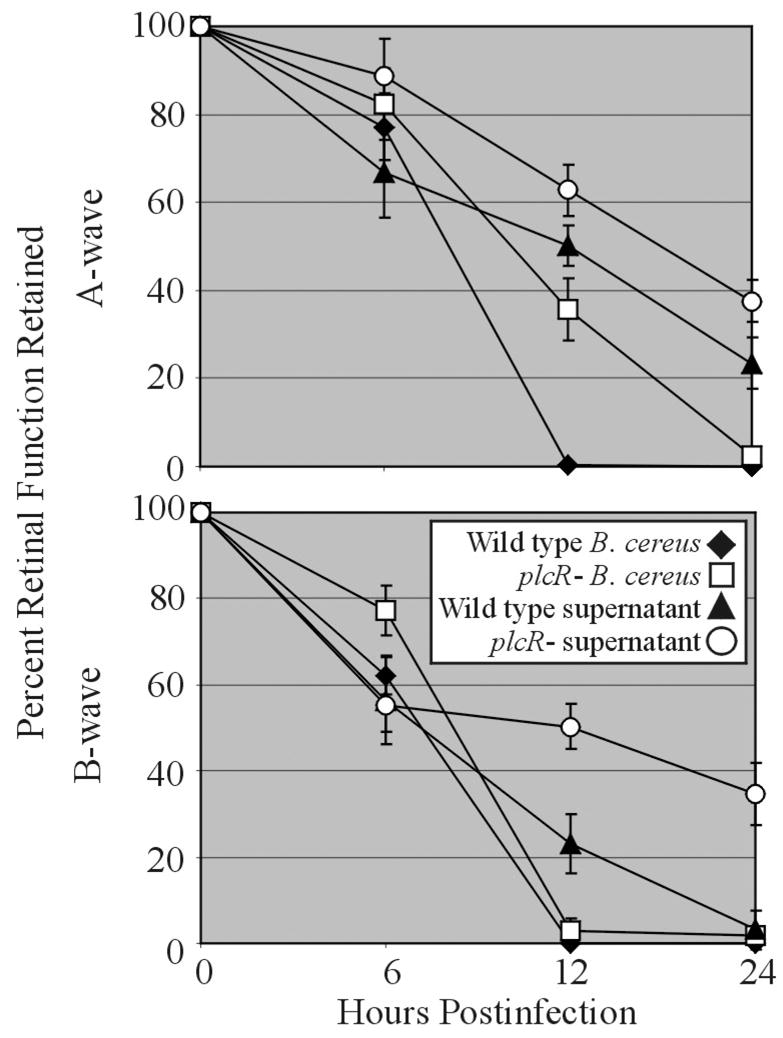
Mouse eyes were injected into the mid-vitreous with approximately 100 CFU/100 μl of wild type (◆) or plcR- (□) B. cereus or 100 μl of a 10-hour sterile filtered culture supernatant of wild type (▲) or plcR- (○) B. cereus. Data at each time point are presented as the percentages of A-wave or B-wave amplitude of retinal function retained compared to baseline and sham-injected controls. Data was averaged for each group and compared using the Student t test. Values and error bars represent means ± standard errors of the means for four or more eyes per group.
3.2. Enterococci
Enterococci are a rarer cause of post-operative endophthalmitis, and it is often associated with filtering bleb surgery. However, expressing fewer virulence traits than B. cereus, it is more amenable to study. The main toxin expressed by strains of E. faecalis, the cytolysin, has been shown to contribute to the severity of infection. Cytolysin is a secreted toxin that can lyse bacteria, erythrocytes, and other mammalian cells. The presence of cytolysin rendered experimental E. faecalis endophthalmitis refractory to antibiotic and anti-inflammatory drug treatment (Jett, et al., 1992, 1998). In terms of toxin regulation, the E. faecalis quorum sensing system fsr has been evaluated for its role in endophthalmitis pathogenicity (Mylonakis, et al., 2002). The fsr quorum sensing system regulates the production of gelatinase (GelE) and a serine protease (SprE) in a density dependent manner (Qin, et al., 2000). When deletions were made in gelE, sprE, or both genes, experimental endophthalmitis was attenuated, with the gelE deletion having the most significant effect. However, these mutations were not able to achieve the same degree of attenuation as an fsr-deficient mutant (Engelbert, et al., 2004; Mylonakis, et al., 2002). These results suggest the importance of the fsr quorum sensing system as a potential therapeutic target. In addition to the toxicity of secreted virulence factors, clinical isolates of enterococci possess an antibiotic resistance profile that renders them resistant to most commonly-used antibiotics. Enterococci have also been shown to produce biofilms on intraocular lens material (Kobayawa, et al., 2005), further highlighting their potential virulence for the eye.
3.3. Streptococcus pneumoniae
S. pneumoniae has been isolated from post-traumatic and post-operative cases of endophthalmitis. Two potential virulence factors (pneumolysin and autolysin) have been analyzed for their contribution to endophthalmitis pathogenicity. Pneumolysin is a cytolytic toxin and can activate the classical complement pathway (Paton, et al., 1997), while autolysin regulates the stability of the pneumococcal cell wall (Ng, et al., 2002). In an experimental rat model of endophthalmitis, infection with a pneumolysin-deficient strain caused less intraocular inflammation than infection with the wild type strain at 24 hours postinfection. By 48 hours postinfection, however, inflammation was similar in eyes infected with the pneumolysin-deficient and wild type parental strains. In contrast, infection with the autolysin-deficient mutant resulted in less intraocular inflammation than infection with the pneumolysin-deficient mutant and wild type pneumococcus at both time points. These results suggest that autolysin may be an important pneumococcal virulence factor in endophthalmitis, perhaps by functioning in the liberation of inflammogenic pneumococcal cell wall fragments during infection (Ng, et al., 2002).
3.4. Staphylococcus
Staphylococci are the predominant organisms recovered from cases of post-traumatic and post-operative endophthalmitis. In addition to the many virulence factors produced by both S. aureus and S. epidermidis, the increased incidence of resistance to several commonly-used antibiotics in clinical isolates threatens to further increase the rate of treatment failures for staphylococcal endophthalmitis. Like enterococci, staphylococci can also readily form biofilms on several types of abiotic surfaces, including intraocular lenses (IOLs), highlighting their potential for contamination and infection of the interior of the eye.
Experimental rat and rabbit models of endophthalmitis have been used to demonstrate the importance of S. aureus cytolytic toxins and global regulation of those toxins. In terms of the significance of individual toxins to pathogenicity, S. aureus alpha-toxin and beta-toxin appear to be important to intraocular virulence. Eyes infected with strains deficient in alpha-toxin, beta-toxin, or both toxins were less inflamed and retained greater retinal function than eyes infected with wild type S. aureus (Callegan, et al., 2002b). The global regulators agr and sar, which control the density-dependent production of S. aureus adhesins and toxins, are critical for the intraocular virulence of S. aureus. Infections in eyes inoculated with strains deficient in agr, sar, or both global regulators were highly attenuated (Booth, et al., 1995, 1997; Giese, et al., 1999), more so than that observed during infection with staphylococcal strains deficient individual toxins.
The majority of experimental studies regarding the contribution of S. epidermidis virulence factors to intraocular infections have analyzed the contribution of adhesins to biofilm formation on lens material. S. epidermidis bound to IOLs may be a source of infecting organisms that seed the posterior segment and result in endophthalmitis or chronic inflammation. The ability of S. epidermidis to form biofilms on IOL materials contributes not only to colonization, but also to circumvention of an immune response designed to eliminate bacteria, and to limitation in the effectiveness of antibiotics (Baillif, et al., 2006). Although S. epidermidis produces few toxins, this organism can readily evade the immune system by biofilm formation, a process mediated by surface proteins such as autolysin, polysaccharide intercellular adhesin, fibrinogen-binding proteins, and accumulation-associated protein (Foster, 2005). The role of these specific adhesins in the formation of intraocular biofilms or the pathogenicity of S. epidermidis endophthalmitis has yet to be determined.
3.5. Gram-negative Bacteria
As stated above, Gram-negative endophthalmitis following surgery or trauma is relatively rare. Although less common than Gram-positive infections, Gram-negative endophthalmitis is of particular concern because these infections are significantly more difficult to treat and visual outcome is typically poor. In western countries, Gram-negative endogenous infections are less common, accounting for approximately 32–37% of endogenous endophthalmitis cases. However, in eastern countries, particularly Taiwan, Gram-negative bacteria were responsible for 70% of endogenous bacterial endophthalmitis cases (Jackson, et al., 2003).
Klebsiella spp. has surpassed E. coli as the most common Gram-negative cause of endogenous bacterial endophthalmitis, accounting for 80–90% of all cases in East Asian countries (Wong, et al., 2003). Over half of K. pneumoniae endogenous endophthalmitis patients were diabetic, and two-thirds had an underlying liver abscess caused by Klebsiella. For K. pneumoniae, over 77 serological types have been classified. The two most common serotypes cultured from cases of endogenous K. pneumoniae endophthalmitis were the K1 and K2 serotypes (Podschun and Ullmann, 1998). K1 serotypes contain two genes that are associated with a hypermucoviscosity phenotype, magA and rmpA (Yu, et al., 2006). This phenotype is strongly associated with immune system avoidance, bacteremia, and liver abscess formation. It is not known whether hypermucoviscosity or other virulence factors contribute to the persistence of K. pneumoniae in the bloodstream and/or its ability to infect the eye, nor whether these factors contribute to inflammation and retinal function loss once K. pneumoniae has reached the interior of the eye. Preliminary studies have shown that K. pneumoniae can replicate quickly in the vitreous, but cause inflammation and retinal function loss at a rate considerably slower compared to than that observed in experimental B. cereus endophthalmitis models (Figure 2, B. J. Wiskur, B. D. Novosad, R. Ramadan, M. C. Callegan. Abstr. Assoc. Res. Vision Ophthalmol Meet. Abstr. 5071, 2005).
Figure 2. Bacterial growth, retinal function loss, and histological analysis of intravitreal injection of Klebsiella pneumoniae.
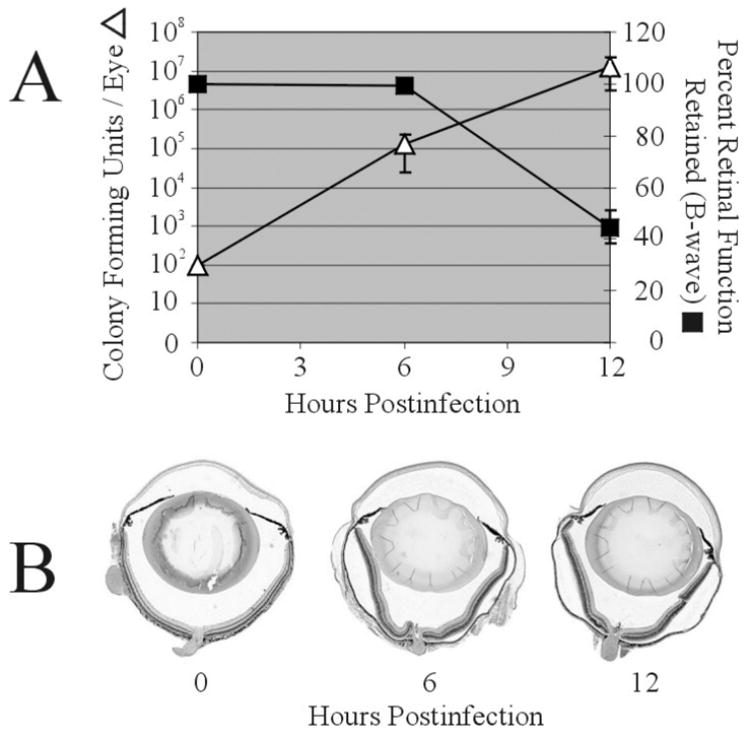
Mouse eyes were injected into the mid-vitreous with approximately 100 CFU/0.5 μl of an ocular isolate of K. pneumoniae. A) Bacterial growth (△) is presented as CFU / whole mouse eye. Retinal function (■) is presented as the percentage of B-wave amplitude of retinal function retained compared to baseline and sham-injected controls. Bacterial counts and retinal function data were averaged for each group and compared using the Student t test. Values and error bars represent means ± standard errors of the means for four or more eyes per group. B) Eyes were harvested at 0, 6, and 12 hours postinfection and following routine histological preparation, were stained with hematoxylin and eosin. By 12 hours postinfection, eyes exhibited only mild posterior and anterior segment inflammation, and retinal layers remained esssentially intact, despite the number of bacteria in the eye and degree of retinal function loss occurring at that time. Magnification, ×5.
3.6. Bacterial Migration in the Eye
The majority of studies analyzing the contribution of bacterial migration to the pathogenesis of endophthalmitis have involved Bacillus. In a comparison of the intraocular behavior of B. cereus to S. aureus and E. faecalis, B. cereus was the only organism isolated from all parts of the eye during the later stages of infection (Callegan, et al., 1999a). B. cereus can migrate througout the eye within a short period of time, replicating and inciting inflammation in all parts of the eye within 12 hours postinfection. Motility contributes significantly to the pathogenesis of B. cereus endophthalmitis. Non-motile B. cereus mutants (rendered incapable of intraocular migration by transposon or allele-replacement mutagenesis) did not cause rapid retinal function loss or explosive inflammation compared with their wild type parental strains (Callegan, et al., 2002c, 2005). Additional attenuation of endophthalmitis was achieved by mutating the quorum sensing regulator plcR in a non-motile strain (Callegan, et al., 2005). These attenuated infections eventually resulted in complete loss of retinal function and severe inflammation, indicating that other virulence factors not associated with motility and not under the control of quorum sensing may also contribute to intraocular virulence. Swarming migration has also been demonstrated to contribute to the ability of B. cereus to migrate inside the eye. Swarming migration is the movement of bacteria on a surface or through a viscous medium (i.e., the vitreous) in response to a chemotactic signal. A defect in swarming prevented migration of B. cereus throughout the eye and resulted in attenuation of anterior chamber inflammation, but did not prevent rapid retinal function loss or severe vitritis (Callegan, et al., 2006b). Taken together, these results suggest that motility is an important virulence factor and its blockade may be an important therapeutic target.
4. The Host Response to Endophthalmitis
4.1. Immune Privilege
The eye is an immune privileged site where multiple mechanisms work together to protect the visual axis from destructive inflammation. Immune privilege was first described by Medawar in the 1940’s as an anatomical site where foreign tissue grafts survived for prolonged periods of time (Medawar 1948). Using this criterion, Streilein et al. 2003 identified several immune privileged sites within the eye: the anterior chamber, the vitreous cavity, and the subretinal space. Both tissue allografts and immunogenic tumor cells experience immune privilege within these sites. Direct experiments demonstrating that bacteria experience immune privilege within the eye have not been performed, but several studies imply that this phenomenon occurs. Hoebe et al. 2005 demonstrated that subcutaneous injection of 5 x 105 CFU of S. aureus was rapidly cleared in C57BL/6 mice within 7 days. By contrast, Engelbert and Gilmore (2005) demonstrated that C57BL/6 mice were unable to clear an intravitreal injection of as few as 5000 CFU of S. aureus, and the bacterial load within the eye increased to 2 x 109 CFU within 72 hours, resulting in its rapid destruction. Taken together, these studies suggested that immune privilege in the eye limits bacterial clearance as well as permitting graft survival and tumor cell proliferation.
A wide array of cell surface (Fas ligand and complement regulatory proteins) and soluble factors (transforming growth factor-β, alpha-melanocyte stimulating hormone, vasoactive intestinal peptide, calcitonin gene-related peptide, soluble Fas ligand, etc.) are involved in creating the immunosuppressive environment within the eye (Streilein, 2003). One effect of ocular immune privilege is the limitation of inflammation within the eye. For example, intraocular tumors that experience immune privilege fail to induce inflammation, while rejection of the same tumors from non-privileged sites is accompanied by vigorous inflammation (Chen, et al., 1998). The extent to which inflammation is limited in response to bacteria in the posterior segment of the eye is less clear. Bacteria in the vitreous appear to benefit from immune privilege and evidenced by the survival and proliferation of even small inocula. However, most bacteria appear nevertheless to trigger vigorous inflammation. The relationship of immune privilege to innate immunity, specifically as it relates to modulating the host-pathogen dynamic, has only begun to be studied and many questions remain to be answered: (i) How is inflammation in response to bacterial stimuli different between immune privileged and non-privileged sites; (ii) Does intraocular inflammation affect bacterial growth; (iii) How long is immune privilege maintained in the presence of an inflammatory response; and (iv) Is the destruction of ocular tissue mediated primarily by bacteria or inflammation? Answers to these questions may lead to improved treatments for endophthalmitis, enabling a rapid clearance of the invading pathogen while protecting fragile ocular tissues.
4.2. Chronic versus Acute Inflammation
The inflammatory response triggered by intraocular pathogens can be acute or chronic. Acute inflammation is most commonly associated with more virulent bacterial infections (B. cereus, E. faecalis, streptococci, gram negative organisms or S. aureus) and an invariably poor visual outcome (Callegan, et al., 2002c). As early as 48 hours postinfection both the anterior and posterior segments are involved, resulting in corneal edema, neutrophil infiltrate within the cornea and aqueous humor, vitritis, and retinal periphelbitis (Mandelbaum and Forster, 1996). By contrast, chronic inflammation is associated with less virulent bacterial infections ( Proprionibacterium acnes and S. epidermidis) and a better visual outcome (Mandelbaum and Forster, 1996). While the onset of chronic inflammation is typically delayed and clinically milder, a failure to treat this infection during its early stage can result in loss of vision. In endophthalmitis with acute or chronic inflammation, early diagnosis and eradication of bacteria are key for successful treatment and preservation of vision.
4.3. Sensors of Innate Immunity
Innate immunity is the first line of defense that coordinates an immediate and rapid immune response to microbial challenge. The first step in the host defense against infection is distinguishing self from non-self and “sensing” an invading microbial pathogen. The innate immune system uses pattern recognition receptors to initiate innate immunity against microbial components, known as pathogen associated molecular patterns (e.g., LPS, peptidoglycan, flagellin, etc.) (Tosi, 2005). These pathogen associated molecular patterns trigger innate immunity through the activation of the complement pathway, and through the Toll-like receptor subfamily of pattern recognition receptors. Toll-like receptors (TLRs) are the human homologues of the toll receptor first discovered in Drosophila melanogaster as a key component of innate immunity (Tosi, 2005). TLRs recognize specific bacterial motifs and are expressed on cells of the innate system such as neutrophils, monocytes, macrophages, dendritic cells, and mast cells. To date, 10 human TLRs have been identified and several are specific for bacteria (Tosi, 2005). Peptidoglycan and lipoteichoic acid from gram-positive bacteria are ligands for TLR-2, LPS from gram-negative bacteria is a ligand for TLR-4, and CpG motifs in bacterial DNA are ligands for TLR-9. Recently, it was demonstrated that TLRs, including TLR-2, TLR-4, and TLR-9, are expressed in the retina and/or the retinal pigment epithelial (RPE) cells, and may serve as the first line of defense against invading bacterial pathogens in the posterior segment of the eye (Kumar, et al., 2004). Triggering of the TLRs expressed on RPE induces the production of multiple proinflammatory cytokines, chemokines, and adhesion molecules including IFN-β, IL-6, IL-8, MCP-1, and sICAM-1 (Kumar, et al., 2004).
4.4. Complement
Components of the bacterial cell wall, such as LPS, can directly activate the complement system via the alternative pathway (Tosi, 2005). The alternative pathway is one of three routes to the activiation of complement in response to the presence of pathogens. Activation of the complement system provides a very effective host defense against invading organisms by generating anaphylatoxins that: (i) trigger inflammation, (ii) attract phagocytes to the site of infection via chemotactic factors, (iii) promote the opsinization and lysis of invading bacteria, and (iv) cause vasodilation and increased vascular permeability (Tosi, 2005). While the complement system is effective at eradicating invading pathogens, activation of complement often results in significant bystander tissue damage. Therefore, while active complement components are present within the eye, specifically C3 convertase and membrane attack complex, the activation of complement is kept in check by complement regulatory proteins (Sohn, et al., 2000). The complement regulatory proteins CD55, CD46, CD59, and Crry are all expressed in the normal eye and have been shown to tightly regulate the activation of the complement system (Sohn, et al., 2000).
Early studies using guinea pigs decomplemented with cobra venom factor (CVF) demonstrated that, in complement depleted animals, guinea pigs displayed impaired host defense to S. aureus endophthalmitis (Giese, et al., 1994). Similar effects were demonstrated with CVF-treated mice (Engelbert and Gilmore, 2005). However, studies using C3 −/− mice that were deficient in the central component of the complement system, the absence of C3 was found to be inconsequential to the outcome of endophthalmitis (Engelbert and Gilmore, 2005). Reasons for these contradictory results most likely lie in that CVF depletes complement by inducing massive, uncontrolled complement activation. Animals treated with CVF experience severe inflammatory side effects, including death of a significant fraction of the population prior to microbe challenge. Hence, CVF treated animals may exhibit a number of pleiotropic effects of CVF in addition to complement depletion. In contrast, the C3 −/− mouse model allows a more direct study of the specific roles of complement without the severe side effects as seen following CVF treatment. It is a formal possibility that C3−/− mice bred for several generations adapt and compensate to the loss of complement function over time, and hence may be upregulated for other clearance mechanisms. Taken together, these studies indicate that while complement may contribute to the early inflammatory response in the eye, it does not appear to play a significant role in the clinical outcome of endophthalmitis.
4.5. Fas Ligand
Fas ligand is constitutively expressed within the normal eye and plays a critical role in maintaining immune privilege by inducing apoptosis in infiltrating T cells and inhibiting adaptive immunity (Griffith, et al., 1995). However, recent studies reveal that the membrane form of FasL can also promote innate mediated inflammation through the activation of neutrophils (Gregory, et al., 2002). Moreover, Engelbert and Gilmore (2005) demonstrated that Fas ligand is critical in the clearance of bacterial endophthalmitis, and may play a direct role in recruiting and activating neutrophils. While normal mice readily cleared an infection with 500 CFU of S. aureus, mice deficient in Fas ligand were unable to clear the same size inoculum. In the absence of Fas ligand, bacteria grew more rapidly and fewer neutrophils were recruited to the site of infection. These findings are best interpreted using recent studies by Gregory et al. 2002 indicating that Fas ligand plays a critical role in the activation of the early innate immune response within the eye. Membrane bound Fas ligand appears to activate innate immunity, whereas soluble Fas ligand is immunosuppressive. The balance between the various forms of Fas ligand may critical in regulating host inflammation triggered by invading bacterial pathogens. The membrane form of Fas ligand may act to amplify the early innate response, by triggering the infiltrating cells to secrete more proinflammatory cytokines such as IL-1β and MIP-2α, resulting in a robust innate response. Once bacteria are cleared, inflammation may be suppressed by the liberation of Fas ligand from the cell surface.
4.6. Cellular Infiltration
Neutrophils are an essential component of innate immunity, and are the primary infiltrating cell type in the early phase of endophthalmitis (Callegan, et al., 2002b; Giese, et al., 2003; Ramadan, et al., 2006). However, the recruitment and activation of neutrophils within the eye represent an important biological dilemma. The generation of toxic reactive oxygen intermediates and other inflammatory mediators by neutrophils may be essential for clearance of bacteria, but may contribute to irreversible bystander tissue damage in the eye. Using an S. aureus model of endophthalmitis, Giese et al. 2003 demonstrated that depletion of neutrophils early in the inflammatory response reduced the severity of host inflammation, yet severely hampered the clearance of bacteria.
Robust inflammation is a hallmark of intraocular infection caused by B. cereus and other types of virulent bacteria. In experimental B. cereus endophthalmitis, inflammatory cells were observed in the posterior chamber in close proximity to the optic nerve head as early as 4 hours postinfection (Callegan, et al., 2005; Ramadan, et al., 2006). Further analysis by myeloperioxidase assays and flow cytometry confirmed that the primary infiltrating cell was the PMN (Ramadan, et al., 2006). The numbers of CD18+/Gr-1+ PMN were minimal at 4 and 6 hours postinfection, but increased significantly thereafter. Histologically, PMN influx into the vitreous near the iris and ciliary body and into the anterior segment increased significantly after 6 hours postinfection. The pathways involved in inducing this inflammation, despite immune privilege, remain to be fully elucidated.
4.7. Cytokines, Chemokines, and Adhesion Molecules
The primary function of the innate immune system is to detect invading pathogens and clear them as quickly as possible. Cytokines and chemokines are signaling molecules that play a central role in detecting the invading pathogen, recruiting inflammatory cells, and clearing the infection. Using a rat model of endophthalmitis, Giese and colleagues (1998) demonstrated that within 6 hours of intravitreal inoculation with S. aureus, TNF-α, IL-1β, and CINC (rat homologue of IL-8) are detected within the vitreous. These were found to contribute to the break down of the blood retinal barrier, and to the recruitment of leukocytes, in particular neutrophils. The adhesion molecules ICAM-1 and E-selectin are also upregulated early in iris, ciliary body, and retinal vessels, serving to enhance the infiltration of leukocytes to the site of infection (Giese, et al., 2000). In contrast, IFN-γ peaks at 24 hours post infection, correlating with an increased infiltration of macrophages and lymphocytes (Giese, et al., 1998). During experimental B. cereus endophthalmitis, the influx of C18+/Gr-1+ PMN into the posterior segment occurred simultaneously with the increase of TNFα in the eye at approximately 4 to 6 hours postinfection (Ramadan, et al., 2006). While the time course of cytokine production has been determined in these studies, the source of these cytokines is currently unknown. Overall, these studies do not address whether these molecules serve in a cause or effect role in the clinical outcome of endophthalmitis.
5. Changes to the Retina in Response to Infection
5.1. Blood Retinal Barrier Permeability
As discussed previously, the transparency of the visual axis is critical for vision, and this seems to be the driver in the evolution of the active immune suppression mechanisms that result in immune privilege in the eye. In addition to the active release of immunosuppressive molecules into the eye, other factors contribute to immune privilege. Among these are the absence of blood vessels and lymphatic drainage pathways from the internal compartments of the eye (except the uveoscleral pathway), and a paucity of local antigen presenting cells. Although the intersection of the conjunctiva and cornea is one of the most immunologically active sites in the eye, the anterior and posterior chambers, retina, and subretinal space are sequestered from the systemic circulation by the blood ocular barrier (Mangone and Whitcup, 1999; Streilein, 1999). The blood aqueous humor barrier is an epithelial barrier formed by the nonpigmented layer of ciliary epithelium and the posterior iridial epithelium, and the endothelium of iridial vessels. The inner blood retinal barrier is formed by intercellular tight junctions between the endothelium of the retinal vessels, preventing seepage of plasma constituents into the retina. The outer blood retinal barrier is formed by tight junctions between the retinal pigment epithelium (RPE), which manages the blood supply from the choroid to the photoreceptors. The blood ocular barrier limits the incursion of macromolecules into the aqueous, vitreous, and the subretinal spaces. These selective barriers allow nutrients into the neural retina, while simultaneously protecting the neural retina from harmful macromolecules and cells (Mangone and Whitcup, 1999; Streilein, 1999).
Multiple causes of blood retinal barrier breakdown have been identified, including infection (Cellini and Baldi, 1991; Pepose, et al., 1985), diabetes (Lobo, et al., 2000; Schalnus and Orloff, 1995; Yoshida, et al., 1993), and intraocular surgery (Men, et al., 2003). Associations have been reported between the breakdown of the blood retinal barrier and almost every retinal disease, particularly vascular retinopathies and pigment epitheliopathies (Forrester and Menamin, 1999). Inflammation-mediated damage to the neurosensory retina and RPE may affect the basic photochemical process of vision. Inflammation has been shown to play a key role in damaging both the inner and outer components of the blood retinal barrier (Cunha-Vaz, 1997, 2004). Ocular inflammation stimulates upregulation of cell adhesion molecules, permitting inflammatory cell and macromolecule extravasation into the vitreous and subretinal spaces (Huber, et al., 2006; Xu, et al., 2003). Inflammatory mediator production in this area likely exacerbates and maintains the inflammatory response, which can result in loss of vision (Koizumi, et al., 2003). During experimental autoimmune uveitis, leukocytes have been shown to have an active role in tight junction disruption and blood retinal barrier breakdown during retinal inflammation (Xu, et al., 2005).
To date, few studies have examined the role of blood retinal barrier breakdown in the pathogenesis of bacterial endophthalmitis. Endophthalmitis induced by E.coli LPS resulted in a dose-dependent breakdown of the inner components of the blood retinal barrier, as demonstrated by MRI (Metrickin, et al., 1995). There was not an active infection in this study, and other elements of inflammation were not analyzed. Significant localization of serum albumin in both the inner and outer blood retinal barriers has been reported in human cases of endophthalmitis (Vinores, et al., 1994). As stated earlier, neutrophils enter the posterior segment at about the time that inflammatory cytokines are detected during experimental bacterial endophthalmitis (Giese, et al., 1998; Ramadan, et al., 2006).
The primary barrier cells of the blood retinal barrier are the RPE, which are polarized cells with basal sides intricately folded and attached to the thin basal lamina, forming the inner layer of Bruch’s membrane (Mangone and Whitcup, 1999; Streilein, 1999). RPE cells, in addition to their barrier function, also contribute to retinal physiological homeostasis. In terms of the potential role of RPE dysfunction in blood retinal barrier permeability during endophthalmitis, efforts are focused on analyzing the interactions between RPE and bacteria and bacterial toxins. RPE are sensitive to B. cereus growth and toxin production. Exposure of RPE to wildtype or quorum sensing deficient Bacillus in vitro resulted in lactate dehydrogenase release, regardless of quorum regulated factors (Figure 3, A. L. Moyer, C. Roach, M. C. Callegan. Assoc. Res. Vision Ophthalmol. Meet. Abstr. 4016, 2004). In vitro, B. cereus infection of RPE monolayers resulted in toxin-dependent degradation of ZO-1 (Figure 4, A. L. Moyer, B. D. Novosad, M. C. Callegan. Abstr. Assoc. Res. Vision Ophthalmol. Meet. Abstr. 5078, 2005). Since RPE function is dependent on the integrity of intercellular tight junction proteins formed between cells, the dysfunction or degradation of proteins contributing to tight junction formation as a result of infection may lead to a breach of the blood retinal barrier.
Figure 3. Changes in membrane and tight junction integrity of retinal pigment epithelial cell (RPE) monolayers in response to B. cereus infection.
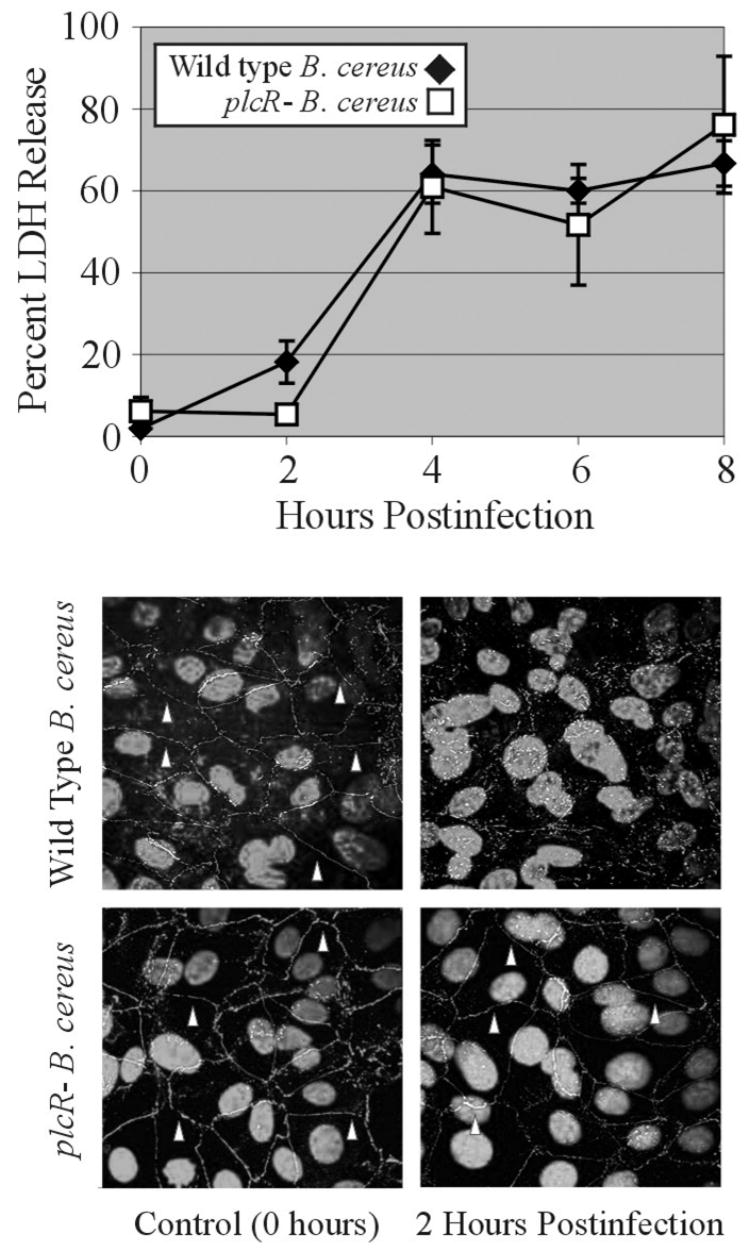
RPE (ARPE19, American Type Culture Collection, Manassas VA) were propagated to confluency in Dulbecco’s Minimal Essential Medium/F-12 (1:1) supplemented with 5% fetal bovine serum and 1% glutamine in 24 well tissue culture plates or on glass coverslips. Monolayers were infected with 105 CFU wild type or quorum sensing-defective plcR- B. cereus. A) Membrane permeability was analyzed detecting the release of lactate dehydrogenase (LDH) release at 0, 2, 4, 6, and 8 hours (CytoToxONE, Promega, Madison WI). Values represent the mean ± standard deviation of six or more samples per time point. B) Integrity of the tight junction protein ZO-1 was analyzed by immunohistochemistry. At 0 or 2 hours postinfection, RPE monolayers on glass coverslips were incubated with mouse anti-ZO-1 (1:200, Zymed®/Invitrogen, Carlsbad CA) and goat-anti-mouse (1:200, Alexafluor 488; Molecular Probes™/Invitrogen). Coverslips were analyzed by confocal microscopy (Olympus FluoView FV500, Center Valley PA). Areas of intact ZO-1 are designated by arrowheads. Loss of ZO-1 integrity was observed after 2 hours of infection with wild type B. cereus, but not the plcR- mutant. Magnification, ×100.
5.2. Retinal Architectural and Function Loss
Retinal detachment is one of the most serious complications of endophthalmitis and occurs with an incidence between 4% to 21% (Abu El-Asrar, et al., 2000; Azad, et al., 2003; Bali, et al., 2003; Doft, et al., 2000; Endophthalmitis Vitrectomy Study, 1995; Lieb, et al., 2003). Retinal structural changes during endophthalmitis, in addition to detachment in its entirety, include photoreceptor layer folding and detachment, and complete dissolution of retinal cell layers (Callegan, et al., 1999a, 1999b, 2002b). Motile organisms, such as Bacillus, have been observed within retinal folds by 8 hours postinfection (Callegan, et al., 1999b, 2002b). Retinal detachment and destruction of the retinal layers has also been observed in endophthalmitis caused by organisms which remain in the midvitreous, such as S. aureus, E. faecalis, or non-motile Bacillus (Callegan, et al., 2002b).
The exact mechanisms of retinal function loss, and loss of retinal architecture during endophthalmitis, are not yet understood. During endophthalmitis, bacteria replicate and produce toxins in close proximity to the delicate cells of the retina. The contribution of bacterial toxin production to the loss of retinal function and architecture has recently been analyzed in an experimental model of B.cereus endophthalmitis (Ramadan, et al., 2006), focusing primarily on the interaction of Bacillus toxins with the Muller cell. Muller cells are elongated neuroglial cells that extend across the thickness of the retina, from the photoreceptor cell layer to the inner limiting membrane abutting the vitreous. Muller cells provide structural and physiological support to the retina, and contribute to the generation of the retinal response (specifically the b-wave and the slow P3 component of the electroretinogram), by regulating K+ distribution across the retina (Reichenbach and Robinson, 1995; Sarthy, et al., 1998; Sarthy and Ripps, 2001). The contribution of Muller cell dysfunction to retinal structural alterations or function loss during endophthalmitis is not yet clear. However, analysis of experimental retinal detachment models suggests that detachment can cause, or be a secondary effect of, Muller cell dysfunction (Francke, et al., 2001; Lewis and Fisher, 2003; Sarthy, et al., 1998; Willbold and Layer, 1998). In detachment models, Muller cells undergo stress and subsequently upregulate glial fibrillary acidic protein (GFAP), an intermediate filament protein. During the progression of experimental B. cereus endophthalmitis in the mouse, slight increases in GFAP immunostaining were detected at 8 hours postinfection, and significant GFAP immunostaining was detected at 16 hours postinfection, suggesting that the retina is undergoing stress during infection (Ramadan, et al., 2006). Whether this stress is attributable to Muller cells or other GFAP-synthesizing cells, such as astrocytes, is presently being investigated. The Muller cell end plates lie in the inner limiting membrane next to the vitreous, in close proximity to bacterial replication and toxin production. The initial decline in retinal function, especially in the b-wave, occurs in parallel with logarithmic growth of B. cereus and maximal toxin production, suggesting that there may be interactions between Bacillus and/or its toxins and the Muller cell.
6. Hypothetical Model of Endophthalmitis
Advances in the understanding of molecular and cellular interactions between the host and pathogen during endophthalmitis, make possible the proposal of a model for the pathogenesis of this infection: Following introduction of bacteria into the posterior segment, endophthalmitis follows one of two general paths: i) infection, mild inflammation, effective treatment, and recovery of vision, or ii) infection, significant inflammation, ineffective treatment, and vision loss. In either case, the immune privileged intraocular environment is generally permissive for bacterial replication, which depending on organism results in inflammation. During the growth of virulent organisms, toxin production results in vision loss. Toxins may 1) interact specifically with the retina, potentially causing dysfunction of cells essential for vision and ocular homeostasis (e.g., induce Muller cell dysfunction, which may affect retinal function and architecture); 2) induce RPE dysfunction leading to breakdown of the blood retinal barrier and unimpeded influx of inflammatory cells and mediators into the posterior segment; and or 3) interact with inflammatory cells resulting in upregulation of inflammatory cytokines, resulting in severe inflammation and bystander damage. The ultimate result is disruption of retinal architecture, retinal cell death, and a breach of the barriers that provide an immune modulated environment within the eye.
7. Future Directions
J. Wayne Streilein (1996) noted that immune privilege in the eye posed a Faustian dilemma: “To maintain accurate vision, the eye must develop strategies that limit its vulnerability to the blinding consequences of trauma, inflammation, neovascularization, regeneration, and autoimmunity.” Yet, like other parts of the body, the eye must confront from time to time the challenge of infection. Because of the importance of vision to survival, natural selection has led to an optimized balance between threats to the host from compromised vision stemming from systemic inflammation and autoimmunity, and vulnerability to infection. As a result, the host-pathogen dynamic as it occurs in the eye, and as it is influenced by various mechanisms of immune privilege, is not likely to be similar to that which occurs at other anatomical sites. This was most evident by the recent unexpected finding that in the eye, complement does not play a measurable role in the clearance of S. aureus (Engelbert and Gilmore, 2005). To understand the pathogenesis of ocular infection toward the goal of optimizing treatment and salvaging vision, then, it will be necessary to test host-pathogen interactions directly in this organ.
Acknowledgments
The authors would like to acknowledge Dr. Didier Lereclus (L’Institut Nationale de la Recherche Agronomique, Paris France) for providing the wild type and plcR- B. cereus strains and Darlene Miller (Bascom Palmer Eye Institute, Miami FL) for providing the Klebsiella ocular isolate used in these studies. We would also like to thank Mark Dittmar (DMEI Animal Research Facility) for his assistance and Paula Pierce (Excalibur Pathology, Oklahoma City OK) for her histology expertise.
Portions of the work presented in this review were supported by U.S. Health Service Grants R01EY12985 (to M.C.C.), R01EY08289 (to M.S.G.), and P30EY12190 (CORE grant to Robert E. Anderson, OUHSC) and a Lew R. Wasserman Award from Research to Prevent Blindness, Inc (to M.C.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu El-Asrar AM, Al-Amro SA, Al-Mosallam AA, Al-Obeidan S. Post-traumatic endophthalmitis: Causative organisms and visual outcome. Eur J Ophthalmol. 1999;9:21–31. doi: 10.1177/112067219900900104. [DOI] [PubMed] [Google Scholar]
- Abu El-Asrar AM, Al-Amro SA, Khan NM, Kangave D. Visual outcome and prognostic factors after vitrectomy for posterior segment foreign bodies. Eur J Ophthalmol. 2000;10:304–311. doi: 10.1177/112067210001000406. [DOI] [PubMed] [Google Scholar]
- Agaisse H, Gominet M, Okstad OA, Kolsto AB, Lereclus D. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol Microbiol. 1999;32:1043–1053. doi: 10.1046/j.1365-2958.1999.01419.x. [DOI] [PubMed] [Google Scholar]
- Aguilar HE, Meredith TA, Drews C, Grossniklaus H, Sawant AD, Gardner S. Comparative treatment of experimental Staphylococcus aureus endophthalmitis. Am J Ophthalmol. 1996;121:310–317. doi: 10.1016/s0002-9394(14)70280-6. [DOI] [PubMed] [Google Scholar]
- Azad R, Ravi K, Talwar D, Rajpal Kumar N. Pars plana vitrectomy with or without silicone oil endoptamponade in post-traumatic endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2003;241:478–83. doi: 10.1007/s00417-003-0670-4. [DOI] [PubMed] [Google Scholar]
- Baillif S, Casoli E, Marion K, Roques C, Pellon G, Hartmann DJ, Freney J, Burillon C, Kodjikian L. A novel in vitro model to study staphylococcal biofilm formation on intraocular lenses under hydrodynamic conditions. Invest Ophthalmol Vis Sci. 2006;47:3410–3416. doi: 10.1167/iovs.05-1070. [DOI] [PubMed] [Google Scholar]
- Bali E, Huyghe P, Caspers L, Libert J. Vitrectomy and silicone oil in the treatment of acute endophthalmitis. Preliminary results. Bull Soc Belge Ophthalmol. 2003;288:9–14. [PubMed] [Google Scholar]
- Baum J, Peyman GA, Barza M. Intravitreal administration of antibiotic in the treatment of bacterial endophthalmitis. III. Consensus. Surv Ophthalmol. 1982;26:204–206. doi: 10.1016/0039-6257(82)90080-7. [DOI] [PubMed] [Google Scholar]
- Benz MS, Scott IU, Flynn HW, Jr, Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: A 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137:38–42. doi: 10.1016/s0002-9394(03)00896-1. [DOI] [PubMed] [Google Scholar]
- Booth MC, Atkuri RV, Nanda SK, Iandolo JJ, Gilmore MS. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Invest Ophthalmol Vis Sci. 1995;36:1828–1836. [PubMed] [Google Scholar]
- Booth MC, Cheung AL, Hatter KL, Jett BD, Callegan MC, Gilmore MS. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun. 1997;65:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton GS, Topping TM, Hyndiuk RA, Aaberg TM, Reeser FH, Abrams GW. Posttraumatic endophthalmitis. Arch Ophthalmol. 1984;102:547–550. doi: 10.1001/archopht.1984.01040030425016. [DOI] [PubMed] [Google Scholar]
- Busbee BG. Endophthalmitis: A reappraisal of incidence and treatment. Curr Opin Ophthalmol. 2006;17:286–291. doi: 10.1097/01.icu.0000193102.21174.a5. [DOI] [PubMed] [Google Scholar]
- Busbee BG. Advances in knowledge and treatment: An update on endophthalmitis. Curr Opin Ophthalmol. 2004;15:232–237. doi: 10.1097/01.icu.0000122123.24016.b8. [DOI] [PubMed] [Google Scholar]
- Callegan MC, Booth MC, Jett BD, Gilmore MS. Pathogenesis of Gram-positive bacterial endophthalmitis. Infect Immun. 1999a;67:3348–3356. doi: 10.1128/iai.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Cochran DC, Kane ST, Gilmore MS, Gominet M, Lereclus D. Contribution of membrane-damaging toxins to Bacillus endophthalmitis pathogenesis. Infect Immun. 2002a;70:5381–5389. doi: 10.1128/IAI.70.10.5381-5389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Engelbert M, Parke DW, 2nd, Jett BD, Gilmore MS. Bacterial endophthalmitis: Epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev. 2002b;15:111–124. doi: 10.1128/CMR.15.1.111-124.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Jett BD, Hancock LE, Gilmore MS. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect Immun. 1999b;67:3357–3366. doi: 10.1128/iai.67.7.3357-3366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Kane ST, Cochran DC, Gilmore MS. Molecular mechanisms of Bacillus endophthalmitis pathogenesis. DNA Cell Biol. 2002c;21:367–373. doi: 10.1089/10445490260099647. [DOI] [PubMed] [Google Scholar]
- Callegan MC, Kane ST, Cochran DC, Gilmore MS, Gominet M, Lereclus D. Relationship of plcR-regulated factors to Bacillus endophthalmitis virulence. Infect Immun. 2003;71:3116–3124. doi: 10.1128/IAI.71.6.3116-3124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Kane ST, Cochran DC, Novosad B, Gilmore MS, Gominet M, Lereclus D. Bacillus endophthalmitis: Roles of bacterial toxins and motility during infection. Invest Ophthalmol Vis Sci. 2005;46:3233–3238. doi: 10.1167/iovs.05-0410. [DOI] [PubMed] [Google Scholar]
- Callegan MC, Kane ST, Cochran DC, Ramadan RT, Chodosh J, McLean C, Stroman D. Virulence factor profiles and antimicrobial susceptibilities of ocular Bacillus isolates. Curr Eye Res. 2006a;31:693–702. doi: 10.1080/02713680600850963. [DOI] [PubMed] [Google Scholar]
- Callegan MC, Novosad B, Ramirez R, Ghelardi E, Senesi S. Role of swarming migration in the pathogenesis of Bacillus endophthalmitis. Invest Ophthalmol Vis Sci. 2006b;47:4461–4467. doi: 10.1167/iovs.06-0301. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Conway BP. Aminogloycoside toxicity -- A survey of retinal specialists. Implications for ocular use. Arch Ophthalmol. 1991;109:946–950. doi: 10.1001/archopht.1991.01080070058035. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Lim JI. Aminoglycoside toxicity in the treatment of endophthalmitis. The Aminoglycoside Toxicity Study Group. Arch Ophthalmol. 1994;112:48–53. doi: 10.1001/archopht.1994.01090130058017. [DOI] [PubMed] [Google Scholar]
- Cellini M, Baldi A. Vitreous fluorophotometric recordings in HIV infection. Int Ophthalmol. 1991;15:37–40. doi: 10.1007/BF00150977. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–1717. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- Costello P, Bakri SJ, Beer PM, Singh RJ, Falk NS, Peters GB, Melendez JA. Vitreous penetration of topical moxifloxacin and gatifloxacin in humans. Retina. 2006;26:191–195. doi: 10.1097/00006982-200602000-00012. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG. The blood-ocular barriers. Past, present, and future. Doc Ophthalmol. 1997;93:149–157. doi: 10.1007/BF02569055. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp Eye Res. 2004;78:715–721. doi: 10.1016/s0014-4835(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Das T, Choudhury K, Sharma S, Jalali S, Nuthethi R The Endophthalmitis Research Group. Clinical profile and outcome in Bacillus endophthalmitis. Ophthalmology. 2001;108:1819–1825. doi: 10.1016/s0161-6420(01)00762-x. [DOI] [PubMed] [Google Scholar]
- Das T, Kunimoto DY, Sharma S, Jalali S, Majji AB, Nagaraja RT, Gopinathan U, Athmanathan S The Endophthalmitis Research Group. Relationship between clinical presentation and visual outcome in postoperative and posttraumatic endophthalmitis in south central India. Indian J Ophthalmol. 2005;53:5–16. doi: 10.4103/0301-4738.15298. [DOI] [PubMed] [Google Scholar]
- Davey RT, Jr, Tauber WB. Posttraumatic endophthalmitis: The emerging role of Bacillus cereus infection. Rev Infect Dis. 1987;9:110–123. doi: 10.1093/clinids/9.1.110. [DOI] [PubMed] [Google Scholar]
- David DB, Kirkby GR, Noble BA. Bacillus cereus endophthalmitis. Br J Ophthalmol. 1994;78:577–580. doi: 10.1136/bjo.78.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doft BM, Kelsey SF, Wisniewski SR. Retinal detachment in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 2000;118:1661–1665. doi: 10.1001/archopht.118.12.1661. [DOI] [PubMed] [Google Scholar]
- Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study: A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496. [PubMed] [Google Scholar]
- Engelbert M, Gilmore MS. Fas ligand but not complement is critical for control of experimental Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci. 2005;46:2479–2486. doi: 10.1167/iovs.04-1139. [DOI] [PubMed] [Google Scholar]
- Engelbert M, Mylonakis E, Ausubel FM, Calderwood SB, Gilmore MS. Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infect Immun. 2004;72:3628–3633. doi: 10.1128/IAI.72.6.3628-3633.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermis SS, Cetinkaya Z, Kiyici H, Ozturk F. Treatment of Staphylococcus epidermidis endophthalmitis with intravitreal moxifloxacin in a rabbit model. Tohoku J Exp Med. 2005;205:223–229. doi: 10.1620/tjem.205.223. [DOI] [PubMed] [Google Scholar]
- Essex RW, Yi Q, Charles PG, Allen PJ. Post-traumatic endophthalmitis. Ophthalmology. 2004;111:2015–2022. doi: 10.1016/j.ophtha.2003.09.041. [DOI] [PubMed] [Google Scholar]
- Francke M, Faude F, Pannicke T, Bringmann A, Eckstein P, Reichelt W, Wiedemann P, Reichenbach A. Electrophysiology of rabbit Muller (glial) cells in experimental retinal detachment and PVR. Invest Ophthal Vis Sci. 2001;42:1072–1079. [PubMed] [Google Scholar]
- Forrester JV, Menamin PG. Immunopathogenic mechanisms in intraocular inflammation. In: Streilein JW, editor. Immune Response and the Eye, Chem Immunol. Vol. 73. Karger; Basel: 1999. pp. 159–185. [DOI] [PubMed] [Google Scholar]
- Forster RK. Experimental postoperative endophthalmitis. Trans Am Ophthalmol Soc. 1992;90:505–559. [PMC free article] [PubMed] [Google Scholar]
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- Fox A, Hammer ME, Lill P, Burch TG, Burrish G. Experimental uveitis. Elicited by peptidoglycan-polysaccharide complexes, lipopolysaccharide, and muramyl dipeptide. Arch Ophthalmol. 1984;102:1063–1067. doi: 10.1001/archopht.1984.01040030857033. [DOI] [PubMed] [Google Scholar]
- Gan IM, Ugahary LC, van Dissel JT, Feron E, Peperkamp E, Veckneer M, Mulder PG, Platenkamp GJ, van Meurs JC. Intravitreal dexamethasone as adjuvant in the treatment of postoperative endophthalmitis: a prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2005;243:1200–1205. doi: 10.1007/s00417-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Giese MJ, Berliner JA, Riesner A, Wagar EA, Mondino BJ. A comparison of the early inflammatory effects of an agr-/sar- versus a wild type strain of Staphylococcus aureus in a rat model of endophthalmitis. Curr Eye Res. 1999;18:177–185. doi: 10.1076/ceyr.18.3.177.5370. [DOI] [PubMed] [Google Scholar]
- Giese MJ, Mondino BJ, Glasgow BJ, Sumner HL, Adamu SA, Halabi HP, Chou HJ. Complement system and host defense against staphylococcal endophthalmitis. Invest Ophthalmol Vis Sci. 1994;35:1026–1032. [PubMed] [Google Scholar]
- Giese MJ, Rayner SA, Fardin BF, Sumner HL, Rozengurt N, Mondino BJ, Gordon LK. Mitigation of neutrophil infiltration in a rat model of early Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci. 2003;44:3077–3082. doi: 10.1167/iovs.02-1250. [DOI] [PubMed] [Google Scholar]
- Giese MJ, Shum DC, Rayner SA, Mondino BJ, Berliner JA. Adhesion molecule expression in a rat model of Staphylococcal aureus endophthalmitis. Invest Ophthalmol Vis Sci. 2000;41:145–153. [PubMed] [Google Scholar]
- Giese MJ, Sumner HL, Berliner JA, Mondino BJ. Cytokine expression in a rat model of Staphylococcus aureus endophthalmitis. Invest Ophthalmol Vis Sci. 1998;39:2785–2790. [PubMed] [Google Scholar]
- Gregory MS, Repp AC, Holhbaum AM, Saff RR, Marshak-Rothstein A, Ksander BR. Membrane Fas ligand activates innate immunity and terminates ocular immune privilege. J Immunol. 2002;169:2727–2735. doi: 10.4049/jimmunol.169.5.2727. [DOI] [PubMed] [Google Scholar]
- Griffith T, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, Kelsey SF. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:1–17. doi: 10.1016/s0002-9394(14)71959-2. [DOI] [PubMed] [Google Scholar]
- Hariprasad SM, Mieler WF, Holz ER. Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch Ophthalmol. 2003;121:345–350. doi: 10.1001/archopht.121.3.345. [DOI] [PubMed] [Google Scholar]
- Ho PC, O'Day DM, Head WS. Fulminating panophthalmitis due to exogenous infection with Bacillus cereus: Report of 4 cases. Br J Ophthalmol. 1982;66:205–208. doi: 10.1136/bjo.66.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- Huber JD, Campos CR, Mark KS, Davis TP. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan induced inflammatory pain. Am J Physiol Heart Circ Physiol. 2006;290:H732–H740. doi: 10.1152/ajpheart.00747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Jett BD, Atkuri RV, Gilmore MS. Enteroccus faecalis localization in experimental endophthalmitis: Role of plasmid-encoded aggregation substance. Infect Immun. 1998;66:843–848. doi: 10.1128/iai.66.2.843-848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett BD, Jensen HG, Nordquist RE, Gilmore MS. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Knorr HL, Budde WM. Prognostic factors in ocular injuries caused by intraocular or retrobulbar foreign bodies. Ophthalmology. 2000;107:823–828. doi: 10.1016/s0161-6420(00)00079-8. [DOI] [PubMed] [Google Scholar]
- Josephberg RG. Endophthalmitis: the latest in current management. Retina. 2006;26:S47–S50. doi: 10.1097/01.iae.0000236457.08393.3f. [DOI] [PubMed] [Google Scholar]
- Kobayakawa S, Jett BD, Gilmore MS. Biofilm formation by Enterococcus faecalis on intraocular lens material. Curr Eye Res. 2005;30:741–745. doi: 10.1080/02713680591005959. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Poulaki V, Doehmen S, Welsandt G, Radetzky S, Lappas A, Kociok N, Kirchhof B, Joussen AM. Contribution of TNFα to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- Kufoy EA, Fox K, Fox A, Parks C, Pakalnis VA. Modulation of the blood-aqueous barrier by gram positive and gram negative bacterial cell wall components in the rat and rabbit. Exp Eye Res. 1990;50:189–195. doi: 10.1016/0014-4835(90)90230-r. [DOI] [PubMed] [Google Scholar]
- Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: Its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003;230:263–290. doi: 10.1016/s0074-7696(03)30005-1. [DOI] [PubMed] [Google Scholar]
- Lieb DF, Scott IU, Flynn HW, Jr, Miller D, Feuer WJ. Open globe injuries with positive intraocular cultures: Factors influencing final visual acuity outcomes. Ophthalmology. 2003;110:1560–1566. doi: 10.1016/S0161-6420(03)00497-4. [DOI] [PubMed] [Google Scholar]
- Liu SM, Way T, Rodrigues M, Steidl SM. Effects of intravitreal corticosteroids in the treatment of Bacillus cereus endophthalmitis. Arch Ophthalmol. 2000;118:803–806. doi: 10.1001/archopht.118.6.803. [DOI] [PubMed] [Google Scholar]
- Lobo CL, Bernardes RC, Cunha-Vaz JG. Alterations of the blood-retinal barrier and retinal thickness in preclinical retinopathy in subjects with type 2 diabetes. Arch Ophthalmol. 2000;118:1364–1369. doi: 10.1001/archopht.118.10.1364. [DOI] [PubMed] [Google Scholar]
- Magone MT, Whitcup SM. Mechanisms of intraocular inflammation. In: Streilein JW, editor. Immune Response and the Eye, Chem Immunol. Vol. 73. Karger; Basel: 1999. pp. 90–119. [DOI] [PubMed] [Google Scholar]
- Mandelbaum S, Forster RK. Exogenous endophthalmitis. In: Pepose JS, Wilhelmus KR, editors. Ocular Infection and Immunity. Mosby; St. Louis MO: 1996. pp. 1298–1320. [Google Scholar]
- Medawar PB. Immunity to homologous grafted skin, III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Men G, Peyman GA, Kuo PC, Ghahramani F, Canakis C, Ratnakaram R, Bezerra Y. The effect of intraoperative retinal manipulation on the underlying retinal pigment epithelium: An experimental study. Retina. 2003;23:475–480. doi: 10.1097/00006982-200308000-00005. [DOI] [PubMed] [Google Scholar]
- Meredith TA. Posttraumatic endophthalmitis. Arch Ophthalmol. 1999;117:520–521. doi: 10.1001/archopht.117.4.520. [DOI] [PubMed] [Google Scholar]
- Meredith TA, Aguilar HE, Drews C, Sawant A, Gardner S, Wilson LA, Grossniklaus HE. Intraocular dexamethasone produces a harmful effect on treatment of experimental Staphylococcus aureus endophthalmitis. Trans Am Ophthalmol Soc. 1996;94:241–252. doi: 10.1016/s0002-9394(14)70164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrickin DC, Wilson CA, Berkowitz BA, Lam MK, Wood GK, Peshock RM. Measurement of blood-retinal barrier breakdown in endotoxin-induced endophthalmitis. Invest Ophthalmol Vis Sci. 1995;36:1361–1370. [PubMed] [Google Scholar]
- Miller D, Flynn PM, Scott IU, Alfonso EC, Flynn HW., Jr In vitro fluoroquinolone resistance in staphylococcal endophthalmitis isolates. Arch Ophthalmol. 2006;124:479–483. doi: 10.1001/archopht.124.4.479. [DOI] [PubMed] [Google Scholar]
- Mylonakis E, Engelbert M, Qin X, Sifri CD, Murray BE, Ausubel FM, Gilmore MS, Calderwood SB. The Enterococcus faecalis fsrB gene, a key component of the fsr quorum-sensing system, is associated with virulence in the rabbit endophthalmitis model. Infect Immun. 2002;70:4678–4681. doi: 10.1128/IAI.70.8.4678-4681.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng EW, Costa JR, Samiy N, Ruoff KL, Connolly E, Cousins FV, D'Amico DJ. Contribution of pneumolysin and autolysin to the pathogenesis of experimental pneumococcal endophthalmitis. Retina. 2002;22:622–632. doi: 10.1097/00006982-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Ng JQ, Morlet N, Pearman JW, Constable IJ, McAllister IL, Kennedy CJ, Isaacs T, Semmens JB, Team EPSWA. Management and outcomes of postoperative endophthalmitis since the endophthalmitis vitrectomy study: The Endophthalmitis Population Study of Western Australia (EPSWA)'s fifth report. Ophthamology. 2005;112:1199–1206. doi: 10.1016/j.ophtha.2005.01.050. [DOI] [PubMed] [Google Scholar]
- O’Brien TP, Choi S. Trauma-related ocular infections. Int Ophthalmol Clin N Am. 1995;8:667–679. [Google Scholar]
- O’Day DM, Smith RS, Gregg CR, Turnbull PC, Head WS, Ives JA, Ho PC. The problem of Bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/s0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Endogenous bacterial endophthalmitis: Report of a ten-year retrospective study. Ophthalmology. 1994;101:832– 838. [PubMed] [Google Scholar]
- Paton JC, Berry AM, Lock RA. Molecular analysis of putative pneumococcal virulence proteins. Microb Drug Resist. 1997;3:1–10. doi: 10.1089/mdr.1997.3.1. [DOI] [PubMed] [Google Scholar]
- Pepose JS, Holland GM, Nestor MS, Cochran AJ, Foos RY. Acquired immune deficiency syndrome: Pathogenic mechanisms of ocular disease. Ophthalmology. 1985;92:472–484. doi: 10.1016/s0161-6420(85)34008-3. [DOI] [PubMed] [Google Scholar]
- Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack JS, Beecher DJ, Pulido JS, Wong ACL. Failure of intravitreal dexamethasone to diminish inflammation or retinal toxicity in an experimental model of Bacillus cereus endophthalmitis. Curr Eye Res. 2004;29:253–259. doi: 10.1080/02713680490516701. [DOI] [PubMed] [Google Scholar]
- Qin X, Singh KV, Weinstock GM, Murray BE. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun. 2000;68:2579–2586. doi: 10.1128/iai.68.5.2579-2586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan R, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006 doi: 10.1080/02713680600976925. in press. [DOI] [PubMed] [Google Scholar]
- Recchia FM, Busbee BG, Pearlman RB, Carvalho-Recchia CA, Ho AC. Changing trends in microbiology of post-cataract endophthalmitis. Arch Ophthalmol. 2005;123:341–346. doi: 10.1001/archopht.123.3.341. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Robinson SR. The involvement of Müller cells in the outer retina. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and Clinical Aspects of the Outer Retina. Chapman & Hall; London: 1995. pp. 395–416. [Google Scholar]
- Robertson SM, Curtis MA, Schlech BA, Rusinko A, Owen GR, Dembinska O, Liao J, Dahlin DC. Ocular pharmacokinetics of moxifloxacin after topical treatment of animals and humans. Surv Ophthalmol. 2005;50:S32–S45. doi: 10.1016/j.survophthal.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Romero CF, Rai MK, Lowder CY, Adal KA. Endogenous endophthalmitis: Case report and brief review. Am Fam Phys. 1999;60:510–514. [PubMed] [Google Scholar]
- Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- Sarthy V, Ripps H. The Retinal Muller Cell: Structure and Function. Plenum Publishers; New York: 2001. [Google Scholar]
- Schalnus R, Orloff C. The blood-ocular barrier in type 1 diabetes without diabetic retinopathy: Permeability measurements using fluorophotometry. Ophthalmic Res. 1995;27:S116–S123. doi: 10.1159/000267856. [DOI] [PubMed] [Google Scholar]
- Shah GK, Stein JD, Sharma S, Sivalingam A, Benson WE, Regillo CD, Brown GC, Tasman W. Visual outcomes following the use of intravitreal steroids in the treatment of postoperative endophthalmitis. Ophthalmology. 2000;107:486–489. doi: 10.1016/s0161-6420(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Smith MA, Sorenson JA, D'Aversa G, Mandelbaum S, Udell I, Harrison W. Treatment of experimental methicillin-resistant Staphylococcus epidermidis endophthalmitis with intravitreal vancomycin and intravitreal dexamethasone. J Infect Dis. 1997;175:462–466. doi: 10.1093/infdis/175.2.462. [DOI] [PubMed] [Google Scholar]
- Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS. Chronic low level of complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci. 2000;41:3492–3502. [PMC free article] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege and the Faustian dilemma. The Proctor Lecture. Invest Ophthalmol Vis Sci. 1996;37:1010, 1940–1950. [PubMed] [Google Scholar]
- Streilein JW. Immunoregulatory mechanisms of the eye. Prog Retin Eye Res. 1999;18:357–370. doi: 10.1016/s1350-9462(98)00022-6. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–888. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Thompson ST, Parver LM, Enger CL, Meiler WF, Liggett PE. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. Ophthalmology. 1993;100:1468–1474. doi: 10.1016/s0161-6420(93)31454-5. [DOI] [PubMed] [Google Scholar]
- Thompson WS, Rubsamen PE, Flynn HW, Jr, Schiffman J, Cousins SW. Endophthalmitis after penetrating trauma. Risk factors and visual acuity outcomes. Ophthalmology. 1995;102:1696–701. doi: 10.1016/s0161-6420(95)30807-x. [DOI] [PubMed] [Google Scholar]
- Tosi MF. Innate immune responses to infection. J Allergy Clin Immunol. 2005;116:241–249. doi: 10.1016/j.jaci.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Amin A, Derevjanik NL, Green WR, Campochiaro PA. Immunohistochemical localization of blood-retinal barrier breakdown sites associated with post-surgical macular oedema. Histochem J. 1994;26:655–665. doi: 10.1007/BF00158291. [DOI] [PubMed] [Google Scholar]
- West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112:1388–1394. doi: 10.1016/j.ophtha.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Willbold E, Layer PG. Muller glia cells and their possible roles during retina differentiation in vivo and in vitro. Histol Histopathol. 1998;13:531–552. doi: 10.14670/HH-13.531. [DOI] [PubMed] [Google Scholar]
- Williams DF, Mieler WF, Abrams GW, Lewis H. Results and prognostic factors in penetrating ocular injuries with retained intraocular foreign bodies. Ophthalmology. 1988;95:911–916. doi: 10.1016/s0161-6420(88)33069-1. [DOI] [PubMed] [Google Scholar]
- Wong JS, Chan TK, Lee HM, Chee SP. Endogneous bacterial endophthalmitis: An East Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2003;107:1483–1491. doi: 10.1016/s0161-6420(00)00216-5. [DOI] [PubMed] [Google Scholar]
- Xu H, Dawson R, Crane I, Liversidge J. Leukocyte diapedesis in vivo induces transient loss of tight junction protein at the blood-retinal barrier. Invest Ophthalmol Vis Sci. 2005;46:2487–2494. doi: 10.1167/iovs.04-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Forrester JV, Liversidge J, Crane IJ. Leukocyte trafficking in experimental autoimmune uveitis: breakdown of blood-retinal barrier and upregulation of cellular adhesion molecules. Invest Ophthalmol Vis Sci. 2003;44:226–234. doi: 10.1167/iovs.01-1202. [DOI] [PubMed] [Google Scholar]
- Yagci R, Oflu Y, Dincel A, Kaya E, Yagci S, Bayar B, Duman S, Bozkurt A. Penetration of second-, third-, and fourth-generation topical fluoroquinolone into aqueous and vitreous humour in a rabbit endophthalmitis model. [May 26];Eye. 2006 doi: 10.1038/sj.eye.6702414. Online http://www.nature.com/eye/journal/vaop/ncurrent/full/6702414a.html. [DOI] [PubMed]
- Yildirim O, Oz O, Aslan G, Cinel L, Delialioglu N, Kanik A. The efficacy of intravitreal levofloxacin and intravitreal dexamethasone in experimental Staphylococcus epidermidis endophthalmitis. Ophthalmic Res. 2002;34:349–356. doi: 10.1159/000067047. [DOI] [PubMed] [Google Scholar]
- Yoon YH, Lee SU, Sohn JH, Lee SE. Result of early vitrectomy for endogenous Klebsiella pneumoniae endophthtalmitis. Retina. 2003;23:366–370. doi: 10.1097/00006982-200306000-00013. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Ishiko S, Kojima M, Ogasawara H. Permeability of the blood-ocular barrier in adolescent and adult diabetic patients. Br J Ophthalmol. 1993;77:158–161. doi: 10.1136/bjo.77.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, Fung CP, Chuang YC. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Investig Dis. 2006;42:1351–1358. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]


