SUMMARY
Tooth eruption is a multifactorial process involving movement of existing tissues and formation of new tissues coordinated by a complex set of genetic events. We have used the model of the unopposed rodent molar to study morphological and genetic mechanisms involved in axial movement of teeth. Following extraction of opposing upper molars, lower molars supererupted by 0.13 mm. Labeled tissue sections revealed significant amounts of new bone and cementum apposition at the root apex of the unopposed side following supereruption for 12 days. Newly apposited cementum and alveolar bone layers were approximately 3-fold thicker in the experimental vs the control group, whereas periodontal ligament width was maintained. Tartrate-resistant acid phosphatase staining indicated bone resorption at the mesial alveolar walls of unopposed molars and provided in tandem with new bone formation at the distal alveolar walls an explanation for the distal drift of molars in this model. Microarray analysis and semiquantitative RT-PCR demonstrated a significant increase in collagen I, integrin β5, and SPARC gene expression as revealed by comparison between the unopposed molar group and the control group. Immunohistochemical verification revealed increased levels of integrin β5 and SPARC labeling in the periodontal ligament of the unopposed molar. Together our findings suggest that posteruptive axial movement of teeth was accomplished by significant formation of new root cementum and alveolar bone at the root apex in tandem with upregulation of collagen I, integrin β5, and SPARC gene expression.
Keywords: tooth movement, tooth eruption, bone resorption, matrix remodeling
The concept that tooth movement is facilitated by extensive bone tissue reorganization was originally proposed by Sandstedt (1904) and Oppenheim (1911–1912). Both authors described the various processes of bone formation and absorption on aspects of the moving tooth and established the bone extracellular matrix (ECM) as a dynamic tissue facilitating tooth movement. In the century that followed Sandstedt’s and Oppenheim’s seminal papers, the process of tissue remodeling as a consequence of orthodontic forces has been extensively studied (reviewed in Sandy et al. 1993; McCulloch et al. 2000). A number of studies have established tooth movement as a complex process involving highly refined interactions between growth factors, transcription factors, and structural proteins (Shimono et al. 2003; Sringkarnboriboon et al. 2003). In addition, remodeling of the collagenous ECM of the periodontal ligament (PDL) and alveolar bone has been identified as a molecular key process facilitating tooth movement (Nakagawa et al. 1994; Karimbux and Nishimura 1995).
Effects of the ECM on cell behavior are often mediated by integrin cell surface receptors and/or matricellular glycoproteins. Integrins are a family of cell surface receptors that attach cells to the matrix and affect cell cycle regulations, directing cells to live or die, to proliferate, or to exit the cell cycle and differentiate (Boudreau and Jones 1999; Giancotti and Ruoslahti 1999). SPARC (osteonectin, BM-40) modulates cellular interaction with the ECM by binding to structural matrix proteins such as collagen I or by interacting with hydroxyapatite (Bornstein 2000; Brekken and Sage 2000; Remelli et al. 2002). Consequentially, signals from matricellular mediators such as integrins or SPARC may regulate and control the manufacture and organization of newly secreted ECM molecules, notably collagen I, by affecting cell behavior. It is therefore conceivable that signals from periodontal pressure or tension receptors, because they are affected by occluding teeth or mechanical stimulation and may trigger matricellular mediators such as integrins and SPARC, in turn would cause a change in ECM synthesis, especially collagen I. This synthesis of new ECM might be one cause for tooth movement to occur.
We have used the model of the unopposed rodent molar to study the effects of axial tooth movement on matrix remodeling and to identify matricellular mediators. As a first step, we have characterized the amount of mouse molar supereruption in this model using skeletal preparations, ultrathin ground sections, fluorescent labels, and histochemical bone markers. These studies demonstrated a significant degree of molar tooth super-eruption associated with new cementum and alveolar bone mineralized tissue deposition. In addition, we have performed tartrate-resistant acid phosphatase (TRAP) staining to detect sites and levels of bone resorption in the periodontium and bony sockets of affected teeth. To reveal the molecular factors that might be involved in matrix remodeling during tooth movement, we have used an ECM gene-based microarray assay to identify candidate genes. The first three candidate genes that emerged from our preliminary studies were collagen I, integrin β5, and SPARC. We then verified these findings using semiquantitative RT-PCR and immunohistochemistry. Together our findings indicated that posteruptive axial movement of teeth involves significant formation of new apical root cementum and alveolar bone in tandem with upregulation of collagen I, integrin β5, and SPARC gene expression.
Materials and Methods
Experimental Animals and Extraction Technique
Swiss-Webster mice, 35 days old (Charles River; Wilmington, MA) were chosen as experimental animals. Animal experiments were approved by and performed according to the guidelines of the UIC Animal Care Committee. Mice were randomly divided into control and experimental groups. Mice were anesthetized using ketamine (100 mg/kg) and xylazine (5 mg/kg). Following successful anesthesia, all three left-side maxillary molars were extracted (Figure 1). After extraction, pressure was applied to achieve hemostasis. Buprenorphine was administered as an analgesic SC at a dose of 0.05 mg/kg before ending the procedure. Doses of analgesic were administered every 12 hr for up to 2 days postprocedure. Mandibles from untreated animals were used as controls. For fluorescent labeling, fluorochromes were applied at concentrations of 50 mg/kg (tetracycline hydrochloride), 25 mg/kg (alizarin red), and 30 mg/kg (calcein blue).
Figure 1.
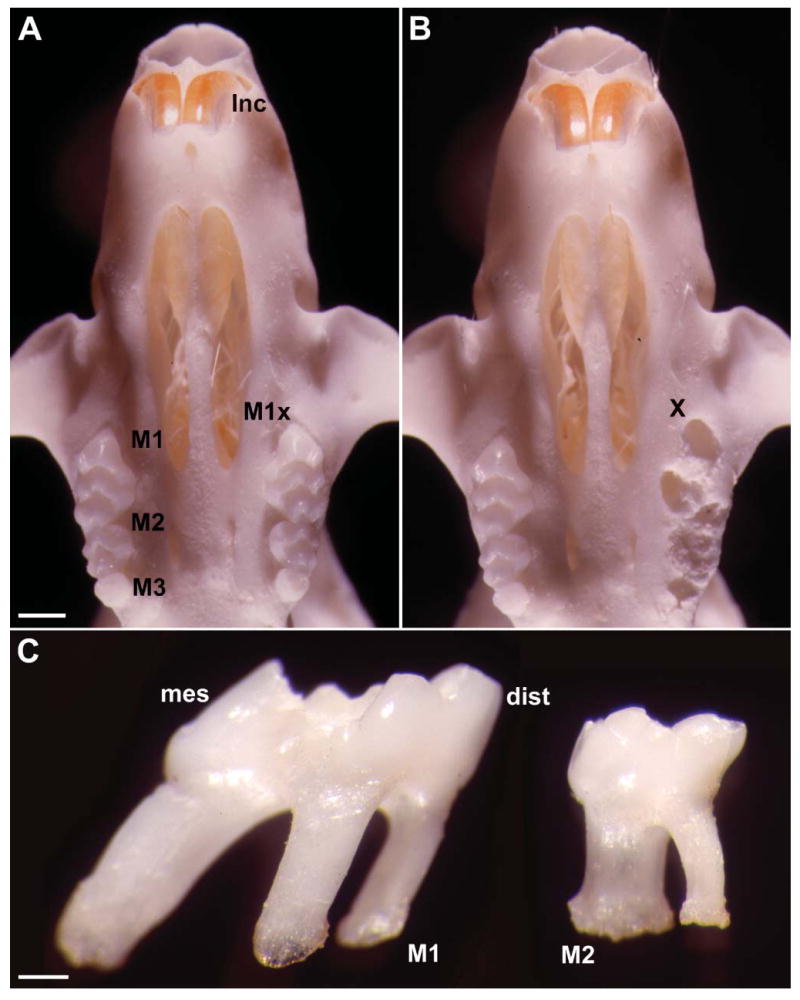
Unopposed mouse molar skull preparations illustrating tooth extraction procedures and unopposed side supereruption. (A) Caudal view onto a skeletonized upper jaw illustrating the position of the incisors (Inc) and maxillary molars (M1, M2, M3). All three left molars were extracted to create an unopposed situation (M1x). (B) Base of the upper jaw following extraction of the left maxillary molars (X). Note the sockets of alveolar bone remaining immediately after extraction (X). (C) Close-up of an extracted first (M1) and second (M2) maxillary mouse molar. Note the sharp inclination of the mesial (mes) root of the first maxillary molar, providing an indication of the distal (dist) tooth drift in the rodent dentition. Bars: A,B = 1 mm; C = 500 μm.
Tissue Processing
A group of 15 mice received an IP injection of tetracycline hydrochloride as a vital stain at the time of extraction. Subsequently, they received injections of alizarin red at 4 days and calcein blue after 8 days. Mice were sacrificed on day 12 by using CO2 gas and stored in 70% ethanol for overnight fixation. Tissues were dehydrated in a graded series of ethanols, followed by acetone, and then infiltrated with resin (Technovit 2000; EXAKT Technologies, Oklahoma City, OK). Subsequently, samples were embedded and prepared for undecalcified ground sections, fluorescent microscopy, and von Kossa’s and Villanueva’s Osteochrome stain.
For immunohistochemistry, tissues of 15 mice were fixed with 10% buffered formalin for 1 week, decalcified in 5% EDTA for 2 weeks, and dehydrated in a graded series of ethanols. Subsequently, tissues were embedded in paraffin wax, cut into 5-μm-thick sections, and prepared for immunohistochemistry.
Morphometry
To determine the total eruption rate over 12 days in an unopposed situation, three mandibles were skeletonized, and the difference between molar heights in the unopposed and opposed situation was determined. Molar height in the unopposed model was compared with molar height in a control group in which opposing molars maintained occlusion. Resulting macrographs were oriented by aligning the base of the mandible parallel to the base of the image while a second parallel was constructed through the occlusal plane of the opposed molar. Mandibles were photographed en face using the posterior prominences of both condyles and ramus to establish a retrograde plane of orientation.
Alveolar bone and cementum apposition were measured using computerized techniques by measuring the distance between the initially stained mineralized zone to the final margin of bone or cementum. Measurements were taken from sagittal sections through the center of the first and second molar. Values were compared between the experimental side and the control side using the paired t-test statistical analysis.
Histochemistry and von Kossa’s Procedure
Plastic-embedded sections were stained with either Villanueva’s Osteochrome stain to detect newly mineralized tissue matrix or von Kossa’s stain as a mineralized tissue marker. For von Kossa’s procedure, tissue sections were treated with 5% silver nitrate solution as previously described (Diekwisch et al. 1995). To detect osteoclasts, paraffin sections were stained for TRAP using the Sigma acid phosphatase detection kit 386A (Sigma; St Louis, MO) according to the manufacturer’s instructions.
Microarray Technology
For microarray analysis, three mandibles from the control and the experimental group (day 6) each were removed and apical regions prepared using surgical instruments. The dissected apical region of each molar contained apical root tip including cementum, PDL, and adjacent alveolar bone. Day 6 was chosen based on preliminary studies indicating high levels of gene expression on day 6.
mRNA was extracted from the tissues using Trizol LS Reagent (Invitrogen; Carlsbad, CA) according to the manufacturer’s instructions. Quality and quantity of the RNA was tested using spectrophotometry and agarose gel electrophoresis. Optimal quality of RNA from one mouse from each group was used, hybridized, and converted to cDNA via reverse transcription. cDNA was labeled by 32P isotope and exposed to a GEArray Q Series Mouse Extracellular Matrix and Adhesion Molecules Gene Array (SuperArray; Frederick, MD). The response of 96 genes associated with the ECM and adhesion molecules, plus positive and negative controls, was evaluated using a software package provided by SuperArray.
Semiquantitative RT-PCR
Total RNA was extracted from apical tissues (apical region including root tip, cementum, PDL, and alveolar bone of all three molars) of three mice (per group) using the Trizol LS Reagent (Invitrogen) and its protocol. Quality and quantity of the RNA was tested with spectrophotometry and agarose gel electrophoresis in the same way samples were prepared for microarray analysis. RNA was then reverse transcribed and the product of cDNA was amplified using selected primers and the PCR technique. Primers were selected for type I collagen, SPARC, and integrin β5 based on results from the microarray analysis. Primer sequences were as follows: collagen I—5′ CGT CGG AGC AGA CGG GAG TTT 3′ (forward) and 5′ CAG AGT TTG GAA CTT ACT GTC 3′ (reverse), integrin β5—5′ TTG CCA AGT TCC AAA GTG A 3′ (forward) and 5′ GCG TGA CCT TTT TAT TTC AT 3′ (reverse), SPARC—5′ CTG CGT GTG AAG AAG ATC CA 3′ (forward) and 5′ CAT GTG GGT TCT GAC TGG TG 3′ (reverse), and β-actin—5′ GAG CAA GAG AGG TAT CCT GAC 3′ (forward) and 5′ TTC ATG GAT GCC ACA GGA TTC 3′. RT-PCR products were then run through agarose gel electrophoresis along with a β-actin internal control and a DNA ladder.
Immunohistochemistry
Tissue sections were prepared from experimental and control molars in sagittal orientation, and mid-sagittal sections were used for further analysis. Slides were deparaffinized and tissues were rehydrated. Immunoreactions were performed using a polyclonal primary antibody for integrin β5 (Santa Cruz Biotechnology; Santa Cruz, CA) and a monoclonal antibody for SPARC (Santa Cruz Biotechnology). Sections were incubated with primary antibody at room temperature for 2 hr. Dilution of the primary antibodies was previously determined in preliminary trials. The integrin β5 primary antibody was diluted using PBS. The SPARC primary antibody was diluted using a buffered solution of sodium chloride, HEPES, and calcium chloride. Sections were washed three times in PBS and incubated for 10 min with the appropriate secondary antibody, anti-mouse antibody for SPARC, and anti-rabbit IgG for integrin β5. After three additional washes in PBS, sections were incubated 10 min with streptavidin–enzyme conjugate and then washed three additional times with PBS. Immunoreaction signals were detected using an AEC substrate–chromogen mixture (color substrate), counterstained with hematoxylin, and mounted with GVA mount. The following controls were performed to test for antibody specificity: (1) tissue controls—specificity of the antibody was evaluated in various tissues, (2) antibody controls by using a dilution series, (3) controls with preadsorbed antibody to exclude unspecific binding, (4) controls with preimmune serum to control for binding to serum components, and (5) omission of primary antibody as a systematic control.
Results
Measurements of relative levels of molar supereruption based on skeletal preparations were performed to determine skeletal dimensions of tooth movement. Whereas in control experiments the occlusal plane of the left and right molar was parallel to the baseline, there was a significant amount of supereruption on the unopposed side of the experimental group (Figure 1). The amount of molar supereruption on the unopposed side in comparison to the control side was 0.13 mm (±0.06 mm) after 12 days.
Changes in mineralized tissue dimensions were assessed using fluorescent bone markers, and changes were measured on mid-root sagittal ultrathin ground sections using fluorescent microscopy. Our results revealed highly significant differences in the thickness of alveolar bone and cementum apposition in the unopposed (experimental) group vs the opposed (control) group in the unopposed mouse molar model (88.26 μm experimental vs 28.64 μm control for new alveolar bone and 78.81 μm experimental vs 30.61 μm control for new cementum). In comparison, levels of mineralized tissue apposition were remarkably similar for alveolar bone and cementum (88.26 μm alveolar bone and 78.81 μm cementum for the experimental group and 28.64 μm alveolar bone and 30.61 μm cementum for the control group) (Figure 2; Table 1).
Figure 2.
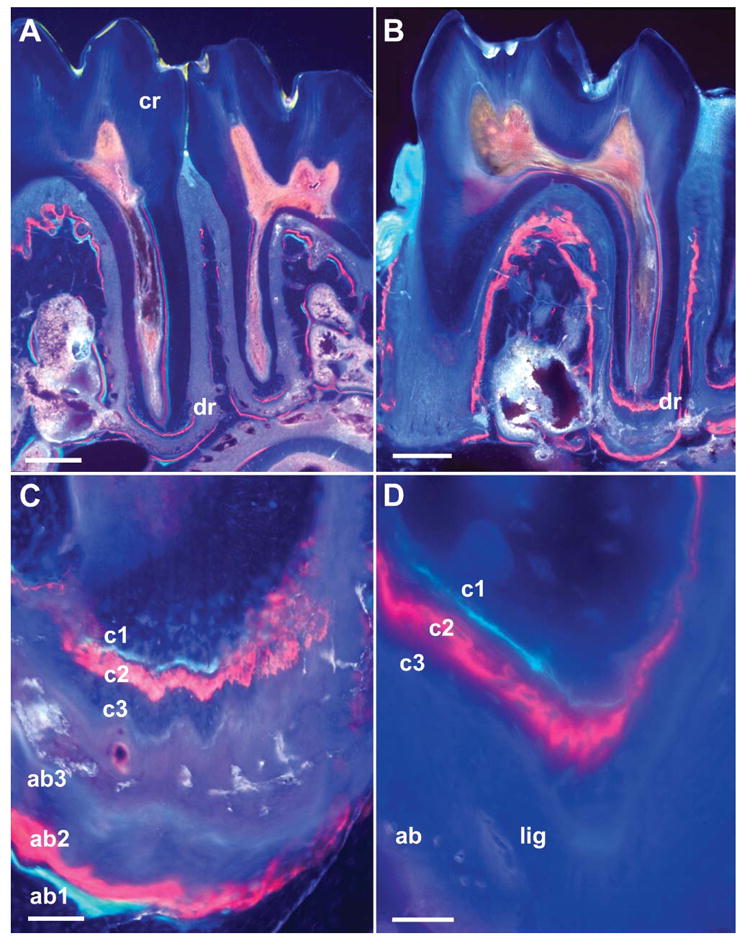
Tissue apposition in unopposed molars as revealed by fluorescent labels and ultrathin ground sections. (A,B) Overview micrographs illustrating ultrathin cross-sections through a control molar (A) and through an unopposed molar (B). Both were labeled using fluorescent markers for tetracycline (green label, after 4 days), alizarin red (red label, after 8 days), and calcein (blue label, after 12 days). Compare the apex of the distal root of the first molar in both images (dr), and note the significant deposition of new root cementum and alveolar bone on the unopposed side (B). The tooth crown (cr) in A is identified for orientation purposes. (C,D) High-magnification images of unopposed molars indicating the position of fluorescent labels: c1 (tetracycline label, cementum), c2 (alizarin red label, cementum), c3 (calcein blue label, cementum), ab1 (tetracycline label, alveolar bone), ab2 (alizarin red label, alveolar bone), and ab3 (calcein blue label, alveolar bone). In D the positions of the periodontal ligament (lig) and alveolar bone (ab) are also indicated. Bars: A,B = 500 μm; C = 100 μm; D = 50 μm.
Table 1.
Tooth apex mineralized tissue apposition and periodontal ligament (PDL) dimensions in unopposed and control molars after 12 daysa
| Experimental | Control | Mean difference | SD | Significance | |
|---|---|---|---|---|---|
| Alveolar bone | 88.26 ± 19.20 | 28.64 ± 5.65 | 59.63 | 17.86 | >0.001*** |
| Cementum | 78.81 ± 20.55 | 30.61 ± 7.45 | 48.20 | 22.23 | >0.001*** |
| PDL width | 83.06 ± 12.08 | 81.57 ± 12.41 | 1.49 | 20.62 | 0.783 |
There were highly significant differences in the thickness of alveolar bone and cementum apposition in the unopposed (experimental) group vs the opposed (control) group in the unopposed mouse molar model (88.26 μm experimental vs 28.64 μm control for new alveolar bone, and 78.81 μm experimental vs 30.61 μm control for new cementum). In comparison, levels of mineral tissue apposition were remarkably similar for alveolar bone and cementum (88.26 μm alveolar bone and 78.81 μm cementum for the experimental group and 28.64 μm alveolar bone and 30.61 μm cementum for the control group). Moreover, PDL width remained unaffected from supereruption in unopposed situations, and total PDL width was about 80 μm in both unopposed and opposed conditions.
Highly significant.
Based on the same sections that were used in the previously described labeling studies, mean PDL diameter was calculated from mid-root cut sagittal sections and compared between experimental and control group. In opposition to the dramatic changes observed in mineralized tissue apposition, PDL width remained unaffected from supereruption in unopposed situations, and total PDL width was ~80 μm in both unopposed and opposed conditions (Figure 3; Table 1).
Figure 3.
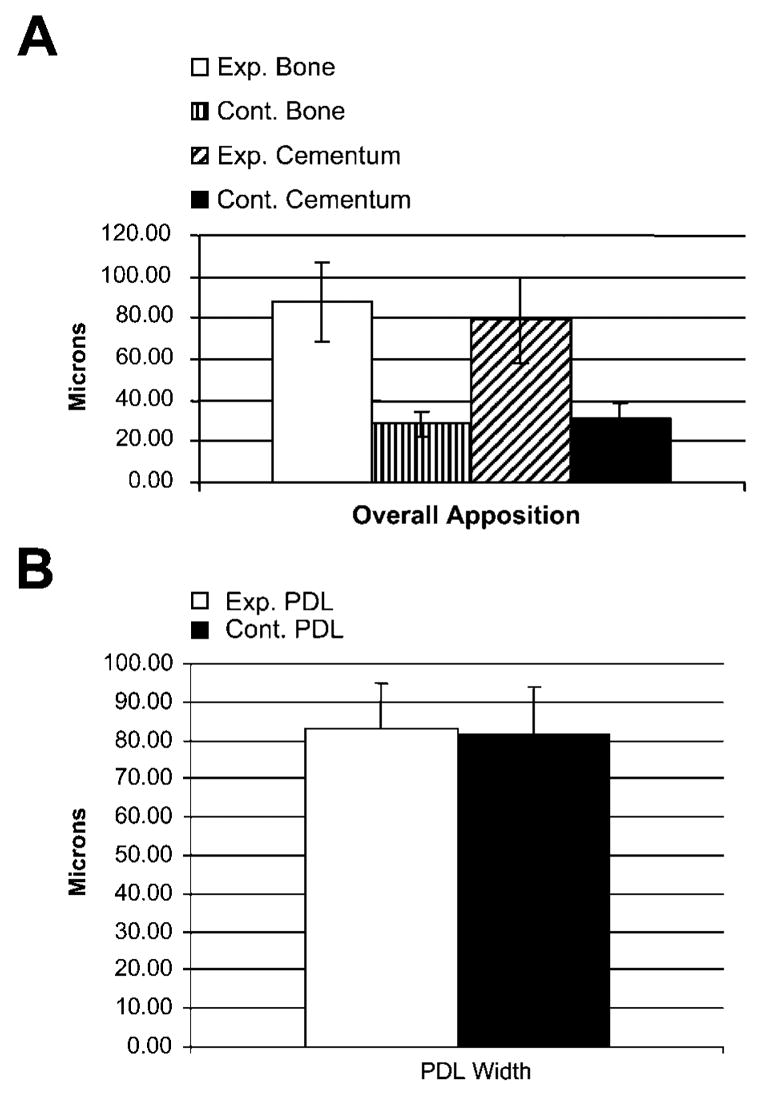
Twelve-day apposition statistics for apical alveolar bone, cementum, and periodontal ligament (PDL) in unopposed and control molars. (A) Thickness of newly apposited cementum and alveolar bone was ~2.5- to 3-fold higher in the unopposed molar group than in the opposed molar group. There was a total of 80–90 μm each of both alveolar bone and cementum apposited in the unopposed group, whereas the apposition values for both bone and cementum were significantly less in the opposed molar group, each totaling ~30 μm. Interestingly, periodontal ligament width remained unaffected from supereruption in unopposed situations, and total PDL width was ~80 μm in both unopposed and opposed conditions (B). Exp., experimental; Cont., control; PDL, periodontal ligament. Exact values are documented in Table 1.
Using the TRAP-staining procedure, distinct TRAP-positive demarcation linings containing multiple osteoclasts were recorded along the mesial alveolar wall of the unopposed molars (Figure 4). In contrast, control molars featured only a few single osteoclasts and no TRAP-positive alveolar linings (Figure 4). There were no osteoclasts or indications of TRAP activity at the apex of unopposed molars (Figure 4). These findings suggest the presence of bone resorption at the mesial alveolar walls.
Figure 4.
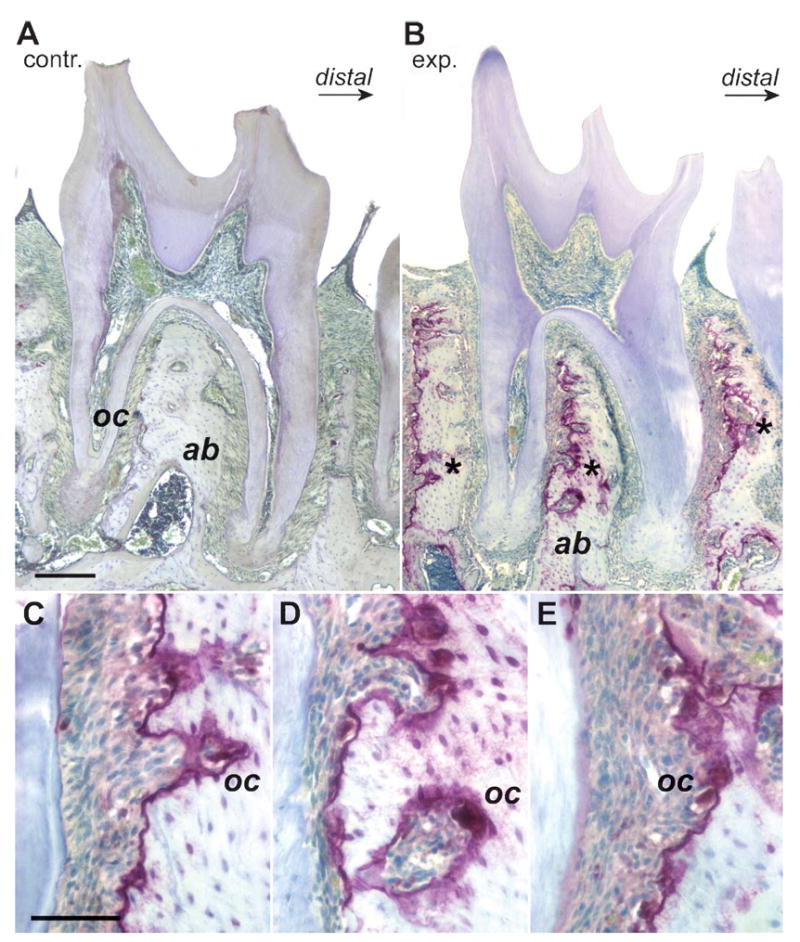
Osteoclast activity as visualized via tartrate-resistant acid phosphatase (TRAP) staining indicates bone resorption at the mesial alveolar walls of unopposed mouse molars. (A,B) Comparable mid-sagittal sections through mouse jaws with the second molar in the center and a small fragment of the third molar at the right margin of the micrograph. A is from a control and B is from a mouse jaw that remained in an unopposed situation for 9 days. (C–E) Higher-magnification images of the three selected root areas marked in B by an asterisk. Note the distinct TRAP-positive lining (TRAP) containing multiple osteoclasts (oc) along the mesial alveolar wall of the unopposed molars. In contrast, control molars featured only a few single osteoclasts (oc). The following were marked for orientation purposes: alveolar bone (ab), control group (contr.), and experimental group (exp.). Bars: A,B = 500 μm; C–E = 100 μm.
To determine whether newly formed apical tissues were mineralized, von Kossa’s technique for the detection of inorganic phosphate was applied. There was a significantly enhanced layer of mineralized cementum in the experimental group in comparison to the control group (Figure 5). The entire cellular cementum region was labeled by von Kossa’s procedure, indicating that the newly formed apical cementum was mineralized (Figure 5). The mineralizing cementoblast contours at the ligament/cementum interface resembled patterns of normal apical cementogenesis (Figure 5).
Figure 5.
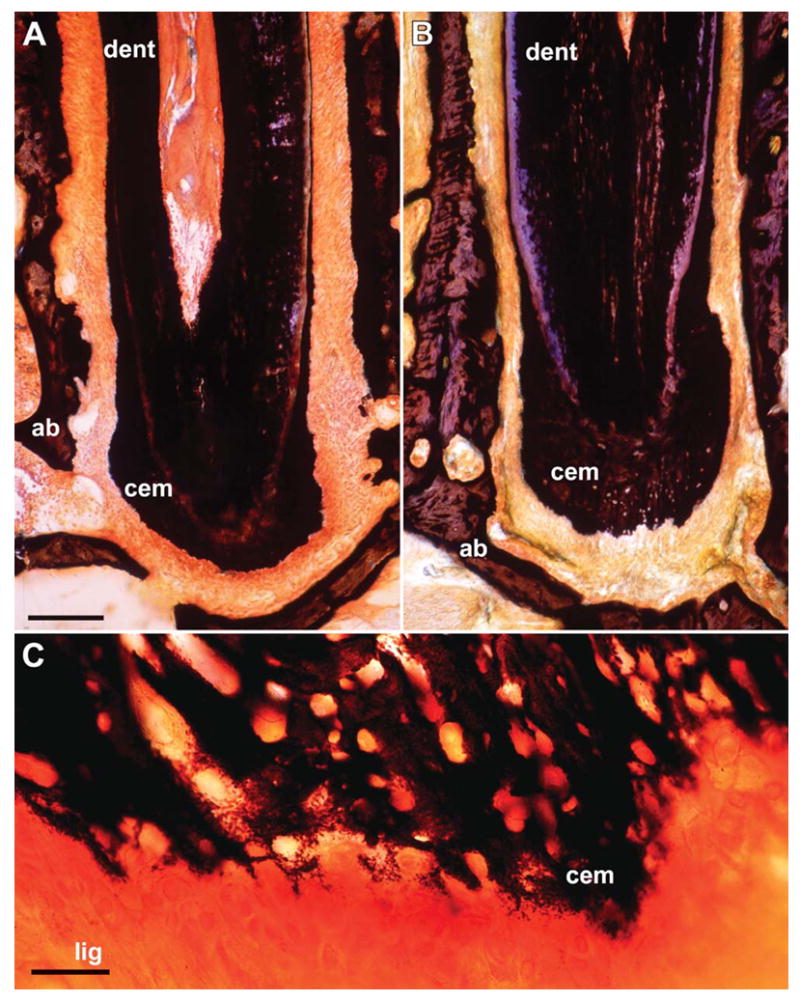
Formation of new mineralized tissues at the apex of unopposed molars. To determine whether newly formed apical tissues were mineralized, von Kossa’s technique for the detection of inorganic phosphate was applied. Note the significantly enhanced layer of mineralized cementum (cem) on the experimental side (B) in comparison to the control side (A). The entire cellular cementum region was labeled by von Kossa’s procedure, indicating that the newly formed apical cementum was mineralized. Root dentin (dent) and alveolar bone (ab) were labeled for orientation purposes. (C) High-magnification image of the interface between periodontal ligament (lig) and apical cementum (cem). The mineralizing cementoblast contours at the ligament/cementum interface resembled patterns of normal apical cementogenesis. Bars: A,B = 100 μm; C = 20 μm.
ECM microarray comparisons were performed using mRNA extracted from root apices of super-erupted and control molars and commercially available ECM gene-specific arrays. Our results based on microarray analysis of 96 selected ECM genes documented a 4.4-fold increase in collagen I gene expression, a 1.9-fold increase in integrin β5 gene expression, and a 2.3-fold increase in osteonectin (SPARC) gene expression, whereas the remainder of the ECM genes investigated in this assay were significantly below the 2-fold threshold (Table 2).
Table 2.
Transcript levels of extracellular matrix-related genes as revealed via microarray analysisa
| Gene | Genbank # | Ratio day 6 vs control |
|---|---|---|
| Collagen I | NM_007742 | 4.4 |
| Integin β5 | NM_010580 | 1.9 |
| Osteonectin (SPARC) | NM_009262 | 2.3 |
Extracellular matrix microarray comparison between 12-day unopposed and control molar apex mRNA revealed differential expression of collagen I, SPARC, and integrin β5 genes before and after axial movement of teeth. Microarray data suggest a significant increase in collagen I, SPARC, and integrin β5 gene expression, a finding that was confirmed using RT-PCR (Figure 6) and immunoreactions (Figure 7 and Figure 8).
Semiquantitative RT-PCR analysis and densitometry were performed to verify microarray data on changes in ECM gene expression in unopposed molar root tissues. Using semiquantitative RT-PCR, all three gene products, collagen I, integrin β5, and SPARC (osteonectin), demonstrated significantly higher expression levels in the unopposed molar group compared with the opposed molar control group, indicating an active involvement of ECM genes in tissue remodeling that occurs during axial tooth movement (Figure 6). There was no difference in β-actin control gene expression levels between the experimental and the control group, and the negative control reaction (Neg) did not yield any product in all four experiments (Figure 6). Densitometric evaluation of RT-PCR results yielded a more than 2-fold increase in collagen I and SPARC gene expression and a slight increase (1.3-fold) in integrin β5 gene expression (Figure 6E).
Figure 6.
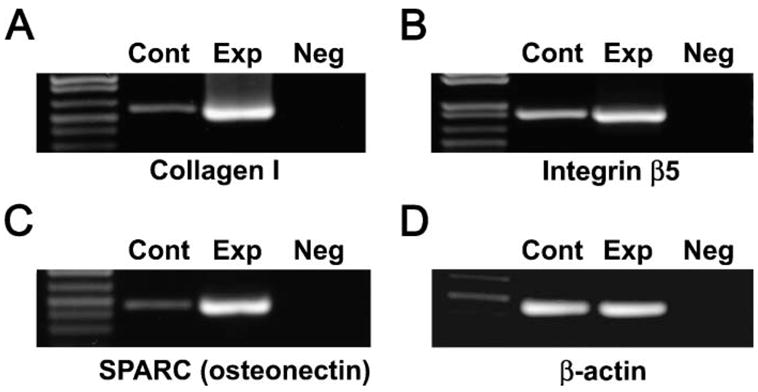
RT-PCR amplification of important extracellular matrix (ECM) gene products collagen I and SPARC (osteonectin) and the integrin β5 cell surface receptor in the unopposed mouse molar model. All three gene products, collagen I (A), integrin β5 (B), and SPARC (osteonectin, C) demonstrated significantly higher expression levels in the unopposed molar group (Exp) compared with opposed molar control group (Cont), indicating an active involvement of ECM genes in the tissue remodeling that occurs during axial tooth movement. There was no difference in β-actin control gene expression levels between the experimental and the control group (D). The negative control reaction (Neg) did not yield any product in all four experiments. (E) Densitometric analysis of our semiquantitative RT-PCR results. Note increased gene expression levels for collagen I, SPARC, and integrin β5 in the 6-day unopposed group as compared with the control group.
To verify semiquantitative RT-PCR and microarray results on a protein level, immunohistochemical studies were performed using same-thickness adjacent sections and identical reaction conditions in a single experiment. Studies were focused on integrin β5 and osteonectin, whereas the ubiquitous collagen I was not used for these studies. Immunoreactions revealed specific labeling reactions for both integrin β5 and osteonectin in the PDL, whereas surrounding dental tissues such as alveolar bone or cementum exhibited little or no reaction products (Figure 7 and Figure 8). Labeling intensity for integrin β5 and osteonectin in the unopposed molar group vs the control group was dramatically enhanced under otherwise identical conditions on adjacent same-thickness sections (Figure 7). Careful study of the micrographs revealed localization of integrin β5 reaction products to the cell surfaces of PDL fibroblasts whereas osteonectin (SPARC) was distributed in ECMs including the adjacent bone and cementum matrix (Figure 8). Control immunoreactions were negative.
Figure 7.
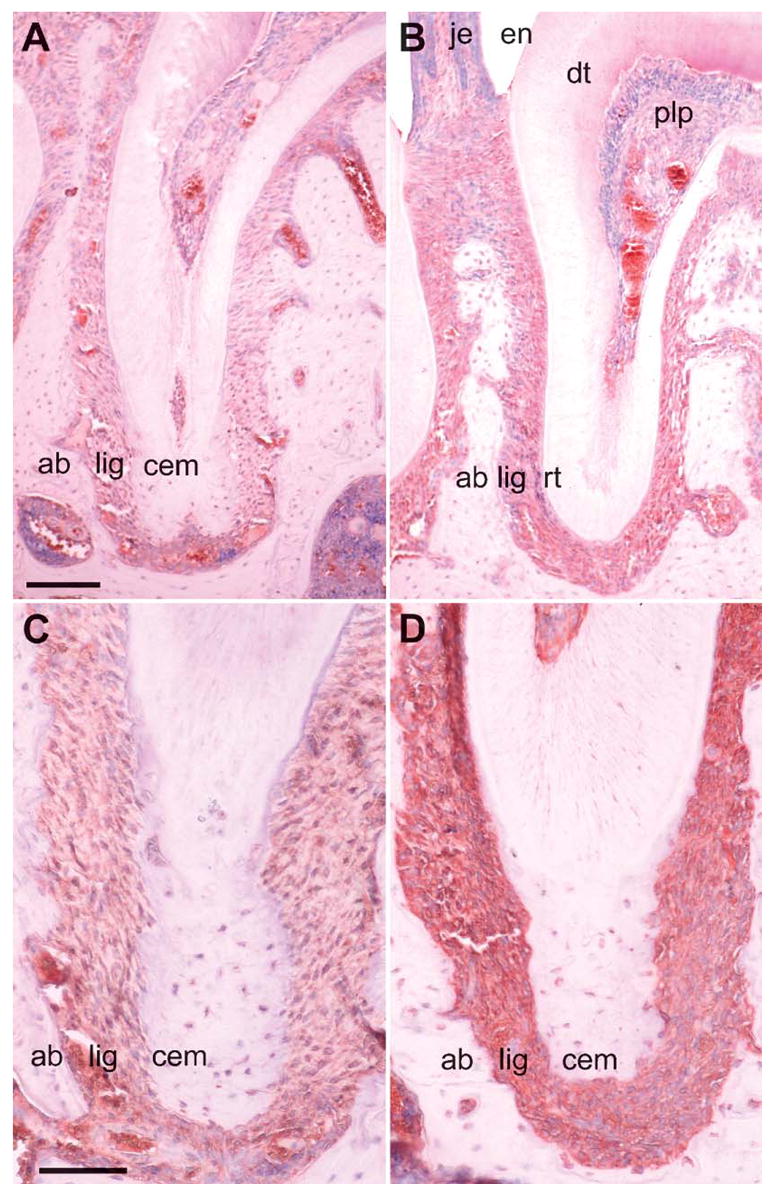
Immunohistochemistry of integrin β5 and osteonectin (SPARC) in the unopposed mouse molar model. In this set of micrographs, localization and relative staining intensity were compared for integrin β5 (A,B) and osteonectin (C,D). Comparisons were performed between the control group (A,C) and the unopposed molar experimental group (B,D). Both integrin β5 and osteonectin were specifically localized in the periodontal ligament (lig), whereas surrounding dental tissues such as alveolar bone or cementum exhibited little or no reaction products. Labeling intensity for integrin β5 and osteonectin in the unopposed molar group vs the control group (B,D vs A,C) was dramatically enhanced under otherwise identical conditions on adjacent same-thickness sections. The following tissues were identified for orientation purposes: alveolar bone (ab), periodontal ligament (lig), root cementum (cem), root (rt), junctional epithelium (je), enamel demineralized space (en), dentin (dt), and dental pulp (plp). This highly specific localization of key factors suggests a pivotal role of the PDL in the ECM-mediated control and modulation of tooth movement. Bars: A,B = 100 μm; C,D = 50 μm.
Figure 8.
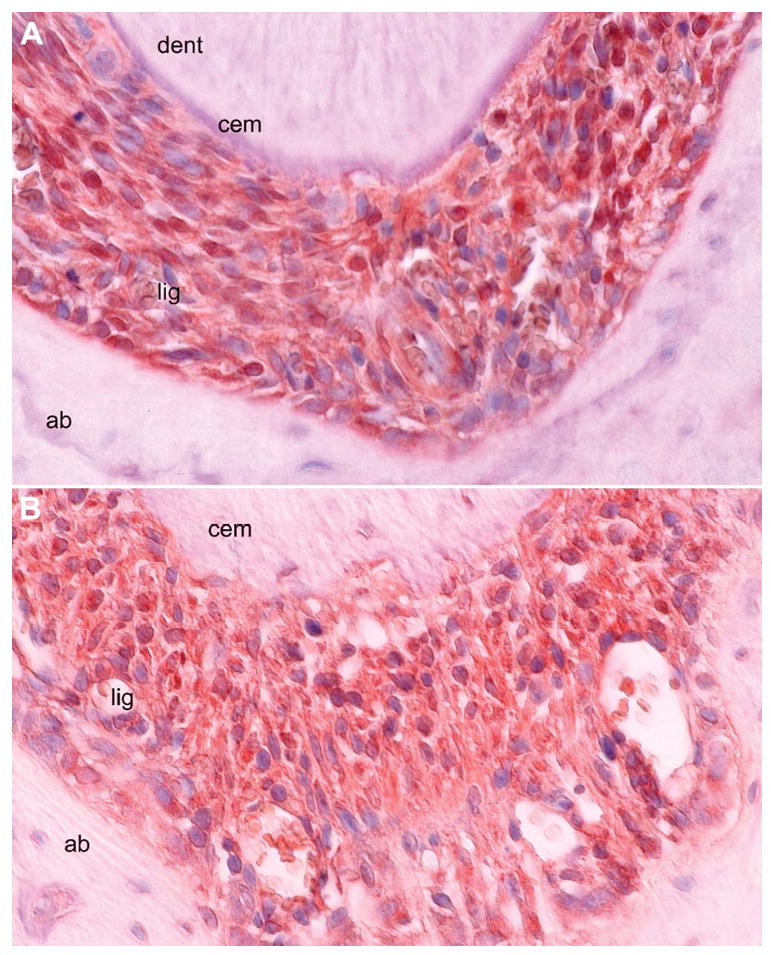
Localization of integrin β5 and osteonectin (SPARC) in the unopposed molar root apex PDL. These are high-magnification images of paraffin sections through the apical tips of unopposed mouse molar root apices treated with anti-integrin β5 (A) and anti-osteonectin (B) antibodies. Strong reaction products were detected in the periodontal ligament (lig), whereas adjacent tissues dentin (den), cementum (cem), and alveolar bone (ab) were devoid of reaction products. Careful study of the micrographs revealed that integrin β5 reaction products were localized to the cell surfaces of periodontal ligament fibroblasts (A), whereas osteonectin (SPARC) was distributed in ECMs including the adjacent bone and cementum matrix (B). Cellular localization findings were consistent with the normal distribution of integrin β5 and osteonectin and suggest a distinct role of these molecules in tissue remodeling that occurs during axial tooth movement. Bar = 50 μm.
Discussion
In the present study we have used the model of the unopposed mouse molar as a model system to investigate tissue dynamics and ECM remodeling following axial movement of teeth. Our data established that in the 12-day unopposed mouse molar model, unopposed molars erupted 0.13 mm above the level of the contralateral side. Historically, the model of the unopposed mouse molar was conceived in the early 1950s at the University of Illinois by Viennese émigrés to distinguish genetic and acquired traits in the functional anatomy of dentition but has been used more recently to assess patterns of posteruptive tooth movement (Schneider and Meyer 1965; Cohn 1966; Anneroth and Ericsson 1967; Levy and Maillard 1980; Compagnon and Woda 1991).
Our results demonstrated that layers of similar thickness of both mineralized tissues, alveolar bone and cementum, were deposited at the root apex following removal of the antagonistic tooth. Morphometrical evaluation of fluorescent labels on ultrathin ground sections revealed that significant amounts of cementum and alveolar bone were gradually deposited at the apex of unopposed molars, indicating that posteruptive axial movement was greatly facilitated by the active deposition of mineralized tissues at the apex of the affected tooth. Whereas some investigators reported lesser amounts of cementum deposition in comparison to alveolar bone deposition (Schneider and Meyer 1965; Cohn 1966) or complete absence of newly formed cementum (Levy and Maillard 1980), other studies (Anneroth and Ericsson 1967; Compagnon and Woda 1991) report similar findings of significant amounts of new cementum deposition after loss of antagonists. Our findings indicated that the rate by which both mineralized tissues were deposited was approximately identical between cementum and alveolar bone, suggesting that similar stimuli might result in similar tissue responses in cementum and alveolar bone.
Using the TRAP-staining procedure, we reported distinct TRAP-positive demarcation linings containing multiple osteoclasts along the mesial alveolar walls of unopposed molars. Secretion of TRAP has been associated with resorptive behavior of osteoclasts (Kirstein et al. 2006) and might in this case suggest the presence of bone resorption at the mesial lining of the alveolar walls in this model. Bone resorption at the mesial alveolar walls would be a logical phenomenon associated with the distal drift of these molars that occurs in an unopposed situation and that has been previously reported (Schneider and Meyer 1965). This finding also corresponds with the new bone apposition at the mesial alveolar walls that was detected in our fluorescent labeling studies using ground sections. Thus, the distal drift of unopposed rodent molars might be explained through alveolar bone resorption at the mesial alveolar walls and new alveolar bone deposition at the distal alveolar walls.
Histochemical analysis using von Kossa’s procedure suggested that the newly formed tissue at the apex of unopposed molars was mineralized cellular cementum. von Kossa’s stain is a routine procedure to detect inorganic calcium phosphate deposits as a marker for mineralized tissues. Staining newly formed cementum via von Kossa’s procedure established distinct boundaries between mineralized tissues and PDL similar to sharp mineralization boundaries present in normal cellular and acellular cementum. This procedure visualized that a new layer of mineralized cellular cementum was formed at the apical tip of the unopposed tooth root.
A number of authors have concluded that the PDL may not be an essential morphological contributor toward tooth eruption, as suggested in earlier studies by Gowgiel (1967) and Cahill and Marks (1980) of dentinal dysplasia type I dentitions. Other studies established a principal role of the PDL as it pertains to the continuous eruption of permanently growing rodent incisors (Berkovitz and Thomas 1969; Berkovitz 1971). Our study resulted in a number of intriguing findings related to the dimensions and possible function of the PDL during posteruptive tooth movement in rodent molars. Morphometrical analysis indicated that using the unopposed molar model, PDL width remained unaffected after axial movement of teeth, in spite of extensive new alveolar bone and cementum deposition. These findings were congruent with previous studies reporting that PDL width was maintained throughout the lifetime of adult mammals (McCulloch and Melcher 1983; Melcher 1986). Thus, one of the most remarkable findings of our study was the extraordinary capacity of the PDL to preserve its width even during tooth movement and even in light of mineralized tissue deposition from two opposing sides. However, width preservation was not the only feature of the PDL that was highlighted during our study. A second feature was the concentration of integrin-β5 and SPARC immunoreactive signals in the PDL and the significant enhancement of reaction intensity for both gene products following axial tooth movement. These findings indicate that the PDL may play a major role in the coordination of the ECM signaling events related to tooth movement while at the same time maintaining its width.
To verify our findings of a potential upregulation of the ECM gene products collagen I, SPARC (osteonectin), and integrin β5, as initially suggested by microarray-based results, we confirmed our findings using semiquanitative RT-PCR and immunohistochemistry (SPARC and integrin β5 only). We have refrained from performing immunoreactions for collagen I because of the abundance of this gene product at the tooth apex and because of inherent specificity issues with collagen antibodies. Our findings confirmed our initial microarray data suggesting that all three ECM gene products were greatly upregulated in the unopposed molar situation and that the ECM may play an important role during tissue remodeling that occurs during tooth movement. Based on these findings, we therefore speculate that matricellular regulators such as SPARC and integrin β5 may act as transducers for mechanical stressors and affect the synthesis of collagen I and other ECM structural gene products, which in turn may be once more functionally modulated by SPARC to bind with collagens or to interact with hydroxyapatite to adapt to constantly changing mechanical stress portfolios imposed on the occluding and non-occluding tooth. Our data suggest that collagen I and SPARC are more concentrated in the unopposed molar PDL, whereas the width of the PDL remains constant. This phenomenon might be explained by spatial changes in other ECM components such as proteoglycans, as previously discussed (Cheng et al. 1999; Sato et al. 2002). Although, at present, this remains a speculative model, our data confirmed our hypothesis about changes in ECM gene expression following loss of antagonists in the unopposed mouse molar model.
Acknowledgments
Support for this study was kindly provided by the National Institute of Dental and Craniofacial Research, National Institutes of Health, Grant R01 DE-15425 (to TGHD) and from the Brodie Endowment to the Department of Orthodontics, UIC College of Dentistry, Chicago, IL.
Literature Cited
- Anneroth G, Ericsson SG. An experimental histological study of monkey teeth without antagonist. Odontol Revy. 1967;18:345–359. [PubMed] [Google Scholar]
- Berkovitz BK. The effect of root transection and partial root resection on the unimpeded eruption rate of the rat incisor. Arch Oral Biol. 1971;16:1033–1043. doi: 10.1016/0003-9969(71)90208-1. [DOI] [PubMed] [Google Scholar]
- Berkovitz BK, Thomas NR. Unimpeded eruption in the root-resected lower incisor of the rat with a preliminary note on root transection. Arch Oral Biol. 1969;14:771–780. doi: 10.1016/0003-9969(69)90168-x. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Matricellular proteins: an overview. Matrix Biol. 2000;19:555–556. doi: 10.1016/s0945-053x(00)00103-7. [DOI] [PubMed] [Google Scholar]
- Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. doi: 10.1016/s0945-053x(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Cahill DR, Marks SC., Jr Tooth eruption: evidence for the central role of the dental follicle. J Oral Pathol. 1980;9:189–200. doi: 10.1111/j.1600-0714.1980.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Cheng H, Caterson B, Yamauchi M. Identification and immunolocalization of chondroitin sulfate proteoglycans in tooth cementum. Connect Tissue Res. 1999;40:37–40. doi: 10.3109/03008209909005276. [DOI] [PubMed] [Google Scholar]
- Cohn SA. Disuse atrophy of the periodontium in mice following partial loss of function. Arch Oral Biol. 1966;11:95–105. doi: 10.1016/0003-9969(66)90120-8. [DOI] [PubMed] [Google Scholar]
- Compagnon D, Woda A. Supraeruption of the unopposed maxillary first molar. J Prosthet Dent. 1991;66:29–34. doi: 10.1016/0022-3913(91)90347-y. [DOI] [PubMed] [Google Scholar]
- Diekwisch TGH, Berman BJ, Gentner S, Slavkin HC. Initial enamel crystals are not spatially associated with mineralized dentine. Cell Tissue Res. 1995;279:149–167. doi: 10.1007/BF00300701. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gowgiel JM. Observations on the phenomena of tooth eruption. J Dent Res. 1967;46:1325–1330. doi: 10.1177/00220345670460063201. [DOI] [PubMed] [Google Scholar]
- Karimbux NY, Nishimura I. Temporal and spatial expressions of type XII collagen in the remodeling periodontal ligament during experimental tooth movement. J Dent Res. 1995;74:313–318. doi: 10.1177/00220345950740010501. [DOI] [PubMed] [Google Scholar]
- Kirstein B, Chambers TJ, Fuller K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J Cell Biochem. 2006;98:1085–1094. doi: 10.1002/jcb.20835. [DOI] [PubMed] [Google Scholar]
- Levy G, Maillard M. Histologic study of the effects of occlusal hypofunction following antagonist tooth extraction in the rat. J Periodontol. 1980;51:393–399. doi: 10.1902/jop.1980.51.7.393. [DOI] [PubMed] [Google Scholar]
- McCulloch CA, Lekic P, McKee MD. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontology. 2000;24:56–72. doi: 10.1034/j.1600-0757.2000.2240104.x. [DOI] [PubMed] [Google Scholar]
- McCulloch CA, Melcher AH. Continuous labeling of the periodontal ligament of mice. J Periodontal Res. 1983;18:231–241. doi: 10.1111/j.1600-0765.1983.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Melcher AH. Periodontal ligament. In: Bhaskar SN, editor. Orban’s Oral Histology and Embryology. 10. Toronto: Mosby; 1986. pp. 198–231. [Google Scholar]
- Nakagawa M, Kukita T, Nakasima A, Kurisu K. Expression of the type I collagen gene in rat periodontal ligament during tooth movement as revealed by in situ hybridization. Arch Oral Biol. 1994;39:289–294. doi: 10.1016/0003-9969(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Oppenheim A. Tissue changes, particularly of the bone, incident to tooth movement. American Orthodontist. 1911–1912;III:57–67. 113–132. [Google Scholar]
- Remelli M, Luczkowski M, Bonna AM, Mackiewicz Z, Conato C, Kozlowski H. Cu (II) ion coordination to SPARC: a model study on short peptide fragments. N J Chem. 2002;27:245–250. [Google Scholar]
- Sandstedt C. Einige Beiträge zur Theorie der Zahnregulierung. Nordisk Tandlakare Tidskrift. 1904;5:236–256. [Google Scholar]
- Sandy JR, Farndale RW, Meikle MC. Recent advances in understanding mechanically induced bone remodeling and their relevance to orthodontic theory and practice. Am J Orthod Dentofacial Orthop. 1993;103:212–222. doi: 10.1016/0889-5406(93)70002-6. [DOI] [PubMed] [Google Scholar]
- Sato R, Yamamoto H, Kasai K, Yamauchi M. Distribution pattern of versican, link protein and hyaluranic acid in the rat periodontal ligament during experimental tooth movement. J Periodontal Res. 2002;37:15–22. doi: 10.1034/j.1600-0765.2002.90770.x. [DOI] [PubMed] [Google Scholar]
- Schneider BJ, Meyer J. Experimental studies on the interrelations of condylar growth and alveolar bone formation. Angle Orthod. 1965;35:187–199. doi: 10.1043/0003-3219(1965)035<0187:ESOTIO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Shimono M, Ishikawa T, Ishikawa H, Matsuzaki H, Hashimoto S, Muramatsu T, Shima K, et al. Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003;60:491–502. doi: 10.1002/jemt.10290. [DOI] [PubMed] [Google Scholar]
- Sringkarnboriboon S, Matsumoto Y, Soma K. Root resorption related to hypofunctional periodontium in experimental tooth movement. J Dent Res. 2003;82:486–490. doi: 10.1177/154405910308200616. [DOI] [PubMed] [Google Scholar]


