Abstract
Loss of pancreatic β-cells in type I diabetes is associated with an increase in T helper 1 (Th1) proinflammatory cytokines in the islet milieu, with a concomitant reduction in Th2 anti-inflammatory cytokines. In animal models, manoeuvres designed to polarize Th1 responses towards Th2, particularly involving interleukin (IL)-4, have been shown to protect against insulitis and diabetes. The aim of this study was to determine whether IL-4 can exert a direct effect on β-cell viability. The rat pancreatic β-cell line, BRIN-BD11, was used. IL-4R mRNA expression was assayed by reverse transcription–polymerase chain reaction and DNA sequencing and protein expression measured using anti-IL-4R antibodies and confocal microscopy. Cells were pretreated in vitro with IL-4, incubated with IL-1β and interferon (IFN)-γ and DNA fragmentation and nitrite production analysed by flow cytometry and Griess assay, respectively. Expression of type I (IL-4R alpha and common γ-chain) and type II (IL-4R alpha, IL-13R alpha-1) IL-4R mRNA transcripts, together with cell surface expression of IL-4R, was demonstrated. Pre-incubation with IL-4 reduced significantly cell death induced by IL-1β alone or by a combination of IL-1β and IFN-γ, although this was not accompanied by a reduced production of nitrite. The protective effect of IL-4 was not seen when all three cytokines were added simultaneously. These results demonstrate, for the first time, expression of IL-4 receptor components on rat pancreatic β-cells and reveal a direct protective effect on the loss of viability mediated by proinflammatory cytokines when β-cells are pre-incubated with IL-4.
Keywords: apoptosis, interferon-γ, interleukin-1β, interleukin-4, islet cells, pancreatic β cells
Introduction
In man, type I diabetes is characterized by a progressive autoimmune-mediated insulitis culminating in the death of pancreatic β-cells [1]. This process is mediated by CD4+ and CD8+ T lymphocytes as well as by macrophages that infiltrate the pancreas and trigger β-cell death either directly by cell-mediated cytotoxicity and/or indirectly due to the release of inflammatory mediators [1–4]. Two types of CD4+ T lymphocytes are now recognized − T helper 1 (Th1) and Th2 cells − and these may exert opposing effects on the disease process in type I diabetes [5]. Th1 cells and macrophages promote cell-mediated immunity by secreting a range of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, interferon (IFN)-γ and tumour necrosis factor (TNF)-α that result in inflammation and cell damage [1–6]. By contrast, Th2 cells promote humoral immunity by secreting cytokines such as IL-4 and IL-10, which have the ability to down-regulate the inflammatory actions of Th1 cells.
Although perhaps an oversimplification, it is often considered that autoimmune diabetes is a Th1-mediated disease that is exacerbated by a concomitant reduction in Th2 responses. In support of this, combinations of proinflammatory cytokines are synergistically cytotoxic to rodent and human β-cells [2–4] while decreased production of IL-4 by Th2 cells also occurs in both humans with type I diabetes and in non-obese diabetic (NOD) mouse models [6–8]. Moreover, patients with elevated levels of IL-4 have been shown to be resistant to the progression of diabetes [9] and, in animal models, IL-4 may also be protective against diabetes development [10,11]. The molecular basis of this protection has not been established fully but it is presumed to be mediated, in part, via actions of IL-4 to down-regulate Th1 cell activity. However, there is also evidence that IL-4 may directly improve the viability of β-cells, although these results were obtained in the simultaneous presence of IL-10 [12]. Thus, it remains unclear whether β-cells are directly responsive to IL-4 in the absence of IL-10, and the aim of the present study was to determine whether IL-4 can exert a direct protective effect on β-cell viability.
Materials and methods
Cells and cell culture
The rat pancreatic β-cell line, BRIN-BD11 [13], was used for this study. BRIN-BD11 β-cells were seeded at a concentration of 105 cells per well in six-well tissue cell culture plates containing RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 400 U/ml penicillin and 200 µg/ml streptomycin (all media and supplements were from Invitrogen, Paisley, UK). The cells were cultured at 37°C in 5% CO2 in the absence or presence of test reagents. Stock solutions of all reagents were prepared in water or dimethylsulphoxide (DMSO) (Sigma, Poole, UK) and the solvent added to the control cultures. IL-1β, IL-4 and IFN-γ were purchased from Sigma.
RNA extraction and reverse transcription–polymerase chain reaction (RT–PCR)
Gene expression of IL-4Rα, common γ-chain, IL-13Rα1 was measured by RT–PCR. Total cellular RNA was extracted from BRIN-BD11 cells with Trizol reagent (Biogenesis, Poole, UK) and was amplified by RT–PCR in a single tube reaction using the Reddy Mix Reverse-RT One Step Kit (ABgene, Epsom, UK). Primers were designed as follows. For rat IL-4Rα, forward: GGGGTGGCTTTGCACCAAGTTCC, reverse: CATTGGTGTGGAGTGTGTGGTTGT. Cycling conditions (35 cycles) were 94°C for 20 s, 62°C for 30 s and 72°C for 60 s. For rat IL-13Rα1, forward: GGACATGGAGTCCTCCTGAGGG, reverse: CTTTCCAGGGAGCCAGGAACACTTC, and rat common γ-chain, forward: GCACTTGGAATAGCAGTTCTGAGC, reverse: CAGCGTTCTATGTATCTGCTTTTC. Cycling conditions were 94°C for 20 s, 59°C for 30 s and 72°C for 60 s. All primers were purchased from MWG, Germany. Products were separated by electrophoresis on 1·4% agarose gels and the DNA extracted for direct sequencing (MWG, Ebersburg, Germany).
Flow cytometric analysis of cell death
BRIN-BD11 cell death was analysed using flow cytometry. Following incubation with test reagents, the culture medium containing detached cells was harvested. Adherent cells were incubated with trypsin (Invitrogen, Paisley, UK)/ethylenediamine tetraacetic acid (EDTA) (0·5 ml per well) for 5 min at 37°C, after which 2 ml of complete cell culture medium was added. Both detached and adherent cells were then centrifuged (300 g; 5 min) and pelleted cells were resuspended in 200 µl of phosphate-buffered saline (PBS) and fixed with ice-cold ethanol : PBS (70 : 30 v:v) for 30 min on ice. After fixation, the cells were centrifuged for 5 min at 300 g and resuspended in 820 µl of PBS. Eighty µl of propidium iodide (0·5 mg/ml) (Sigma) and 5 µl of DNase free ribonuclease (Roche, Lewes, UK) were added and incubation continued for 30 min at 37°C. Subsequently, cellular DNA was analysed using a flow cytometer [fluorescence activated cell sorter (FACScan), Becton Dickinson, Oxford, UK] and the extent of DNA fragmentation determined as an index of loss of viability. Experiments were performed in triplicate and were repeated on at least three occasions.
Confocal microscopy
Cell surface IL-4Rα, IL-13Rα1 and common γ-chain expression was measured using specific anti-rat polyclonal antibodies (Santa Cruz Biotechnology, Calne, UK) and confocal microscopy. BRIN-BD11 cells were grown on a coverslip and fixed at − 20°C for 10 min with ice-cold methanol. Fixed cells were then washed with 1 × PBS/1% bovine serum albumin (BSA) and blocked using 10% goat serum for 15 min. After incubation cells were washed in 1% BSA/PBS and incubated with primary antibody for 1 h, washed and secondary goat anti-rabbit fluorescein isothiocyanate (FITC) antibody added (Santa Cruz Biotechnology) for 30 min. After final washing in PBS/1% BSA, cells were viewed on a Zeiss LSM 510-meta confocal microscope to monitor receptor expression.
Measurements of nitrite using the Griess assay
BRIN-BD11 cells were incubated in a total volume of 1 ml medium per well in six-well tissue culture plates. After incubation with appropriate cytokines, the supernatant was removed and 100 µl solution containing 0·1% napthylethylenediamine in H2O and 1% sulphanilamide in 5% orthophosphoric acid (all from Sigma) mixed in a 1 : 1 ratio was added to 100 µl of sampled medium or 100 µl of sodium nitrite standard (0–100 µM). The mixture was incubated at room temperature for 10 min and the absorbance measured at 550 nm. Nitrite concentrations of experimental samples were determined by reference to a standard curve constructed in parallel.
Statistical analysis
Statistical analysis was performed by the unpaired Student's t-test and the results were considered significant when P < 0·05.
Results
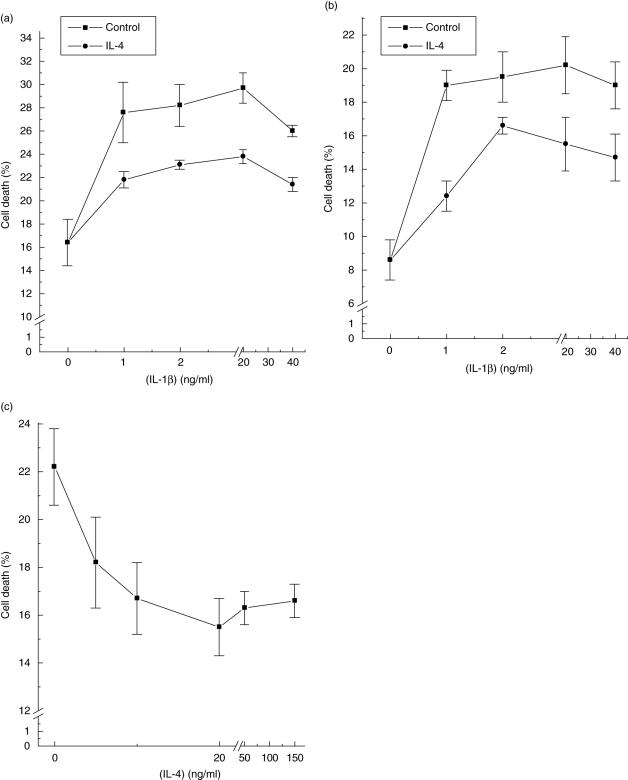
It is well known that exposure of rodent pancreatic β-cells to the cytokine IL-1β leads to a reduction in viability [2–4], and this was confirmed in the present studies (Fig. 1a). However, when β-cells were pretreated with the Th2-derived cytokine, IL-4, for 48 h prior to addition of IL-1β, the extent of cell death induced by the proinflammatory cytokine was attenuated markedly (Fig. 1a). This was observed across the whole range of IL-1β concentrations tested and resulted in a mean reduction in cytotoxicity of between 40 and 60%. Similar results were obtained when a combination of IL-1β plus IFN-γ was employed as the cytotoxic stimulus (Fig. 1b). Further analysis of the protective effects of IL-4 revealed that the response was dose-dependent (Fig. 1c), with maximal inhibition observed when 20 ng/ml IL-4 was employed (EC50 ∼7·5 ng/ml). The inhibition of cell death following exposure of β-cells to IL-4 was not accompanied by a reduction in IL-1β/IFN-γ-induced nitrite formation (control: 1·6 µM nitrite; IL-4 alone: 1·8 µM; IL-1β/IFN-γ: 11·7 µM; IL-1β/IFN-γ/IL-4: 12·4 µM; mean values obtained in two experiments each performed in triplicate), suggesting that its effects were not due to a reduction in β-cell nitric oxide production.
Fig. 1.
Suppression of β-cell death by interleukin (IL-4). BRIN-BD11 cells were preincubated for 48 h with IL-4 (20 ng/ml) and then treated with different concentrations of IL-1β alone (a) or in the presence of 2 ng/ml interferon (IFN)-γ (b). In (c) cells were preincubated with increasing concentrations of IL-4 for 48 h and then treated with IL-1β (2 ng/ml) and IFN-γ (2 ng/ml). Twenty-four h after addition of the proinflammatory cytokines, cells were harvested and their viability analysed by flow cytometry. Each point is the mean (± s.e.m.) of at least three different experiments each performed in triplicate. In (a) and (b) IL-4 reduced significantly the proinflammatory cytokine response at all concentrations of IL-1β tested (P < 0·01). In (c) significant inhibition (P < 0·05) was achieved at 10 ng/ml IL-4 and above.
The capacity of IL-4 to attenuate the cytotoxic effects of IL-1/IFN-γ were not seen if the three cytokines were added to β-cells simultaneously and cell death was then monitored 24 or 48 h later. Indeed, under these conditions, the presence of IL-4 tended to enhance further the loss of viability, although this did not achieve statistical significance (untreated cells: 6·5 ± 0·5% non-viable after 24 h; IL-1/IFN-γ: 23·7 ± 5·4%; IL-1/IFN-γ/IL-4: 32·6 ± 7·3%; mean data from three separate experiments performed in triplicate). Thus, it appears that prior exposure of β-cells to IL-4 is required in order for its protective actions to become manifest.
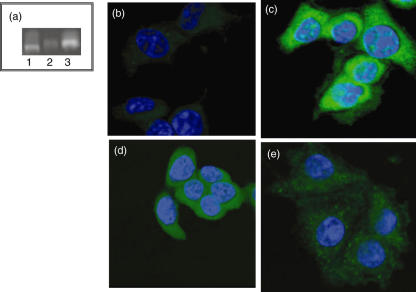
IL-4 normally exerts its effects by interaction with one of two types of cell surface receptor, comprising a unique IL-4 binding domain (IL-4Rα) in combination with either the cytokine receptor common γ-chain (in type I IL-4 receptors) or the α1-chain of IL-13 receptors (in type II IL-4 receptors). Therefore, the expression of each of these components was examined at both the RNA and protein level in β-cells (Fig. 2). Analysis of RNA extracted from cultured BRIN-BD11 β-cells revealed the expression of all three receptor components, IL-4Rα, IL-13Rα1 and the common γ-chain (Fig. 2a). Confirmation of the identity of the IL-4Rα and IL-13Rα1 components was achieved by excision from the gel and direct sequencing of the amplified DNA. In each case, this was more than 99% identical with the predicted sequence of the relevant rat genes.
Fig. 2.
Expression of interleukin (IL)-4 receptors in β-cells. The expression of mRNA transcripts encoding IL-4Rα (a; lane 1), common γ-chain (a; lane 2) and IL-13Rα1 (a; lane 3) was confirmed by reverse transcription–polymerase chain reaction after extraction of total RNA from BRIN-BD11 cells. The presence of IL-4Rα (c), common γ-chain (d) and IL-13Rα1 (e) protein on BRIN-BD11 cells was confirmed by confocal microscopy (green) after staining with relevant antibodies. Controls from which primary antibodies were omitted were not stained (b). Nuclei were stained with 4,6-diamine-2-phenylindole (blue).
To confirm that β-cells express IL-4 receptor protein, immunocytochemical analysis was performed using antibodies raised against each component. Antibodies against IL-4Rα (Fig. 2c), common γ-chain (Fig. 2d) and IL-13Rα1 (Fig. 2e) revealed the presence of the relevant proteins in cultured BRIN-BD11 cells. The staining intensity was reduced markedly when the primary antibodies were omitted or replaced with non-specific rabbit IgG. As the antibodies used were cross-reactive with human receptors, the specificity of labelling was also confirmed using a human Jurkat T cell line (not presented).
Taken together, these results confirm that the components of IL-4 receptor are present in pancreatic β-cells and that IL-4 can attenuate the cytotoxic actions of proinflammatory cytokines.
Discussion
Previous studies in non-obese diabetic mice have shown that systemic administration of IL-4 or delivery of recombinant adenoviral vectors harbouring IL-4 mRNA reduce insulitis and protect from diabetes [10,11]. Partial protection has also been observed in diabetes-prone BioBreeding (BB) rats treated with IL-4 via retrovirally transduced lymphocytes [14]. Thus, there is evidence that enhanced secretion of IL-4 may be protective against islet cell destruction. The present results confirm this and show, for the first time, the expression of both type I and type II IL-4 receptors on rat pancreatic β-cells. Moreover, they reveal a direct protective effect of IL-4 in vitro on the loss of β-cell viability mediated by proinflammatory cytokines, suggesting that these receptors are functionally active.
Until recently, it has been assumed that any protective effects of IL-4 against β-cell death in diabetes are likely to be mediated by regulation of the respective functional activities of Th1 and Th2 cell subsets [11]. However, our results show that IL-4 has a direct protective effect on rat β-cells, mediated probably through IL-4 receptors expressed on these cells. This is consistent with other studies demonstrating a direct protective effect of a combination of Th2 cytokines on human islet cells [12], as well as IL-4 inhibition of apoptosis in human conjunctival fibroblasts [15], human synoviocytes [16] and porcine endothelial cells [17].
An important question arising from the present data concerns the mechanism(s) by which the protective effect of IL-4 is exerted. IL-4 has been shown to modulate the synthesis of NO in macrophages [18] and as NO forms one component of the mechanism by which proinflammatory cytokines promote β-cell death [2–4], a similar action could underlie the protective actions of IL-4 in β-cells. However, the inhibition of IL-1β/IFN-γ-induced cell death by IL-4 shown in our study was not accompanied by a reduction in the nitrite concentration of the incubation medium, suggesting that it was not associated with impaired β-cell nitric oxide production. Similar findings were reported previously when human islet cells were exposed to the combination of IL-4 and IL-10 [12], and the results suggest that IL-4 may exert its action via an NO independent pathway. This conclusion differs from that of another study in which a partial reduction of IL-1β-induced production of NO by IL-4 was reported in rat islets [19].
In certain cell types, IL-4 can induce the synthesis of a naturally occurring IL-1R-antagonist that competes with IL-1β for binding to the receptor, thereby attenuating the ability of IL-1β to activate its receptor [20]. Because the IL-1β receptor antagonist is known to be expressed in β-cells under certain conditions [21], this represents one possible mechanism by which IL-4 could mediate an anti-apoptotic action in the presence of IL-1β. However, we find that IL-4 did not antagonize the ability of IL-1β to promote nitrite formation in β-cells and this implies that the anti-apoptotic actions of IL-4 are not mediated by alterations in the synthesis of an IL-1R-antagonist. This is because any such effect would lead to reduced IL-1β-signalling and thereby cause a reduction in IL-1β-mediated NO (and nitrite) formation. This was not observed. Thus, we conclude that the cytoprotective actions of IL-4 must be mediated downstream of the IL-1β receptor.
Interestingly, in our study, the protective effect of IL-4 on IL-1/IFN-γ-induced cell death was not seen if the three cytokines were added to β-cells simultaneously. Indeed, under these conditions, the presence of IL-4 tended to enhance further the loss of viability. This suggests that prior exposure of β-cells to IL-4 is required in order for its protective actions to become manifest and implies that time-dependent alterations in the downstream signalling events initiated by proinflammatory cytokine are targeted. If similar considerations apply in vivo, then it seems probable that a critical window of opportunity might exist for IL-4 to exert a protective effect during the induction of insulitis and diabetes. In this context, it is interesting to note that a temporal relationship in the secretion of cytokines such as IL-4 and IFN-γ has been demonstrated in T cells infiltrating the islet cells of diabetic NOD mice [22].
In summary, the present results demonstrate, for the first time, expression of type I and type II IL-4 receptor components on rat pancreatic β-cells and they reveal a direct protective effect against loss of viability when cells are exposed to IL-4 before the addition of proinflammatory cytokines. This information is relevant to a full understanding of the immunological mechanisms that influence the development of diabetes and might also have implications for the handling of pancreatic β-cells in vitro prior to transplantation. It is known that β-cell loss can occur by apoptosis when isolated islets are maintained in vitro [23], and the possibility that exposure to IL-4 during this period might exert a protective influence is worthy of further evaluation.
Acknowledgments
This study was supported by a Plymouth Hospitals NHS Trust Research Encouragement Award.
References
- 1.Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580–91. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 2.Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996;39:1005–29. doi: 10.1007/BF00400649. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitch A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1998;14:129–51. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Eizirik DL, Mandrup-Poulsen T. A choice of death − the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–33. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 5.Juedes AE, von Herrath MG. Regulatory T-cells in type 1 diabetes. Diabetes Metab Res Rev. 2004;20:446–51. doi: 10.1002/dmrr.508. [DOI] [PubMed] [Google Scholar]
- 6.Berman MA, Sandborg CI, Wang Z, et al. Decreased IL-4 production in new onset type I insulin-dependent diabetes mellitus. J Immunol. 1996;157:4690–6. [PubMed] [Google Scholar]
- 7.Szelachowska M, Kretowski A, Kinalska I. Decreased in vitro IL-4 and IL-10 production by peripheral blood lymphocytes in first degree relatives at high risk of diabetes type-I. Horm Metab Res. 1998;30:526–30. doi: 10.1055/s-2007-978926. [DOI] [PubMed] [Google Scholar]
- 8.Rapoport MJ, Mor A, Vardi P, et al. Decreased secretion of Th2 cytokines precedes up-regulated and delayed secretion of Th1 cytokines in activated peripheral blood mononuclear cells from patients with insulin-dependent diabetes mellitus. J Autoimmun. 1998;11:635–42. doi: 10.1006/jaut.1998.0240. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SB, Kent SC, Patton KT, et al. Extreme TH1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 1998;391:177–81. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport MJ, Jaramillo A, Zipris D, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron MJ, Arreaza GA, Waldhauser L, Gauldie J, Delovitch TL. Immunotherapy of spontaneous type 1 diabetes in nonobese diabetic mice by systemic interleukin-4 treatment employing adenovirus vector-mediated gene transfer. Gene Ther. 2000;7:1840–6. doi: 10.1038/sj.gt.3301309. [DOI] [PubMed] [Google Scholar]
- 12.Marselli L, Dotta F, Piro S, et al. Th2 cytokines have a partial, direct protective effect on the function and survival of isolated human islets exposed to combined proinflammatory and Th1 cytokines. J Clin Endocrinol Metab. 2001;86:4974–8. doi: 10.1210/jcem.86.10.7938. [DOI] [PubMed] [Google Scholar]
- 13.McClenaghan NH, Barnett CR, O'Harte FPM, Flatt PR. Mechanism of amino-acid-induced insulin secretion from the glucose-responsive BRIN-BD11 pancreatic beta-cell line. J Endocrinol. 1996;15:349–57. doi: 10.1677/joe.0.1510349. [DOI] [PubMed] [Google Scholar]
- 14.Zipris D, Karnieli E. A single treatment with IL-4 via retrovirally transduced lymphocytes partially protects against diabetes in BioBreeding (BB) rats. J Pancreas. 2002;3:76–82. [PubMed] [Google Scholar]
- 15.Fujitsu Y, Fukuda K, Kimura K, Seki K, Kumagai N, Nishida T. Protection of human conjunctival fibroblasts from NO-induced apoptosis by interleukin-4 or interleukin-13. Invest Ophtalmol Vis Sci. 2005;46:797–802. doi: 10.1167/iovs.04-1016. [DOI] [PubMed] [Google Scholar]
- 16.Relic B, Guicheux J, Mezin F, et al. IL-4 and IL-13, but not IL-10, protect human synoviocytes from apoptosis. J Immunol. 2001;166:2775–82. doi: 10.4049/jimmunol.166.4.2775. [DOI] [PubMed] [Google Scholar]
- 17.Grhan JF, Levay-Young BK, Fogelson JL, Francois-Bongarcon V, Benson BA, Dalmasso AP. IL-4 and IL-13 induce protection of porcine endothelial cells from killing by human complement and from apoptosis through activation of a phosphatidylinositide 3-kinase/Akt pathway. J Immunol. 2005;175:1903–10. doi: 10.4049/jimmunol.175.3.1903. [DOI] [PubMed] [Google Scholar]
- 18.Liew FY, Li Y, Severn A, et al. A possible novel pathway of regulation by murine T helper-2 (Th2) cells of a T1 cell activity via the modulation of the induction of nitric acid synthase on macrophages. Eur J Immunol. 1991;21:2489–94. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- 19.Sandler S, Sternesjo J. Interleukin 4 impairs rat pancreatic islet function in vitro by an action different to that of interleukin 1. Cytokine. 1995;7:296–300. doi: 10.1006/cyto.1995.0036. [DOI] [PubMed] [Google Scholar]
- 20.Wong HL, Costa GL, Lotze MT, Wahl SM. Interleukin-4 diferentially regulates monocyte IL-1 family gene expression and synthesis in vitro and in vivo. J Exp Med. 1993;177:775–81. doi: 10.1084/jem.177.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maedler K, Sergeev P, Ehses JA, et al. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci USA. 2004;101:8138–43. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy S, Karanam M, Krissansen G, et al. Temporal relationship between immune cell influx and the expression of inducible nitric oxide synthase, interleukin-4 and interferon-gamma in pancreatic islets of NOD mice following adoptive transfer of diabetic spleen cells. Histochem J. 2000;32:195–206. doi: 10.1023/a:1004084232446. [DOI] [PubMed] [Google Scholar]
- 23.Emamaullee JA, Shapiro AM. Interventional strategies to prevent beta-cell apoptosis in islet transplantation. Diabetes. 2006;55:1907–14. doi: 10.2337/db05-1254. [DOI] [PubMed] [Google Scholar]