Abstract
Background
This study examined the influence of increased cardiac size on maximal lung volumes, forced expiratory airflows, and the diffusing capacity of the lungs in heart failure (HF) patients compared to controls.
Methods and Results
Forty-one HF patients of New York Heart Association (NYHA) class: Group A=class I/II (n=26) and Group B=class III/IV (n=15) and an equal number matched controls (CTL) were recruited. Participants underwent echocardiography (ECHO), spirometry, and posterioanterior and lateral chest radiographic evaluation (RAD) for volumetric estimation of the total thoracic cavity (TTC), diaphragm, heart, and lungs. ANOVA demonstrated no difference between groups for TTC volume (p=0.63). RAD cardiac volumes (% TTC volume) were significantly different among all groups (p<0.001). ECHO determined left ventricular mass was elevated in the HF groups compared to the CTL group (p<0.001) with no difference between HF groups. Lung volume (% TTC volume) was reduced as a function of disease severity (p<0.001). RAD measures of cardiac volume demonstrated the strongest relationship with restrictive lung alterations (t-statistic=-5.627, p<0.001 and t-statistic=-4.378, p<0.001 for FVC and FEV1, respectively).
Conclusions
These results suggest cardiac size may pose significant constraints on the lungs and likely plays a major role in the restrictive breathing patterns often reported in HF patients.
Keywords: Cardiomegaly, Roentenography, Lung volume, Heart Failure
INTRODUCTION
Chronic heart failure (HF) is associated with mild to moderate changes in pulmonary function, including restrictive and obstructive changes as well as a reduction in lung diffusing capacity (DLCO) 1-3. Although heart failure induced causes of altered lung function remain unclear, they have been attributed to respiratory muscle weakness, pulmonary hypertension, changes in lung fluid balance, and chronic neurohumoral changes 4-6. Because the lungs and heart both reside in a common enclosure (chest wall) and the cardiac muscle is less compliant than the lungs another potential contributor to the changes in pulmonary function in HF relates to progressive cardiac enlargement within the thoracic cavity. Such changes in cardiac volume would be expected to result in primarily restrictive lung changes manifested as reductions in total lung volume and vital capacity 2.
In addition, it may be expected that a relationship also exists between cardiac volume and maximal expiratory airflows as well as the DLCO. As cardiac filling pressures increase and pulmonary congestion progressively develops, blood flow may back up into the bronchial circulation and influence airway caliber resulting in airflow limitations 7. Further, the reduction in DLCO with disease severity is likely related to lung fluid imbalance and chronic changes at the alveolar-capillary membrane 3.
Epidemiological studies have shown a link between pulmonary function and mortality, particularly related to cardiovascular events 8-10. Although the causal link between lung function and cardiac mortality remains unclear, it may be associated with the progressive changes in cardiac size. Studies have implied a marginal link between cardiac size and lung function in HF 1,11-14; however, these studies are limited by echocardiographic measurement of left ventricular mass as opposed to total heart size and one-dimensional estimates of the cardio-thoracic relationship. Importantly, these studies may have inadequately represented the importance of changes in total cardiac size on lung function in relation to the constraints imposed by the thoracic cavity.
The focus of this study was to examine the relationship between radiographically determined cardiac volume and maximal lung volumes, maximal expiratory airflows, and DLCO in patients with long standing, but stable HF. Further, we sought to determine if a commonly obtained echocardiographic measure of cardiac size in this population might be as predictive of lung function changes. We hypothesized that increased competition for intrathoracic space caused by changes in cardiac volume associated with chronic HF contributes to changes observed in pulmonary function and the commonly derived echocardiographic measures of cardiac dimension would inadequately predict these changes.
METHODS
Population Characteristics
Forty-one HF patients were recruited from the Mayo Clinic Heart Failure Service and the Cardiovascular Health Clinic (a preventive and rehabilitative center) over the period of 2000 to 2004 (Table 1). Patients included those with a history of ischemic or dilated cardiomyopathy, stable HF symptoms (>3 months), duration of HF symptoms >1 year, left ventricular ejection fraction (EF) ≤35%, body mass index (BMI) <35 kg/m2, and current non-smokers (past 15 yrs) with a smoking history <10 pack-years. Patients were treated with standard optimized medications for heart failure at the time of the study. An equal number of control participants were recruited via advertisement from the surrounding area and were matched with the HF group for age, gender, and height. Control participants had normal cardiac function (EF>50%), without history of hypertension, lung disease or coronary artery disease (CAD). All participants gave written informed consent after being provided a description of study requirements. The study protocol was approved by the Mayo Clinic Institutional Review Board; all procedures followed institutional and HIPAA guidelines.
TABLE 1.
Participant Characteristics and Patient Medications
| Control | Group A | Group B | p-value | |
|---|---|---|---|---|
| N (% Female) | 41 (39) | 26 (54) | 15 (40) | 0.39 |
| Age (yr) | 57.8 ± 13.1 | 56.6 ± 12.6 | 55.4 ± 12.7 | 0.81 |
| Height (cm) | 171.0 ± 9.4 | 170.6 ± 9.4 | 173.2 ± 10.6 | 0.68 |
| Weight (kg) | 75.1 ± 13.6 | 81.0 ± 15.1 | 87.2 ± 14.0* | 0.02 |
| BMI (kg/m2) | 25.5 ± 3.0 | 27.9 ± 4.8 | 29.2 ± 5.6* | 0.01 |
| BSA (m2) | 1.87 ± 0.21 | 1.93 ± 0.21 | 2.01 ± 0.18 | 0.08 |
| Smoking History (pack yrs) | 3.02 ± 7.07 | 2.90 ± 6.26 | 2.33 ± 4.48 | 0.94 |
| Exercise History (min/week) | 187.6 ± 168.7 | 62.9 ± 87.8* | 56.0 ± 60.3* | <0.001 |
| LV Ejection Fraction (%) | 63.2 ± 7.9 | 30.5 ± 11.6* | 26.3 ± 10.9* | <0.001 |
| NYHA Class | class I, n=15
class II, n=11 |
class III, n=7
class IV, n=8 |
<0.001 | |
| Medications | Number (% of total) | Number (% of total) | ||
| ACE Inhibitors | - | 16 (62) | 13 (87) | 0.15 |
| A II Receptor Blockers | - | 4 (15) | 1 (7) | 0.64 |
| β-Blockers | - | 14 (54) | 12 (80) | 0.18 |
| Digitalis | - | 16 (62) | 9 (60) | 0.99 |
| Diuretics | - | 16 (62) | 12 (80) | 0.30 |
Data are presented as mean±SD unless otherwise noted. ACE, Angiotensin Converting Enzyme; BMI, Body Mass Index; BSA, Body Surface Area; NYHA, New York Heart Association.
=p<0.05 vs. control group.
=p<0.05 vs. group A.
Overview of Protocol
Participants underwent posterioanterior (PA) and lateral (LAT) chest radiographs, echocardiography, and spirometry. The HF patients were divided into two groups by New York Heart Association (NYHA) class as follows: class I and II, n = 26 (Group A) and class III and IV, n= 15 (Group B).
Radiographic Volumetric Evaluation
The PA and LAT radiographic views were used to make volumetric estimations of the total thoracic cavity (TTC), diaphragm, cardiac, and lungs (TLCR) based on the assumptions of a partial ellipsoid as initially described by Barnhard and colleagues 15 and later by Glenn and Greene as well as others 16-18. This methodology has been shown to be valid and reliable 16,17. Details of this technique from our laboratory, in a companion cohort of HF and matched controls, are published elsewhere 19. Briefly, the inner most edge of the intra-thoracic cavity and outer most edge of the cardiac silhouette on both radiographic views were manually traced on a digitizing tablet (AccuGrid A43BL, Numonics Corp, Montgomeryville, Pennsylvania) with data exported to a digitizing software program (Didger 3, Golden Software Inc, Golden, Colorado) on a personal computer for offline analysis. Coordinate data were used to make linear measurements for the volumetric computation. The volumetric measures for total thoracic cavity volume (TTCV), cardiac volume (CV), and the total radiographic lung volume (TLCR) were calculated as follow: TTCV=(1/4 π)*D1*D2*D3 where Dn represents width, depth and height of the PA and LAT views, CV=(1/6π)*D1*D2*D3 where Dn represents diameters of the atrium and ventricles in the PA and LAT views and TLC=TTCV=(CV+DV+PBV+PTV) where DV represents diaphragm volume, PBV pulmonary blood volume and PTV, parenchymal tissue volume (for details see reference 19).
Echocardiographic Evaluation
Doppler and 2D echocardiographic measurements were performed according to the recommendations of the American Society of Echocardiography 20. Left atrial (LA) dimension, left ventricular (LV) mass, LV internal dimension during systole and diastole (LVIDs and LVIDd, respectively), interventricular septal thickness (IVST), LV posterior wall thickness and left atrial end-diastolic dimension were measured. Left ventricular mass was calculated using the formula of Troy and colleagues 21. Left ventricular mass index was calculated as left ventricular mass divided by body surface area. The LV ejection fraction (EF%) was calculated using the modified Simpson’s rule 22. Transmitral inflow velocity was obtained from a 2-dimensional apical window with the pulsed wave Doppler function facilitating the calculation of maximal early flow velocity (E), maximal late flow velocity (A), the ratio of maximal early to late flow velocity (E/A), and deceleration time of the early diastolic filling.
Pulmonary Function Evaluation
Participants underwent spirometry evaluation including, forced vital capacity (FVC) and assessment of maximal expiratory airflows including forced expiratory volume in one second (FEV1), mean forced expiratory flow between 25% and 75% of the FVC (FEF25-75) and maximal FEF (FEFmax). Participants also underwent assessment of the diffusing capacity of the lung for carbon monoxide (DLCO) and measurement of alveolar volume (TLCVA) using the single breath method. Spirometry and DLCO measures were collected in accordance with the American Thoracic Society (ATS) standards 23,24.
Statistical Analysis
Statistical analysis and graphic presentation were accomplished using SPSS (v 12.0, Chicago, IL) and Graphpad Prism (v 4.0, San Diego, CA). One-way analysis of variance (ANOVA) was used to test means across the groups with Bonferonni post-hoc analysis where appropriate. Unpaired t-tests were used to compare the control and the entire CHF group. Partial correlations were calculated between radiographic measures and measures of HF severity adjusting for age, height, weight, body surface area, smoking history, and systolic and diastolic blood pressure. Standardized beta coefficients were calculated from linear regression. Fischer’s exact test was used to test for differences in categorical variables. Statistical significance was set at p<0.05 for all analyses. Data are presented as mean ± standard deviation (SD) or number and percent of the group.
RESULTS
Population Characteristics
The clinical characteristics of each study group are reported in Table 1. Notable differences include a lower body mass index for the control group compared to Group B (p<0.05) due to a difference in body weight between groups (p<0.05) as opposed to a difference in height. These differences contributed to the trend for elevated BSA in Group B (p=0.08). By definition Group A demonstrated a greater NYHA class compared to Group B (p<0.05) although there were no differences between the HF groups for medication use. Also, the control group had greater exercise history compared to either Group A (p<0.05) or Group B (p<0.05) with no differences between the HF groups.
Radiographic Evaluation
The radiographic volumetric measurements are reported in Table 2. There were no differences between the groups for TTC volume (p=0.63). The groups differed significantly in blood and parenchymal tissue, cardiac, and lung volumes expressed in absolute terms (p<0.05 for all). There were differences between the groups for all measurements (p<0.05) when examining these measurements as a % of the TTC volume. Importantly, the radiographic measures delineated differences between the HF groups for absolute cardiac volume (p<0.05) and both cardiac volume and lung volume as a % of the TTC volume (p<0.05 for both).
TABLE 2.
Differences in Radiographically Determined Volumes and Structural Echocardiographic Measures across Groups
| Control | Group A | Group B | p-value | |
|---|---|---|---|---|
| Absolute Volumetric Estimations | ||||
| Total Thoracic Volume (cm3) | 8445.0 ± 1496.8 | 8070.8 ± 1716. 6 | 8252.0 ± 1493.5 | 0.63 |
| Blood and Tissue Volume (cm3) | 937.6± 134.2 | 997.5± 160.9 | 1049.4 ± 121.2* | 0.03 |
| Diaphragm Volume (cm3) | 942.6 ± 295.2 | 936.3± 331.4 | 1161.3 ± 493.2 | 0.09 |
| Cardiac Volume (cm3) | 608.6 ± 156.7 | 978.7 ± 350.7* | 1337.5 ± 384.0*† | 0.001 |
| Lung Volume (cm3) | 5956.3 ± 1197.2 | 5158.5 ± 1422.4 | 4703.8 ± 1361.3* | 0.003 |
| Percent of Total Thoracic Cavity Volume | ||||
| Blood and Tissue (%) | 11.3 ± 2.1 | 12.8 ± 3.4 | 13.1 ± 2.6* | 0.03 |
| Diaphragm (%) | 11.1 ± 2.7 | 11.8± 4.0 | 13.9 ± 5.1* | 0.04 |
| Cardiac (%) | 7.3 ± 1.7 | 12.3 ± 4.4* | 16.8 ± 6.0*† | <0.001 |
| Lung (%) | 70.3 ± 3.6 | 63.1 ± 9.5* | 56.2 ± 7.2*† | <0.001 |
| Structural Echocardiographic Measures | ||||
| LA Dimension (mm) | 33.6 ± 4.7 | 45.5 ± 9.8* | 49.7 ± 6.5* | <0.001 |
| LV Mass (g) | 163.4 ± 45.5 | 271.5 ± 103.4* | 271.8 ± 68.8* | <0.001 |
| LV Mass Index (g/m2) | 82.2 ± 22.9 | 139.2 ± 44.3* | 134.2 ± 35.9* | 0.001 |
Data are presented as mean±SD unless otherwise noted. LA, left atrial; LV, left ventricular.
=p<0.05 vs. control group.
=p<0.05 vs. group A.
Echocardiographic Evaluation
The results of the primary structural echocardiographic evaluation are also shown in Table 2. The LV mass was greater in both HF groups compared to the CTL group (p<0.05 for both CHF groups) as was the LV mass index (p<0.05 for both CHF groups). Also, there was a difference in left atrial dimension between the HF groups and CTL group (p<0.05 for both HF groups). Although there was no difference among the groups for LV posterior wall thickness (CTL=9.1±2.0 vs. Group A=10.0±2.0 vs. Group B=9.2±1.2 mm, p=0.17), there was a difference between the HF groups compared to the CTL group for LV dimension during systole (CTL=29.5±5.2 vs. Group A=53.5±11.3 vs. Group B=56.6±14.0 mm, ANOVA p<0.001 and p<0.05 for both HF groups) and diastole (CTL=48.4±5.4 vs. Group A=64.0±9.2 vs. Group B=65.9±11.6 mm, p<0.001 and p<0.05 for both HF groups). Interestingly, as opposed to the radiographic evaluation, the echocardiographic measures of LV structure (i.e. LV mass, LV mass index, and LV dimension during systole or diastole) did not differ between the HF groups relative to disease severity. The ANOVA demonstrated no significant differences between the groups for peak A-wave velocity (CTL=0.6±0.1 vs. Group A=0.7±0.3 vs. Group B=0.6±0.2 m/sec, p=0.12), E/A ratio (CTL=1.3±0.5 vs. Group A=1.4±1.1 vs. Group B=1.6±1.1 mm, p=0.51), or E-wave deceleration time (CTL=205.9±49.8 vs. Group A=212.7±81.7 vs. Group B=183.8±42.1 msec, p=0.40). There was, however, a significant difference between the CTL group and Group B for peak E-wave velocity (CTL=0.7±0.2 vs. Group B=0.9±0.3 m/sec, p<0.05) whereas Group A (0.8±0.3 m/sec) was not statistically different from either the CTL group or Group B.
Pulmonary Function Evaluation
The results of the pulmonary function evaluation are detailed in Table 3. The HF group demonstrated primarily restrictive changes compared to the CTL group, noted by the reduction in the % predicted FVC, and FEV1 with a comparable FEV1/FVC ratio. Group A demonstrated a reduced FEF25-75 compared to the CTL group (p<0.05 for both absolute and % predicted). Both HF groups demonstrated reduced % predicted DLCO (p<0.05 for both groups) whereas only Group B differed from the CTL group for the % predicted VA (p<0.05).
TABLE 3.
Differences in Pulmonary Function Measures across Groups
| Control | Group A | Group B | p-value | |
|---|---|---|---|---|
| FVC (L) | 4.24 ± 1.05 | 3.43 ± 1.03* | 3.44 ± 1.42* | 0.003 |
| %Pred | 105.9 ± 13.1 | 85.2 ± 16.8* | 78.9 ± 21.9* | <0.001 |
| FEV1 (L/sec) | 3.32 ± 0.76 | 2.62 ± 0.73* | 2.73 ± 1.14 | 0.002 |
| %Pred | 104.7 ± 15.2 | 82.7 ± 16.2* | 76.5 ± 24.7* | <0.001 |
| FEV1/FVC | 78.4 ± 7.1 | 77.4 ± 6.0 | 78.9 ± 4.4 | 0.73 |
| FEF25-75 (L/sec) | 2.88 ± 1.06 | 2.24 ± 0.71* | 2.46 ± 1.08 | 0.03 |
| %Pred | 99.4 ± 31.5 | 79.2 ± 24.7* | 77.6 ± 30.7 | 0.008 |
| DLCO (mL/min/mm Hg) | 24.6 ± 6.0 | 21.5 ± 6.3 | 22.2 ± 5.8 | 0.11 |
| %Pred | 96.3 ± 14.3 | 86.3 ± 14.4* | 83.2 ± 18.0* | 0.004 |
| VA (L) | 5.79 ± 1.04 | 5.27 ± 1.17* | 5.37 ± 1.62 | 0.18 |
| %Pred | 99.2 ± 8.3 | 89.2 ± 11.3 | 85.8 ± 18.2* | <0.001 |
Data are presented as mean±SD. VC, Vital Capacity; FVC, Forced Vital Capacity; FEV1, Forced Expiratory Volume in one second; FEF, Forced Expiratory Flow; DLCO, Diffusion Capacity of the Lung for Carbon Monoxide; VA, Alveolar Volume.
=p<0.05 vs. control group.
=p<0.05 vs. group A.
Comparison of Radiographic and Echocardiographic Measures of Cardiac Size in Predicting Lung Volumes and Restrictive Pulmonary Changes
Table 4 details the partial correlation coefficients between the TLCR, TLCVA, and DLCO and the radiographically and echocardiographically determined cardiac size. This analysis highlights the negative relationship between lung volume and cardiac size, although the echocardiographically determined measures of cardiac size did not correlate with the % predicted TLCVA. There also was a significant relationship between DLCO and left atrial dimension and the absolute cardiac volume. The relationship with the absolute cardiac volume however was no longer significant but rather demonstrated a trend after correcting for TTC volume.
TABLE 4.
Regression Analysis for Lung Volume and Diffusion Capacity with Measures of Cardiac Size
| Partial Correlation | p-value | Standardized Beta Coefficient | t-statistic | p-value | |
|---|---|---|---|---|---|
| TLCR | |||||
| LA Dimension (mm) | -0.52 | <0.001 | -0.09 | -0.68 | 0.50 |
| LV Mass Index (g/m2) | -0.47 | 0.002 | 0.65 | 1.88 | 0.07 |
| Cardiac Volume (cm3)* | -0.70 | <0.001 | 0.69 | 3.03 | 0.004 |
| Cardiac (%)* | -0.78 | <0.001 | -1.33 | -7.40 | <0.001 |
| TLCVA | |||||
| LA Dimension (mm) | -0.19 | 0.23 | -0.24 | -1.51 | 0.14 |
| LV Mass Index (g/m2) | -0.18 | 0.26 | 0.40 | 0.91 | 0.37 |
| Cardiac Volume (cm3)* | -0.28 | 0.07 | 1.28 | 4.41 | <0.001 |
| Cardiac (%)* | -0.49 | <0.001 | -1.48 | -6.39 | <0.001 |
| DLCO | |||||
| LA Dimension (mm) | -0.41 | 0.007 | -0.52 | -2.47 | 0.02 |
| LV Mass Index (g/m2) | -0.06 | 0.68 | -0.33 | -0.56 | 0.58 |
| Cardiac Volume (cm3)* | -0.11 | 0.48 | 1.02 | 2.67 | 0.01 |
| Cardiac (%)* | -0.23 | 0.15 | -0.57 | -1.88 | 0.07 |
Data are presented as mean ± SD. Regression analysis were adjusted for age, height, weight, body surface area, smoking history, and blood pressure. LA, Left Atria; LV, Left Ventricle.
indicates radiographic measures.
Table 5 outlines the relationship between FVC, FEV1, and FEF25-75 and radiographically and echocardiographically determined cardiac size. The partial correlation coefficient suggests a close relationship between both the radiographically determined cardiac volume and FVC. There was also a close correlation between the echocardiographically determined LV Mass and LV Mass Index and FVC. There were no significant relationships between radiographically and echocardiographically determined cardiac dimensions and FEF25-75. The partial correlation analysis demonstrated a minor trend towards a significant relationship between FEF25-75 and left atrial dimension although there were no other significant relationships.
TABLE 5.
Regression Analysis for Restrictive Pulmonary Changes with Measures of Cardiac Size
| Partial Correlation | p-value | Standardized Beta Coefficient | t-statistic | p-value | |
|---|---|---|---|---|---|
| FVC | |||||
| LA Dimension (mm) | -0.42 | 0.005 | -0.30 | -2.11 | 0.04 |
| LV Mass Index (g/m2) | -0.31 | 0.05 | 0.95 | 2.46 | 0.02 |
| Cardiac Volume (cm3)* | -0.40 | 0.008 | 0.92 | 3.59 | <0.001 |
| Cardiac (%)* | -0.59 | <0.001 | -1.14 | -5.63 | <0.001 |
| FEV1 | |||||
| LA Dimension (mm) | -0.43 | 0.004 | -0.38 | -2.41 | 0.02 |
| LV Mass Index (g/m2) | -0.22 | 0.16 | 0.93 | 2.11 | 0.04 |
| Cardiac Volume (cm3)* | -0.33 | 0.03 | 0.87 | 3.01 | 0.004 |
| Cardiac (%)* | -0.47 | 0.002 | -1.01 | -4.38 | <0.001 |
| FEF 25-75 | |||||
| LA Dimension (mm) | -0.26 | 0.09 | -0.35 | -1.60 | 0.12 |
| LV Mass Index (g/m2) | 0.03 | 0.86 | 0.72 | 1.19 | 0.24 |
| Cardiac Volume (cm3)* | -0.06 | 0.72 | 0.39 | 0.99 | 0.33 |
| Cardiac (%)* | -0.07 | 0.68 | -0.25 | -0.79 | 0.43 |
Data are presented as mean ± SD. Regression analysis were adjusted for age, height, weight, body surface area, smoking history, and blood pressure. LA, Left Atria; LV, Left Ventricle.
indicates radiographic measures.
Figure 1 graphically depicts key relationships outlined in tables 4 and 5 separated by groups. These figures demonstrate that the elevation in cardiac mass is closely related to the reduction in lung volumes and restrictive pulmonary changes in this population of HF patients.
FIGURE 1.
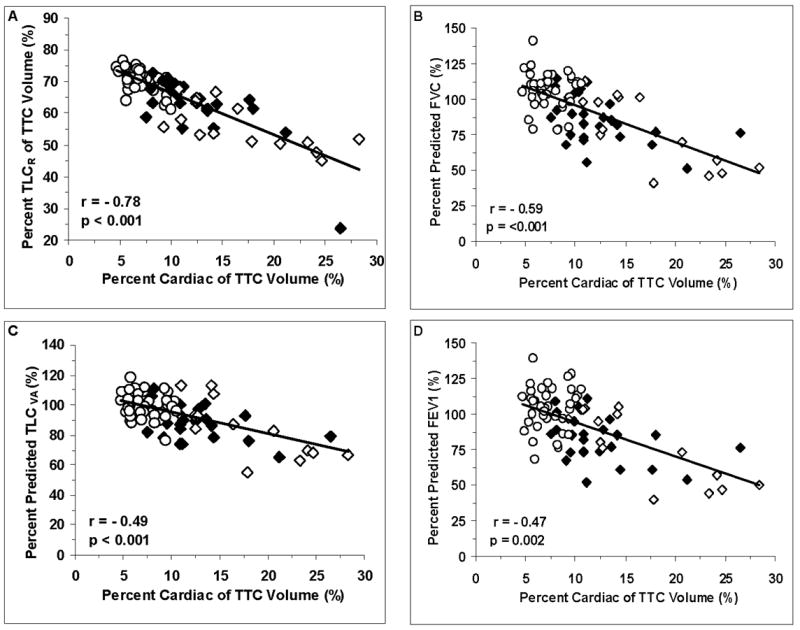
A. Correlation between the percent of the TTC volume that is taken up by the heart and the radiographically determined TLC. B. Correlation between the percent of the TTC volume that is taken up by the heart and the spirometrically determined percent predicted FVC. C. Correlation between the TTC volume that is taken up by the heart and the TLC measured as the percent predicted alveolar volume. D. Correlation between the percent of the TTC volume that is taken up by the heart and the spirometrically determined percent predicted FEV1. Control=O. Group A=■. Group B=□.
DISCUSSION
Primary Findings
Chronic heart failure often results in restrictive and to a lesser degree obstructive changes in pulmonary function. Heart failure also is associated with gas exchange abnormalities including reductions in DLCO. Reasons for these changes in lung function are likely multifactorial, particularly during times of decompensation. However, in stable, well managed HF patients who are not morbidly obese and who have a limited smoking history, increased cardiac volume may play an important role in reducing maximal lung volumes and to a lesser extent maximal airflows. It is possible that the association observed between lung function and cardiovascular mortality in large epidemiological studies, including those associated with the Cardiovascular Health Study 11 and Framingham Heart Study 25,26, may be partially due to this link with cardiac size, which is also associated with severity of heart failure 1. The findings of the present study suggest that although newer echocardiographic derived measures of cardiac dimensions appear to be associated with lung function, these measures are less predictive than radiographically determined cardiac volume. The reasons for the more predictive nature of the radiographic estimation of cardiac size may be due to the incorporation of the entire cardiac mass as opposed to individual chamber assessment as well as the normalization of the heart and lung estimations for the total thoracic volume.
Results of Epidemiological Studies in Relation to Pulmonary Function
In a prospective follow-up of approximately 2,500 individuals over 5 years, Beaty and colleagues 10 demonstrated that pulmonary function impairment is a significant risk factor for short- and long-term morbidity and mortality, despite adjustment for potential confounding factors such as age, gender, and smoking status. These authors suggest that impairment of pulmonary function not only contributes to morbidity and mortality independently but also does so through its pathogenic contribution to several non-respiratory diseases. This relationship also has been documented in patients at risk for myocardial infarction and sudden death 27, those with obstructive airway disease 28, and those with lung cancer 29. Thus it is apparent that pulmonary limitations, including those of a restrictive nature, not only act as a marker of underlying disease but also significantly elevate an individuals risk for morbidity and mortality independently.
Pulmonary Function Changes in Chronic Heart Failure
A number of studies have examined the baseline changes in pulmonary function in patients with CHF. These include relatively minimal change compared to age and height predicted measures 30, primarily restrictive abnormalities 31, obstructive changes 7, and combined restrictive and obstructive alterations 1. It is apparent that disorders of the heart frequently contribute detrimentally to the pulmonary system. Although the specific mechanisms causing altered lung function in HF are not entirely clear, these changes have been ascribed to respiratory muscle weakness 4,32, chronic pulmonary congestion and hypertension 33, changes in lung fluid balance 5, as well as neurohumoral changes 4-6. However, because the pulmonary and cardiac systems are hemodynamically and mechanically linked, it would be expected that progressive increases in cardiac volume within a closed thoracic cavity may contribute to the pulmonary function abnormalities in HF patients. One would expect such changes in cardiac size to result in primarily restrictive lung changes manifested as reductions in TLC as well as VC 2.
Influence of Heart Size on Restrictive and Obstructive Pulmonary Function Changes
Cardiac enlargement, commonly seen in HF, leads to reductions in intrathoracic space and limits the ability of the lungs to fill adequately. This could potentially reduce the effectiveness of the elastic recoil component of exhalation due to insufficient stretch of the lungs and result in reduced maximal expiratory flows 12,13. The inability of the lungs to fill due to a mechanical limitation of space would be represented by primarily restrictive changes exhibited as reduced TLC, FVC, and FEV1. As such, the present study demonstrated significantly reduced TLC in HF patients, measured either as the radiographically estimated volume and reported as a percent of the total thoracic cavity or total alveolar volume from single breath gas dilution. Also, our results suggest that both TLC measures were closely related to the radiographically determined absolute cardiac size and the cardiac size in relation to the thoracic cavity. Similar to the findings of Ulrik and colleagues 14 who demonstrated no relationship between LV end-diastolic volume and indices of pulmonary function, the results of this study do not demonstrate a significant relationship with TLC and the echocardiographically determined measures of cardiac size.
In support of the relationship between cardiac size and restrictive alterations of pulmonary function, we noted significantly reduced FVC and FEV1in the HF patients as compared to the control group. Further, as is typical of a restrictive pattern of pulmonary dysfunction, both the FVC and FEV1 were reduced proportionately resulting in a normal FEV1/FVC ratio 23. When examining the reduction of FVC and FEV1 in relation to the radiographically and echocardiographically determined cardiac size it was apparent that the strongest predictor of reduced FVC and FEV1 was the radiographically determined cardiac size reported as a percentage of the total thoracic cavity volume. These results are consistent with a previous study by Hosenpud and colleagues who have shown a significant relationship between the difference in heart size before and after transplantation and the change in FVC as well as significant proportional improvements in FVC and FEV1 associated with cardiac transplantation, in HF patients 13. However, this relationship described by Hosenpud et al. could also have been related to factors other than cardiac size, such as reduced lung congestion and lower pulmonary vascular pressures. The relationship with lung function would also be influenced by factors such as the size of the donor heart relative to the previous heart size and the general size of the thoracic cavity. The present study focused on the impact of cardiac size alone and controlled for the size of the thoracic cavity. In addition, the potential for bias related to the size of the post-transplant heart or post-transplant changes in hemodynamic status is minimized by evaluating hemodynamically stable and optimally managed patients. With this, there was relatively little difference between the three groups with regard to LV filling pressures. Although there was a significant difference in the early mitral inflow velocity (E-wave) between Group B and the control participants these results do not account for the differences in heart size or relationships between heart size and pulmonary function as seen between the two heart failure groups. These results would suggest that the cardiac volume, as opposed to LV filling pressure, plays a larger role in the reduction of pulmonary function in this population. Further, in contrast to previous reports, the present study also demonstrates the relationship between heart size and maximal airflows and DLCO. The present study uses a more comprehensive radiographic volumetric assessment of cardiac size and compares this to the more commonly reported echocardiographic measure of LV mass. The echocardiographic LA dimension, LV mass and LV mass index also showed a significant correlation (albeit less than the total radiographically determined cardiac size) with the reductions in FVC and FEV1, supporting the relationship between heart size and lung volumes.
The results of this study also demonstrate that the percent predicted mean forced expiratory flow during the mid portion of the FVC (FEF25-75) as well as the DLCO were significantly reduced in our HF patients as compared to the control group. The relatively similar reduction in FEF25-75 compared to the reduction in FEV1 suggests a pure restrictive breathing pattern, with no evidence of significant airway involvement. This is consistent with previous findings from our group in which we found minimal obstructive changes in a stable, non-smoking HF population 34. Importantly the forced expiratory airflows were not apparently related to cardiac size measured either by radiographic or echocardiographic methods. In contrast, the radiographically determined cardiac size and echocardiographically determined LA diameter were related to the DLCO. Agostoni and colleagues 12 have shown that the cardiothoracic index is an independent predictor of DLCO; however when corrected for alveolar volume these relationships were lost or greatly reduced. Thus, elevated cardiac size likely plays a role in the reduced DLCO by a direct mechanical compression of the lung resulting in reduced alveolar volume and overall TLC with subsequent limitation of membrane diffusion capacity. Conversely, It has also been suggested that an acute reduction in cardiac size by transplantation results in either no change or significant reduction in DLCO 12,14,35, however, these findings have been attributed to significant pulmonary vascular structural changes associated with disease severity 14.
Potential Limitations
A potential limitation of this study is the relationship between total body weight and pulmonary function. It is well known that obesity can result in similar pulmonary function changes as those observed in this study 36. The HF patients in this study were significantly heavier than the control group. In an effort to control for this difference, as well as other potentially confounding factors, the regression analyses were adjusted for age, height, weight, body surface area, systolic blood pressure, and diastolic blood pressure. Thus, the results presented demonstrate relationships which are independent of these potentially confounding influences.
Clinical Implications
It is clear that the HF associated changes in cardiac size within a closed thoracic cavity pose significant constraints on the lungs and result in reductions in lung volumes and contributes to the overall restrictive breathing pattern often reported in heart failure patients 37. In addition, the cardiomegaly associated changes in lung function may contribute to the inspiratory load, result in low lung volume breathing, limit the encroachment on the inspiratory reserve volume during times of increased ventilatory demand, and contribute to symptoms of dyspnea 37.
Acknowledgments
The authors thank Jacob Johnson, Kathy O’Malley, and Minelle Hulsebus for help with data acquisition and management. This work was supported in part by: National Institutes of Health grants HL71478 and HL07111.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dimopoulou I, Daganou M, Tsintzas OK, Tzelepis GE. Effects of severity of longstanding congestive heart failure on pulmonary function. Respir Med. 1998;92:1321–1325. doi: 10.1016/s0954-6111(98)90136-6. [DOI] [PubMed] [Google Scholar]
- 2.Light RW, George RB. Serial pulmonary function in patients with acute heart failure. Arch Intern Med. 1983;143:429–433. [PubMed] [Google Scholar]
- 3.Wright RS, Levine MS, Bellamy PE, Simmons MS, Batra PSL, Walden JA, Laks H, Tashkin DP. Ventilatory and diffusion abnormalities in potential heart transplant recipients. Chest. 1990;98:816–820. doi: 10.1378/chest.98.4.816. [DOI] [PubMed] [Google Scholar]
- 4.Daganou M, Dimopoulou I, Alivizatos PA, Tzelepis GE. Pulmonary function and respiratory muscle strength in chronic heart failure: comparison between ischaemic and idiopathic dilated cardiomyopathy. Heart. 1999;81:618–620. doi: 10.1136/hrt.81.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puri S, Baker BL, Oakley CM, Hughes JM, Cleland JG. Increased alveolar/capillary membrane resistance to gas transfer in patients with chronic heart failure. Br Heart J. 1994;72:140–144. doi: 10.1136/hrt.72.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham MR, Olson LJ, Joyner MJ, Turner ST, Beck KC, Johnson BD. Angiotensin-converting enzyme genotype modulates pulmonary function and exercise capacity in treated patients with congestive stable heart failure. Circulation. 2002;106:1794–1799. doi: 10.1161/01.cir.0000031735.86021.79. [DOI] [PubMed] [Google Scholar]
- 7.Petermann W, Barth J, Entzian P. Heart failure and airway obstruction. Int J Cardiol. 1987;17:207–209. doi: 10.1016/0167-5273(87)90132-x. [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. Journal of Internal Medicine. 2003;254:540–547. doi: 10.1111/j.1365-2796.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 9.Thomason MJ, Strachan DP. Which spirometric indices best predict subsequent death from chronic obstructive pulmonary disease? Thorax. 2000;55:785–788. doi: 10.1136/thorax.55.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaty TH, Cohen BH, Newill CA, Menkes HA, Diamond EL, Chen CJ. Impaired pulmonary function as a risk factor for mortality. Am J Epidemiol. 1982;116:102–113. doi: 10.1093/oxfordjournals.aje.a113385. [DOI] [PubMed] [Google Scholar]
- 11.Enright PL, Kronmal RA, Smith VE, Gardin JM, Schenker MB, Manolio TA. Reduced vital capacity in elderly persions with hypertension, coronary heart disease, or left ventricular hypertrophy: The Cardiovascular Health Study. Chest. 1995;107:28–35. doi: 10.1378/chest.107.1.28. [DOI] [PubMed] [Google Scholar]
- 12.Agostoni P, Cattadori G, Guazzi M, Palermo P, Bussotti M, Marenzi G. Cardiomegaly as a possible cause of lung dysfunction in patients with heart failure. Am Heart J. 2000;140:e24. doi: 10.1067/mhj.2000.110282. [DOI] [PubMed] [Google Scholar]
- 13.Hosenpud JD, Stibolt TA, Atwal K, Shelley D. Adnormal pulmonary function specifically related to congestive heart failure: Comparison of patients before and after cardiac transplantation. Am J Med. 1990;88:493–496. doi: 10.1016/0002-9343(90)90428-g. [DOI] [PubMed] [Google Scholar]
- 14.Ulrik CS, Carlsen J, Arendrup H, Aldershvile J. Pulmonary function in chronic heart failure. Changes after heart transplantation. Scand Cardiovasc J. 1999;33:131–6. doi: 10.1080/14017439950141740. [DOI] [PubMed] [Google Scholar]
- 15.Barnhard HJ, Pierce JA, Joyce JW, Bates JH. Roentgenographic determination of total lung capacity. Am J Med. 1960;28:51–60. doi: 10.1016/0002-9343(60)90222-9. [DOI] [PubMed] [Google Scholar]
- 16.Miller RD, Offord K. Roenthenologic determination of total lung capacity. Mayo Clin Proc. 1980;55:694–699. [PubMed] [Google Scholar]
- 17.Glenn WV, Greene R. Rapid computer-aided radiographic calculation of total lung capacity (TLC) Radiology. 1975;117:269–273. doi: 10.1148/117.2.269. [DOI] [PubMed] [Google Scholar]
- 18.Keats TE, Enge IP. Cardiac mensuration by the cardiac volume method. Radiology. 1965;85:850–855. doi: 10.1148/85.5.850. [DOI] [PubMed] [Google Scholar]
- 19.Olson TP, Beck KC, Johnson JB, Johnson BD. Competition for Intrathoracic Space Reduces Lung Capacity in Patients with Chronic Heart Failure: A Radiographic Study. Chest. 2006;130:64–71. doi: 10.1378/chest.130.1.164. [DOI] [PubMed] [Google Scholar]
- 20.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, feigenbaum H, Gutgesell H, Reichek N, Shan D, Schnittger I. Recommendations for quantification of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 21.Troy BL, Pombo J, Rackley CE. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation. 1972;45:602–611. doi: 10.1161/01.cir.45.3.602. [DOI] [PubMed] [Google Scholar]
- 22.Parisi AF, Moynihan PF, Feldman CL, Folland ED. Approaches to determination of left ventricular volume and ejection fraction by real-time two-dimensional echocardiography. Clin Cardiol. 1979;2:257–263. doi: 10.1002/clc.4960020404. [DOI] [PubMed] [Google Scholar]
- 23.ATS. Standardization of spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 24.ATS. Single breath carbon monoxide diffusing capacity (transfer factor). Recommendatins for a standard technique. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136:1299–1307. doi: 10.1164/ajrccm/136.5.1299. [DOI] [PubMed] [Google Scholar]
- 25.Ashley F, Kannel W, Sorlie PD, Masson R. Pulmonary function: Relation to aging, cigarette habit, and mortality. Ann Intern Med. 1975;82:739–745. doi: 10.7326/0003-4819-82-6-739. [DOI] [PubMed] [Google Scholar]
- 26.Sorlie PD, Kannel W, G OC. Mortality associated with respiratory function and symptoms in advancing age. The Framingham Study. Am Rev Respir Dis. 1989;140:379–384. doi: 10.1164/ajrccm/140.2.379. [DOI] [PubMed] [Google Scholar]
- 27.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1976;13:1071–1075. doi: 10.1056/NEJM197605132942001. [DOI] [PubMed] [Google Scholar]
- 28.Menkes HA, Cohen BH, Beaty TH, Newill CA, Khoury MJ. Risk factors, pulmonary function, and mortality. Prog Clin Biol Res. 1984;147:501–521. [PubMed] [Google Scholar]
- 29.Eberly LE, Ockene J, Sherwin R, Yang L, Kuller L. Pulmonary function as a predictor of lung cancer mortality in continuing cigarette smokers and in quitters. Int J Epidemiol. 2003;32:592–599. doi: 10.1093/ije/dyg177. [DOI] [PubMed] [Google Scholar]
- 30.Collins JV, Clark TJ, Brown DJ. Ariway function in healthy subjects and patients with left heart disease. Clin Sci Mol Med. 1975;49:217–228. doi: 10.1042/cs0490217. [DOI] [PubMed] [Google Scholar]
- 31.Cabanes LR, Weber SN, Matran R, Regnard J, Richard MO, Degeorges ME, Lockhart A. Bronchial hyperresponsiveness to methacholine in patients with imparied left ventricular function. N Engl J Med. 1989;321:1756–1758. doi: 10.1056/NEJM198905183202005. [DOI] [PubMed] [Google Scholar]
- 32.Hammond MD, Bauer KA, Sharp JT, Rocha RD. Respiratory muscle strength in congestive heart failure. Chest. 1990;98:1091–1094. doi: 10.1378/chest.98.5.1091. [DOI] [PubMed] [Google Scholar]
- 33.Dembinski R, Henzler D, Rossaint R. Modulating the pulmonary circulation: an update. Minerva Anestesiol. 2004;70:239–243. [PubMed] [Google Scholar]
- 34.Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, Gau GT. Pulmonary function in patients with reduced left ventricular function: Influence of smoking and cardiac surgery. Chest. 2001;120:1869–1876. doi: 10.1378/chest.120.6.1869. [DOI] [PubMed] [Google Scholar]
- 35.Ravenscraft SA, Gross CR, Kubo SH, Olivari MT, Shumway SJ, Bolman RM, Hertz MI. Pulmonary function after successful heart transplantation: One year follow-up. Chest. 1993;103:54–58. doi: 10.1378/chest.103.1.54. [DOI] [PubMed] [Google Scholar]
- 36.Koenig SM. Pulmonary Complications of Obesity. Am J Med Sci. 2001;321:249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, Gau GT. Ventilatory Constraints During Exercise in Patients With Chronic Heart Failure. Chest. 2000;117:321–332. doi: 10.1378/chest.117.2.321. [DOI] [PubMed] [Google Scholar]


