Abstract
Peripheral initiators of muscle pain are virtually unknown, but likely key to development of chronic pain after muscle insult. The current study tested the hypothesis that ASIC3 in muscle is necessary for development of cutaneous mechanical, but not heat hyperalgesia induced by muscle inflammation. Using mechanical and heat stimuli, we assessed behavioral responses in ASIC3−/− and ASIC3+/+ mice after induction of carrageenan muscle inflammation. ASIC3−/−mice did not develop cutaneous mechanical hyperalgesia after muscle inflammation when compared to ASIC3+/+ mice; heat hyperalgesia developed similarly between groups. We then tested if the phenotype could be rescued in ASIC3−/− mice by using a recombinant herpes virus vector to express ASIC3 in skin (where testing occurred) or muscle (where inflammation occurred). Infection of mouse DRG neurons with ASIC3-encoding virus resulted in functional expression of ASICs. Injection of ASIC3-encoding virus into muscle or skin of ASIC3−/− mice resulted in ASIC3 mRNA in DRG and protein expression in DRG and the peripheral injection site. Injection of ASIC3-encoding virus into muscle, but not skin, resulted in development of mechanical hyperalgesia similar to that observed in ASIC3+/+ mice. Thus, ASIC3 in primary afferent fibers innervating muscle is critical to development of hyperalgesia that results from muscle insult.
Keywords: pain, acid, proton, ion, nociceptor, carrageenan
1. Introduction
Chronic musculoskeletal pain is disabling and difficult to treat, and includes both inflammatory and non-inflammatory components. Chronic musculoskeletal pain conditions such as repetitive strain injury and tendonitis are clearly associated with peripheral tissue damage that includes inflammation of the muscle (Barr and Barbe, 2002; Barr et al., 2004; Stauber, 2004). Data from the Bureau of Labor and Statistics states that 53% of the private workforce had musculoskeletal pain over the last 2 weeks, and 13% of these lost productive work time with losses estimated at $61 billion per year.
As a model of chronic inflammatory muscle pain, injection of carrageenan into muscle produces a local inflammatory response and hyperalgesia of the paw that lasts for weeks, spreading to the contralateral side when the inflammation becomes chronic (Radhakrishnan et al., 2003). Inflammation of muscle sensitizes neurons in both the peripheral and central nervous system (Mense, 1993). Muscle nociceptors increase their resting activity and responses to mechanical stimuli following muscle inflammation (Berberich et al., 1988; Diehl et al., 1988). Similarly dorsal horn neurons show increased background activity and decreased threshold to noxious stimulation in response to muscle inflammation (Hoheisel et al., 1994; 1997). These changes in central neurons are thought to underlie the hyperalgesia that occurs outside the site of injury.
A decrease in pH is observed following inflammation, hematomas and isometric exercise (Hood et al., 1988; Issberner et al., 1996; Pan et al., 1988; Revici et al., 1949), and decreases in pH in muscle produce pain in humans (Issberner et al., 1996). Peripheral initiators of muscle pain are virtually unknown, but are likely key to development of chronic pain after muscle insult. Previously, we showed that mechanical hyperalgesia and dorsal horn neuron sensitization induced by repeated intramuscular acid injections, a model of non-inflammatory muscle pain, do not develop in mice with a null mutation of the acid-sensing ion channel ASIC3 (Sluka et al., 2003). Mechanical hyperalgesia is enhanced, and heat hyperalgesia is unchanged after carrageenan inflammation of the paw (Price et al., 2001). ASIC3 is a member of the DEG/ENaC family that is known to mediate mechanical responsiveness in Caenorhabditis elegens including those sensations related to deep tissue, proprioception and muscle stretch (Driscoll and Chalfie, 1991; Huang and Chalfie, 1994; Liu et al., 1996; Tavernarakis et al., 1997). Further, ASIC3 plays a role in normal visceral mechanosensation to stretch and in mechanical sensitization of primary afferents after visceral inflammation (Jones et al., 2005). In contrast, TRPV1 appears to mediate heat hyperalgesia associated with inflammation (Keeble et al., 2005; Honore et al., 2005; Caterina et al., 2000). Since in ASIC3−/− mice ASIC3 is removed from the entire animal it is not clear whether the reduction in mechanical hyperalgesia is a result of a loss of ASIC3 in muscle where the carrageenan is injected, or ASIC3 in paw where the mechanical hyperalgesia is tested. It is also not clear from these earlier experiments if this loss of mechanical hyperalgesia is specific or if it also includes a loss of heat hyperalgesia. Therefore, we hypothesized that ASIC3 in the muscle is a key factor for development of cutaneous mechanical hyperalgesia, but not heat hyperalgesia, induced by muscle insult.
2. Materials and Methods
2.1 Mice
All experiments were approved by the institutional Animal Care and Use Committee and are in accordance with National Institutes of Health guidelines. For initial characterization of behavioral response to carrageenan, ASIC3−/− (n=17) mice that are congenic on a C57BL/6J background were bred at the University of Iowa Animal Care facility and were compared to C57BL/6J mice (n=15, Jackson Laboratories) housed at the University of Iowa. Both males and female mice were used in each group. F2 generation ASIC3−/− (n=6) and ASIC3+/+ (n=7) mice were also utilized in the initial experiment examining mechanical and heat sensitivity after carrageenan muscle inflammation. The original ASIC3−/− was made by homologous recombination in an ES cell line of 129sVJ origin, and the original ASIC3−/− were a mixture of 129SVJ and C57BL/6J. The congenic ASIC3−/− mice were backcrossed for 10 generations on C57BL/6J mice. Preliminary experiments show that behavioral assessments in congenic ASIC3−/− mice when comparing to C57BL/6J mice show the same pattern with loss of mechanical hyperalgesia after muscle inflammation as ASIC3−/− F2 generation when compared to wild-type littermates. Importantly the behavioral effects in F2 generation ASIC3−/− mice and the congenic ASIC3−/− mice are not statistically different, and thus the data from the F2 generation and the congenic ASIC3−/− mice were combined. There was no effect for sex (ASIC3+/+ WT male=11, female=9; ASIC3−/− male=12, female=9). All subsequent experiments were performed with congenic ASIC3−/− mice and compared to C57BL/6J mice.
2.2 Induction of inflammation
The gastrocnemius muscle of mice was injected with 3% carrageenan, 20 μl, while the mouse was anesthetized with 2–4% halothane. Mice were allowed to recover prior to the first behavioral test 24 h later.
2.3 Virus infection
These experiments utilized the herpes simplex virus (HSV-1) as a vector to express ASIC3 in muscle or skin. HSV-1 was chosen as a vector since it is taken up by peripheral primary afferent terminals, transported to the DRG, stays localized to the primary afferent fibers that take up the virus (i.e. no transsynaptic transport), and has been utilized effectively to infect peripheral neurons (Jones et al., 2003; Wilson and Yeomans, 2002; Wilson et al., 1999).
Recombinant herpes simplex virus (HSV-1) vectors were constructed by standard techniques. To construct the control virus PZ, a shuttle plasmid was constructed that contained an expression cassette consisting of the hCMV immediate-early enhancer-promoter, the lacZ gene flanked by Pac I restriction sites and an SV40 polyadenylation sequence inserted into a segment of HSV-1 DNA at a unique site in the viral thymidine kinase gene. This linearized shuttle plasmid and HSV-1 (strain KOS) DNA were co-tranfected into Vero cells. A recombinant virus expressing β-galactosidase (β-gal) was purified by 3 rounds of limiting dilution and the insertion site of the expression cassette in the viral DNA verified by Southern blotting. Insertion of the expression cassette into the viral thymidine kinase gene prevents viral replication in non-dividing cells such as neurons and prevents reactivation from latency (Tenser et al., 1989). A viral vector map is shown in Figure 1.
Figure 1.
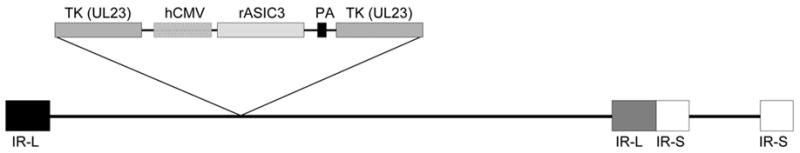
Schematic diagram of recombinant rASIC 3 HSV vector. This vector does not express thymidine kinase (TK, insertional inactivation). Expression of rat ASIC3 is driven by the hCMV immediate-early enhancer-promoter. PA, SV40 polyadenyation signal; IR, internal repeats flanking the unique long (L) and unique short (S) viral sequences.
Similar techniques were used to construct a recombinant virus that contains the full-length rat ASIC3 cDNA (PrASIC). A shuttle plasmid containing the ASIC3 cDNA was transfected with PZ DNA that had been digested with Pac I to excise the lacZ sequence. This technique results in a high percentage of recombinants (Krisky et al., 1997).
Muscle
Four weeks prior to beginning the experiment, 10 μl of a recombinant herpes simplex virus (HSV-1) designed to express rat ASIC3 (sense; 107 plaque forming units (pfu)) or a control HSV-1 expressing β-galactosidase was injected into the gastrocnemius muscle while the rat was anesthetized with 2–4% halothane. These injections were made into congenic ASIC3−/− mice. For a control, HSV-1 expressing ASIC3 was injected into the muscle from C57BL/6J mice. The animals were tested before and 4 weeks after virus injection. There was no difference in behavioral responses 4 weeks after injection of HSV-1 expressing ASIC3 or lacZ compared to baseline responses prior to injection of virus. Four weeks was chosen since we obtain adequate expression of ASIC3 at this time and to minimize any confounding factors attributed to an initial immune response to the injection of HSV-1 during the first week.
Skin
Four weeks prior to the beginning of the experiment, mice were anesthetized with 2–4% halothane. The plantar surface of the skin of the ipsilateral hindpaw was injected with 2, 10 μl injections (15 min apart) of HSV-1 expressing rat ASIC3 (sense; 107 plaque forming units (pfu)/ml) or HSV-1 expressing β-galactosidase. These injections were made into congenic ASIC3−/− mice.
2.4 Behavior Testing
Heat Testing
Mice were tested for withdrawal latency to radiant heat. Mice were placed in small cubicles on a glass platform. The radiant heat source was directed to the plantar surface of the paw until the mouse withdrew its paw. The latency (in seconds) was recorded for each mouse and each hindpaw. An average of 3 trials was taken at each testing time.
Mechanical Testing
Mice were also tested for response frequency to a von Frey filament with a 0.4 mN bending force. The mice were placed in small cubicles on a screen platform. The von Frey filament was applied to the plantar surface of the paw 5 times and the number of withdrawals to the stimulus was counted. An average of 10 trials was taken at each testing time.
2.5 Electrophysiology of cultured DRG neurons
Thoracic DRG neurons were collected and dissociated from a 5 month-old congenic ASIC3−/− mouse. Briefly, DRG were dissociated with papain, collagenase and dispase, plated on poly-D-lysine/laminin-coated 35 mm plastic dishes, and stored at 37°C in F12 medium supplemented with 50 nM nerve growth factor. On day 1, the recombinant HSV-1 vector expressing rat ASIC3 (PrASIC) was diluted with F12 medium to 1×106 pfu/ml and added to the neurons. For non-transfected neurons, the medium was exchanged without virus. Neurons 20–35 μm in diameter where studied on day 3 after plating.
Whole-cell patch-clamp recordings (at −70 mV) from DRG neurons were performed with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and acquired and analyzed with Pulse/Pulsefit 8.30 (HEKA Electronics, Lambrecht, Germany). Currents were filtered at 5 kHz and sampled at 2 kHz. Desensitization kinetics were measured by filtering the upslope of currents with single experimental equations and time constants (τ) reported. Micropipettes (3–5 MΩ) were filled with internal solution (mM): 100 KCl, 10 EGTA, 40 HEPES, and 5 MgCl2, pH 7.4 with KOH. External solution contained (mM): 120 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 10 MES, pH adjusted with tetramethylammonium hydroxide, and osmolarity adjusted with tetramethylammonium chloride. Extracellular solutions were changed using a computer-driven solenoid valve system.
2.6 Reverse transcriptase-polymerase chain reaction (RT-PCR)
L3–L6 DRG were removed from congenic ASIC−/− mice injected with HSV-1 expressing ASIC3, ipsilaterally and contralaterally to the side injected with virus, stabilized with RNAlater RNA stabilization reagent, and stored at −70°C until processing. Tissue was homogenized and total RNA isolated according to animal tissue protocol with RNeasy Mini Kit (Qiagen). The RNA were then placed in reverse transcriptase (Omniscript Reverse Transcriptase kit, Qiagen) and run with standard PCR protocols. Primers directed to rat ASIC3 were used to measure expression of mRNA following injection of ASIC3 expression virus into muscle of ASIC3−/−mice. Controls were added as follows: control comparison to mice injected with HSV-1 expressing β-galactosidase, rat positive control for the ASIC3 rat primers, reagents without the ASIC primers, reagents without reverse transcriptase. Ethidium bromide gels will be imaged and stored to a computer for off-line analysis. For ASIC3 expression (sense) experiments, the primers for ASIC3 PCR (5′-TCTTTGGATCCCGACGAC-3′; 5′-ACTATAAGGGCTGGGT-3′) were designed to recognize rat but not mouse ASIC3 transcript.
2.7 Enzyme-Linked Immunosorbent Assay (ELISA) for ASIC3 in muscle or skin
At the end of each experiment, C57BL/6J or congenic ASIC3−/− mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p) and decapitated prior to removal of tissue. The inflamed gastrocnemius muscle and ispilateral skin of the hindpaw were removed, weighed, and placed in Triton-X 100 solubilization buffer. Triton-X solubilization buffer contained 1% Triton-X, 10 mM Tris, 10 mM EGTA, 10 mM EDTA, 150 mM NaCl, with 5ul/ml of pepA, leupetin, and PMSF. Tissues were then homogenized and centrifuged to remove the supernatant for analysis by ELISA. Direct ELISA was used to determine the concentration of ASIC3 in muscle tissue. On day 1, plates were coated with supernatant diluted in coating buffer, covered, and stored at 8°C. Day 2 plates were washed and blocked for 2 h. Plates were then washed and incubated in rabbit polyclonal antibody to ASIC3 (Chemicon, 1:200) for 2 h. After washing, a goat anti-guinea pig IgG (Vector, 1:500) was added to each well for 2 h followed by streptavidin-HRP (Vector, 1:1000) incubation for 2 h. Visualization was done with tetramethylbenzidine for 20 min (TMB), the reaction stopped with 1.8 N sulfuric acid, and results read on a plate reader at 450 nm. The concentration of ASIC3 was calculated from a standard curve on each plate. Samples and standards were run in duplicate. Data are represented as mg ASIC3/ml of solution/gram of tissue (mg/ml/g).
2.8 Immunohistochemistry examined protein expression of ASIC3 in DRG (Sluka et al., 2003)
L4–L6 DRG were removed fresh and postfixed overnight in 2% paraformaldehyde and 15% sucrose from C57BL/6J or congenic ASIC3 mice. DRG were frozen and cut the following day at 10 μm on a cryostat. Standard immunohistochemical techniques were used with tissue sections incubated in primary antibody to ASIC3 (1:500, Chemicon, guinea pig) overnight. The secondary antibody was conjugated to a fluorescent marker Alexa Fluor 546 (1:1000, red). Sections were imaged with an Olympus BX-51 fluorescent microscopy in the Microscopy Core Facility within the College of Medicine. This methodology results in staining of ASIC3 in DRG of wild-type animals but not ASIC3−/− mice (Sluka et al., 2003).
2.9 Measurement of inflammation
Circumference of the inflamed muscle was measured before and 24h (n=15 ASIC3−/−; n=15 ASIC3+/+), 1 week (n=11 ASIC3−/−; n=12 ASIC3+/+) and 2 weeks (n=8 ASIC3−/−; n=8 ASIC3+/+) after induction of muscle inflammation with carrageenan. Circumference was measured around the greatest portion of the muscle belly with a flexible tape measure. After measurement of circumference mice were deeply anesthetized with sodium pentobarbital, guillotined, and the muscle removed and processed for neutrophilic activity using myeloperoxidase assay (Desser et al., 1972). We analyzed muscle 24 h (n=8 ASIC3−/−; n=7 ASIC3+/+), 1 week (n=7 ASIC3−/−; n=8 ASIC3+/+), and 2 weeks (n=7 ASIC3−/−; n=7 ASIC3+/+) after induction of inflammation. The muscle was homogenized in 0.5% hexadecyltrimethylammonium bromide (Sigma, St Louis, MO) to temporarily neutralize enzyme activity. Samples then underwent three cycles of freezing and thawing followed by centrifugation to remove debris. Samples were reacted with hydrogen peroxide and o-dianisidine dihydrochloride at ambient temperature. Absorbance was measured every minute for up to 10 min with a Spectramax 190 (Molecular Devices, Sunnyvale, CA) plate reader at a wavelength of 450 nm. Maximal enzymel velocity (Vmax) was used to determine the units of myeloperoxidase per gram of tissue from the standard curve. An enzyme unit was defined as the amount of enzyme required to produce an increase of one absorbance unit in one minute. Samples were prepared in triplicate and averaged. Myeloperoxidase obtained from humans (Sigma, St Louis, MO) was used as a standard. The assay showed a 3 % coefficient of variance between days and a limit of quantification of 0.07 units/gram tissue.
2.10 Experimental Protocol
Experiment 1 (Behavior)
ASIC3−/− mice (n=23) were tested against wild-type controls (n=22) for responses to mechanical and heat stimuli before and up to 2 weeks after injection of carrageenan. Data were analyzed with a repeated measures analysis of variance for differences between groups. Post hoc testing between groups at individual times was with an independent t-test, and paired t-tests compared pre-inflammation values to post-inflammation values for each group.
Experiment 2 (Inflammation)
Muscle circumference and myeloperoxidase activity of the inflamed muscle were measured in congenic ASIC3−/− mice and ASIC3+/+ mice 24h, 1 week and 2 weeks after induction of muscle inflammation with carrageenan. A one way ANOVA with post hoc testing with a Tukey’s test compared differences between groups.
Experiment 3 (Rescue)
Congenic ASIC3−/− mice were injected with HSV-1 expressing ASIC3 into either the muscle (n=10) or skin (n=7). As a control, congenic ASIC3−/− mice were injected with HSV-1 expressing β-galactosidase (LacZ) into either muscle (n=7) or skin (n=6). Mice were tested for mechanical and heat sensitivity before and 4 weeks after injection of virus. Carrageenan was then injected into the muscle and mice were tested again for mechanical and heat sensitivity 24 h, 72 h, 1 week, and 2 weeks later. Data were analyzed with a repeated measures analysis of variance for differences between groups followed by post hoc testing with a Tukey’s test to compare ASIC3−/− injected with HSV-1 expressing ASIC3 and ASIC3−/− mice injected with HSV-1 expressing β-galactosidase. For comparison ASIC3+/+ mice (n=7) were injected with HSV-1 expressing ASIC3 and behavioral responses to mechanical stimuli were measured 24 h, 72 h, and 1 week after injection of carrageenan.
3. Results
Following muscle inflammation induced by carrageenan injection there is a decrease in the withdrawal latency to heat (indicating heat hyperalgesia), and an increase in the number of withdrawals to mechanical stimuli (indicating mechanical hyperalgesia) applied to the paw ipsilateral to the side of inflammation (Figure 2). In ASIC3−/− mice, this mechanical hyperalgesia is attenuated: the number of withdrawals in the ASIC3−/− mice is significantly less than those in the ASIC3+/+ mice (F1,35=12.1, p=0.001). Differences between groups occurred at 24 h (p=0.0001), 72 h (p=0.001), 1 week (p=0.0001), and 2 weeks (p=0.003)(independent t-test). Significant increases in mechanical responses occurred in the ASIC3−/− 24 h, 72 h, and 1 week, and in ASIC3+/+ occurred 24 h, 72 h, 1 week and 2 weeks after injection of carrageenan (paired t-test, p<0.05). In contrast, the decreased withdrawal latency to heat is similar between the ASIC3−/− and the ASIC +/+ mice (F1,41=0.83; p=0.54) (Figure 2). No significant changes in mechanical (F1,41=2.7, p=0.11) or heat (F1,41=0.83; p=0.54) responses occurred on the contralateral hindlimb.
Figure 2.
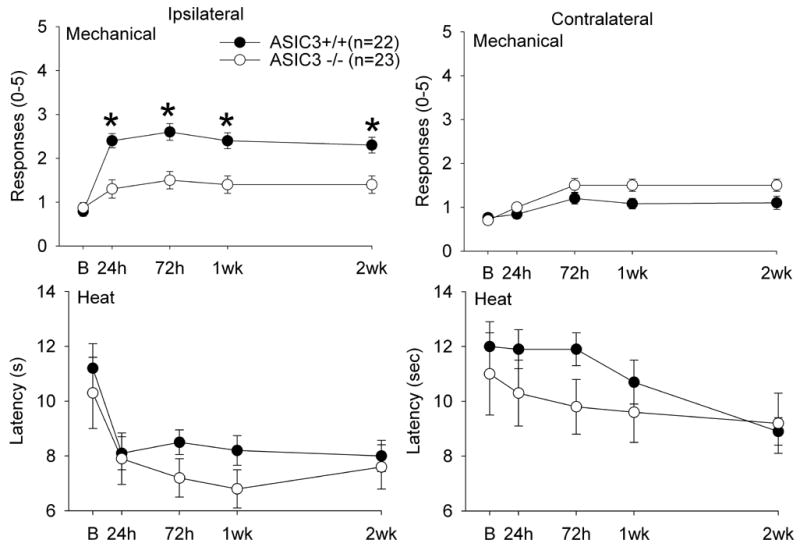
Graphs represent the responses of the paw to mechanical and heat stimuli in ASIC3−/− and ASIC3+/+ mice before and after induction muscle inflammation with 3% carrageenan. ASIC3+/+ mice developed an increased response to mechanical stimuli (mechanical hyperalgesia) and a decreased latency to withdrawal from heat stimuli (heat hyperalgesia) ipsilateral to the side of injection, but not contralateral to the side of injection. ASIC3−/− mice did not develop an increased response to mechanical stimuli, but still developed a decreased latency to heat applied to the paw. Values are mean ± S.E.M. *, p=0.01, significantly different between groups
To test if ASIC3 plays a role in the development of inflammation, we assessed circumference of the muscle, and neutrophilic activity 24 h, 1 week and 2 weeks after induction of inflammation. Baseline joint circumferences averaged 2.4 +/− 0.19 cm and increased approximately 0.4–0.5 cm after inflammation. These increases in circumference in ASIC3−/− and ASIC3+/+ mice were similar between groups measured 24 h, 1 week, and 2 weeks after induction of inflammation (Figure 3A). Neutrophilic activity, measured by myeloperoxidase activity, is also similar between ASIC3+/+ and ASIC3−/− mice 24 h and 1 week after induction of inflammation (Figure 3B). Consistent with neutrophil infiltration during the acute phase of inflammation, the greatest increases in myeloperoxidase activity occurred 24 h after induction and was significantly greater than that measured 1 and 2 weeks after induction of inflammation (p=0.008, p=0.001, respectively), and there was virtually no myeloperoxidase activity noted by 2 weeks.
Figure 3.
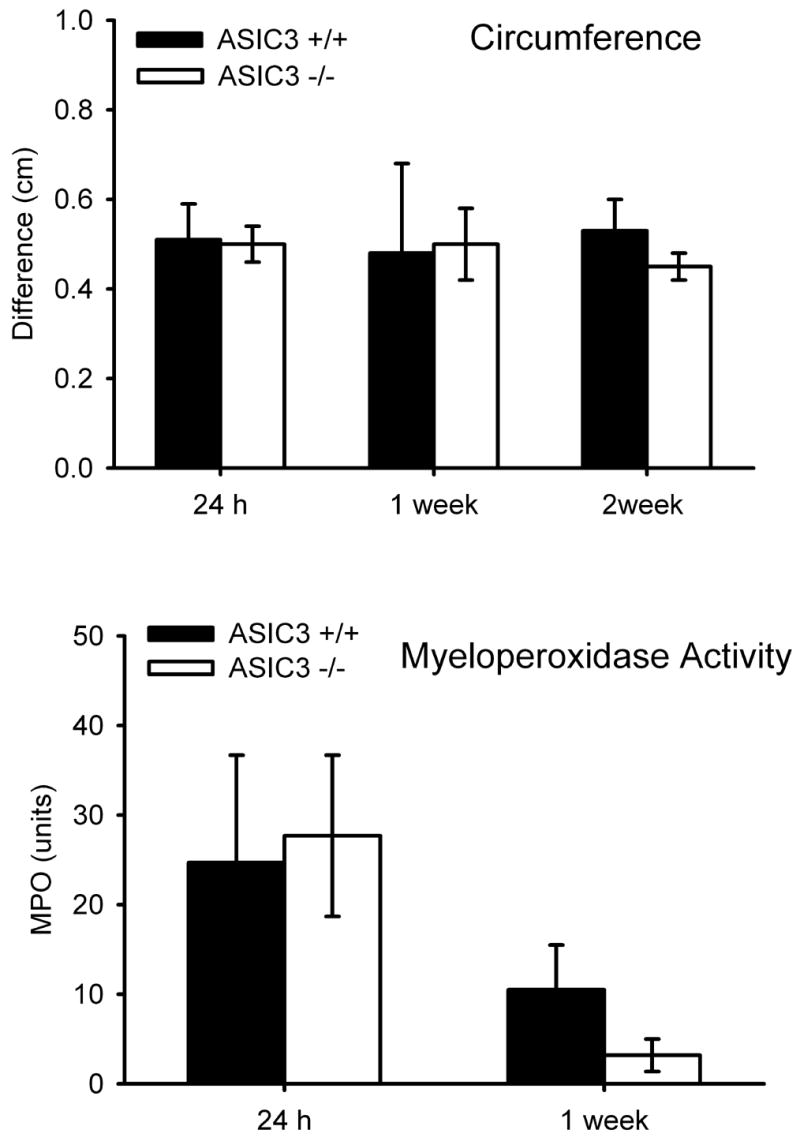
A. Bar graphs represent the difference in muscle circumference (ipsilateral-contralateral) before and 24h, 1 week, and 2 weeks after induction of muscle inflammation. There was no difference between ASIC3−/− and ASIC3+/+ mice. B. The myeloperoxidase activity of the gastrocnemius muscle was measured 24h, 1 week and 2 weeks after induction of muscle inflammation. As expected, the greatest activity occurred at 24h when there was an acute inflammation, and was reduced by 2 weeks to nearly 0 when the inflammation was chronic. There was no difference between ASIC3−/− and ASIC3+/+ mice.
To show that rat ASIC3 forms functional currents in mouse DRG neurons we utilized whole cell patch clamp to record acid-evoked currents in DRG from adult ASIC3−/− mice infected with HSV-1 expressing ASIC3 (n=8) or non-infected DRG neurons as controls (n=7). Figure 4A shows an example of pH 5-evoked currents from DRG infected with PrASIC compared to non-infected cells. Five of 8 neurons exposed to PrASIC expressed transient pH-evoked currents, typical for ASIC3 (Waldmann et al., 1997); whereas 0/7 non-infected neurons displayed transient pH activated currents. The fast desensitization kinetics of transient pH 6-evoked currents (τ=0.22 ± 0.05 S.E.M.) confirms the contribution of ASIC3 (Benson et al., 2002).
Figure 4.
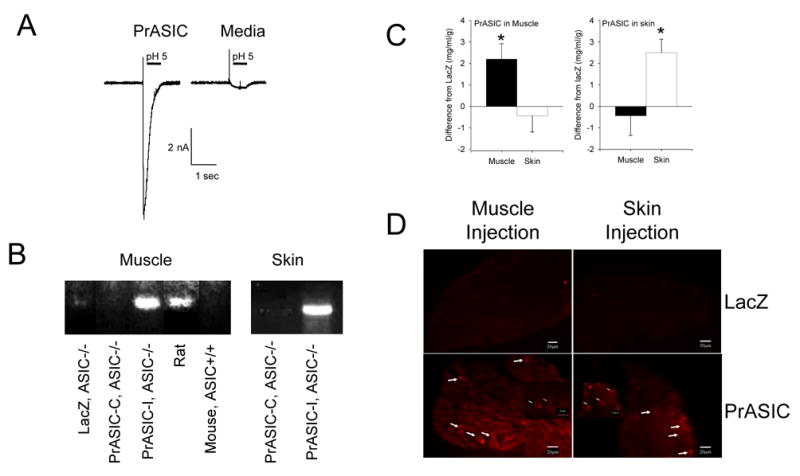
A. Whole cell patch clamp shows that DRG neurons from ASIC3−/− mice infected with PrASIC restores acid currents with a similar profile to that of ASIC3. Controls in which media was exchanged instead of adding PrASIC had no ASIC current. Sample traces and summary responses (mean +/− S.E.M.) of all cells are shown. B. RT-PCR of DRG was performed with primers specific for rat. Notice the primers recognize rat mRNA but not mouse mRNA for ASIC3. Expression of ASIC3 mRNA in ASIC3−/− mice was observed when HSV-1 expressing ASIC3 (PrASIC) was injected into muscle or skin ipsilaterally, but not contralaterally. There was also no expression when the control lacZ virus was injected into ASIC3−/− mice. C. ELISA for ASIC3 of the muscle or skin shows increased expression of protein when HSV-1 expressing ASIC3 (PrASIC) was injected into the muscle or skin or ASIC3−/− mice, respectively, Data were compared to muscle or skin from ASIC3−/− mice injected with HSV-1 expressing β-galactosidase (lacZ) as a control. Values are mean ± S.E.M. D. DRG from ASIC3−/− mice were immunoreactive for ASIC3 after injection of HSV-1 expressing ASIC3 was injected into the muscle or the skin. No immunoreactivity was observed in DRG from ASIC3−/− mice after injection of HSV-1 expressing β-galactosidase (lacZ).
To show that the virus produced mRNA in DRG after infection, we performed RT-PCR of DRG after infections with HSV-1 expressing ASIC3 or β-galactosidase for control. RT-PCR of DRG 4 weeks after injection of virus into muscle or skin (Figure 4B) confirmed expression of ASIC3 mRNA in the DRG ipsilateral, but not contralateral, to the side of injection.
To show the virus produced measurable protein peripherally in the injected tissues, we performed an ELISA for ASIC3 of muscle or skin from mice after infection with HSV-1 expressing ASIC3 (PrASIC) or β-galactosidase (LacZ) as a control. ELISA of muscle or skin from ASIC3−/− mice injected with HSV-1 expressing ASIC3 showed an increased concentration of ASIC3 compared to muscle or skin after injection of HSV-1 expressing β-galactosidase (Figure 4C). Significant increases in concentrations of ASIC3 were observed for the muscle of ASIC3−/− mice when the muscle was injected with HSV-1 expressing ASIC3 when compared to the skin from the same mice (p<0.05, t-test). Similarly, significant increases in concentrations of ASIC3 were observed from the skin of ASIC3−/− mice when the skin was injected with HSV-1 expressing ASIC3 when compared to the muscle from the same mice (p<0.05, t-test). Muscle from ASIC3+/+ mice show an average of 4.5 +/− 0.96 mg/ml/g (n=10). Muscle from animals injected with HSV-1 expressing ASIC3 into muscle produced an average of 2.7 +/− 0.84 mg/ml/g compared to muscle from animals injected with HSV-1 expressing β-galactosidase into muscle which showed 0.74 +/− 0.80 mg/ml/g. Skin from ASIC3+/+ mice averaged 7.2 +/− 1.9 mg/ml/g (n=6). Skin from animals injected with HSV-1 expressing ASIC3 into skin produced an average of 6.3 +/− 0.62 mg/ml/g compared to skin from animals injected with HSV-1 expressing β-galactosidase into skin which showed 3.7 +/− 0.65 mg/ml/g.
To show the increase in protein occurred in primary afferent fibers, we performed immunohistochemistry of DRG after infection with HSV-1 expressing ASIC3 or β-galactosidase. Immunohistochemistry of DRG 4 weeks after infection with virus confirmed ASIC3 protein was expressed in DRG neurons from ASIC3−/− mice previously injected into the muscle or the skin with HSV-1 expressing PrASIC, but not ASIC3−/− previously injected with HSV-1 expressing β-galactosidase (Figure 4D).
Once we were able to show expression of ASIC3 mRNA and protein and ASIC3 function in ASIC3−/− mice after infection with an ASIC3-expressing virus, we performed behavioral experiments with viral expression of ASIC3 in muscle or skin of these ASIC3−/− mice. Importantly, injection of HSV-1 expressing either ASIC3 or β-galactosidase (before induction of inflammation) produced no difference in baseline behavioral responses to heat and mechanical stimuli. Animals showed 0.35 +/− 0.09 responses (out of 5) to mechanical stimuli before and 0.42 +/− 0.14 responses after injection of virus into muscle. Withdrawal latencies to heat were 10.1 +/−0.9 s before and 9.3 +/− 0.5 after injection of virus into muscle.
In ASIC3−/− mice injected with HSV-1 expressing ASIC3 (PrASIC) into muscle, which would restore ASIC3 to primary afferent fibers innervating muscle, carrageenan muscle inflammation produced a similar increase in responsiveness to mechanical stimulation of the paw when compared to ASIC3+/+ mice (Figure 5). When HSV-1 expressing ASIC3 was injected into the skin of the hindpaw of ASIC3−/− mice, which would restore ASIC3 to primary afferent fibers innervating the skin, there was no difference in the number of responses to mechanical stimuli after carrageenan muscle inflammation, and those responses were similar to ASIC3−/−injected with HSV-1 expressing β-galactosidase. The responses to mechanical stimuli were significantly increased in ASIC3−/− injected with HSV-1 expressing ASIC3 into the gastrocnemius muscle after carrageenan muscle inflammation (F3,25=4.6, p=0.01) when compared to those injected with HSV-1 expressing β-galactosidase in the muscle (p=0.05, Tukey’s test), or those when the skin was injected with either HSV-1 expressing ASIC3 (p=0.02, Tukey’s test) or β-galactosidase (p=0.04; Tukey’s test). On the other hand, the withdrawal latency to heat after carrageenan muscle inflammation was decreased similarly in ASIC3−/− mice injected with HSV-1 expressing ASIC3 or β-galactosidase into either the muscle or the skin. As a control, HSV-1 expressing ASIC3 was injected into ASIC3+/+ mice. In these mice, there was a similar increase in response to mechanical stimuli of the paw, and a similar decrease in heat stimuli, to that observed in wild-type mice or ASIC3−/− mice injected with HSV-1 expressing ASIC3. Thus, expression of ASIC3 in muscle, but not in skin, of ASIC3−/− mice restores the development of mechanical hyperalgesia that normally occurs after carrageenan muscle inflammation.
Figure 5.
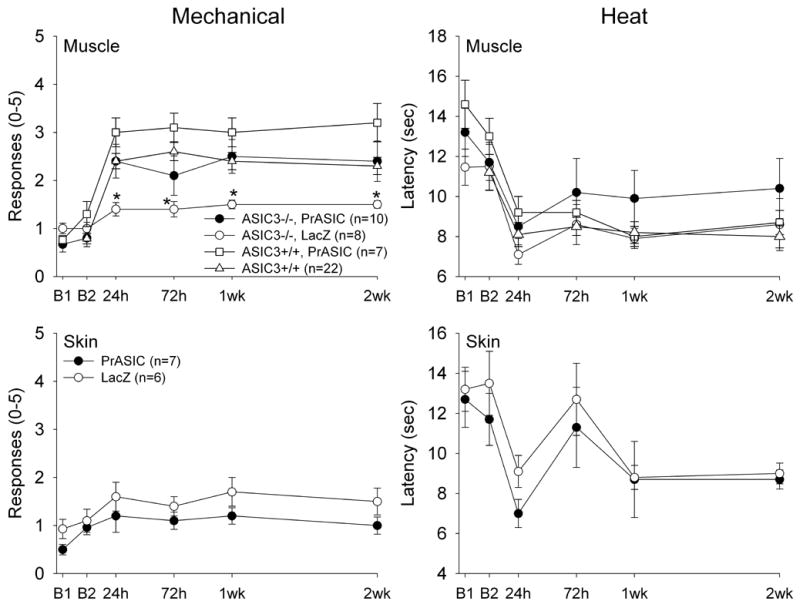
The response to mechanical and heat stimuli applied to the paw before and after induction of muscle inflammation in ASIC3−/− mice infected with HSV-1 expressing ASIC3 injected into muscle or skin, and in ASIC3−/− mice infected with HSV-1 expressing β-galactosidase (lacZ) injected into muscle or skin. Increased mechanical sensitivity was restored after expression of ASIC3 in muscle, but not in skin. There was no effect for ASIC3 expression on heat sensitivity before or after carrageenan inflammation. For comparison, the data from ASIC3+/+ mice for mechanical and heat responses were added to the muscle injection graphs. We also show that in animals injected with HSV-1 expressing ASIC3 into muscle of ASIC3+/+ mice there is no increased mechanical sensitivity after injection of carrageenan for up to 2 weeks (open squares). Values are mean ± S.E.M.
4. Discussion
4.1 ASIC3 in muscle mediates mechanical hyperalgesia induced by muscle insult
The current study shows that mechanical hyperalgesia induced by carrageenan muscle inflammation is attenuated in ASIC3−/− mice when compared to ASIC3+/+ mice. These data agree with prior data showing that ASIC3−/− mice do not develop mechanical hyperalgesia after repeated injections of acidic saline in the muscle, a model of chronic non-inflammatory muscle pain (Sluka et al., 2003). Together the data suggest a role of ASIC3 in muscle-induced hyperalgesia.
The current study also shows that expression of ASIC3 in primary afferent fibers innervating muscle of ASIC3−/− mice restores mechanical hyperalgesia of the paw after muscle insult. In contrast, expression of ASIC3 in primary afferent fibers innervating skin has no effect on mechanical responses. Similarly, intramuscular injection of amiloride, a non-selective ASIC blocker, prevents the mechanical hyperalgesia produced by repeated intramuscular acid injections in mice (Sluka et al., 2003). These data therefore show, for the first time, that ASIC3 in primary afferent fibers innervating muscle is critical to the development of hyperalgesia after muscle insult.
The current data, showing a loss of hyperalgesia in ASIC3−/− mice, are distinctly different from prior results from ASIC3−/− mice (Price et al., 2001; Chen et al., 2002), or dominant-negative ASIC3 transgenic mice that inactivates all ASIC-like currents (Mogil et al., 2005). These prior studies tested responses to cutaneous insult, i.e. carrageenan, capsaicin, and acid and show either no or enhanced response to nociceptive stimuli after injury (Price et al., 2001; Chen et al., 2002; Mogil et al., 2005). Our prior study, and the current study tested responses to muscle insult and show a loss of mechanical hyperalgesia after insult (Sluka et al., 2003). Mogil and colleagues attempted to test the effects of muscle insult in their dominant-negative ASIC3 transgenic mice; however, the wild-type controls for ASIC3 transgenic mice do not develop mechanical hyperalgesia to repeated intramuscular acid injections (Mogil et al., 2005), as we previously described (Sluka et al., 2004). Differences are likely related to the strain of the mouse since prior studies demonstrate strain differences in mechanical sensitivity and in different injury-induced animal models of pain (Mogil et al., 1999a; Mogil et al., 1999b). In the current study we utilize congenic mice on a C57BL/6J background, while Mogil and colleagues (2005) utilize mice on an FVB background. In support of our results Mogil and colleagues (2005) showed development of mechanical hyperalgesia similar to that observed in our prior studies in ASIC3+/+ mice using C57BL/6J mice. Recently, McCleskey and colleagues (Molliver et al., 2005) show that ASIC3 is expressed in 51% of small nociceptive DRG neurons that innervate muscle compared to only 28% of small nociceptive DRG neurons that innervate skin. Further, 80% of ASIC3-positive DRG neurons express calcitonin gene-related peptide, and nerve terminals co-expressing ASIC3 and CGRP are found in primary afferents innervating muscle arterioles (Molliver et al., 2005). In contrast, few proprioceptors express ASIC3 (Molliver et al., 2005). Together, these data suggest that ASIC3 in primary afferent fibers innervating muscle is critical to development of mechanical hyperalgesia as a result of muscle insult. We further hypothesize that ASIC3 in primary afferent fibers innervating skin plays a minimal role in mechanical sensitivity to tissue injury; and that this role is opposite of that after muscle insult. It should be noted, however, there are acid-evoked currents in a skeletal muscle cell line that are blocked by amiloride and APETx2, a more sensitive ASIC3 blocker (Gitterman et al., 2005). Further mRNA for ASIC3 was found in skeletal muscle preparations (Gitterman et al., 2005). Thus, it is possible that both skeletal muscle and primary afferent fibers innervating skeletal muscle utilize ASIC3 to sense changes in the pH of the microenvironment to result in alterations in muscle function, as well as activation of nociceptors.
Responses to cutaneous mechanical stimuli of the paw in ASIC3−/− mice without tissue injury show mixed results. There is no difference in mechanical sensitivity when measuring the number of responses to repeated application of a von Frey filament in ASIC3−/− mice on a mixed 129 x C57BL/6J background (Sluka et al., 2003; Price et al., 2001), but mechanical sensitivity may be increased in dominant-negative ASIC3 trangenic mice on a FVB background (Mogil et al., 2005). Mechanical thresholds, however, were lower in ASIC3−/− mice (Chen et al., 2002), and in dominant-negative ASIC3 transgenic mice (Mogil et al., 2005). The current study shows that expression of ASIC3 in the primary afferent fibers innervating skin of ASIC3−/− mice does not alter the responses to repeated mechanical stimulation. The direction of change in the ASIC3−/− and transgenic mice, when present, shows increased sensitivity, and in several studies shows no difference in mechanical sensitivity, thus arguing against ASIC3 as a mechanosensor in primary afferents innervating skin. Expression of ASIC3 in primary afferent fibers innervating muscle of ASIC3−/− mice restores the increased responses to mechanical stimulation after carrageenan inflammation similar to that observed in ASIC3+/+ mice. These data suggest that ASIC3 in primary afferents innervating muscle, but not skin, is necessary for development of secondary mechanical hyperalgesia and likely serves as a pH sensor.
In the current study, we show that heat hyperalgesia induced by carrageenan muscle inflammation develops similarly between ASIC3−/− mice when compared to ASIC3+/+ mice. This is similar to that observed for heat responsiveness after paw inflammation with carrageenan or zymosan in mice with a null mutation of ASIC3−/− (Price et al., 2001; Chen et al., 2002; Mogil et al., 2005). Normal response to heat applied to the paw is also unaltered in ASIC3−/−mice (Price et al., 2001; Chen et al., 2002; Mogil et al., 2005). Further, ASIC3 plays no role in the development of inflammation since ASIC3−/− mice were similar to ASIC3+/+ mice. Thus, ASIC3 appears to play no role in processing of cutaneous heat stimuli, or in the inflammatory process.
4.2 Acid, pain, and ASICs
A decrease in pH is observed following inflammation, hematomas and isometric exercise (Hood et al., 1988; Issberner et al., 1996; Pan et al., 1988; Revici et al., 1949). In fact, there is a positive correlation between pain and local acidity (Issberner et al., 1996). Constant infusion of pH 5.2 phosphate buffer into the flexor carpi radialis muscle produces a rating of 20% on the visual analogue scale that shows no adaptation during infusion (Issberner et al., 1996). Continuous infusion of pH 6.1–6.9 saline activates approximately 40% of C-polymodal primary afferent fibers (Steen et al., 1992). Thus, decreases in pH occur after tissue injury, activate nociceptors and produce pain.
Low pH activates ASICs including ASIC1, ASIC2, and ASIC3, and TRPV1. All three ASICs and TRPV1 are found on primary afferent neurons (Waldmann and Lazdunski, 1998). ASIC3 is found in large and small diameter primary afferents, free nerve endings of the skin, co-localizes with substance P in small DRG neurons, and is found in DRG innervating muscle (Sluka et al., 2003; Price et al., 2001). Further, dorsal horn neurons do not sensitize to the second intramuscular injection of acid in ASIC3−/− mice suggesting that activation of ASIC3 is important for the development of central sensitization after muscle insult (Sluka et al., 2003).
ASIC3 mRNA is upregulated in DRG after complete Freund’s adjuvant inflammation of the paw (Voilley et al., 2001) suggesting there may be an increase in ASIC3 protein after inflammation. Application of inflammatory mediators to DRG neurons (nerve growth factor, serotonin, interleukin-1 and bradykinin), mimics this increased expression of ASIC3 mRNA after inflammation. Application of these inflammatory mediators to DRG neurons results in an increase in the number of neurons expressing ASIC currents, including ASIC3-like currents, and increased co-expression of ASIC3 and TRPV1. For ASIC3-like currents there is an increase in current density and an increased in the number of neurons expressing ASIC3 (Mamet et al., 2002). These changes in ASIC3 current should increase acid-induced excitation of ASIC3 neurons and increase the number of neurons responding to acid. ASIC3−/− mice are significantly less responsive to colorectal distension, an indicator of visceral nociception, with an average response magnitude of only 50% that of C57BL/6J mice (Jones et al., 2005). Further, enhancement of stretch-evoked afferent fiber responses in control mice evoked by inflammatory mediators is impaired in ASIC3−/− mice compared to C57BL/6J mice (Jones et al., 2005), suggesting a role for ASIC3 in visceral nociception. Thus, muscle inflammation should result in release of inflammatory mediators that would sensitize primary afferent fibers, increasing expression of ASIC3 in muscle. The decreased pH that occurs after inflammation would activate ASIC3 on primary afferent fibers innervating muscle, increasing the input to the spinal cord, resulting in central sensitization manifested behaviorally as secondary hyperalgesia of the paw.
In summary, we suggest that ASIC3 does not play a role in the development of hyperalgesia after cutaneous insult, but rather in hyperalgesia induced by deep tissue injury. We further show that ASIC3 does not play a role in the development of muscle inflammation. The current data support a role for ASIC3 as a pH sensor in muscle in the development of mechanical hyperalgesia after muscle insult.
Acknowledgments
Supported by a grant from the Carver College of Medicine, and National Institutetes of Health K0202201 (KAS). The authors wish to thank Tammy Lisi and Sandra Kolker (The University of Iowa) for excellent technical assistance, and Michael Welsh (University of Iowa) for advice and critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barr AE, Barbe MF. Pathophysiological tissue changes associated with repetitive movement: A review of the evidence. Phys Ther. 2002;82:173–187. doi: 10.1093/ptj/82.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AE, Barbe MF, Clark BD. Work-related musculoskeletal disorders of the hand and wrist: Epidemiology, pathophysiology, and sensorimotor changes. J Orthop Sports Phys Ther. 2004;34:610–627. doi: 10.2519/jospt.2004.34.10.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich P, Hoheisel U, Mense S. Effects of a carrageenan induced myositis on the discharge properties of group III and IV muscle receptors in the cat. J Neurophysiol. 1988;59:1395–1409. doi: 10.1152/jn.1988.59.5.1395. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:241–2. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desser RK, Himmelhoch SR, Evans WH, Januska M, Mage M, Shelton E. Guinea pig heterophil and eosinophil peroxidase. Arch Biochem Biophys. 1972;148:452–465. doi: 10.1016/0003-9861(72)90164-6. [DOI] [PubMed] [Google Scholar]
- Diehl B, Hoheisel U, Mense S. Histological and neurophysiological changes induced by carrageenan in skeletal muscle of cat and rat. Agents Actions. 1988;25:210–213. doi: 10.1007/BF01965013. [DOI] [PubMed] [Google Scholar]
- Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–93. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- Gitterman DP, Wilson J, Randall AD. Functional properties and pharmacological inhibition of ASIC channels in the human SJ-RH30 skeletal muscle cell line. J Physiol London. 2005;562:759–769. doi: 10.1113/jphysiol.2004.075069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel U, Koch K, Mense S. Functional reorganization in the rat dorsal horn during an experimental myositis. Pain. 1994;59:111–118. doi: 10.1016/0304-3959(94)90054-X. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Sander B, Mense S. Myositis-induced functional reorganization of the rat dorsal horn: effects of spinal superfusion with antagonists to neurokinin and glutamate receptors. Pain. 1997;69:219–230. doi: 10.1016/S0304-3959(96)03276-9. [DOI] [PubMed] [Google Scholar]
- Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, Kouhen RE, Lee C-H, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314:410–21. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- Hood VL, Chubert C, Keller U, Muller S. Effect of systemic pH on pHi and lactic acid generation in exhaustive forearm exercise. Am J Physiol. 1988;255:F479–485. doi: 10.1152/ajprenal.1988.255.3.F479. [DOI] [PubMed] [Google Scholar]
- Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature. 1994;367:467–70. doi: 10.1038/367467a0. [DOI] [PubMed] [Google Scholar]
- Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208:191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- Jones RCW, Xu LJ, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TL, Sweitzer SM, Wilson SP, Yeomans DC. Afferent fiber selective shift in opiate potency following targeted opioid receptor knockdown. Pain. 2003;106:365–371. doi: 10.1016/j.pain.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Keeble J, Russell F, Curtis B, Starr A, Pinter E, Brain SD. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005;52:3248–56. doi: 10.1002/art.21297. [DOI] [PubMed] [Google Scholar]
- Krisky DM, Marconi PC, Oligino T, Rouse RJD, Fink DJ, Glorioso JC. Rapid method for construction of recombinant HSV gene transfer vectors. Gene Ther. 1997;4:1120–1125. doi: 10.1038/sj.gt.3300497. [DOI] [PubMed] [Google Scholar]
- Liu J, Schrank B, Waterston RH. Interaction between a putative mechanosensory membrane channel and a collagen. Science. 1996;273:361–364. doi: 10.1126/science.273.5273.361. [DOI] [PubMed] [Google Scholar]
- Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Breese NM, Witty M-F, Ritchie J, Rainville M-L, Ase A, Abbadi N, Stucky CL, Séguéla P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25:9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999a;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception II. ‘Types’ of nociception revealed by genetic correlation analysis. Pain. 1999b;80:83–93. doi: 10.1016/s0304-3959(98)00196-1. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Hamm JR, Rotham DL, Shulman RG. Intracellular pH in human skeletal muscle by 1H NMR. Proc Natl Acad Sci. 1988;85:7836–7839. doi: 10.1073/pnas.85.21.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104:567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revici E, Stoopen E, Frenk E, Ravich RA. The painful focus. II. The relation of pain to local physiochemical changes. Bull Inst Appl Biol. 1949;1:21–38. [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003a;2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Wemmie JA, Welsh MJ. ASIC3, but not ASIC1, channels are involved in the development of chronic muscle pain. Proceedings of the 10th World Congress on Pain; IASP Press, Seattle. 2004. [Google Scholar]
- Stauber WT. Factors involved in strain-induced injury in skeletal muscles and outcomes of prolonged exposures. J Electromyogr Kinesiol. 2004;14:61–70. doi: 10.1016/j.jelekin.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N, Shreffler W, Wang S, Driscoll M. unc-8, a DEG/ENaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. elegans locomotion Neuron. 1997;18:107–119. doi: 10.1016/s0896-6273(01)80050-7. [DOI] [PubMed] [Google Scholar]
- Tenser RB, Hay KA, Edris WA. Latency-associated transcript by not reactivatable virus is present in sensory ganglion neuorns after inoculation of thymadine kinase-negative mutants of herpes simplex virus type 1. J Virol. 1989;63:2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Wilson SP, Yeomans DC. Virally mediated delivery of enkephalin and other neuropeptide transgenes in experimental pain models. Ann NY Acad Sci. 2002;971:515–521. doi: 10.1111/j.1749-6632.2002.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Wilson SP, Yeomans DC, Bender MA, Lu Y, Glorioso J. Antihyperalgisic effects of delivery of enkephalins to mouse nociceptive neurons by a herpes virus encoding proenkephalin. Proc Nat Acad Sci USA. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]


