Abstract
Longitudinal growth of long bones occurs at the growth plate by endochondral ossification. In the embryonic mouse, this process is regulated by Wnt signaling. Little is known about which members of the Wnt family of secreted signaling proteins might be involved in the regulation of the postnatal growth plate. We used microdissection and real-time PCR to study mRNA expression of Wnt genes in the mouse growth plate. Of the 19 known members of the Wnt family, only six were expressed in postnatal growth plate. Of these, Wnts -2b, -4, and -10b signal through the canonical β-catenin pathway and Wnts -5a, -5b, and -11 signal through the noncanonical calcium pathway. The spatial expression for these six Wnts was remarkably similar, showing low mRNA expression in the resting zone, increasing expression as the chondrocytes differentiated into the proliferative and prehypertrophic state and then (except Wnt-2b) decreasing expression as the chondrocytes underwent hypertrophic differentiation This overall pattern is broadly consistent with previous studies of embryonic mouse growth cartilage suggesting that Wnt signaling modulates chondrocyte proliferation and hypertrophic differentiation. We also found that mRNA expression of these Wnt genes persisted at similar levels at 4-weeks, when longitudinal bone growth is waning. In conclusion, we have identified for the first time the specific Wnt genes that are expressed in the postnatal mammalian growth plate. The six identified Wnt genes showed a similar pattern of expression during chondrocyte differentiation, suggesting overlapping or interacting roles in postnatal endochondral bone formation.
Keywords: Wnt, growth plate, chondrocyte, cartilage
Introduction
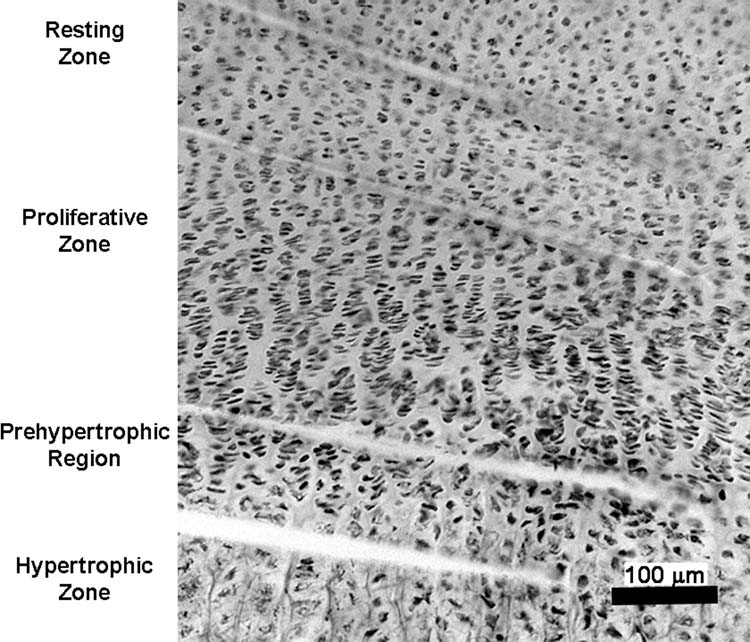
Longitudinal growth of long bones occurs by endochondral ossification, a process in which bone is formed over a cartilaginous template. In postnatal life, this process occurs at the growth plate, a thin layer of cartilage found between the epiphysis and the metaphysis. The growth plate is organized into four distinct zones with different patterns of proliferation rate, differentiation state, and cell morphology. The resting zone, adjacent to the epiphyseal bone, contains small, roughly spherical chondrocytes distributed singly or in pairs, which divide infrequently and are considered "stem-like cells" capable of generating new clones of proliferative chondrocytes (1-3). The proliferative zone, which lies farther from the epiphysis, contains chondrocytes which have become flattened and are arrayed in columns parallel to the long axis of the bone. As its name implies, the rate of chondrocyte proliferation is greatest in this zone. As cells progress into the prehypertrophic region and then the hypertrophic zone, the chondrocytes maintain their columnar arrangement but proliferation ceases, the cells enlarge and there is a shift in matrix composition, including an increase in type X collagen production. The hypertrophic cartilage is then invaded by blood vessels and bone cells from the adjacent metaphysis that remodel the newly formed cartilage into bone tissue.
The rate of endochondral bone formation at the growth plate is regulated by a complex endocrine network, involving growth hormone, IGF-I, thyroid hormone, glucocorticoid and sex steroids (4-6). In addition, the multistep process of endochondral bone formation is coordinated by paracrine factors that regulate chondrocyte proliferation and differentiation and ossification. Several of these local molecular regulatory mechanisms have been defined in the last several years, primarily through identification of mutations in human skeletal dysplasias and genetic manipulation in mice (7). For instance, Indian hedgehog and parathyroid hormone-related protein participate in a negative feedback loop that regulates chondrocyte proliferation and hypertrophy (8-11). In addition, fibroblast growth factors, primarily produced in perichondrium, and bone morphogenetic proteins, expressed primarily in hypertrophic zone, also regulate chondrocyte proliferation and differentiation and osteogenesis (12-15).
Endochondral bone formation also appears to be regulated by members of the Wnt family of secreted signaling proteins. In vertebrates, there are 19 known members of the Wnt family. They act through receptors called Frizzled (Fzd) and co-receptors, Lrp5 and Lrp6, to mediate several effectors in a localized signaling cascade resulting in the regulation of cell adhesion and/or gene expression (16-20). Wnts can signal through a canonical pathway that involves accumulation of β-catenin and through non-canonical pathways. One non-canonical pathway that involves release of intracellular calcium leading to activation of protein kinase C may actually antagonize the canonical pathway by promoting degradation of β-catenin (21;22).
During embryogenesis, differentiation of chondrocytes from mesenchymal progenitors in the developing limb appears to require non-canonical Wnt signaling and to be inhibited by the canonical β-catenin pathway. Later in embryonic development, both pathways appear to be needed for normal proliferation and differentiation of chondrocytes (23-27). Inactivation of β-catenin in chondrocytes of mouse embryos causes decreased chondrocyte proliferation and hypertrophic differentiation (28;29). Ablation of Wnt-5a causes decreased proliferation in the columnar proliferative zone and delayed hypertrophic differentiation. Overexpression of Wnts -5a or -5b, which act through the calcium pathway, in mice also produces complex effects on chondrocyte proliferation and differentiation in the growth plate (30;31).
Although Wnt signaling in general appears to be important in growth cartilage, little is known about which specific Wnts are responsible for these effects. This gap in our understanding is particularly evident in the postnatal growth plate; most previous work has focused on the embryonic skeleton. This information is needed in order to understand how Wnt signaling may be regulating proliferation and differentiation in the different zones of the growth plate. We therefore examined the expression profile of Wnt family members in growth plate, perichondrium and metaphyseal bone. Wnt expression in developing bone has generally been studied using in situ hybridization, which provides precise localization but is primarily non-quantitative and may lack adequate sensitivity in growth plate cartilage. To circumvent these difficulties and complement previous studies, we chose to assess gene expression of all members of the Wnt family in distinct zones of growth plate using microdissection followed by real-time PCR, a quantitative method with greater sensitivity and specificity than previous techniques.
Materials and Methods
Animal procedures and tissue processing
C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used in accordance with the Guide for the Care and Use of Laboratory Animals (32). The protocol was approved by the Animal Care and Use Committee, National Institute of Child Health and Human Development, National Institutes of Health.
One and 4-week-old male mice (n = 6, at each age) were killed by CO2 inhalation. Proximal tibial epiphyses were rapidly excised, embedded in Tissue-Tek O.C.T. Compound (Sakur Finetek, Torrance, CA) and stored at -80°C for subsequent processing. The entire tissue collecting process was completed within 5 min for each animal to minimize RNA degradation. As a positive control for the real-time PCR assays, embryos (at E18.5, n = 3) were killed and immediately homogenized in 5ml Trizol (Invitrogen, Carlsbad, CA) and stored at -80°C for later RNA isolation.
Growth plate microdissection
Frozen longitudinal sections, 40μm thick, of proximal tibial growth plates from each 1 and 4-week-old mouse (n = 6, each age) were mounted on Superfrost Plus slides (Fisher Scientific, Chicago, IL) and stored at -80°C. Sections were thawed 30s, fixed in 70% ethanol, 95% ethanol and 99% methanol, stained with eosin (Sigma-Aldrich, St Louis, MO), dehydrated in graded ethanol solutions (70%, 100%, 100%) for 1 min each and then stored in xylene for subsequent microdissection. Standard RNase precautions were employed. Manual microdissection from each section was performed using an inverted microscope, hypodermic needles, and razor blades. Each region (resting, proliferative, prehypertrophic and hypertrophic) was separated, based on histological landmarks, and placed in 100μl of a solution containing 4M guanidine, thiocyanate, 25mM sodium citrate, pH 7.0, 0.1M β-mercaptoethanol (Sigma-Aldrich, St Louis, MO).
To screen for mRNA expression of all 19 known Wnts in growth plate and adjacent tissues, the whole growth plate, perichondrium and metaphyseal bone were individually microdissected from 1-week-old mice (n = 3). To characterize the expression of the Wnts in different zones, growth plates from additional 1-week-old mice (n = 6) were separated into resting zone, proliferative zone, prehypertrophic region, and hypertrophic zone, which were readily distinguished visually using an inverted microscope (Fig 1). To minimize cross-contamination, we discarded a narrow segment of tissue between the resting zone and the proliferative zone, between the proliferative and prehypertrophic regions, and between the hypertrophic zone and the metaphyseal bone. Due to decreasing growth plate height in 4-week-old mice (n = 6), only two major regions (resting/proliferative and hypertrophic/prehypertrophic) were microdissected. At both ages, for each zone, approximately 20 sections from a single animal were pooled prior to RNA isolation. At later time points, the growth plate becomes so thin that we were unable to reliably microdissect the different zones, and thus we were not able to assess Wnt expression during the final stages of decelerating longitudinal bone growth.
Figure 1.
Embryo and growth plate RNA extraction and quantitation
Total RNA from whole growth plate and each specific zone, as well metaphyseal bone and perichondrium were isolated as previously described (33), except that the procedure was modified to use one fifth of each volume and to omit the LiCl precipitation. The final pellet was resuspended in 20μl DEPC-treated water. For all samples, the integrity, quality and concentration of total RNA was assessed using RNA Pico Chips in a Bioanalyzer 2100, version B.02.02 software (Agilent Technologies, Palo Alto, CA), following the manufacturer's instructions. Approximately 40-100ng of total RNA was obtained from each sample.
Total RNA was extracted and purified from embryos using Trizol reagent (Invitrogen, Carlsbad, CA) followed by RNeasy MiniKit (QIAGEN, Valencia, CA) according to the manufacturers' instructions.
Real-time quantitative RT-PCR
Total RNA (40-100ng) was reversed-transcribed into cDNA by adding random hexamers and Superscript III Reverse Transcriptase (Invitrogen) according to manufacturer's instructions. The resulting cDNA solution was stored at -20°C.
Expression of Wnt mRNAs was quantified by real-time quantitative PCR using an ABI Prism 7300 sequence Detector (Applied Biosystems, Foster City, CA). mRNA expression markers for hypertrophic zone (type X procollagen [Col10a1]); prehypertrophic region (Indian hedgehog [Ihh]); and bone (type I collagen [Col1a1]) were also assayed. The following assays were used (Applied Biosystems, Foster City, CA): Wnt1:Mm0130055-g1; Wnt2:Mm00470018-m1; Wnt2b:Mm00437330-m1; Wnt3:Mm00437336-m1; Wnt3a:Mm00437337-m1; Wnt4:Mm00437341-m1; Wnt5a:Mm00437347-m1; Wnt5b:Mm00437350-m1; Wnt6:Mm00437351-m1; Wnt7a:Mm00437355-m1; Wnt7b:Mm00437357-m1; Wnt8a:Mm00436822-m1; Wnt8b:Mm00442107-m1; Wnt9a:Mm00460518-m1; Wnt9b:Mm00457102-m1; Wnt10a:Mm00437325-m1; Wnt10b:Mm00442104-m1; Wnt11:Mm00437328-m1; Wnt16:Mm00446420-m1; Col10a1:Mm00487041-m1; Col1a1:Mm00801645-m1; Ihh:Mm00439613-m1; and 18S-4319413E. Each assay uses primers and specific FAM-labelled TaqMan probes.
Non-template controls were run to exclude formation of primer dimers or contamination of PCR reagents. Quantitative PCR conditions were as follows: one cycle at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C.
Each sample, which represents a single region from a single animal, was run in triplicate for every assay along with 18S ribosomal RNA as a reference gene. 18S rRNA reportedly shows similar expression levels in different tissues and is therefore useful as an internal standard for quantitative comparison of mRNA levels (34;35). Triplicate threshold cycle (Ct) values were averaged and then normalized to that of 18S rRNA from the same sample to account for variability in cDNA loading using the formula: Relative Expressioni = (2)(Ctr-Cti) where Ctr represents the threshold cycle for 18S rRNA and Cti represents the threshold cycle for the gene of interest. Serial 10-fold dilutions of cDNA were used to confirm near-theoretical efficiencies of assays.
Statistical analysis
PCR data are presented as mean ± SEM. mRNA expression values were log transformed to achieve a normal distribution. Wnt mRNA expression between different zones of 1-week-old growth plate were analyzed by one-way analysis of variance (ANOVA) followed by pair-wise comparison using the Holm-Sidak method. Wnt mRNA expression between two major regions in 4-week-old growth plate were analyzed by Student's t test. For the type X collagen, type I collagen and Ihh markers, comparison between the region of greatest expected expression and an appropriate comparison region was made by Student's t test. When the data failed a test for normality, Kruskal-Wallis (pair-wise comparison by Tukey), and Mann-Whitney tests were used instead of ANOVA or Student's t test, respectively. All P values were two-tailed, and significance was recognized at P ≤ 0.05.
Results
Accuracy of microdissection: expression of markers in growth plate and surrounding bone
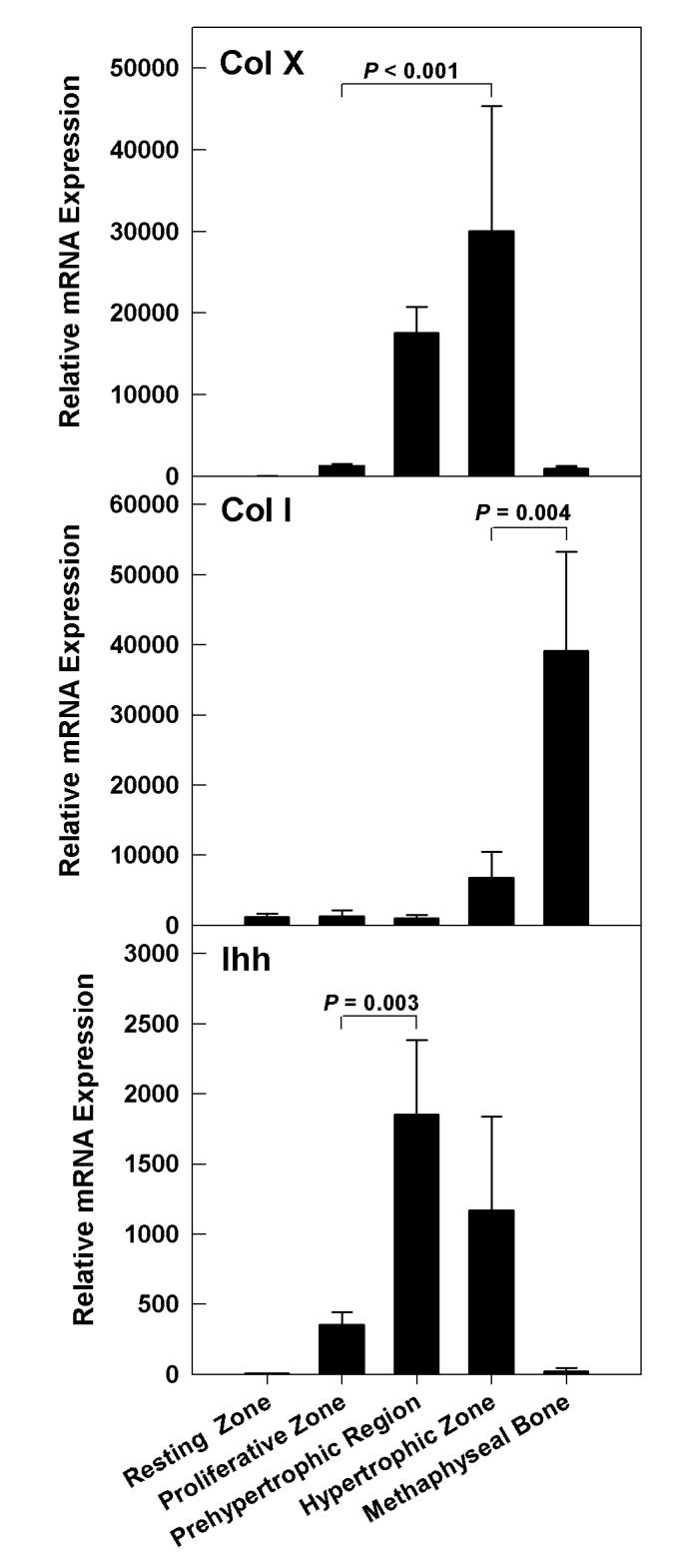
To confirm the accuracy of the microdissection, we analyzed mRNA expression for type X collagen, Indian hedgehog (Ihh) and type I collagen which are considered markers for hypertrophic chondrocytes, prehypertrophic chondrocytes, and bone, respectively (36-38). mRNA expression of markers was analyzed in metaphyseal bone and all zones of growth plate from 1-week-old mice. As expected, type X collagen was primarily expressed in hypertrophic cartilage, Ihh in the prehypertrophic region, and type I collagen in the bone (Fig 2). Type X collagen mRNA levels in hypertrophic zone were 23-fold higher than in proliferative zone (P < 0.001). Ihh mRNA levels were 5-fold higher in prehypertrophic region compared to proliferative zone (P = 0.003). Type I collagen mRNA was 6-fold higher (P = 0.004) in metaphyseal bone than in hypertrophic zone.
Figure 2.
Wnt mRNA expression in whole growth plate, perichondrium, and metaphyseal bone of 1-week-old mice
We analyzed mRNA expression of all Wnts in the whole growth plate and adjacent tissues (metaphyseal bone and perichondrium) from proximal tibias from 1-week-old mice. Of the 19 known members of the Wnt family, we found nine Wnts expressed in growth plate (Table 1). Of these, Wnts -4, -5a, -5b, -10b and -11 were expressed at higher levels whereas Wnts -2b, -7b, -9a, and -10a were expressed only at very low levels (Table 1). Of the Wnts expressed in growth plate, most were also expressed in both perichondrium and metaphyseal bone, except for Wnt-10a and Wnt-7b which were detected at very low levels in growth plate but not detected in bone or perichondrium. In addition to the nine Wnts present in growth plate, we also detected several other Wnts in adjacent structures: Wnt-1 in metaphyseal bone, Wnt-2 in perichondrium and Wnt-16 in both perichondrium and metaphyseal bone. Perichondrium showed higher mRNA expression of Wnts -9a (30-fold), -2b (7.8-fold), -10b (5-fold), and -5a (4-fold) than did growth plate. Metaphyseal bone showed higher mRNA expression of Wnt-2b (9-fold) and -9a (3-fold) than did growth plate. Wnts -4, -5b and -11 showed higher expression levels in growth plate than in bone and perichondrium (Table 1).
Table 1.
Normalized mRNA expression of Wnt genes in skeletal tissues of 1-week-old mice and whole E18.5 d embryos.
| Gene | Whole Growth Plate | Perichondrium | Metaphyseal Bone | Whole Embryo |
|---|---|---|---|---|
| Wnt-1 | ND | ND | 2.7 (2.3) | 0.7 (0.1) |
| Wnt-2 | ND | 0.58 (0.4) | ND | 5.8 (0.9) |
| Wnt-2b | 0.1 (0.1) | 0.9 (0.4) | 1.0 (0.5) | 5.0 (0.8) |
| Wnt-3 | ND | ND | ND | 4.6 (0.5) |
| Wnt-3a | ND | ND | ND | 0.8 (0.1) |
| Wnt-4 | 112.4 (55.7) | 14.7 (3.5) | 39.2 (17.1) | 54.6 (9.7) |
| Wnt-5a | 18.0 (8.0) | 72.5 (36.2) | 22.5 (10.2) | 45.4 (6.1) |
| Wnt-5b | 144.3 (64.6) | 60.8 (15.9) | 33.9 (11.8) | 14.1 (2.0) |
| Wnt-6 | ND | ND | ND | 6.0 (0.4) |
| Wnt-7a | ND | ND | ND | 2.8 (0.6) |
| Wnt-7b | 0.5 (0.2) | ND | ND | 13.1 (1.9) |
| Wnt-8a | ND | ND | ND | ND |
| Wnt-8b | ND | ND | ND | 0.7 (0.1) |
| Wnt-9a | 0.1 (0.1) | 2.7 (1.0) | 0.3 (0.1) | 3.5 (1.3) |
| Wnt-9b | ND | ND | ND | 0.9 (0.1) |
| Wnt-10a | 0.1 (0.1) | ND | ND | 10.9 (3.6) |
| Wnt-10b | 3.8 (1.6) | 20.2 (9.9) | 4.0 (1.0) | 27.0 (9.0) |
| Wnt-11 | 11.2 (2.3) | 7.5 (5.0) | 1.2 (0.3) | 12.5 (1.6) |
| Wnt-16 | ND | 31.6 (13.7) | 7.1 (1.5) | 13.0 (1.9) |
mRNA expression was normalized to 18S rRNA, and, for convenience, multiplied by 107.
Mean (SEM). ND = not detected.
As a positive control for the real-time PCR assays, we measured Wnt expression in RNA from whole mouse embryos (E18.5). All Wnt members were detected (Table 1), except for Wnt-8a, which has been shown to be rapidly down-regulated during early somitogenesis, being restricted to early stages of mouse embryogenesis (39).
Differential expression of Wnts at different stages of chondrocyte maturation in growth plate
The resting zone, proliferative zone, prehypertrophic region, and hypertrophic zone contain chondrocytes in successive stages of differentiation. To explore the role of Wnt signaling in this progressive differentiation, we analyzed expression of Wnts in each zone of growth plates of 1-week-old mice.
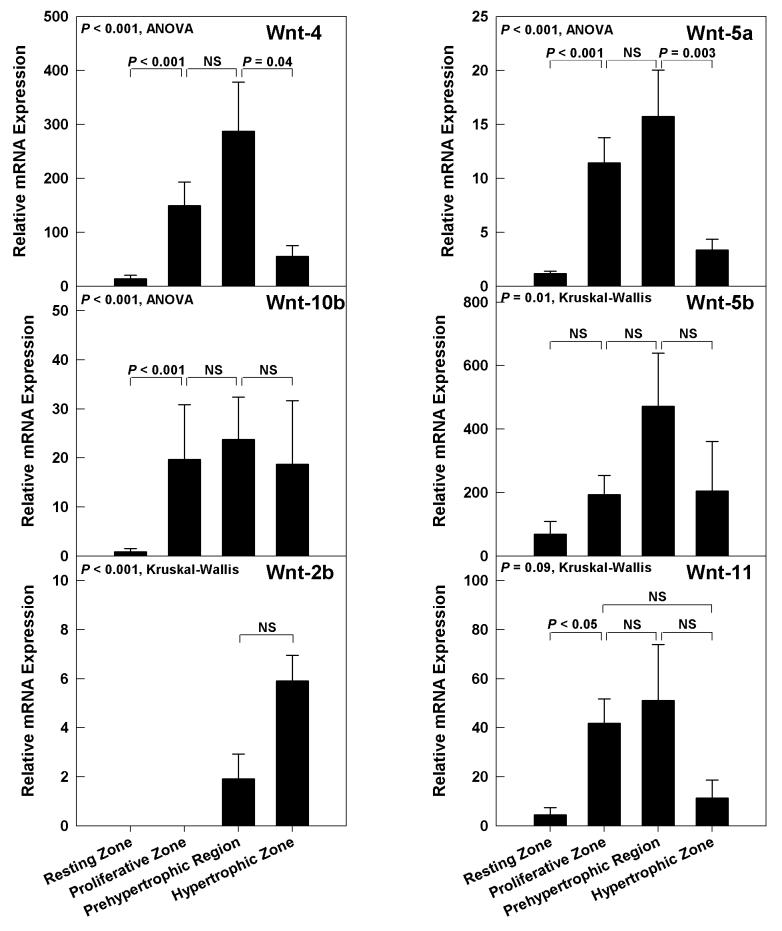
Of the Wnts that had shown higher levels of expression in whole growth plate, all showed a generally similar spatial pattern of expression. Specifically, Wnts -4, -5a, -5b, -10b, and -11, all showed relatively low expression in resting zone (Fig 3). As the chondrocytes passed into the proliferative state and the prehypertrophic state, expression of these Wnts appeared to increase. As the cells underwent terminal differentiation to the hypertrophic state, Wnt expression tended to decline. Although this general pattern was observed for Wnts -4, -5a, -5b, -10b, and -11, not all pairwise comparisons between adjacent zones were statistically significant (Fig 3).
Figure 3.
Wnt-2b, which showed a low expression in whole growth plate, was detected primarily in the hypertrophic zone and less in the prehypertrophic region, and was not detectable in the resting or proliferative zone (Fig 3). Wnts -7b, -9a and -10a, which had been detected only at very low levels in whole growth plate, were below the detection limit in the individual zones of the growth plate in many animals (data not shown).
Persistence of Wnt mRNA expression in growth plates of older mice
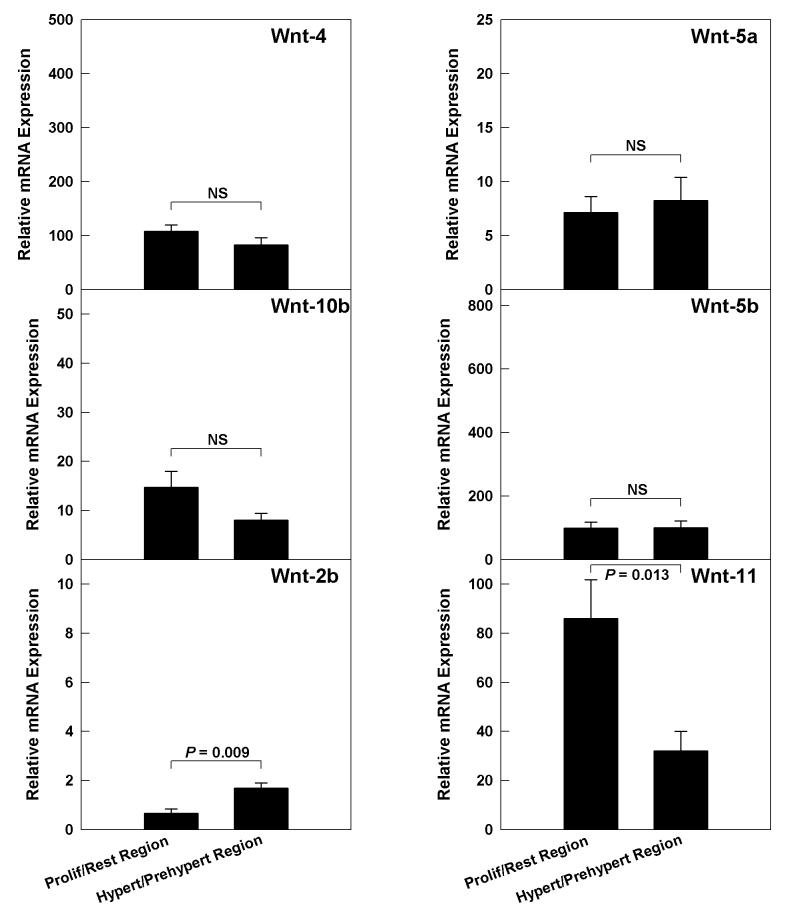
The rate of longitudinal bone growth in mice declines markedly with age due, in large part, to a decline in the rate of chondrocyte proliferation. To determine whether Wnt expression persists into this phase of decelerating postnatal growth, we assessed Wnt mRNA expression in 4-week-old mice. Because the growth plate height is diminished at this age, we were only able to microdissect the growth plates into two regions, the resting/proliferative and prehypertrophic /hypertrophic regions. In general, those Wnts that were expressed at higher levels in growth plate, i.e. Wnts -4, -5a, -5b, -10b, and -11, continued to be expressed at similar levels in the 4-week-old animals (Fig 4). This persistent expression was observed both in the resting-proliferative region and the prehypertrophic/hypertrophic region (Fig 4).
Figure 4.
Discussion
To explore the role of Wnt signaling in postnatal endochondral bone formation, we determined the mRNA expression of all known Wnt family members in mouse growth plate and surrounding tissues. For this purpose, we used microdissection followed by real time PCR, a technique that is quantitative and highly sensitive. In general, the accuracy of the microdissection was confirmed by the observations that type X collagen expression was preferentially expressed in the hypertrophic zone, type I collagen in bone, and Ihh in the prehypertrophic region. However, there is likely to be some cross-contamination between adjacent samples, particularly in the prehypertrophic zone which anatomically represents a narrow transition zone between the proliferative and hypertrophic zones without sharp boundaries. Thus, the sample labeled prehypertrophic probably does contain some hypertrophic cells, explaining the apparent expression of type X collagen in this region. Consequently, the actual differences in expression of Wnt mRNAs among different zones could, in fact, be sharper than the observed differences.
Wnt mRNA expression during chondrocyte differentiation in the 1-week mouse growth plate
Of the 19 known members of the Wnt family, only nine were expressed at the mRNA level in the postnatal mouse growth plate. However, three of these, Wnts -7b, -9a and -10a were detected in whole growth plate only at very low levels and, in individual zones, were near the assay detection limit and thus not consistently detected in all specimens, suggesting that Wnts -7b, -9a and -10a may not have an important biological role in postnatal growth plate. The remaining six members of the Wnt family, Wnts -2b, -4, -5a, -5b, -10b, and -11 were detected both in whole growth plate and individual zones. Of these, Wnts -4, -5a and -5b have been shown to be expressed in embryonic growth cartilage of mice and chicks (40;41), and Wnt-11 was found in a subset of prehypertrophic chondrocytes just beneath the perichondrium of embryonic chick cartilage (42), but reported as absent in human embryonic growth plate and restricted to perichondrium (43). To our knowledge, Wnts -2b and -10b have not been previously implicated in chondrocyte regulation.
Of the six members of the Wnt family that were expressed in growth plate cartilage, three, Wnts -2b, -4 and -10b, act through the β-catenin pathway. In the β-catenin pathway, also called the canonical pathway, receptor activation by Wnts leads to stabilization and accumulation of β-catenin in the cytosol, followed by its translocation to the nucleus where it regulates gene transcription. During early development, this pathway appears to inhibit chondrocyte differentiation. In mice that overexpress constitutively active β-catenin in cartilage, typical growth plates do not form, whereas genetic inactivation of β-catenin stimulates ectopic formation of chondrocytes (44). In contrast, β-catenin overexpression in more mature chondrocytes appears to stimulate hypertrophic differentiation (45). Genetic inactivation of β-catenin in chondrocytes decreases chondrocyte proliferation and delays hypertrophic differentiation (46).
Among the Wnt family members that signal through β-catenin, Wnt-4 had the highest mRNA expression in the growth plate. Wnt-4 mRNA levels were low in resting zone, but increased as chondrocytes differentiated into the proliferative and prehypertrophic regions and then decreased as cells proceeded to hypertrophic differentiation. Previous studies using in situ hybridization reported Wnt-4 only in prehypertrophic region of growth cartilage of embryonic mice (47). The more widespread detection of mRNA in the current study might be related to the greater sensitivity of our technique or could reflect a difference between embryonic cartilage and the postnatal growth plate. The observed expression pattern is compatible with previous studies showing that Wnt-4 overexpression accelerates hypertrophy of chondrocytes (48). However, mice null for Wnt-4 have been reported to have either no growth plate phenotype (49) or a slight delay in chondrocyte maturation (50). Our data suggest that this mild phenotype, which contrasts with the more severe delayed hypertrophic differentiation and decreased chondrocyte proliferation of mice lacking β-catenin, may be attributable to redundancy between Wnt-4 and Wnt-10b, which also acts through the β-catenin pathway and shows a similar spatial expression pattern. Consistent with this hypothesized redundancy in growth plate, mice lacking Wnt-10b display decreased trabecular bone but have not been reported to have abnormal endochondral bone formation at the growth plate (51). Wnt-2b, although expressed at low levels, may also contribute to redundancy. In our study, Wnt-2b, which is required for limb initiation in zebrafish and chick (52;53), was absent in resting and proliferative chondrocytes, but consistently present in hypertrophic and prehypertrophic regions. There is no study involving ablation or overexpression of Wnt-2b in growth plate to add insights about its role in growth plate regulation.
In addition to the Wnts that act though the β-catenin pathway, we also identified three Wnts expressed in postnatal growth plate, Wnts -5a, -5b and -11, that act through the calcium pathway (54). This noncanonical pathway can antagonize the canonical Wnt/β-catenin pathway by promoting the degradation of β-catenin (55;56). The calcium pathway appears to have an important role in growth plate formation and function. Wnt-5a null mice display a severe skeletal phenotype with limb truncation. In growth plates, chondrocyte hypertrophy is delayed, suggesting that Wnt-5a promotes chondrocyte hypertrophy (57;58). However, Wnt-5a overexpression also delays hypertrophy, although this effect may be secondary to delayed formation of proliferative chondrocytes (59). Wnt-5b overexpression appears to have different effects, promoting proliferative zone formation and inhibiting cell cycle withdrawal and chondrocyte hypertrophy (60).
We found that Wnts -5a and -5b expression was low in resting zone, increased as the cells differentiated in the proliferative and prehypertrophic regions, and then decreased as cells further differentiated into hypertrophic chondrocytes. This pattern is consistent with the findings from mouse models, described above, suggesting that Wnts -5a and -5b may modulate production of proliferative zone chondrocytes and their conversion to hypertrophic chondrocytes. This expression pattern is also similar to, though perhaps not identical to, the expression pattern observed by in situ hybridization in the embryonic skeleton; Wnt-5a was observed in proliferative and prehypertrophic chondrocytes and Wnt-5b in the region between prehypertrophic and hypertrophic chondrocytes (61).
In addition to Wnts -5a and -5b, we found that Wnt-11, which also signals through the calcium pathway, was also expressed in growth plate with a similar spatial pattern of expression. In the embryonic chick, Wnt-11 expression in the growth plate, assessed by in situ hybridization, was found to be restricted to the prehypertrophic region. The wider expression pattern observed in the current study could be related to differences in species, stage of development, or the sensitivity of the technique. Overexpression of Wnt-11 in the developing chick limb results in slightly truncated limbs and joint fusion but does not appear to delay chondrocyte differentiation (62). Little is known about Wnt-11 function in the mammalian growth plate. Our study, showing similar expression patterns for Wnts -5a, -5b, and -11, all of which signal through the calcium pathway, suggest that these three members of the Wnt family may have redundant, overlapping, or interacting roles in the growth plate. Therefore, ablation of these genes in combinations may reveal a growth plate phenotype that is more severe and perhaps qualitatively different than occurs with ablation of any single one of these genes.
Interestingly, the general pattern of Wnt expression was similar to the pattern of Ihh expression in the growth plates of 1-week-old mice in that both showed low expression in resting zone, high expression in the prehypertrophic region and lower expression in hypertrophic zone. This similarity suggests a possible interaction. In cultures of mesenchymal cells from chick wing bud, no interaction between Ihh and Wnts -5b or -11 was detected (63). However, in embryonic mouse long bones, Wnt-9a ablation caused downregulation of Ihh expression and signaling (64). Whether Wnts, either those signaling through the canonical pathway or those signaling through the calcium pathway, interact with Ihh in the postnatal growth plate is unknown.
Some of the Wnt genes that we found to be expressed in the postnatal growth plate have also been implicated in other models of postnatal chondrogenesis. Human dermal fibroblasts cultured in the presence of chondroinductive demineralized bone powder show increased expression of Wnt-2b, Wnt-5b, and Wnt-10b (65), whereas Wnts -4, -5a and -5b are up-regulated during bone repair in vivo (66;67).
It is noteworthy that Wnts that are known to be associated with certain skeletal diseases, such as Wnts -3 and -7a, were not found in postnatal growth plate and surrounding tissues of mice. Tetra-amelia, a congenital syndrome with complete limb agenesis, is associated with homozygous mutations in Wnt-3 (68), and, in mice, postaxial hemimelia results from a mutation in Wnt-7a (69). Our findings therefore suggest that Wnts -3 and -7a are essential in early stages of limb formation, but not essential for postnatal chondrocyte differentiation.
Temporal regulation of Wnt expression in growth plate
Growth plate structure and function do not remain constant during postnatal life. Rather, growth plate function declines and eventually ceases, due, in large part, to a decline in chondrocyte proliferation (70). This decline in function is accompanied by changes in growth plate structure; the number of chondrocytes in the resting, proliferative and hypertrophic zones decreases as does the size of the individual hypertrophic cells (71). The mechanisms responsible for this programmed senescence of the growth plate are only partially understood (72-74).
We hypothesized that changes in Wnt gene expression might contribute to growth plate senescence. We therefore studied Wnt expression in the growth plate at 4 weeks, an age by which longitudinal bone growth has slowed markedly, approximately two-fold, in the mouse (75). Contrary to our hypothesis, we found that all Wnts that had been readily detected in the growth plates of 1-week-old mice were still expressed in growth plate cartilage of 4-week-old mice, at similar levels. However, because the growth plates of 4-week-old mice were thinner, we were not able to microdissect individual zones but instead grouped the resting and proliferative zones together and also grouped the prehypertrophic and hypertrophic regions together. As a result, our comparisons to 1-week-old mice were less precise. We conclude only that expression persisted and remained at similar levels, but we cannot exclude modest changes in gene expression of Wnts with age.
Wnt-2b mRNA was not detected in the resting or proliferative zones in 1-week old mice but was detected in the resting/proliferative region in 4-week old mice. However, the level of expression was near the detection limit, and thus it is not clear whether this represents a true change in gene expression. In 1-week old mice, Wnt-11 mRNA levels were similar in the proliferative zone and prehypertrophic zone whereas in 4-week old mice, Wnt-11 mRNA levels were higher in the proliferative/resting region than in the hypertrophic/prehypertrophic region, suggesting that Wnt-11 expression may shift to an earlier stage of chondrocyte differentiation as the animal ages and longitudinal bone growth slows.
In summary, we used manual microdissection and real-time PCR to study mRNA expression of Wnt genes in growth plate cartilage, including both spatial and temporal regulation of expression. We found that only six of the 19 known Wnts were expressed in postnatal growth plate cartilage. Although ours is the first study to investigate extensively Wnt expression in the postnatal mammalian growth plate, some of these members of the Wnt family, Wnts -4, -5a and -5b, had previously been implicated in embryonic mammalian cartilage, whereas Wnts -2b, -10b, and -11 had not previously been implicated in the regulation of the mammalian growth plate at any stage of development. Of the six Wnts that we found to be expressed in the growth plate, three (Wnts -2b, -4, and -10b) signal through the canonical, β-catenin pathway and three (Wnts -5a, -5b, and -11) signal through the noncanonical, calcium pathway. The spatial expression for all these Wnts was remarkably similar, showing low mRNA expression in the resting zone, increasing expression as the chondrocytes differentiated into the proliferative and prehypertrophic region and then decreasing expression as the chondrocytes underwent hypertrophic differentiation. Wnt-2b may be a partial exception to this generalization in that expression did not seem to decrease in the hypertrophic zone. This overall pattern of gene expression is broadly consistent with previous studies in the mouse embryo suggesting that Wnts, both those that signal through the canonical and those that signal through noncanonical pathways modulate chondrocyte hypertrophic differentiation, and perhaps, for Wnts -5a and -5b, differentiation into the proliferative state. We also found that mRNA expression of all these members of the Wnt family persisted at similar levels at 4-weeks, an age at which longitudinal bone growth is slowing in the mouse.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH. Ola Nilsson was supported by grants from the Swedish Research Council (K2003-72PK-15191-01A) and the Swedish Society for Medical Research. The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Correspondence and requests for reprints to: Anenisia C Andrade Rua Castro Alves, 279, Apto133, Aclimação São Paulo, Brasil ZIP CODE: 01532-001 Voice: +55 (11) 3205-1938 Fax: +55 (11) 3205-1938 anenisia@gmail.com
Reference List
- 1.Schrier L, Ferns SP, Barnes KM, Emons JA, Newman EI, Nilsson O, Baron J. Depletion of resting zone chondrocytes during growth plate senescence. J Endocrinol. 2006;189:27–36. doi: 10.1677/joe.1.06489. [DOI] [PubMed] [Google Scholar]
- 2.Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech. 1994;28:505–519. doi: 10.1002/jemt.1070280606. [DOI] [PubMed] [Google Scholar]
- 3.Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J. The role of the resting zone in growth plate chondrogenesis. Endocrinology. 2002;143:1851–1857. doi: 10.1210/endo.143.5.8776. [DOI] [PubMed] [Google Scholar]
- 4.van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev. 2003;24:782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson O, Marino R, De LF, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64:157–165. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 6.Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech. 1994;28:505–519. doi: 10.1002/jemt.1070280606. [DOI] [PubMed] [Google Scholar]
- 7.Horton WA. Skeletal development: insights from targeting the mouse genome. Lancet. 2003;362:560–569. doi: 10.1016/S0140-6736(03)14119-0. [DOI] [PubMed] [Google Scholar]
- 8.van Donkelaar CC, Huiskes R. The PTHrP-Ihh Feedback Loop in the Embryonic Growth Plate Allows PTHrP to Control Hypertrophy and Ihh to Regulate Proliferation. Biomech Model Mechanobiol. 2006 doi: 10.1007/s10237-006-0035-0. [DOI] [PubMed] [Google Scholar]
- 9.Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115:1734–1742. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiyama H, Shigeno C, Iyama K, Ito H, Hiraki Y, Konishi J, Nakamura T. Indian hedgehog in the late-phase differentiation in mouse chondrogenic EC cells, ATDC5: upregulation of type X collagen and osteoprotegerin ligand mRNAs. Biochem Biophys Res Commun. 1999;257:814–820. doi: 10.1006/bbrc.1999.0494. [DOI] [PubMed] [Google Scholar]
- 12.Su WC, Kitagawa M, Xue N, Xie B, Garofalo S, Cho J, Deng C, Horton WA, Fu XY. Activation of Stat1 by mutant fibroblast growth-factor receptor in thanatophoric dysplasia type II dwarfism. Nature. 1997;386:288–292. doi: 10.1038/386288a0. [DOI] [PubMed] [Google Scholar]
- 13.Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005;16:205–213. doi: 10.1016/j.cytogfr.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minina E, Schneider S, Rosowski M, Lauster R, Vortkamp A. Expression of Fgf and Tgfbeta signaling related genes during embryonic endochondral ossification. Gene Expr Patterns. 2005;6:102–109. doi: 10.1016/j.modgep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 221996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 16.Yates KE, Shortkroff S, Reish RG. Wnt influence on chondrocyte differentiation and cartilage function. DNA Cell Biol. 2005;24:446–457. doi: 10.1089/dna.2005.24.446. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y. Wnts and wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res C Embryo Today. 2003;69:305–317. doi: 10.1002/bdrc.10026. [DOI] [PubMed] [Google Scholar]
- 18.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 19.Church VL, Francis-West P. Wnt signalling during limb development. Int J Dev Biol. 2002;46:927–936. [PubMed] [Google Scholar]
- 20.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 21.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 23.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann C. Wnt-signaling and skeletogenesis. J Musculoskelet Neuronal Interact. 2002;2:274–276. [PubMed] [Google Scholar]
- 25.Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006;16:151–158. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y. Wnts and wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res C Embryo Today. 2003;69:305–317. doi: 10.1002/bdrc.10026. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. de CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council . National Academy Press; Washington, D.C.: 2003. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 33.Heinrichs C, Yanovski JA, Roth AH, Yu YM, Domene HM, Yano K, Cutler GB, Jr., Baron J. Dexamethasone increases growth hormone receptor messenger ribonucleic acid levels in liver and growth plate. Endocrinology. 1994;135:1113–1118. doi: 10.1210/endo.135.3.8070354. [DOI] [PubMed] [Google Scholar]
- 34.Goidin D, Mamessier A, Staquet MJ, Schmitt D, Berthier-Vergnes O. Ribosomal 18S RNA prevails over glyceraldehyde-3-phosphate dehydrogenase and beta-actin genes as internal standard for quantitative comparison of mRNA levels in invasive and noninvasive human melanoma cell subpopulations. Anal Biochem. 2001;295:17–21. doi: 10.1006/abio.2001.5171. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji N, Kamagata C, Furuya M, Kobayashi D, Yagihashi A, Morita T, Horita S, Watanabe N. Selection of an internal control gene for quantitation of mRNA in colonic tissues. Anticancer Res. 2002;22:4173–4178. [PubMed] [Google Scholar]
- 36.Gerstenfeld LC, Landis WJ. Gene expression and extracellular matrix ultrastructure of a mineralizing chondrocyte cell culture system. J Cell Biol. 1991;112:501–513. doi: 10.1083/jcb.112.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid TM, Linsenmayer TF. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol. 1985;100:598–605. doi: 10.1083/jcb.100.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Middleton F, Horton JA, Reichel L, Farnum CE, Damron TA. Microarray analysis of proliferative and hypertrophic growth plate zones identifies differentiation markers and signal pathways. Bone. 2004;35:1273–1293. doi: 10.1016/j.bone.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Bouillet P, Oulad-Abdelghani M, Ward SJ, Bronner S, Chambon P, Dolle P. A new mouse member of the Wnt gene family, mWnt-8, is expressed during early embryogenesis and is ectopically induced by retinoic acid. Mech Dev. 1996;58:141–152. doi: 10.1016/s0925-4773(96)00569-2. [DOI] [PubMed] [Google Scholar]
- 40.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 42.Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 43.Lako M, Strachan T, Bullen P, Wilson DI, Robson SC, Lindsay S. Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene. 1998;219:101–110. doi: 10.1016/s0378-1119(98)00393-x. [DOI] [PubMed] [Google Scholar]
- 44.Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 45.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. de CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spater D, Hill TP, O'sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 49.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 50.Spater D, Hill TP, O'sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 51.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, Macdougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawakami Y, Capdevila J, Buscher D, Itoh T, Rodriguez EC, Izpisua Belmonte JC. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 53.Ng JK, Kawakami Y, Buscher D, Raya A, Itoh T, Koth CM, Rodriguez EC, Rodriguez-Leon J, Garrity DM, Fishman MC, Izpisua Belmonte JC. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development. 2002;129:5161–5170. doi: 10.1242/dev.129.22.5161. [DOI] [PubMed] [Google Scholar]
- 54.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 55.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 56.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 62.Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 63.Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 64.Spater D, Hill TP, O'sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–3049. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- 65.Yates KE. Demineralized bone alters expression of Wnt network components during chondroinduction of post-natal fibroblasts. Osteoarthritis Cartilage. 2004;12:497–505. doi: 10.1016/j.joca.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002;277:30177–30182. doi: 10.1074/jbc.M203171200. [DOI] [PubMed] [Google Scholar]
- 67.Zhong N, Gersch RP, Hadjiargyrou M. Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone. 2006 doi: 10.1016/j.bone.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 68.Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, Weber JL, Muller U. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet. 2004;74:558–563. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parr BA, Avery EJ, Cygan JA, McMahon AP. The classical mouse mutant postaxial hemimelia results from a mutation in the Wnt 7a gene. Dev Biol. 1998;202:228–234. doi: 10.1006/dbio.1998.9007. [DOI] [PubMed] [Google Scholar]
- 70.Kember NF. Proliferation controls in a linear growth system: theoretical studies of cell division in the cartilage growth plate. J Theor Biol. 1979;78:365–374. doi: 10.1016/0022-5193(79)90336-9. [DOI] [PubMed] [Google Scholar]
- 71.Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci U S A. 2001;98:6871–6876. doi: 10.1073/pnas.121180498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emons JA, Boersma B, Baron J, Wit JM. Catch-up growth: testing the hypothesis of delayed growth plate senescence in humans. J Pediatr. 2005;147:843–846. doi: 10.1016/j.jpeds.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 73.Nilsson O, Baron J. Fundamental limits on longitudinal bone growth: growth plate senescence and epiphyseal fusion. Trends Endocrinol Metab. 2004;15:370–374. doi: 10.1016/j.tem.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Schrier L, Ferns SP, Barnes KM, Emons JA, Newman EI, Nilsson O, Baron J. Depletion of resting zone chondrocytes during growth plate senescence. J Endocrinol. 2006;189:27–36. doi: 10.1677/joe.1.06489. [DOI] [PubMed] [Google Scholar]
- 75.van BS, Van den BJ. The Snell-dwarfmouse. I. General growth pattern, before and during growth hormone and thyroxine therapy. Acta Endocrinol (Copenh) 1978;89:632–645. [PubMed] [Google Scholar]