Abstract
Activated interleukin (IL)-4Rα stimulates production of IgE through signal transducer and activator of transcription-6 (Stat6) activation in lymphocytes. Genetic studies have shown an association between polymorphisms in the genes encoding IL-4Rα and Stat6 and elevated serum IgE in patients with atopic disease. Some authors, including us, have reported an association of Graves' disease and elevated serum IgE. To analyse the relationship between IL-4Rα and Stat6 polymorphisms and elevated serum IgE in patients with Graves' disease, 169 patients with Graves' disease were studied. We investigated whether these polymorphisms affect IL-4Rα–Stat6 signalling in cultured human lymphocytes. A high frequency of both the Ile50 polymorphism in IL-4Rα and 13GT repeat variants of the Stat6 gene was observed in patients with Graves' disease and elevated serum IgE (Ile50 allele; P < 0·05, 13GT allele; P < 0·01 versus controls) but not in subjects with normal IgE. Cultured human lymphocytes with the Ile50 IL-4Rα polymorphism and the 13GT repeat variant of Stat6 showed increased IL-4 (and/or IL-13)-induced Stat6 activation (2·7-fold; P < 0·05 and 2·2-fold; P < 0·05, respectively). These findings suggest that polymorphisms in the IL-4Rα and Stat6 genes play an important role in elevation of serum IgE through increased Stat6 action in patients with Graves' disease.
Keywords: Graves' disease, IgE, interleukin-4 receptor alpha, polymorphisms, Stat6
Introduction
Elevated circulating immunoglobulin E (IgE) is a hallmark of allergic disease [1,2]. IgE is a key regulator of allergic reactions such as asthma and atopy through various mediators [3]. IgE production in lymphocytes is regulated by cytokines including interleukin (IL)-4 and IL-13; activation of these receptors by IL-4 or -13 stimulates IgE production through the activation of signal transducer and activator of transcription-6 (Stat6) [4–6]. The IL-4 receptor consists of the 140-kDa IL-4Rα chain and the common γ-chain (γc), a shared component of the receptors for IL-2, IL-7, IL-9 and IL-15 [7,8]. The IL-13 receptor consists of the IL-4Rα chain, IL-13Rα1 and IL-13Rα2 [9]. Once IL-4 or -13 activate these receptors, activated IL-4Rα leads to tyrosine-phosphorylation of Stat6 through Janus kinase (Jak). Phosphorylated Stat6 molecules subsequently form homodimers, translocate to the nucleus, and then induce IgE production by promoting gene transcription [10–13]. Inhibition of IL-4Rα results in decreased IL-4/IL-13-driven Stat6 activation and reduced IgE synthesis in cultured lymphocytes [14]. Similarly, lymphocytes from Stat6-deficient animals fail to produce IgE [13]. Additionally, consistent with the above data, circulating IgE is completely diminished in IL-4Rα–/– or Stat6–/– mice [4,5,15]. These findings show that IL-4Rα–Stat6 signalling in lymphocytes is critical for IgE production. Genetic studies have suggested that polymorphisms of IL-4Rα or Stat6 may associate with allergic diseases and elevated serum IgE. A high frequency of the IL-4Rα Ile50 polymorphism was found in patients with atopic asthma [16], while a high frequency of the IL-4Rα Arg551 polymorphism was found in patients with hyper-IgE syndrome [17,18]; however, some studies were unable to confirm these associations [19,20]. Besides IL-4Rα, recent studies have reported that a high frequency of the Stat6 13GT repeat variant is found in patients with allergic disease [21,22]. In cultured lymphocytes, the substitution of isoleucine (Ile) for valine (Val) at amino acid 50 of human IL-4Rα augments IL-4 stimulated Stat6 activation [16]. Moreover, the 13GT repeat variant of Stat6 might enhance the transcriptional function of Stat6 [22]. The findings of these studies suggest that these polymorphisms may cause elevation of IgE through augmentation of IL-4Rα–Stat6 signalling in lymphocytes, leading subsequently to the pathogenesis of allergic diseases.
Graves' disease is an autoimmune disorder characterized by increased thyroid receptor antibodies (TRAb) [23]. We have reported that about 30% of patients with untreated Graves' disease have increased concentrations of serum IgE [24–26]. Graves' patients with elevated IgE show a low rate of remission after treatment with thiamazole and a high recurrence rate even after remission [26]. This may be due to a close cross-talk between the allergic and immune reactions in Graves' disease.
Therefore, the aim of this study was to determine whether these polymorphisms in the genes encoding IL-4Rα and/or Stat6 affect IL-4Rα–Stat6 signalling in lymphocytes and cause an elevation in the level of serum IgE in Graves' patients.
Materials and methods
Subjects
One hundred and sixty-nine patients with untreated Graves' disease (34 men and 135 women, aged 18–79 years) were analysed in this study. None of the patients had other autoimmune diseases. One hundred and sixty-four normal subjects (67 men and 97 women, aged 22–72 years) were also analysed. All participants gave their written informed consent before participating in the study. This study was approved by the ethics committee of the Ryukyu Medical University.
Biochemical measurements
The diagnosis of Graves' disease was based on clinical symptoms and signs of hyperthyroidism with the presence of ophthalmopathy, diffuse goitre, elevated levels of serum thyroxine (T4) and triiodothyronine (T3), and an elevation of thyroid stimulating hormone (TSH)-binding inhibiting immunoglobulin (TBII) or thyroid-stimulating antibody (TSAb). Blood samples were obtained at the time of diagnosis to measure the levels of T3, T4, TSH, anti-thyroid peroxidase (TPO) antibody, thyroid stimulating hormone binding inhibitory immunoglobulin (TBII), thyroid stimulating antibody (TSAb) and immunoglobulin E (IgE) as reported previously [24–26]. Levels of the anti-TPO antibody, TBII, TSAb and IgE were measured at the Mitsubishi Kagaku Bio-Clinical Laboratory (Tokyo, Japan). Under normal conditions, TBII is < 10%, and TSAb is < 180% [24–26]. An anti-TPO antibody level of > 0·2 IU/ml was considered to be positive. A normal IgE concentration was defined as < 170 IU/ml based on previous reports [24,26].
DNA analysis
Genomic DNA was prepared from peripheral blood cells using a standard column extraction technique (Qiagen, Heiden, Germany). The Ile50Val genotypes of IL-4Rα and the GT repeat variants in exon 1 of Stat6 gene were determined by direct sequencing. The sequence of the sense oligonucleotide primer for the IL-4Rα gene was 5′-AGTCTGATGCGGTTCCTGGAG-3′ and that of the anti-sense primer was 5′-CAGCTGTGGGAACACACCCA-3′; these primers were designed to flank Ile50Val variant region allowing the detection of a 297-base pairs (bp) fragment. The sequences of the oligonucleotide primer designed to flank the GT repeat variant region in first exon of Stat6 gene were: sense 5′-GAGGGACCTGGGTAGAAGGA-3′; and anti-sense 5′-CACCCCCATGCACTCATAG-3′; these primers enabled the detection of a 324–332-bp fragment. For genotyping the Arg551Gln variant of the IL-4Rα gene, polymerase chain reaction (PCR) single-stranded conformational polymorphism (SSCP) analysis was performed using the following primers, as described previously: sense 5′-CTCTCTGAGCCAACCACTGT-3′; anti-sense 5′-CTTGTAACCAGCCTCTCCTG-3′ [17,21]. The accuracy of this method was confirmed by direct sequencing of samples from each genotype (Arg/Arg, Arg/Gln, Gln/Gln).
Lymphocyte isolation and cell culture
Heparinized blood samples were collected from 20 Graves' patients. All of these subjects were confirmed to have homozygous Ile50Val and GT variants by direct sequencing. Peripheral blood mononuclear cells (PBMCs) were isolated subsequently by Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation and separated into human B or T cells using a MagCellect T Cell/B Cell Isolation Kit (Funakoshi, Tokyo, Japan). Each cell preparation was cultured in RPMI-1640 medium containing 10% fetal calf serum (FCS), 5 × 10−5 M β-mercaptoethanol, 100 U/ml of penicillin and 100 µg/ml of streptomycin. For Western blotting, B and T cells were either unstimulated or stimulated with 1 nM of recombinant human (rh) IL-4, 5 nM of rhIL-13 or a combination of rhIL-4 and rhIL-13, for the indicated period. RhIL-4 and rhIL-13 were purchased from Wako Chemicals (Osaka, Japan).
Western blot analysis
Cells were lysed at 4°C with NP-40 lysis buffer (1% NP-40, 50 mM Tris-HCl, 150 mM NaCl) containing proteinase and phosphatase inhibitors (Sigma, Tokyo, Japan). Subsequently, total protein (20 µg) from each sample were subjected to 10% denaturing sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Millipore, Billerica, MA, USA). After blocking with Tris-buffered saline containing 5% non-fat milk, the membrane was reacted with an anti-phosphotyrosine Stat6 (Tyr641) antibody (Cell Signalling Technology, Beverly, MA, USA) and detected with horseradish peroxidase-conjugated anti-rabbit IgG using an enhanced chemiluminescence (ECL) system (Amersham, Little Chalfont, UK). The membrane was then stripped of the bound antibody and reprobed with an anti-Stat6 antibody (s-20; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) to reveal the location and intensity of the 119-kDa-labelled band.
Statistical analysis
Results are presented as numbers (%) or means ± standard deviation (s.d.). The proportion of genotypes or alleles were compared by one way analysis of variance (anova), χ2 test with Yates' correction or Fisher's exact probability test. A P-value of less than 0·05 was considered to indicate statistical significance. These analyses were performed using the StatView program for Windows (version 5·01; SAS Institute, Cary, NC, USA).
Results
Clinical characteristics of Graves' patients
Elevation of serum IgE (≥ 170 IU/ml) was found in 70 (41·4%) of the 169 untreated hyperthyroid patients with Graves' disease (Table 1). The serum levels of T3, T4, anti-TPO antibody positivity, concentrations of TBII and TRAb were almost the same in the 70 Graves' patients with elevated IgE and the 99 Graves' patients with normal IgE.
Table 1.
Serum immunoglobulin E (IgE) and thyroid function in untreated Graves' patients.
| Graves' disease with elevated IgE | Graves' disease with normal IgE | Normal controls | |
|---|---|---|---|
| No. of subjects | 70 | 99 | 164 |
| Gender (M : F) | 14 : 56 | 20 : 79 | 67 : 97 |
| Age (years) | 42 ± 13 | 41 ± 13 | 40 ± 7 |
| Anti-TPO antibody positivity | 65% | 63% | – |
| Thyroxine (μg/dl) | 18·4 ± 1·4 | 19·5 ± 1·3 | – |
| Triiodothyronine (ng/dl) | 327 ± 22 | 354 ± 25 | – |
| TSH (μIU/ml) | 0·001 ± 0·02 | 0·02 ± 0·01 | – |
| TBII (%) | 53·3 ± 12·3 | 43·7 ± 12·5 | – |
| Tsab (%) | 560 ± 81 | 540 ± 73 | – |
| IgE (IU/ml) | 352 ± 168 | 56 ± 36 | – |
Data are means ± s.d. Anti-TPO antibody: anti-thyroid peroxidase antibody; TSGL thyroid stimulating hormone; TBII: thyrotropin-binding inhibiting immunoglobulin; TSab: thyroid stimulating antibody. Conversion factors to SI unit: TSH (mU/l), 1·0; thyroxine (nmol/l), 12·87; triiodothyronine (nmol/l), 0·01536.
Polymorphisms of IL-4Rα and Stat6 associate with elevated serum IgE in patients with Graves' disease
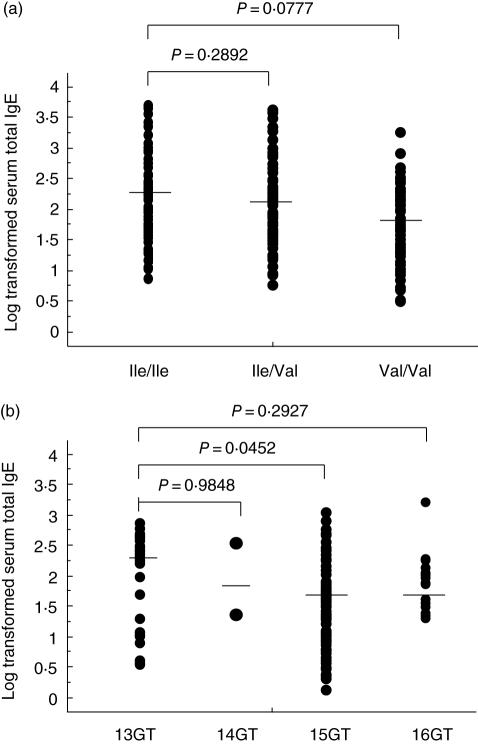
To examine a possible genetic correlation between polymorphisms in the IL-4Rα gene and Graves' disease we determined the genotype of the Ile50Val and Arg551Gln polymorphism in the above subjects (Table 2). Although there was no difference in the frequency of the Ile50 allele among controls (34·8%) and Graves' patients with normal IgE (33·3%), the frequency of the Ile50 allele (46·4%) was significantly higher in Graves' patients with elevated IgE than in other subject groups (P= 0·0174 and 0·0149, respectively). Indeed, the odds ratio for the non-Val50 genotype was significantly higher in Graves' patients with elevated IgE than in controls [odds ratio (OR) 2·26, P= 0·0269]. On the other hand, another polymorphism Arg551Gln did not achieve statistical significance. We next genotyped the dinucleotide (GT) repeat variant of exon 1 of the Stat6 gene in the same subjects. The frequency of 13GT repeat allele was significantly higher in Graves' patients with elevated IgE, compared with those with normal IgE and control subjects (Table 3, P = 0·0011 and 0·0015, respectively). The logarithmic levels of IgE in hyperthyroid Graves' patients are shown in Fig. 1. A trend was obtained for the average IgE concentration (log-transformed) between Ile/Ile and the other genotypes [Ile/Ile; 407·12 IU/ml (2·20), n = 64, Ile/Val; 370·30 IU/ml (2·04), n = 81, Val/Val; 239·43 IU/ml (1·93), n = 25], but did not achieve statistical significance (Ile/Ile versus Ile/Val; P = 0·2892, Ile/Ile versus Val/Val; P = 0·0777, respectively) (Fig. 1a). Similarly, there was no significant difference in the levels of IgE between Arg/Arg and the other genotype (Arg/Gln and Gln/Gln) in the same gene (data not shown). On the other hand, a significant association between the 13GT allele and IgE level was demonstrated by comparing the IgE levels between subjects with the 13GT allele and those with the 15GT allele (13GT allele versus 14GT allele; P = 0·9848, 13GT allele versus 15GT allele; P = 0·0452, 13GT allele versus 16GT allele; P = 0·2927) (Fig. 1b); however, this association was not seen in the homozygous state (13GT/GT versus 15GT/GT; P = 0·0954), due probably to the small number of subjects homozygous for 13GT (n = 12).
Table 2.
Frequencies of genes and alleles of interleukin (IL)-4Rα in Graces' patients and normal controls.
| Graves' disease (GD) | ||||
|---|---|---|---|---|
| Total | With elevated IgE (≥ 170 IU/ml) | With normal IgE (< 170 IU/ml) | Controls | |
| Val50/Val50 | 64/169 (37·9) | 22/70 (31·4) | 42/99 (42·4) | 69/164 (42·1) |
| Ile50/Val50 | 81/169 (47·9) | 32/70 (45·7) | 48/99 (48·5) | 76/164 (46·3) |
| Ile50/Ile50 | 25/169 (14·8) | 16/70 (22·9) | 9/99 (9·1) | 19/164 (11·6) |
| P value*† | P = 0·0268 | P = 0·5251 | ||
| Odds ratio (95% CI) *† | 2·26 (1·09–4·72) | 0·76 (0·33–1·76) | 1·0 | |
| Ile50 allele | 131/338 (38·8) | 65/140 (46·4) | 66/198 (33·3) | 114/328 (34·8) |
| P value*‡ | P = 0·0174 | P = 0·7390 | ||
| Odds ratio (95% CI)†‡ | 1·63 (1·09–2·43) | 0·94 (0·65–1·36) | 1·0 | |
| Arg551/Arg551 | 3/169 (1·8) | 2/70 (2·9) | 1/99 (1·0) | 7/164 (4·3) |
| Arg551/Gln551 | 49/169 (29·0) | 20/70 (28·6) | 29/99 (29·3) | 52/164 (31·7) |
| Gln551/Gln551 | 117/169 (69·2) | 48/70 (68·6) | 69/99 (69·7) | 105/164 (64·0) |
| P value†§ | P = 0·886 | P = 0·2627 | ||
| Odds ratio (95% CI)*† | 0·66 (0·13–3·26) | 0·23 (0·03–1·89) | 1·0 | |
| Arg551 allele | 55/338 (16·3) | 24/140 (17·1) | 31/198 (15·7) | 66/328 (20·1) |
| P value†¶ | P = 0·5450 | P = 0·2008 | ||
| Odds ratio (95% CI)†¶ | 0·82 (0·49–1·38) | 0·74 (0·46–1·18) | ||
Data are no. of subjects (%). Statistical analysis using χ2 test or Fisher's exact probability test.
Ile50/ILE50 versus Ile50/Val50 + Val50/Val50.
Controls versus each group of Graves' disease patients.
Ile50 versus Val50.
Arg551/Arg551 versus Arg551/Gln551 + Gln551.
Arg551 versus Gln551.
Table 3.
Frequencies of GT repeat alleles of the signal transducer and activator of transcription-6 gene in Graves' patients and normal controls.
| Graves' disease (GD) | ||||
|---|---|---|---|---|
| Allele | Total | With elevated IgE (≥ 170 IU/ml) | With normal IgE (< 170 IU/ml) | Controls |
| 13GT | 68/338 (20·1) | 40/140 (28·6) | 28/198 (14·1) | 52/198 (15·9) |
| P value* | P = 0·0015 | P = 0·5963 | ||
| Odds ratio (95% CI)* | 2·12 (1·33–3·40) | 0·87 (0·53–1·44) | 1·0 | |
| 14GT | 4/338 (1·2) | 4/140 (2·9) | 0/198 (0·0) | 2/328 (0·6) |
| P value* | P = 0·1260 | – | – | |
| Odds ratio (95% CI)* | 4·79 (0·86–26·48) | – | 1·0 | |
| 15GT | 227/338 (67·2) | 83/140 (59·3) | 144/198 (72·5) | 222/328 (67·7) |
| P value | P = 0·0808 | P = 0·2231 | ||
| Odds ratio (95% CI)* | 0·70 (0·46–1·05) | 1·27 (0·086–1·88) | 1·0 | |
| 16GT | 39/338 (11·5) | 13/140 (9·3) | 26/198 (13·1) | 52/328 (15·9) |
| P value* | P = 0·0599 | P = 0·3947 | ||
| Odds ratio (95% CI)* | 0·54 (0·29–1·03) | 0·80 (0·48–1·33) | 1·0 | |
Data are no. of subjects (%). Statistical analysis using χ2 test or Fisher's exact probability test.
Controls versus each group of Graves' disease patients.
Fig. 1.
Comparison of total immunoglobulin E (IgE) level in the group of Graves' patients. Relationship between serum IgE concentration and the Ile50Val genotype of interleukin (IL)-4Rα (a) or homozygous GT repeat variants of exon 1 of signal transducer and activator of transcription-6 (Stat6) (b) in Graves' patients. The levels of total IgE are log-transformed. Solid bars indicate geometric mean IgE levels for each group.
Polymorphisms in IL-4Rα and Stat6 are associated with increased Stat6 activation in cultured human lymphocytes
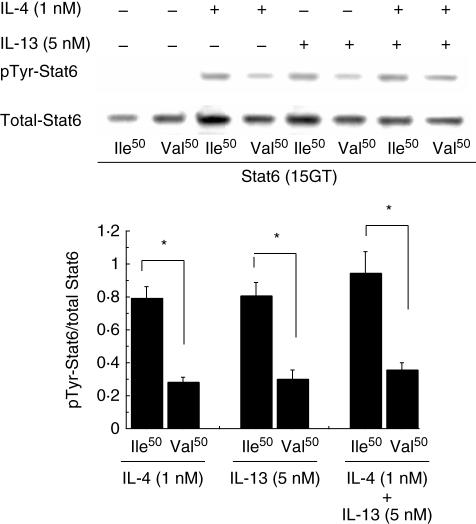
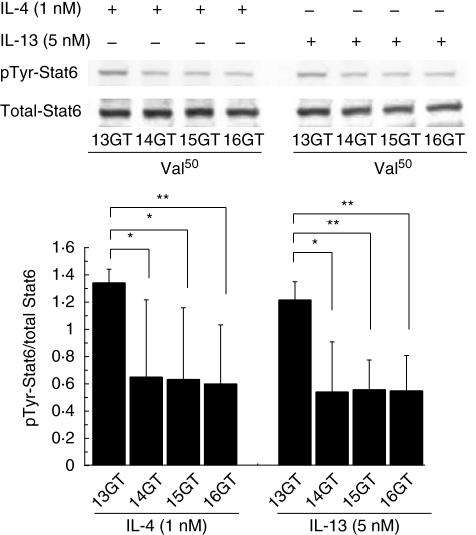
To investigate the effects of the Ile50Val substitution and the GT repeat, Stat6 phosphorylation was assessed by Western blotting using B and T cells separated from human monocytes. Band density was measured using NIH Image J; the relative phosphorylation in each band was calculated from the ratio of the density obtained from probing the membrane with the anti-phosphotyrosine antibody to that obtained from probing the same membrane with the anti-Stat6 antibody. As shown in Fig. 2, when stimulating B cells by rhIL-4 or rhIL-13 the total Stat6 level in cells with the Ile/Ile genotype was slightly higher than that in cells with the Val/Val genotype; however, no significant difference was observed by image analysis. However, the phosphorylated/total Stat6 ratio was substantially different between these genotypes (approximately 2·7-fold). A similar result was obtained even upon co-treatment with rhIL-4 (1 nM) plus rhIL-13 (5 nM), suggesting that IL-4 and IL-13 are unlikely to compete for the binding of their common receptor, IL4Rα. In T cells, rhIL-4 induced phosphorylation of Stat6 to a similar level as seen in B cells (data not shown); expectedly, rhIL-13 failed to promote Stat6 activation in T cells as IL-13, in contrast to rhIL-4, does not act on human T cells [27]. In the next series of experiments investigating the effect of the GT repeat variant, Western blot analysis revealed that the amount of Stat6 phosphorylation stimulated by rhIL-4 or rhIL-13 was markedly enhanced in human B cells with a homozygous 13GT genotype, in the presence of the same level of total Stat6 protein. The phosphorylated/total Stat6 ratio was significantly higher in cells with this genotype than in cells with other genotypes (Fig. 3).
Fig. 2.
Western blot analysis of total and phosphotyrosine (pTyr)-signal transducer and activator of transcription-6 (Stat6) in cultured human B cells. Human B cells bearing the 15GT repeat variant of Stat6 and either the Ile50 allele or Val50 allele of interleukin (IL)-4Rα, from each of five donors with Graves' disease, were stimulated with (+) or without (–) 1 nM of hIL-4 and/or 5 nM of hIL-13 for 30 min, lysed, separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and subjected to Western blotting. Levels of pTyr–Stat6 were normalized to the expression levels of the respective total Stat6 proteins. The phospho/total Stat6 ratio was significantly higher in the Ile50-bearing B cells compared with the Val50-bearing B cells. Values are presented as means ± standard deviation. *Statistically significant difference at P < 0·05.
Fig. 3.
Western blot analysis of total and phosphotyrosine (pTyr)-signal transducer and activator of transcription-6 (Stat6) in cultured human B cells. Human B cells bearing the Val/Val genotype of interleukin (IL)-4Rα and the 13GT, 15GT or 16GT repeat variant of Stat6 from each of five donors with Graves' disease, and human B cells bearing the Val/Val genotype of IL-4Rα and the 14GT repeat variant of Stat6, from each of two donors with Graves' disease, were stimulated with 1 nM of hIL-4 or 5 nMM of hIL-13 for 30 min, lysed, separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and subjected to Western blotting. Levels of pTyr–Stat6 were normalized to the expression levels of the respective total Stat6 proteins. The phospho/total Stat6 ratio was significantly higher in 13GT-bearing B cells compared with B cells bearing the other variants. Values are presented as means ± standard deviation. *P < 0·05; **P < 0·01.
Discussion
IgE is a key regulator of the allergic reactions that are important in the pathogenesis of allergic diseases such as asthma and atopy [1–3]. The IL-4Rα chain of the IL-4 or IL-13 receptor, and Stat6, play a critical role in regulating serum concentration of IgE. There is growing evidence that polymorphisms in IL-4Rα and Stat6 might be associated with allergic diseases mediated by IgE. We have reported previously that Graves' patients with elevated serum IgE have a lower rate of remission after treatment with thiamazole, and a high recurrence even after remission [26]. This is the first analysis of polymorphisms in the IL-4Rα and Stat6 genes in Graves' patients. We compared the frequency of these polymorphisms between Graves' patients with normal IgE, those with elevated IgE and normal control subjects. In addition, we investigated the functional effects of these polymorphisms on IL-4Rα–Stat6 signalling in lymphocytes.
We found a high frequency of the Ile50 allele of IL-4Rα in Graves' patients with elevated serum IgE. However, we found no increased in the frequency of this or other polymorphisms in Graves' patients with normal IgE. Similarly, the frequency of the 13GT allele, one of the GT repeat variants inthe first exon of the Stat6 gene, was significantly higher in Graves' patients with elevated IgE. We also postulated that IgE concentrations were fracturing in Graves' patients. However, in contrast to GT variants, the Ile50Val genotype was not shown to have an obvious correlation with the level of total serum IgE in our patients. It is difficult to account for these results, and therefore further studies are warranted.
There have been several reports about the functional aspects of the Ile50 allele, and a gain-of-function of the Ile50 variant has been shown [16,28]. The Ile50 substitution occurs in the extracellular domain of human IL-4Rα next to Cys49; therefore, a conformational change or an alteration in binding affinity is hypothesized on the basis that a similar extracellular variant, Thr49Ile, in the IL-4Rα chain of BALB/c mice, might alter the ligand-binding affinity of IL-4R [29]. Using an electrophoretic mobility-shift assay (EMSA), Mitsuyasu and co-workers demonstrated that the Ile50 variant augments the activation of Stat6 by 1·6–1·8 fold, compared with the Val50 variant [16,28]. The Ile50 variant of IL-4Rα significantly up-regulates the response to IL-4 and/or IL-13, with resultant increased activation of Stat6, and hence increased IgE production. However, some other investigators deny the association between Ile50 and elevated IgE [19].
On the other hand, the human Stat6 gene is located at 12q13·3–q14·1 and it is also associated with atopy and total serum IgE concentration in various populations [30]. Dinucleotide repeats are distributed widely throughout the human genome and their high flexibility has the potential to alter DNA structures. Indeed, some of them have been shown to affect transcriptional activity in different genes and cell types [31–33]. The GT repeat in exon 1 of Stat6 is located near transcription sites, which makes it possible that this repeat can modulate transcriptional activity in human B and T cells.
We have demonstrated marked Stat6 phosphorylation in lymphocytes from individuals with the Ile50 genotype compared with those from individuals with the Val50 genotype. Furthermore, among the GT repeats, the 13GT variant was associated with a higher degree of Stat6 phosphorylation compared with the other variants under the same conditions. These results from our present studies suggest that polymorphisms in the genes encoding IL-4Rα and Stat6 contribute independently to the regulation of immune responses in Graves' disease through phosphorylation of Stat6.
Gain-of-function mutations in IL-4Rα and Stat6 have been reported to be associated with atopic asthma patients with elevated IgE [16,22,28]. These mutations might be present in Graves' patients with elevated IgE, even if peripheral IL-4 or IL-13 is within the normal range or not detected [26]. Although these mechanisms need further investigation, it can be concluded that the elevation in the level of IgE in Graves' patients is caused by increased levels of IL-4 and/or IL-13-induced Stat6 activation.
In conclusion, this is the first analysis of polymorphisms in the IL-4Rα and Stat6 genes in Graves' patients. We showed an association between these polymorphisms and Stat6 action in lymphocytes.
References
- 1.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 2.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–71. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 3.Sutton BJ, Gould HJ. The human IgE network. Nature. 1993;366:421–8. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Tanaka T, Shi W, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–30. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 5.Shimoda K, van Deursen J, Sangster MY, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–3. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 6.Grunig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowenthal JW, Castle BE, Christiansen J, et al. Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J Immunol. 1988;140:456–64. [PubMed] [Google Scholar]
- 8.Murata T, Taguchi J, Puri RK. Interleukin-13 receptor alpha but not alpha chain: a functional component of interleukin-4 receptors. Blood. 1998;91:3884–91. [PubMed] [Google Scholar]
- 9.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Wilson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotanides H, Reich NC. Requirement of tyrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science. 1993;262:1265–7. doi: 10.1126/science.7694370. [DOI] [PubMed] [Google Scholar]
- 11.Fenghao X, Saxon A, Nguyen A, Ke Z, Diaz-Sanchez D, Nel A. Interleukin 4 activates a signal transducer and activator of transcription (Stat) protein which interacts with an interferon-gamma activation site-like sequence upstream of the I epsilon exon in a human B cell line. Evidence for the involvement of janus kinase 3 and interleukin-4 Stat. J Clin Invest. 1995;96:907–14. doi: 10.1172/JCI118138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JX, Migone TS, Tsang M, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotrophy and redundancy of IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–9. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 14.Carballido JM, Schols D, Namikawa R, et al. IL-4 induces human B cell maturation and IgE synthesis in SCID-hu mice. Inhibition of ongoing IgE production by in vivo treatment with an IL-4/IL-13 receptor antagonist. J Immunol. 1995;155:4162–70. [Google Scholar]
- 15.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–48. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsuyasu H, Izuhara K, Mao X-Q, et al. Ile50Val variant of IL4R alpha upregulates IgE synthesis and associated with atopic asthma. Nature Genet. 1998;19:119–20. doi: 10.1038/472. [DOI] [PubMed] [Google Scholar]
- 17.Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. 1997;337:1720–5. doi: 10.1056/NEJM199712113372403. [DOI] [PubMed] [Google Scholar]
- 18.Hackstein H, Hecker M, Kruse S, Bohnert A, Ober C, Deichmann KA, Bein G. A novel polymorphism in the 5′ promoter region of the human interleukin-4 receptor alpha-chain gene is associated with decreased soluble interleukin-4 receptor protein levels. Immunogenetics. 2001;53:264–9. doi: 10.1007/s002510100324. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi E, Shibasaki M, Arinami T, et al. No association between atopy/asthma and the Ile50Val polymorphism of IL-4 receptor. Am J Resp Crit Care Med. 1999;160:342–5. doi: 10.1164/ajrccm.160.1.9807130. [DOI] [PubMed] [Google Scholar]
- 20.Youn J, Hwang SH, Cho CS, et al. Association of the interleukin-4 receptor alpha variant Q576R with Th1/Th2 imbalance in connective tissue disease. Immunogenetics. 2000;51:743–6. doi: 10.1007/s002510000196. [DOI] [PubMed] [Google Scholar]
- 21.Tamura K, Arakawa H, Suzuki M, et al. Novel dinucleotide repeat polymorphism in the first exon of the STAT-6 gene is associated with allergic diseases. Clin Exp Allergy. 2001;31:1509–14. doi: 10.1046/j.1365-2222.2001.01191.x. [DOI] [PubMed] [Google Scholar]
- 22.Gao PS, Heller NM, Walker W, et al. Variation in dinucleotide (GT) repeat sequence in the first exon of the STAT6 gene is associated with atopic asthma and differentially regulates the promoter activity in vitro. J Med Genet. 2004;41:535–9. doi: 10.1136/jmg.2003.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriss JP. Inactivation of long-acting thyroid stimulator (LATS) by anti-kappa and anti-lambda antisera. J Clin Endocrinol Metab. 1968;28:1440–4. doi: 10.1210/jcem-28-10-1440. [DOI] [PubMed] [Google Scholar]
- 24.Sato A, Yamada T, Takemura Y, et al. A possible role of immunoglobulin E in patients with hyperthyroid Graves' disease. J Clin Endocrinol Metab. 1999;84:3602–5. doi: 10.1210/jcem.84.10.6038. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T, Sato A, Komiya I, et al. An elevation of serum immunoglobulin E provides a new aspect of hyperthyroid Graves' disease. J Clin Endocrinol Metab. 2000;85:2775–8. doi: 10.1210/jcem.85.8.6741. [DOI] [PubMed] [Google Scholar]
- 26.Komiya I, Yamada T, Sato A, Kouki T, Nishimori T, Takasu N. Remission and recurrence of hyperthyroid Graves' disease during and after thiamazole treatment when assessed by IgE and interleukin 13. J Clin Endocrinol Metab. 2001;86:3540–4. doi: 10.1210/jcem.86.8.7734. [DOI] [PubMed] [Google Scholar]
- 27.Zurawski SM, Vega F, Jr, Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993;12:2663–70. doi: 10.1002/j.1460-2075.1993.tb05927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsuyasu H, Yanagihara Y, Mao XQ, et al. Dominant effect of Ile50Val variant of human IL-4 receptor alpha-chain in IgE synthesis. J Immunol. 1999;162:1227–31. [PubMed] [Google Scholar]
- 29.Schulte T, Kurrle RR, Röllingholff M, Gessner A. Molecular characterization and functional analysis of murine interleukin 4 receptor allotypes. J Exp Med. 1997;186:1419–29. doi: 10.1084/jem.186.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel BK, Keck CL, O'Leary RS, Popescu NC, LaRochelle WJ. Localization of the human stat6 gene to chromosome 12q13·3-q14·1, a region implicated in multiple solid tumors. Genomics. 1998;52:192–200. doi: 10.1006/geno.1998.5436. [DOI] [PubMed] [Google Scholar]
- 31.Gebhardt F, Zanker KS, Brandt B. Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem. 1999;274:13176–80. doi: 10.1074/jbc.274.19.13176. [DOI] [PubMed] [Google Scholar]
- 32.Okladnova O, Syagailo YV, St Tranitz M, et al. A promoter associated polymorphic repeat modulates PAX-6 expression in human brain. Biochem Biophys Res Comms. 1998;248:402–5. doi: 10.1006/bbrc.1998.8972. [DOI] [PubMed] [Google Scholar]
- 33.Searle S, Blackwell JM. Evidence for a functional repeat polymorphism in the promoter of the human NRAMP1 gene that correlates with autoimmune verses infectious disease susceptibility. J Med Genet. 1999;36:295–9. [PMC free article] [PubMed] [Google Scholar]