Abstract
Glucocorticoid-induced tumour necrosis factor receptor (TNFR)-related protein (GITR) is one of the T cell co-stimulatory molecules and is associated with the pathogenesis of a number of autoimmune diseases. We investigated the expression patterns of GITR in human arthritic synovium and the role of GITR in the pathogenesis of rheumatoid arthritis (RA). Immunohistochemical analyses revealed the expression of GITR and its cognate ligand, GITRL, in macrophages in RA, but not in osteoarthritis (OA), synovium. To investigate the role of GITR in macrophage functions, primary macrophages from RA patients and a human macrophage cell line, THP-1, were analysed. Stimulation of the macrophages with anti-GITR monoclonal antibody induced up-regulation of intercellular adhesion molecule (ICAM)-1 and subsequent aggregation/adhesion, which was enhanced by the presence of extracellular matrix proteins and blocked by anti-ICAM-1 monoclonal antibody. The validity of these in vitro observations was confirmed by immunohistochemical analyses of RA synovium, which showed strong expression of ICAM-1 in GITR-positive macrophages. Additionally, GITR stimulation induced expression of proinflammatory cytokines/chemokines and matrix metalloproteinase-9 in synovial macrophages. These data indicate that GITR, expressed on macrophages in human RA synovium, may enhance inflammatory activation of macrophages by promoting cytokine gene expression and adhesion between cells and to extracellular matrix in RA synovium.
Keywords: adhesion, inflammation, arthritis, macrophages
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease resulting in the destruction of cartilage and bone through synovial inflammation. Synovial inflammation involves lining layer thickening and infiltration of inflammatory cells into the sublining areas [1,2]. The number of macrophages, resident cells covering the synovial layer in normal joints, greatly increases in RA synovium [3], and the degree of increase is correlated strongly with the development of severe cartilage destruction [4,5]. Consequently, selective depletion of macrophages from the synovial lining before the induction of experimental arthritis prevented both joint inflammation and cartilage destruction [6,7].
Glucocorticoid-induced tumour necrosis factor receptor (TNFR) family-related protein (GITR), also known as AITR or TNFRSF18, is expressed in naive T cells, CD4+ CD25+ T regulatory (Treg) cells and activated CD25– effector T cells [8,9]. In these T cells, the interaction of GITR with its ligand provides an early co-stimulatory signal, enhancing proliferation and cytokine production [10,11]. The ligand of GITR (GITRL) (TNFSF18/AITRL/TL6) is expressed in endothelial cells, dendritic cells (DCs), macrophages and B cells, but not in T cells [10,12,13]. GITR has also been implicated in RA pathogenesis through studies involving the murine model of arthritis [14,15]. In GITR-deficient mice the severity of collagen-induced arthritis was reduced, demonstrating that GITR-mediated responses are involved in pathogenesis. The authors also demonstrated that GITR triggering abrogated Treg suppressor activity and co-stimulated CD4+ CD25– effector T cells in vitro. These data indicate that GITR-mediated signalling in both regulatory and effector T cells promote the inflammatory processes associated with the development of arthritis. This interpretation was supported further by observations that Treg cells and effector T cells in RA synovial fluid (SF) expressed GITR [16,17] and administration of agonistic anti-GITR antibody exacerbated joint inflammation with elevated production of cytokines and anti-collagen antibody in vivo [15].
Although these previous results support the potential role of GITR as a modulator of both regulatory and effector T cell function during the development of experimentally induced arthritis, the expression patterns of this molecule in human arthritic tissues have not yet been reported. The current study investigated the expression patterns of GITR and GITRL in human RA and osteoarthritis (OA) synovium and the possible role of GITR-mediated macrophage activation in RA pathogenesis.
Materials and methods
Synovial tissue samples and cell fractionation from synovial fluid and peripheral blood
Synovial fluid and peripheral blood were obtained from RA patients during therapeutic arthrocentesis. Fluids and blood were collected in sterile tubes containing preservative-free heparin. Mononuclear cells were isolated from synovial fluid and peripheral blood by density gradient centrifugation using Histopaque (Sigma-Aldrich, St Louis, MO, USA). Subsequently, macrophages were incubated in culture dishes for 1 h and non-adherent cells were removed to obtain adherent cells, which are mainly monocyte/macrophage cells. Macrophage cell purity (> 95% CD14+ cells) was then confirmed using flow cytometry. Synovial fluid macrophages were used directly and peripheral blood monocytes were differentiated into macrophages by incubating the cells for 1 week. Synovial tissue samples were collected from RA/OA patients who were undergoing joint replacement therapy and were snap-frozen in optimum cutting temperature (OCT) compound and stored at −80°C until use. The current study was approved by an institutional review committee and the subjects gave informed consent. RA/OA was diagnosed according to the criteria of the American College of Rheumatology.
Monoclonal antibodies and immunohistochemistry
Monoclonal antibodies (MoAb) for GITR (clone 621) [18] and GITRL (clone EB11) were purchased from Immunomics (Ulsan, Korea); endotoxin levels in the anti-GITR/GITRL stock solution (2 mg/ml) were below 20 pg/ml (tested with the QCL-1000 chromogenic Limulus amebolyte lysate test method; Bio-Whittaker, Walkersville, MD, USA); MoAb for CD68 (KP1) and CD3 (F7·2.38), and rabbit polyclonal antibody to von Willebrand factor (vWF) (N1505) from Dako (Glostrup, Denmark); monoclonal antibody to intracellular adhesion molecule-1 (ICAM-1) (BBIG-1), mouse IgG1 and recombinant human GITRL (rhGITRL) from R&D Systems, Inc. (Minneapolis, MN, USA); and anti-CD11a (HI111) antibody from Becton-Dickinson (Mountain View, CA, USA). For immunohistochemical analysis, frozen synovial tissues were cut into 5-µm sections and were stained using a labelled streptavidin-biotin (LSAB) kit (Dako, Copenhagen, Denmark) according to the manufacturer's manual. Double immunohistochemical analysis was performed as described previously [19]. Briefly, each specimen was treated sequentially with anti-α-actin, anti-GITR or anti-GITRL monoclonal antibody, alkaline phosphatase-labelled secondary reagents and fuchsin for visualization of α-actin, GITR or GITRL staining (red colour). The slides were mounted and pictures were taken at this point to record the staining pattern in the case of GITR and GITRL staining. The same sections were then unmounted and treated sequentially with anti-CD68 monoclonal antibody which was preconjugated with horseradish peroxidase using an Animal Research Kit (Dako Copenhagen, Denmark) according to the manufacturer's manual and diaminobenzidine (DAB) for visualization of CD68 (coloured brown) and finally counterstained with haematoxylin.
Flow cytometric analysis
Flow cytometric analysis was performed on a fluorescence activated cell sorter (FACSCalibur) (Becton-Dickinson, Mountain View, CA, USA). For the analysis of THP-1 cells and SF macrophages, 1 × 106 cells were used per sample. For staining, cells were incubated sequentially with either 1 µg of monoclonal antibodies, 0·5 µg of fluorescein isothiocyanate (FITC)-labelled rat anti-mouse IgG (Caltag Laboratories, Burlingame, CA, USA) and 0·5 µg of phycoerythrin (PE)-labelled anti-CD14 antibody (Caltag Laboratories) in the case of SF macrophages. For the background fluorescence profiles, isotype-matching mouse IgG1 was used for staining. The fluorescence profile of 1 × 104 cells was obtained. For the analysis of cells SF macrophages, CD14+ cells were gated to obtain the GITR/GITRL fluorescence profiles.
Adhesion and aggregation assay
To visualize the aggregation between cells, THP-1 cells were incubated for 10 min with 10 µm of carboxyl fluorescein diacetate succinimidyl ester (CFSE). CFSE-labelled cells were then stimulated with anti-GITR MoAb or mouse IgG which were added to the culture medium at 1–20 µg/ml concentrations. Two days after the stimulation, cellular aggregation was observed with a fluorescence microscope. To measure the adhesion, THP-1 cells (1 × 105 cells/well) were activated by anti-GITR MoAb in 96-well flat-bottomed culture plates that had been precoated with 10 µg/ml of collagen, fibronectin or laminin. As a negative control, cells were stimulated with isotype-matching control antibody (mouse IgG1) in place of anti-GITR MoAb. After 3 days, unbound cells were removed by washing three times with washing solution [fetal calf serum (FCS)-free RPMI-1640]. A redox indicator, Alamar blue (Serotec), diluted in culture media (10% v/v) was added to the culture, followed by incubation for 4 h. The plates were read on an enzyme-linked immunosorbent assay (ELISA) reader at 540 nm (measurement) and 600 nm (reference). The specific absorbance values were calculated using the following equation: the specific OD (540–600) = sample OD (540–600) − media OD (540–600).
Enzyme-linked immunosorbent assay (ELISA) and gelatin zymogram
For the activation utilizing MoAb, SF macrophages were seeded (1 × 105/well) in a 96-well plate and anti-GITR MoAb was added in 1–10 µg/ml concentrations. Supernatants were collected 24 h after the activation and used for ELISA. Cytokines [interleukin (IL)-8, monocyte chemoattractant protein (MCP)-1, IL-6 and tumour necrosis factor (TNF)-α] were measured by sandwich ELISA (R&D Systems). The detection limits of ELISA were < 10 pg/ml for all the cytokines. Data are expressed as mean ± standard deviation (s.d.) of triplicate measurements. The matrix metalloproteinase (MMP) activity in the culture supernatant was determined by performing substrate gel electrophoresis as described previously [20].
Statistical analysis
Statistical significance of difference was evaluated by means of the two-sided Student's t-test, assuming equal variances. Differences were considered significant when P < 0·05.
Results
Expression of GITR and GITRL was detected in RA, but not in OA, synovium
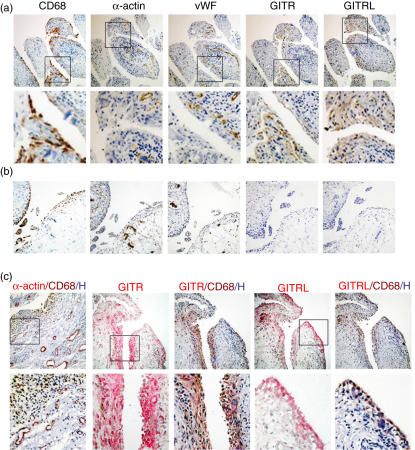
We analysed the expression patterns of GITR and GITRL in human RA and OA synovial tissues. First, we compared the staining patterns of GITR and its ligand with those of CD68 (a marker for macrophages), α-actin (a smooth muscle cell marker) or vWF (a marker for endothelial cells) in RA and OA synovium. The staining patterns of GITR and GITRL in RA synovium (Fig. 1a) matched with that of CD68, but not with those of α-actin and vWF. Both GITR and GITRL were expressed at the highest level in macrophages present in the lining layers. In the sublining areas, the expression of GITR and GITRL was detected in perivascular areas where macrophages and a small number of lymphocytes were mixed to form diffuse cellular aggregates. Few cells were positive for GITR and GITRL in follicular areas, where the majority of the cells are lymphocytes. We detected GITR and GITRL expression in all 10 RA synovial tissues tested. Synovial tissues from OA lacked cellular infiltration in sublining areas (Fig. 1b). CD68-positive macrophages, found mainly in the lining layers, were present in small numbers. The expression of GITR and GITRL was not detected in all seven OA synovial tissues tested.
Fig. 1.
Expression of glucocorticoid-induced tumour necrosis factor receptor (TNFR)-related protein (GITR) and GITR ligand (GITRL) was detected in synovial tissue macrophages of rheumatoid arthritis (RA) patients. RA (a) and osteoarthritis (OA) (b) synovial tissue sections were stained for CD68, α-actin, von Willebrand's factor (vWF), GITR and GITRL. The boxes in the low magnification picture [100×, upper panel in (a) and (b)] indicate the area magnified in high magnification pictures [400×, lower panel in (a)]. (c) RA synovial tissues sections were first stained for α-actin, GITR or GITRL (coloured red) as indicated. Pictures were taken for the GITR or GITRL staining and the same sections were then stained for CD68 in brown. Nuclei were stained in blue. The boxes in the low-magnification picture (100×, upper panel) indicate the area magnified in high-magnification pictures (400×, lower panel).
Because previous reports on mouse arthritis models have indicated that GITR play roles in both Treg and effector T cell functions, we searched for T cell-rich areas for the expression of GITR and GITRL. Expression of GITR was detected in CD3 positive T cell-rich areas, but the staining patterns of GITR and CD3 only partially overlapped. In the case of CD3 staining, positive cells were accumulated focally in the follicular areas of the RA synovium, while the staining pattern of GITR was diffuse in the stroma. In addition, the GITRL-staining pattern was also diffuse in the stroma, resembling the staining pattern of CD68. This indicates that synovial T cells express GITR while macrophages express both GITR and GITRL. Sections of the synovial tissue in which CD3 positive cells were absent were stained with both CD68 and GITR (or GITRL), further strengthening the observation that macrophages express both GITR and GITRL in RA synovium.
In order to confirm whether the cells expressing GITR and GITRL are macrophages, we performed a double immunohistochemistry analysis. RA synovial tissue sections were stained sequentially with anti-GITR or anti-GITRL MoAb and then with anti-CD68 MoAb (Fig. 1c). The same cells stained for GITR or GITRL were also stained for CD68, indicating that both the ligand GITRL and the receptor GITR are expressed in RA synovial macrophages. The specificity of our double staining was confirmed by double-staining a section for CD68 and α-actin. As shown in Fig. 1c, the staining patterns of these two markers did not overlap.
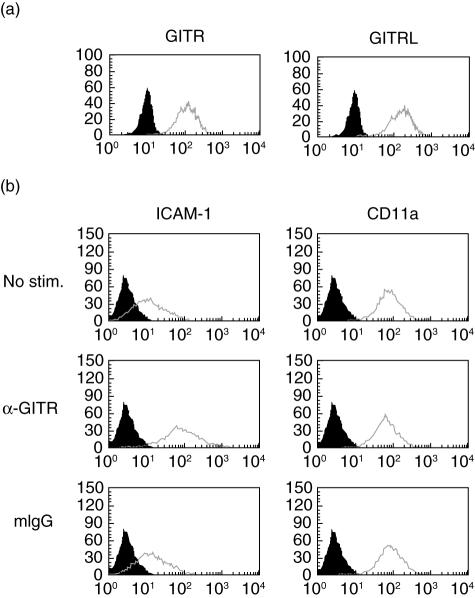
In order to ensure that this ligand–receptor co-expression is a common feature in macrophages, we tested the human macrophage cell line, THP-1, for the cell surface expression of GITR and GITRL. As shown in Fig. 2a, THP-1 cells expressed both GITR and GITRL on the cell surface.
Fig. 2.
THP-1 cells express both glucocorticoid-induced tumour necrosis factor receptor (TNFR)-related protein (GITR) and GITR ligand (GITRL) and the stimulation of GITR induces intracellular adhesion molecule-1 (ICAM-1) expression. (a) THP-1 cells were stained with anti-GITR and anti-GITRL and the fluorescence profiles (empty lines) were overlayed with background staining (filled area, stained with isotype-matching control antibody). (b) THP-1 cells were stimulated with 10 µg/ml anti-GITR monoclonal antibody or isotype-matching control antibody for 2 days. Expression levels of CD54 (ICAM-1) and CD11a were measured using flow cytometry. Histograms from specific staining (empty lines) and background staining (filled area) are compared. These experiments were repeated more than three times with essentially the same results.
GITR stimulation in THP-1 cells induces up-regulation of ICAM-1 expression levels and subsequent aggregation and adhesion
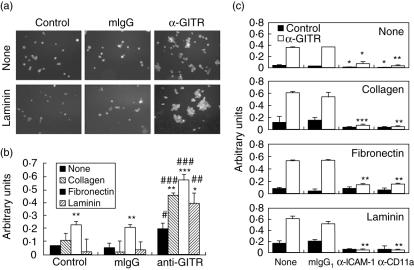
Because synovial macrophages expressed GITR and its ligand, we then investigated the role of GITR in macrophage function in relation to the pathogenesis of RA. Macrophages in RA synovium function mainly as producers of various inflammatory mediators that contribute to the degradation of cartilage and bone. Inflammatory activation of macrophages involves changes in the expression patterns of adhesion molecules which mediate cell–cell and/or cell–extracellular matrix interactions. Additionally, an increase in adhesion molecule expression levels is responsible for the transendothelial migration and tissue infiltration of inflammatory cells [21,22]. Therefore, we investigated the effect of GITR-mediated signalling in the expression levels of various adhesion molecules in the human macrophage cell line, THP-1. Stimulation of GITR was achieved by anti-GITR MoAb treatment which had been reported to have a stimulatory effect on macrophages [18]. We stimulated THP-1 cells with anti-GITR MoAb and analysed the expression levels of various adhesion molecules using flow cytometry. ICAM-1 was included because synovial macrophages have been reported to express this molecule [23,24] and the concentration of soluble ICAM-1 (CD54) has been reported to be elevated in sera and synovial fluids in RA patients [25–27]. Stimulation of GITR caused up-regulation of ICAM-1 expression levels in THP-1 cells, while the expression levels of CD11a were unaffected (Fig. 2b). As ICAM-1 is known to interact with LFA-1 (heterodimer of CD11a and CD18), it was expected that the increase in ICAM-1 expression would induce cellular aggregation. THP-1 cells, labelled with fluorescent dye, were stimulated with anti-GITR MoAband cellular aggregations were visualized by fluorescence microscope. As expected, cellular aggregation was induced in cells stimulated with anti-GITR MoAb, but not in cells treated with isotype-matching control antibody (Fig. 3a). Because anti-GITR MoAb is not the natural ligand of GITR, we used recombinant human GITRL (rhGITRL) to stimulate the cells. The expression levels of ICAM-1 were not changed after rhGITRL treatment (data not shown).
Fig. 3.
Stimulation of glucocorticoid-induced tumour necrosis factor receptor (TNFR)-related protein (GITR) induces intracellular adhesion molecule-1 (ICAM-1)-dependent cellular aggregation/adhesion that was enhanced by extracellular matrix proteins (ECM) proteins. (a) carboxyl fluorescein diacetate succinimidyl ester-labelled THP-1 cells were stimulated with 10 µg/ml anti-GITR monoclonal antibody (MoAb) or control antibody for 2 days in the presence or absence of laminin (10 µg/ml) that had been precoated on the culture plate. Cellular aggregates were visualized using fluorescence microscope. (b) THP-1 cells were stimulated with 10 µg/ml anti-GITR MoAb or isotype matching control antibody (mIgG) for 2 days in the presence or absence of collagen, fibronectin and laminin (10 µg/ml) which had been precoated on the culture plate. After the removal of non-adherent cells, the amount of adherent cells were measured as described in Materials and methods. Unstimulated THP-1 cells were also treated in the same way as a negative control. #P < 0·05, ##P < 0·01, ###P < 0·001 when compared with control. *P < 0·05, **P < 0·01, ***P < 0·001 when compared with cells stimulated without ECM proteins. (c) Experiments were performed as in (b) with the addition of anti-ICAM-1 or anti-CD11a MoAb (10 µg/ml). The same amounts of isotype-matching mouse antibodies were added as a control. **P < 0·01, ***P < 0·001 when compared with positive control.
Tissues and organ structures are maintained by extracellular matrix proteins (ECMs) and the cells within are in constant contact with ECM proteins. These ECM proteins also modulate the activities of cells with which they are in contact [21,22]. When the aggregation assay was performed in the presence of laminin, cellular aggregation and subsequent adhesion to the laminin-coated culture plate was enhanced (Fig. 3a). Up-regulation of ICAM-1 expression levels was not responsible for this enhancement as ICAM-1 expression levels were unaffected by the presence or absence of the laminin (data not shown). Because laminin enhanced aggregation and adhesion, we investigated the effect of other ECM proteins, such as collagen and fibronectin. In these adhesion assays, THP-1 cells were stimulated with anti-GITR MoAb and cellular interactions were allowed in the culture plates precoated with the ECM proteins. Non-adherent cells were then washed out and the amount of remaining adherent cells was measured. As shown in Fig. 3b, anti-GITR MoAb alone increased adhesion of cells and the presence of ECM proteins further enhanced cell adhesion.
In order to confirm whether this aggregation/adhesion was mediated by the interaction between ICAM-1 and CD11a, we performed the same experiments in the presence of blocking anti-ICAM-1 and anti-CD11a MoAb (Fig. 3c). These blocking antibodies inhibit the adhesion of cells stimulated with the anti-GITR MoAb. In the presence of ECM proteins, both anti-ICAM-1 and anti-CD11a MoAb, but not the isotype-matching mouse IgG, almost completely blocked cellular adhesion with statistical significance.
Synovial macrophages and primary macrophages derived from RA patients express GITR and stimulation of GITR-induced proinflammatory cytokines, matrix metalloproteinase-9 and/or ICAM-1
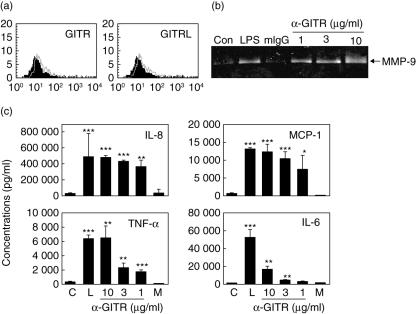
Our observations showing the up-regulation of ICAM-1 after activation of GITR were obtained in experiments using a cultured cell line. In order to support the validity of our in vitro experiment, we tested synovial fluid macrophages isolated from synovial fluids of three RA patients. Synovial fluid macrophages expressed both GITR and GITRL, albeit at low levels (Fig. 4a). We reported recently that GITRstimulation on macrophages, using anti-GITR MoAb, induce expression of inflammatory mediators including various proinflammatory cytokines and MMP-9 [18]. In order to confirm that the GITR in synovial macrophages have a similar function, synovial macrophages were isolated and stimulated with anti-GITR MoAb. Induction of matrix metalloproteinase (MMP)-9 and cytokines/chemokines such as TNF-α, IL-6, IL-8, and MCP-1 were observed in a dose-dependent manner (Fig. 4b,c). We observed the expression of GITR in two of three RA patients tested, and synovial fluid macrophages from these patients responded to anti-GITR MoAb treatment with respect to cytokine induction and MMP-9 expression. However, stimulation of the cells with rhGITRL failed to induce these responses (data not shown).
Fig. 4.
Synovial fluid macrophages express glucocorticoid-induced tumour necrosis factor receptor (TNFR)-related protein (GITR) and GITR ligand (GITRL) and stimulation of GITR induces inflammatory mediators. (a) Synovial fluid cells were double-stained with CD14 and GITR (or GITRL). CD14+ macrophages were gated in flow cytometric analysis and analysed for the expression of GITR and GITRL (open area). For the background staining, isotype-matching mouse IgG was used (filled area). (b) Macrophages isolated from synovial fluids were stimulated with 1 µg/ml lipopolysaccharide (LPS), 10 µg/ml mouse IgG, 1, 3 or 10 µg/ml anti-GITR monoclonal antibody. Culture supernatants were collected after 48 h and analysed for the matrix metalloproteinase (MMP)-9 activity. (c) Synovial macrophages were treated as in (b) and the cytokine levels were measured using sandwich enzyme-linked immunosorbent assay. (c) No treatment control; M, mouse IgG as an isotype-matching control antibody; L, LPS.
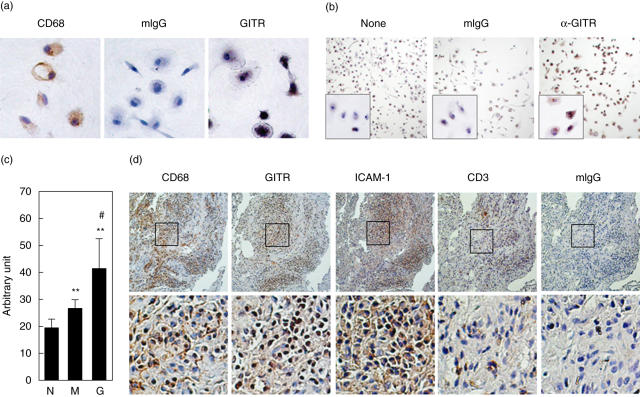
Monocytes from the peripheral blood of RA patients were isolated and incubated for a week to induce macrophage differentiation [28,29]. These cells were confirmed to be macrophages by staining with anti-CD68 MoAb (Fig. 5a). They were also stained with anti-GITR MoAb but not with isotype-matching mouse IgG, indicating that primary macrophages from RA patients expressed GITR (Fig. 5a). These macrophages expressed a low level of ICAM-1 (Fig. 5b). When these cells were activated with anti-GITR MoAb, ICAM-1 expression levels were up-regulated (Fig. 5b) with statistical significance when compared with both no-treatment control and isotype-matching control antibody treatment (Fig. 5c). A low-level induction of ICAM-1 in cells stimulated with mouse IgG was detected with statistical significance (Fig. 5c). This could be mediated by interaction between the Fc portion of the antibody with the Fc receptors, which are known to be up-regulated in macrophages.
Fig. 5.
Intracellular adhesion molecule-1 (ICAM-1) expression can be induced in primary macrophages and synovial macrophages express ICAM-1. (a) Primary macrophages derived from rheumatoid arthritis (RA) peripheral blood monocytes were stained with anti-CD68 monoclonal antibodies (MoAb), mouse IgG or anti-glucocorticoid-induced tumour necrosis factor receptor (TNFR)-related protein (GITR) MoAb. (b) Primary macrophages were stimulated with or without 10 µg/ml of anti-GITR MoAb for 24 h. Ten µg/ml of mouse IgG (mIgG) was used to stimulate the cells as negative control. ICAM-1 expression levels were measured by immunocytochemistry analysis using anti-ICAM-1 MoAb. (c) ICAM-1 expression levels in primary macrophages from two RA patients were tested as in (b). Staining intensities were then measured in three different high-power fields with a total of 80–90 cells in each sample. Mean ± s.d. N, no treatment control; M, stimulated with mIgG; G, stimulated with anti-GITR MoAb. **P < 0·01 when compared with no treatment control, #P < 0·05 when compared with samples treated with mIgG. (d) Consecutive sections of a RA synovium were stained for CD68, GITR and ICAM-1. Isotype-matching control antibody was used as a negative control. The boxes in the low-magnification picture (100×, upper panel) indicate the area magnified in high-magnification pictures (400×, lower panel).
The staining patterns of GITR and ICAM-1 co-localized with that of CD68 in RA synovium
Expression of ICAM-1 in RA synovial macrophages was also tested using immunohistochemical analysis. As shown in Fig. 5d, a strong ICAM-1 expression was detected in areas rich in macrophages, which were also GITR-positive.
Discussion
Previous experiments utilizing collagen-induced arthritis models indicate that the lack of GITR decreased the development of arthritis [14,15]. These reports further demonstrated that stimulation of GITR abrogated suppressor activity in Treg cells, while GITR triggering enhanced the induction of cytokine production in effector T cells. These data indicate that GITR expressed on the surface of Treg cells and effector T cells mediate inflammatory activation associated with the development of arthritis. Although these activities of GITR in T cells could explain the lowered arthritic scores in GITR-deficient mice, our data provide an additional explanation. According to our immunohistochemical data and in vitro experiments, GITR is expressed in RA synovial macrophages, and stimulation of GITR in macrophages induced cellular adhesion and cytokine/chemokine expression, which are the key events in inflammation. These data suggest that lack of GITR on macrophages reduces inflammatory activation of these cells, which consequently inhibits the development of arthritis.
Our immunohistochemical analyses and flow cytometric analyses demonstrated that macrophages express both GITR and GITRL in RA synovium. In contrast, synovial T cells express only GITR, according to our immunohistochemical data and previous analysis of SF Treg and effector T cells [16,17]. These data indicate that macrophages are the cells providing GITRL for the stimulation of GITR on the surface of T cells, as T cells do not express GITRL [10,12,13].
Recently, Lee et al. reported that the treatment of murine monocytic cells with recombinant soluble GITR (rsGITR) resulted in the activation of NOS, COX-2 and MMP-9 [30–32]. Although the cellular receptor for rsGITR and the signalling pathway induced by rsGITR has not yet been elucidated, it is highly likely that rsGITR exerts its effect through reverse signalling via GITRL. Our data showing co-expression of GITR and GITRL in SF macrophages raise the possibility that interaction between these two molecules causes the inflammatory activation of macrophages through GITRL, which will contribute to the cartilage and bone destruction associated with RA pathogenesis.
ICAM-1 is an adhesion molecule expressed primarily by endothelial cells that capture circulating leucocytes and mediate their migration across the endothelium [33]. Unlike this general expression pattern of ICAM-1, various tissue macrophages, including RA synovial macrophages, have been reported to express this molecule [23,24,34]. In agreement with previous observations, our immunohistochemical data revealed strong expression of ICAM-1 in GITR-positive macrophages in RA synovium, and that stimulation of GITR on the surface of macrophages resulted in up-regulation of ICAM-1 expression levels. These observations suggest that ICAM-1 expression levels on the synovial macrophages are also induced or up-regulated by GITR-mediated stimulation. As a consequence of this GITR-mediated increase in ICAM-1 expression levels, THP-1 cells formed cellular aggregates and their adhesion to ECM-coated culture plates was enhanced. The essential role of ICAM-1 in the formation of this cellular aggregate/adhesion was demonstrated by blocking antibodies against ICAM-1 and CD11a, which in turn blocked the adhesion almost completely. The macrophages in RA synovium tend to form aggregates and this could be mediated, at least partially, by interaction between ICAM-1 and its ligands expressed on the surface of these cells. Furthermore, a GITR-mediated increase in ICAM-1 expression in macrophages may contribute to the elevated soluble ICAM-1 levels in the sera and synovial fluid of RA patients [25–27].
It is interesting that cellular aggregation and adhesion were enhanced in the presence of ECM proteins and this was blocked by anti-ICAM-1 or anti-CD11a MoAb. Additional increase in ICAM-1 expression levels cannot explain this enhancement in aggregation/adhesion, as ICAM-1 expression levels were not affected by the presence or absence of ECM proteins (data not shown). Therefore, we then tested whether the enhancement of cellular aggregation/adhesion is mediated by interaction between ECM proteins and integrins on the surface of THP-1 cells. In the case of fibronectin, integrin β1 is one of the principal cell surface molecules involved in interaction with fibronectin. The addition of blocking anti-integrin β1 (CD29) MoAb or synthetic blocking peptide [35] failed to block the aggregation/adhesion of the cells (data not shown). It is possible that the stimulation of GITR may have induced as-yet unidentified adhesion molecule(s). Whatever this adhesion molecule is, it must have played a minor role in the overall adhesion. Instead, aggregation mediated by the interaction between ICAM-1 and LFA-1 must have stabilized the interaction with ECM proteins.
Primary macrophages and THP-1 cells responded to stimulation using anti-GITR MoAb, while rhGITRL failed to induce these responses. This raises the possibility that cells exposed to GITRL require additional secondary stimulation to respond. To identify this possible secondary signal, we tested various stimulants including interferon (IFN)-γ, various ligands of Toll-like receptors, phorbol ester and lysophosphatidylcholine, which were added in suboptimal levels in combination with rhGITRL. None of these stimulants induced responses to GITRL. Our immunohistochemical data indicated that cells expressing GITR/GITRL are found in large cellular aggregates in RA synovium, suggesting that stimulation of GITR on macrophages may require GITRL and other stimulatory molecule(s) to be present on the surface of contacting cells, and that these stimulatory molecules work in concert to induce inflammatory activation of macrophages. Another possibility is that rhGITRL used in our research may not cross-link, and thus stimulate, GITRs on the cell surface. Because the rhGITRL used in our experiments is isolated from cells expressing only the extracellular portion of GITRL, it is possible that it may not form trimers as efficiently as the full-length GITRL. Monomers of GITRL will not be able to cross-link the receptors, and this could be responsible for the lack of response in cells treated with rhGITRL. Our observation showing that the F(ab′)2 fragment, but not the Fab fragment, of anti-GITR MoAb can induce MMP-9 and IL-8 expression in THP-1 cells demonstrates the importance of the cross-linking in stimulating GITR. Finally, we cannot exclude the possibility that there could be other, as-yet unknown, ligand(s) of GITR which can induce, through GITR, inflammatory activation of the cells.
Our data provide the first evidence demonstrating that both GITR and GITRL are expressed in RA synovial macrophages and GITR may play a role in RA pathogenesis through the inflammatory activation of macrophages. Further studies are required to definitely confirm the proposed role of GITR in macrophage function in RA pathogenesis.
Acknowledgments
This work was supported by a grant (no. R01-2003-000-10887-0) from the Basic Research Program of the Korea Science and Engineering Foundation (KOSEF) and by an SRC fund to IRC, University of Ulsan, by KOSEF.
References
- 1.Cunnane G, Hummel KM, Muller-Ladner U, Gay RE, Gay S. Mechanism of joint destruction in rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 1998;46:1–7. [PubMed] [Google Scholar]
- 2.Vervoordeldonk MJ, Tak PP. Cytokines in rheumatoid arthritis. Curr Rheumatol Rep. 2002;4:208–17. doi: 10.1007/s11926-002-0067-0. [DOI] [PubMed] [Google Scholar]
- 3.Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994;53:39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–24. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 6.Van Lent PL, Van den Hoek AE, Van den Bersselaar LA, et al. In vivo role of phagocytic synovial lining cells in onset of experimental arthritis. Am J Pathol. 1993;143:1226–37. [PMC free article] [PubMed] [Google Scholar]
- 7.Van Lent PL, Holthuysen AE, Van Rooijen N, Van De Putte LB, Van Den Berg WB. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type II arthritis. Ann Rheum Dis. 1998;57:408–13. doi: 10.1136/ard.57.7.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4(+) CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+) CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 10.Tone M, Tone Y, Adams E, et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100:15059–64. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamaru F, Youngnak P, Hashiguchi M, et al. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–14. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 12.Kwon B, Yu KY, Ni J, et al. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J Biol Chem. 1999;274:6056–61. doi: 10.1074/jbc.274.10.6056. [DOI] [PubMed] [Google Scholar]
- 13.Kim JD, Choi BK, Bae JS, et al. Cloning and characterization of GITR ligand. Genes Immun. 2003;4:564–9. doi: 10.1038/sj.gene.6364026. [DOI] [PubMed] [Google Scholar]
- 14.Cuzzocrea S, Ayroldi E, Di Paola R, et al. Role of glucocorticoid-induced TNF receptor family gene (GITR) in collagen-induced arthritis. FASEB J. 2005;19:1253–65. doi: 10.1096/fj.04-3556com. [DOI] [PubMed] [Google Scholar]
- 15.Patel M, Xu D, Kewin P, et al. Glucocorticoid-induced TNFR family-related protein (GITR) activation exacerbates murine asthma and collagen-induced arthritis. Eur J Immunol. 2005;35:3581–90. doi: 10.1002/eji.200535421. [DOI] [PubMed] [Google Scholar]
- 16.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+) CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 17.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–7. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WJ, Bae EM, Kang YJ, et al. Glucocorticoid-induced TNFR family-related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques. Immunology. 2006;119:421–9. doi: 10.1111/j.1365-2567.2006.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WJ, Lee WH. LIGHT is expressed in foam cells and involved in destabilization of atherosclerotic plaques through induction of matrix metalloproteinase-9 and IL-8. Immune Network. 2004;4:116–22. [Google Scholar]
- 20.Birkedal-Hansen H, Taylor RE. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982;107:1173–8. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- 21.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- 22.Muller WA. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–34. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 23.Kawanaka N, Yamamura M, Aita T, et al. CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002;46:2578–86. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- 24.Cutolo M, Capellino S, Montagna P, Sulli A, Seriolo B, Villaggio B. Anti-inflammatory effects of leflunomide in combination with methotrexate on co-culture of T lymphocytes and synovial macrophages from rheumatoid arthritis patients. Ann Rheum Dis. 2006;65:728–35. doi: 10.1136/ard.2005.045641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki S, Imai K, Yachi A. Soluble intercellular adhesion molecule-1 (ICAM-1) antigen in patients with rheumatoid arthritis. Scand J Immunol. 1993;38:485–90. doi: 10.1111/j.1365-3083.1993.tb02592.x. [DOI] [PubMed] [Google Scholar]
- 26.Koch AE, Shah MR, Harlow LA, Lovis RM, Pope RM. Soluble intercellular adhesion molecule-1 in arthritis. Clin Immunol Immunopathol. 1994;71:208–15. doi: 10.1006/clin.1994.1074. [DOI] [PubMed] [Google Scholar]
- 27.Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. [Serum soluble adhesion molecules − sICAM-1, sVCAM-1, sE-selectin − in patients with systemic rheumatoid arthritis] Pol Merkuriusz Lek. 2004;17:353–6. [PubMed] [Google Scholar]
- 28.Colli S, Eligini S, Lalli M, Camera M, Paoletti R, Tremoli E. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol. 1997;17:265–72. doi: 10.1161/01.atv.17.2.265. [DOI] [PubMed] [Google Scholar]
- 29.Lesnik P, Rouis M, Skarlatos S, Kruth HS, Chapman MJ. Uptake of exogenous free cholesterol induces upregulation of tissue factor expression in human monocyte-derived macrophages. Proc Natl Acad Sci USA. 1992;89:10370–4. doi: 10.1073/pnas.89.21.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HS, Shin HH, Kwon BS, Choi HS. Soluble glucocorticoid-induced tumor necrosis factor receptor (sGITR) increased MMP-9 activity in murine macrophage. J Cell Biochem. 2003;88:1048–56. doi: 10.1002/jcb.10456. [DOI] [PubMed] [Google Scholar]
- 31.Shin HH, Lee HW, Choi HS. Induction of nitric oxide synthase (NOS) by soluble glucocorticoid induced tumor necrosis factor receptor (sGITR) is modulated by IFN-gamma in murine macrophage. Exp Mol Med. 2003;35:175–80. doi: 10.1038/emm.2003.24. [DOI] [PubMed] [Google Scholar]
- 32.Shin HH, Kwon BS, Choi HS. Recombinant glucocorticoid induced tumour necrosis factor receptor (rGITR) induced COX-2 activity in murine macrophage Raw 264.7 cells. Cytokine. 2002;19:187–92. doi: 10.1006/cyto.2002.1962. [DOI] [PubMed] [Google Scholar]
- 33.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 34.Pouniotis DS, Plebanski M, Apostolopoulos V, McDonald CF. Alveolar macrophage function is altered in patients with lung cancer. Clin Exp Immunol. 2006;143:363–72. doi: 10.1111/j.1365-2249.2006.02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Q, Lai D, Nguyen TT, Chan V, Matsuda T, Hirst SJ. Multiple beta 1 integrins mediate enhancement of human airway smooth muscle cytokine secretion by fibronectin and type I collagen. J Immunol. 2005;174:2258–64. doi: 10.4049/jimmunol.174.4.2258. [DOI] [PubMed] [Google Scholar]