Abstract
Monocyte-derived dendritic cells (MoDCs) are a promising cellular adjuvant for effector immune responses against tumours and chronic viral infections, including hepatitis C virus (HCV). If autologous DC therapeutic approaches are to be applied in persistent HCV infections in patients, it is important to have an unambiguous understanding of the functional status of the cell type used, namely MoDCs from patients with chronic hepatitis C (CHC) infection. Because of conflicting published reports of either impaired or normal MoDC function in CHC infection, we re-examined the ability of MoDCs from CHC and normal healthy donors (NHD) to mature to an inflammatory stimulus [tumour necrosis factor (TNF)-α] and their subsequent functional capabilities. Expression of maturation-associated phenotypic markers [human leucocyte antigen (HLA)-DR, CD83, CD86, CD40], allostimulatory capacity in mixed lymphocyte reactions (MLRs) and CD40-ligand-induced cytokine and chemokine generation were compared in CHC- versus NHD-MoDCs. TNF-α-stimulated CHC-MoDCs up-regulated phenotypic markers, but to significantly lower levels than NHD-MoDCs. At physiological ratios of DCs to T cells, CHC-MoDCs were less allostimulatory than NHD-MoDCs, but not when DC numbers were substantially increased. CHC- and NHD-MoDCs generated equivalent amounts of cytokines [TNF-α, interleukin (IL)-1β, IL-6, IL-12p70, IL-15, IL-10] and chemokines [interferon-inducible protein (IP)-10, macrophage inflammatory protein (MIP)-1α, regulated upon activation, normal T expressed and secreted (RANTES)] after CD40 ligation. Because the functional defect was not apparent at high MoDC : T cell ratios, autologous MoDC therapy with sufficiently high numbers of DCs could, in theory, overcome any impairment of MoDC function in CHC.
Keywords: cell therapy, dendritic cell, HCV, hepatitis C, interleukin
Introduction
Hepatitis C virus (HCV) infection is a major world health problem estimated to infect 170 million people; 3–4 million individuals are newly infected each year, of whom about 80% progress to chronic infection. Persistence of HCV infection results from a combination of the mutability of the virus genome and the ability of the virus to evade cells of the immune system. In those individuals who clear the acute infection a robust and, crucially, sustained CD4+ and CD8+ T cell response to multiple HCV antigens is apparent [1]. In contrast, although subjects who develop chronic infection can mount early, vigorous effector T cell responses against a variety of HCV peptide epitopes [2], these responses are transient. Gradually, the frequency of HCV-specific CD4+ and CD8+ T cells declines and the range of peptide epitopes recognized becomes restricted. In addition, the HCV-specific CD4+ T cells secrete less interferon (IFN)-γ and more regulatory cytokines [3,4] and the HCV-specific CD8+ T cells become less cytolytic [5–7].
The prime initiators of acquired immune responses are dendritic cells (DCs) because of their highly effective antigen presentation to naive T cells [8]. It has been hypothesized that the weak cellular immunity to HCV seen in individuals with chronic HCV infection (CHC) could be due to alteration of DC function by the virus leading to weak, absent or inappropriate T cell responses to HCV. This suggestion arose from studies on circulating blood DCs, where the majority of reports have observed functional impairment in myeloid and plasmacytoid DCs in CHC infection compared with healthy non-HCV-infected individuals [9–15], although there are also two studies reporting no defect in plasmacytoid DCs [16,17] or myeloid DCs [17] from CHC subjects. Blood DCs are scarce and cannot be expanded readily in vitro. More appropriate for DC therapy is the easily cultured monocyte-derived DC (MoDC). Similar to studies on blood DCs, there is controversy about the presence or otherwise of HCV-associated defects in MoDCs. Three groups have reported maturational and allostimulatory defects in MoDCs cultured from CHC compared with NHD subjects [10,18,19], while two studies observed no such impairment [20,21].
In light of the conflicting results obtained in previous investigations, the aim of the present study was to re-evaluate the maturational and functional capabilities of MoDCs from CHC patients compared with NHD subjects, with particular emphasis on cytokine and chemokine products of these cells. Autologous MoDCs are being used as cellular adjuvants in clinical trials for the treatment of tumours [22], and recently in experimental therapy of chronic HIV-1 infection [23]. A recent review concluded that DC vaccination could be effective in CHC infection provided that the MoDCs are appropriately antigen-loaded and matured ex vivo [24]. Encouraging results have been obtained in a mouse model of DC vaccination where cultured, bone marrow-derived DCs, pulsed with recombinant HCV core antigen and injected into naive mice induced anti-core CD8+ T cell responses. The cellular response was associated with enhanced protection against implanted melanoma tumours expressing HCV core protein [25]. If such cell therapy approaches are to be applied in CHC infections in patients, it is important to have an unambiguous understanding of the functional status of the cell type used in DC therapy, namely MoDCs, during chronic HCV infection.
Materials and methods
Human subjects
Ethical approval was obtained from Southampton and South-west Hampshire Joint Research Ethics Committee and all patients gave informed consent in writing prior to participating in the study. A total of 23 patients with chronic hepatitis C virus infection (CHC, 19 male and four female, median age 47 years, range 29–71 years) were recruited from the hepatology clinics run by Southampton University Hospitals National Health Service Trust. All patients had detectable HCV RNA (13 genotype 1a/b, 10 non-genotype 1), which was quantified in 17 individuals using the Cobas Amplicor HCV Monitor test (version 2·0; Roche Molecular Systems, Branchburg, NJ, USA). Nineteen of 20 patients with a known risk factor for HCV acquired the infection via intravenous drug use. In 17 of 23 patients tested, viral loads ranged from 1·9 × 104 to 2·3 × 107 IU/ml (median 4·6 × 106 IU/ml). The median alanine aminotransferase level in CHC donors was 60·0 (range 20·0–265·0). Patients were excluded if they had received treatment for HCV infections 6 months or less prior to the study or tested positive with hepatitis B virus or HIV. Twenty uninfected normal healthy donors (NHD, 13 male and seven female, median age 49 years, range 26–79 years) with no known risk factors for blood-borne virus infection consented to give blood for this study. When DC numbers were limited, DCs could not be used in all assays; however, 10 of 13 NHD and 10 of 10 CHC donor MoDCs were used in both mixed lymphocyte reaction and phenotyping experiments.
Culture of MoDC
Fifty ml of freshly drawn blood was obtained from NHD subjects (n = 20) or CHC patients (n = 23). Blood was collected into K3-ethylenediamine tetraacetic acid (EDTA) and separated immediately by centrifugation over Lymphoprep (Robbins Scientific, Solihull, UK). Peripheral blood mononuclear cells (PBMCs) were recovered and monocytes then isolated using the MACS CD14+ isolation kit (Miltenyi Biotec, Bisley, UK), according to the manufacturer's recommended protocol. CD14+ cells were positively selected from PBMC using an AutoMACS machine (Miltenyi Biotec). Monocytes (106 cells/ml) were cultured in six-well plates (Greiner Bio-One, Stonehouse, UK) in 3 ml per well of complete RPMI medium [RPMI-1640 without phenol red (Invitrogen, Paisley, UK), with 2 mmol/l l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (Sigma, Poole, UK) and 10% heat-inactivated fetal calf serum (FCS) (Hyclone, Perbio Science UK Ltd, Tattenhall, UK)], containing 50 ng/ml recombinant human granulocyte–macrophage colony-stimulating factor (rhGM-CSF) and 1000 IU/ml rhIL-4 (both from R&D Systems, Abingdon, UK). After 2·5 days and 5 days 0·5 ml of culture medium was replaced with fresh cytokines. On day 5, half the immature DCs (iDCs) were additionally given 50 ng/ml rhTNF-α (R&D Systems), resulting in mature DCs (mDCs) 2 days later. The purity of DCs after 7 days was typically 95–97%, as assessed by fluorescence activated cell sorter (FACS) analysis for human leucocyte antigen D-related (HLA-DR+), CD1a+, CD3–, cells (anti-human CD1a; Dako, Ely, UK; anti-human CD3, HLA-DR; BD Biosciences, Cowley, UK).
Immunostaining MoDCs following maturation with rhTNF-α
Fifty ml of peripheral blood was collected into heparin from 10 CHC and 10 NHD subjects. To identify MoDCs and their cell surface phenotype, MoDCs were stained for two-colour flow cytometry. For each condition, 5 × 105 MoDCs were incubated on ice for 30 min in 50 µl wash buffer [phosphate-buffered saline (PBS)/0·05% NaN3/0·5% bovine serum albumin (BSA)] containing 100 µg/ml human Fcγ fragments (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) (‘blocking buffer’). Next, cells were incubated on ice for 30 min with 50 µl of antigen-presenting cell (APC)-labelled anti-human CD83, CD86 or CD40 (BD Biosciences) or fluorescein isothiocyanate (FITC)-labelled anti-human HLA-DR (BD Biosciences), all diluted to their optimal concentrations in blocking buffer or APC- or FITC-labelled isotype controls (murine IgG1 and IgG2b; BD Biosciences) at equivalent dilutions. Cells were washed three times in wash buffer, then fixed in 1% formaldehyde and stored on ice in the dark pending analysis.
Flow cytometric analysis of MoDCs
Events were acquired using a FACSCalibur dual laser flow cytometer (BD Biosciences) and analysed using CellQuest software (BD Biosciences). In MoDC cultures, MoDCs were identified as large cells having strong labelling for HLA-DR. Using two-colour analysis, the presence of the surface markers, HLA-DR, CD83, CD86 and CD40 on immature and mature MoDCs was determined by collecting ≥ 40 000 events in the total cell population. All positive staining was compared with appropriate isotype controls.
Allogeneic mixed lymphocyte reaction (MLR)
Responder CD4+ T cells were purified by negative selection from 10 allogeneic normal healthy donors using a CD4+ T cell Isolation Kit II (Miltenyi Biotec), according to the manufacturer's recommended protocol. All the T cell donors gave blood on more than one occasion to give a total of 23 MLR tests of MoDC function (13 NHD, 10 CHC). Purified CD4+ T cells were then incubated for 10 min at 37°C with 5 µM 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Inc., Eugene, OR, USA). Incorporation of CFSE was stopped by addition of 5 volumes of ice-cold complete RPMI-1640 medium and excess CFSE was removed by washing the cells three times in the same diluent. CFSE-labelled responder T cells (2 × 105) were mixed with serial dilutions of mDCs or, for comparison, iDCs in U-bottomed 96-well plates in complete RPMI-1640. After 6 days, cells were harvested, fixed in 1% formaldehyde and analysed by flow cytometry. As a measure of T cell proliferation, the percentage of CD4+ T cells in which CFSE fluorescence had decayed was determined.
Stimulation of MoDCs using CD40 ligand-transfected cells
To measure cytokine and chemokine release in response to CD40 ligation, 2 × 105 washed immature or TNF-α-matured MoDCs were added to 2 × 105 human CD40 ligand-transfected Chinese hamster ovary cells (hCD40L-CHO) or wild-type cells (wt-CHO) (both gifts from Professor M. Glennie, Tenovus Laboratories, Southampton, UK) adhering to the wells of 48-well cell culture plates in a final culture volume of 0·5 ml complete RPMI-1640 medium. Further wells contained MoDCs, hCD40L-CHO or wt-CHO cells alone. After 24 h, supernatants were harvested and centrifuged for 10 min at 2500 g, then frozen pending further analysis.
Quantifying cytokine production
Multiple cytokines [TNF-α, IL-1β, IL-12p70, IL-10, IL-6, IL-15, regulated upon activation, normal T expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α and interferon-inducible (IP)-10 (CXCL10)] were measured in culture supernatants from MoDCs using Beadlyte® reagents and Luminex xMAP instrumentation (Upstate, Dundee, UK).
Statistical analysis
Unpaired t-tests and, where appropriate (data in Fig. 2), Mann–Whitney tests were used to compare means. Analyses were performed using Prism version 4·0 for Windows (GraphPad Software, San Diego, CA, USA).
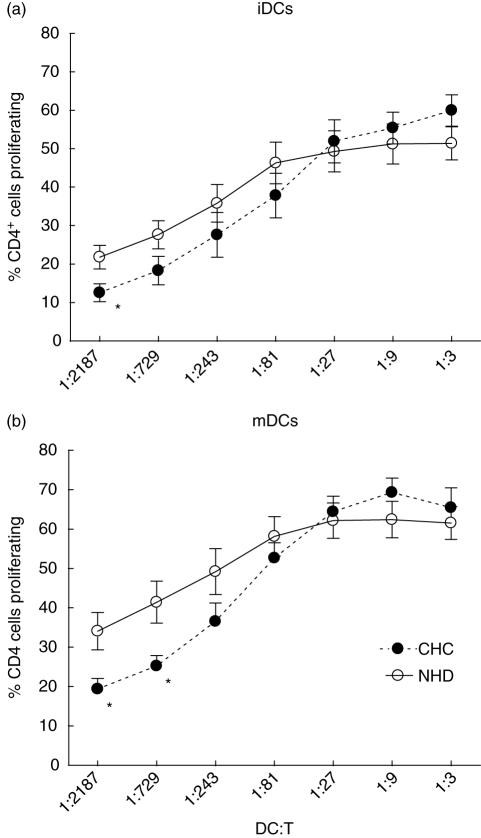
Fig. 2.
The allostimulatory capacity of monocyte-derived dendritic cells (MoDCs) cultured from patients with chronic hepatitis infection (CHC) is impaired at low DC : T cell ratios compared with MoDCs from normal healthy donors (NHD). The percentage of allogeneic CD4+ T cells that proliferated in response to the presence of varying numbers of (a) immature MoDCs (iDCs) or (b) tumour necrosis factor (TNF)-α-matured MoDCs (mDCs) is shown. Each point represents the mean ( s.d.) of results from 10 (CHC) or 13 (NHD) individuals. *P < 0·05.
Results
MoDCs from CHC donors mature less well than NHD-MoDCs in response to TNF-α
Before conducting any functional studies on MoDCs from CHC donors, it was first important to determine whether the cells were able to respond appropriately to a DC maturation stimulus (TNF-α 50 ng/ml). A moderate maturation stimulus was chosen rather than a potent one [e.g. lipopolysaccharide (LPS)] that might have masked any subtle defects in the responses of CHC MoDCs. Using FACS analysis, we measured expression of surface molecules that are characteristically up-regulated in normal DCs after treatment with TNF-α (CD83, HLA-DR, CD40, CD86, Fig. 1). To normalize variations between FACS analyses performed over several months, and because we were comparing the ability to mature between NHD- and CHC-MoDCs, MoDC maturation was expressed as the fold-increase in mean fluorescence intensities (MFIs) pre- and post-treatment with TNF-α. Prior to maturation, there were no significant differences in the MFIs of phenotypic markers between NHD- and CHC-MoDCs [mean ± standard deviation (s.d.) of MFIs of NHD-MoDCs versus CHC-MoDCs were as follows: CD83, 2·3 ± 0·6 versus 3·1 ± 0·9; HLA-DR, 2·4 ± 0·3 versus 1·7 ± 0·1; CD40, 18·8 ± 2·3 versus 19·5 ± 5·4; CD86, 21·8 ± 6·6 versus 57·5 ± 16·2]. MFI values are net amounts, after subtraction of the MFI of the relevant isotype control.
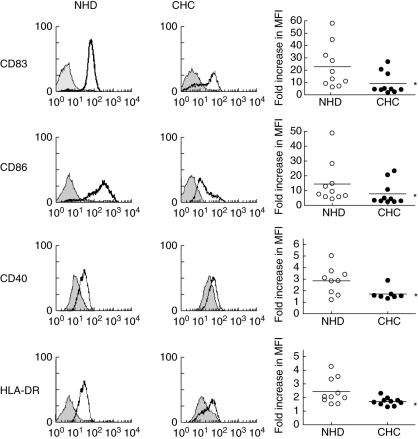
Fig. 1.
Impaired up-regulation of maturation markers following tumour necrosis factor (TNF)-α treatment of monocyte-derived dendritic cells (MoDCs) from chronic hepatitis C (CHC, n= 10) patients compared with normal healthy donor MoDCs (NHD, n= 10, right column of graphs). The left and middle columns of graphs show representative fluorescence activated cell sorter analysis histograms of NHD and CHC donors, respectively; x-axes represent fluorescence intensity and event counts are indicated on the y-axes. Grey-shaded histograms are immature MoDCs and open histograms are TNF-α-matured MoDCs. The graphs in the right column summarize the group results. Values are expressed as fold-increase in mean fluorescence intensity after TNF-α treatment. *P < 0·05.
Although MFIs of all four phenotypic markers that we tested after TNF-α treatment increased in both NHD- and CHC-MoDCs, the increase in expression of the markers was significantly lower (P < 0·05) in CHC-MoDCs compared with NHD-MoDCs. Representative examples of phenotypic marker expression by NHD- and CHC-MoDCs pre- and post-TNF-α treatment are shown in Fig. 1. There were no significant differences in the percentages of TNF-α-matured CHC versus NHD MoDCs positive for HLA-DR (100% versus 100%), CD86 (mean ± s.d. = 94·4 ± 2·5% versus 96·3 ± 1·6%), CD83 (89·6 ± 7·9% versus 90·7 ± 11·3%) or CD40 (96·9 ± 1·0% versus 85·5 ± 10·8%).
MoDCs from CHC donors are less allostimulatory than NHD-MoDCs at low DC : T ratios
Up-regulation of MHC class II and co-stimulatory molecules such as CD86 and CD40 following exposure to endogenous inflammatory stimuli such as TNF-α is critically important for effective antigen presentation by DCs to naive CD4+ T cells. We used the mixed lymphocyte reaction (MLR) assay as one way of assessing the antigen-presenting (in this case allogeneic antigens) capacity of NHD- versus CHC-MoDCs. Results of the MLR experiments indicated that, at higher DC : T ratios, CHC-MoDCs did not show any defect in their ability to stimulate proliferation of allogeneic CD4+ T cells (Fig. 2). Immature DCs from both CHC and NHD groups were equally active in stimulating allogeneic T cell proliferation except at the lowest DC : T cell ratio (1 : 2187). After maturation with TNF-α, there was no statistical difference in the allostimulatory capacities of NHD- and CHC-MoDCs at five of seven DC : T cell ratios tested (1 : 3–1 : 243). At the optimal DC : T ratios for CD4+ T cell proliferation (1 : 9–1 : 27) in the MLR assays, there was no difference between the allostimulatory capacities of TNF-α-matured MoDCs from either study group. However, at DC : T ratios of less than 1 : 81, at what could be regarded as more physiologically relevant numbers of DCs [26,27], there was a distinct trend for weaker allostimulation by CHC- compared with NHD-MoDCs which was statistically significant at DC : T cell ratios of 1 : 729 and 1 : 2187 (P < 0·05).
MoDCs from CHC donors are fully competent at generating cytokines and chemokines in response to a physiological stimulus
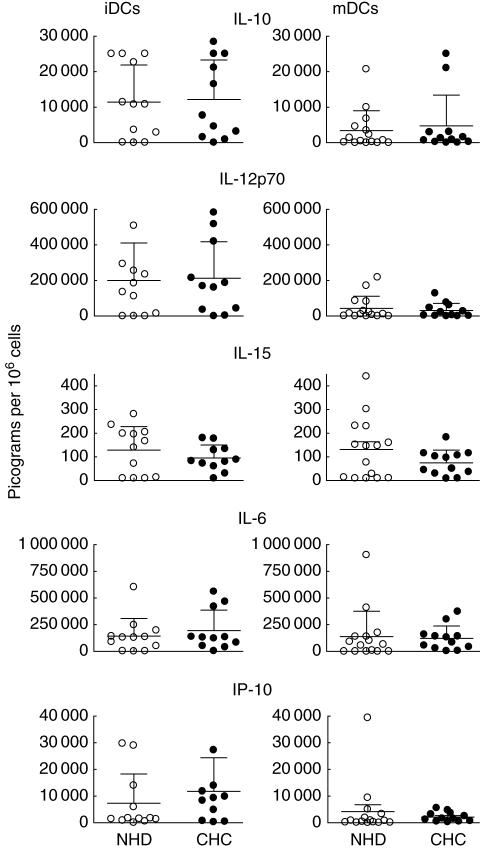
In order to assess whether CHC-MoDCs are defective in cytokine or chemokine production, we compared the ability of immature and TNF-α-matured MoDCs from CHC and NHD subjects to generate a range of key proinflammatory and regulatory cytokines and chemokines in response to ligation with CD40 ligand (CD40L). Specifically, we measured amounts of: (1) a cytokine implicated in T helper 1 (Th1) differentiation (IL-12 p70); (2) chemokines that attract Th1 cells (IP-10, MIP-1α, RANTES); (3) a cytokine involved in inducing CTL and natural killer (NK) cell responses (IL-15); (4) cytokines associated with the induction of tolerance or regulatory T cells (IL-10 and IL-6); and (5) proinflammatory cytokines (e.g. IL-1β, IL-6 and TNF-α).
Amounts of five of the nine key products assayed are shown in Fig. 3. There were no significant differences in IL-10, IL-12p70, IL-15, IL-6 or IP-10 generation by CHC- or NHD-MoDCs after CD40 ligation. TNF-α, IL-1β, MIP-1α and RANTES generation were also equivalent between both groups (data not shown). Wild-type CHO cells did not stimulate measurable cytokine or chemokine secretion from MoDCs (data not shown).
Fig. 3.
Monocyte-derived dendritic cells (MoDCs) cultured from chronic hepatitis C (CHC, n = 11 or 12) patients show no impaired cytokine or chemokine response to CD40 ligation compared with MoDCs from normal healthy donors (NHD, n = 11–15). Immature MoDCs (iDCs) or tumour necrosis factor (TNF)-α-matured DCs (mDCs) were stimulated with CHO cells transfected with human CD40 ligand. Wild-type CHO cells served as a control treatment and stimulated no detectable cytokine or chemokine response (data not shown).
Discussion
The results presented here suggest that monocyte-derived DCs cultured from patients with chronic HCV infection are significantly defective in their capacity to mature in response to a relatively weak stimulus (50 ng/ml TNF-α) in terms of: (1) up-regulating the expression of surface molecules involved in antigen presentation (HLA-DR, CD86, CD40) and a classical marker of DC activation (CD83) and (2) at physiological ratios of DCs to T cells, their ability to present antigen in MLR assays. However, after a strong stimulus (cell-bound CD40L), CHC-MoDCs were as competent as NHD-MoDCs at generating a broad repertoire of effector cytokines and chemokines.
Previous studies of NHD- versus CHC-MoDC activity have shown either a significantly impaired allostimulatory activity of CHC-MoDCs [18,19] or no significant defect [20,21] at all ratios of DC : T cells tested. Unlike both the latter analyses, we used purified CD4+ T cells as responders in the MLR assay and also the FACS-based CFSE method rather than tritiated thymidine incorporation to measure cell proliferation of that specific cell type. These differences in methodology could account for the subtle distinction in our MLR findings. However, in support of our data, Longman et al. [20] showed a trend, although not statistically significant, for lower allostimulatory activity of CHC- versus NHD-MoDCs at the lowest ratios of DC : purified T cells used (1 : 270 and 1 : 810). Imaging studies in intact mouse lymph nodes suggest that an antigen-loaded DC can interact with somewhere between 500 [26] and 5000 [27] T cells in 1 h.
In the present study, although there was a significant defect in the allostimulatory activity of TNF-α-matured CHC-MoDCs when DC numbers were limited, the deficiency was restored at higher DC : T cell ratios when, presumably, higher densities of DC co-stimulatory molecules were achieved This is encouraging for cell therapeutic approaches that would administer in vitro-cultured autologous MoDCs to CHC patients in order to restore defective T cell immunity to HCV because it suggests that T cell responses could be driven provided enough DCs are present, although we did not test HCV-specific responses in the present study.
Our data suggest that despite evidence of an impaired allostimulatory capacity at low DC : T cell ratios, CHC-MoDCs are fully capable of generating a range of proinflammatory cytokines and chemokines that are critical for Th1-induced viral clearance (IL-12p70, IL-15, IP-10) and, furthermore, have no evidence of enhanced immunosuppressive (IL-10) or Th2-inducing (IL-6) cytokine production. There were no statistical associations between amounts of MoDC cytokines and chemokines and the viral loads or disease severity of the donors (data not shown). The weakened cellular immunity to HCV antigens described previously in chronic HCV infection [3–7], therefore, is not attributable to any defect in Th1-inducing (IL-12p70) or enhanced immunosuppressive (IL-10) cytokine production by antigen-presenting cells as represented by cultured MoDCs. We have measured a wider range of MoDC products than in previous studies and also used CD40 ligation as a stimulus rather than LPS. However, with respect to equivalent IL-12p70 generation by CHC versus NHD MoDCs, our data are consistent with those of Bain et al. [19] and Piccioli et al. [21], and also our finding of comparable IL-10 and TNF-α production between MoDCs cultured from both study groups [21].
In conclusion, our data suggest that if CHC-MoDC function is defective, rather than the gross impairment of function reported in some other studies [10,18,19], the defect is subtle and can be overcome by increasing DC numbers (as shown in the MLR assay) or by the magnitude of the maturational stimulus (as shown by cytokine release after stimulation by cell-bound CD40L). Indeed, consistent with the lack of a gross defect in DC function, patients with chronic HCV infection show no evidence of being less able to clear other infections compared with non-HCV-infected individuals. Our results bode well for the possible use of auologous MoDC therapy for chronic HCV infection where large numbers (several millions) of DCs are given that can be preactivated by suitable maturation factors before administration to the patient.
Acknowledgments
We are grateful to the research nurses (Karen Gamble, Jo Cooper, Kirsty Tull and Liz Burge) for venesection. We thank Dr Mike Whelan for critical review of the manuscript. We are especially indebted to all the patients and volunteers who agreed to give blood for this study. This work was supported by iQur Ltd and utilized the Wellcome Trust Clinical Research Facility at Southampton General Hospital.
References
- 1.Rehermann B, Chisari FV. Cell mediated immune response to the hepatitis C virus. Curr Top Microbiol Immunol. 2000;242:299–325. doi: 10.1007/978-3-642-59605-6_14. [DOI] [PubMed] [Google Scholar]
- 2.Lechner F, Gruener NH, Urbani S, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–87. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Ulsenheimer A, Gerlach JT, Gruener NH, et al. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology. 2003;37:1189–98. doi: 10.1053/jhep.2003.50194. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald AJ, Duffy M, Brady MT, et al. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J Infect Dis. 2002;185:720–7. doi: 10.1086/339340. [DOI] [PubMed] [Google Scholar]
- 5.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 6.Lancaster T, Sanders E, Christie JM, Brooks C, Green S, Rosenberg WM. Quantitative and functional differences in CD8+ lymphocyte responses in resolved acute and chronic hepatitis C virus infection. J Viral Hepatol. 2002;9:18–28. doi: 10.1046/j.1365-2893.2002.00330.x. [DOI] [PubMed] [Google Scholar]
- 7.Gruener NH, Lechner F, Jung MC, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–8. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Anthony DD, Yonkers NL, Post AB, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 10.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 11.Murakami H, Akbar SM, Matsui H, Horiike N, Onji M. Decreased interferon-alpha production and impaired T helper 1 polarization by dendritic cells from patients with chronic hepatitis C. Clin Exp Immunol. 2004;137:559–65. doi: 10.1111/j.1365-2249.2004.02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsubouchi E, Akbar SM, Horiike N, Onji M. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J Gastroenterol. 2004;39:754–62. doi: 10.1007/s00535-003-1385-3. [DOI] [PubMed] [Google Scholar]
- 13.Wertheimer AM, Bakke A, Rosen HR. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology. 2004;40:335–45. doi: 10.1002/hep.20306. [DOI] [PubMed] [Google Scholar]
- 14.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237–47. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Ulsenheimer A, Gerlach JT, Jung MC, et al. Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology. 2005;41:643–51. doi: 10.1002/hep.20592. [DOI] [PubMed] [Google Scholar]
- 16.Goutagny N, Vieux C, Decullier E, et al. Quantification and functional analysis of plasmacytoid dendritic cells in patients with chronic hepatitis C virus infection. J Infect Dis. 2004;189:1646–55. doi: 10.1086/383248. [DOI] [PubMed] [Google Scholar]
- 17.Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 18.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–6. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 19.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 20.Longman RS, Talal AH, Jacobson IM, Albert ML, Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–9. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 21.Piccioli D, Tavarini S, Nuti S, et al. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61–7. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–65. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 24.Gowans EJ, Jones KL, Bharadwaj M, Jackson DC. Prospects for dendritic cell vaccination in persistent infection with hepatitis C virus. J Clin Virol. 2004;30:283–90. doi: 10.1016/j.jcv.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Encke J, Findeklee J, Geib J, Pfaff E, Stremmel W. Prophylactic and therapeutic vaccination with dendritic cells against hepatitis C infection. Clin Exp Immunol. 2005;142:362–9. doi: 10.1111/j.1365-2249.2005.02919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–85. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 27.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic cell behaviour and random T cell motility in the lymph node. Proc Natl Acad Sci USA. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]