Abstract
The mechanisms involved in impaired immunity in malnourished children are not well understood. CD4+ CD62L– and CD8+ CD28– do not express the naive cell markers CD62L and CD28, suggesting that they function as effector T cells. Using a flow cytometry-based analysis we examined the proportions of CD4+ CD62L– and CD8+ CD28– T cell subsets in well-nourished infected (WNI) and malnourished infected (MNI) children. Here we report that WNI children had a higher percentage of CD4+ CD62L– (11·1 ± 1·0) and CD8+ D28– (40·2 ± 5·0) T cell subsets than healthy (6·5 ± 1·0 and 23·9 ± 4·8) and MNI children (7·4 ± 1·1 and 23·1 ± 6·2, respectively) (P < 0·5). Data suggest that WNI children respond efficiently against pathogenic microbes. In contrast, relatively low numbers of circulating of CD4+ CD62L– and CD8+ CD28– T cells in MNI children may represent an ineffective response to infection. Levels of effector T cells in children with gastrointestinal infections versus those suffering from respiratory infections were also significantly different within the WNI group. While WNI children with gastrointestinal infections had higher absolute and relative values of CD8+, and CD8+ CD28– T subsets, by those with respiratory infections had higher values of CD4+ lymphocytes. However, due to the small number of subjects examined, our results in WNI children should be interpreted with caution and confirmed using a larger sample size. Our data suggest that altered expression of CD62L and CD28 receptors may contribute to impaired T cell function observed in MNI children.
Keywords: bacterial infections, CD4+ CD62L–, CD8+ CD28–, effector T cells, lymphocytes, malnutrition
Introduction
Malnourished children are immunodeficient and therefore more susceptible to infections compared to well-nourished children [1]. Previous reports have indicated that the impaired immune response observed in severely malnourished infected children is related mainly to altered lymphocyte function rather than decreased numbers of T cell subsets [2,3]. It has also been suggested that T lymphocytes are unable to secrete normal quantities of cytokines which mediate and regulate the activation, differentiation and proliferation of lymphocytes required to achieve an adequate immunological response [4]. In contrast to cells from malnourished children with infection (MNI), those from well-nourished children also with infection (WNI) respond adequately to in vitro activation. Additionally, compared to MNI children, WNI children have an increased percentage of memory T cells [2,5].
Infection-mediated T cell activation results in proliferation and acquisition of a variety of effector functions that ultimately produce an array of effector and memory cells. In their ability to proliferate in response to an antigen, T cells display an extensive diversity of phenotypes, functions and tissue migration patterns [6]. Effector and memory T cells progress through a series of stages where the expression of cell-surface markers within CD4+ and CD8+ populations includes adhesion molecules, cytokine receptors and other markers [7,8]. Naive T cells express homing receptors such as CD28 and CD62L (l-selectin, Leu-8). CD28 is a key co-stimulatory molecule involved in lymphocyte activation, whereas CD62L mediates the binding of lymphocytes to high endothelial venules (HEV) and is also involved in lymphocyte attachment to vascular endothelium at sites of inflammation [9,10]. Both molecules are down-regulated in effector T cells and their absence prime cytolytic functions in CD4+ and CD8+ cell subsets [7,9].
Several studies have shown that CD4+ CD62L– and CD8+ CD28– T cells are essential to mount an immunological response to pathogens [7,9]. The aim of the present study was to analyse the status of CD4+ CD62L– and CD8+ CD28– T cell subsets in WNI and MNI children, as well as in well-nourished, non-infected children (WN).
Materials and methods
Study subjects
Blood samples were obtained from 11 well-nourished healthy children (WN), and 10 WNI and eight MNI children suffering from several primary bacterial infections. The bacterial infections were respiratory and/or gastrointestinal and diagnosis was based on clinical symptoms and laboratory tests. The WN and WNI children had normal weight and height according to their age. The gender and age of children included in the three groups were as follows: (a) WN children were nine boys and two girls, age range 8–44 months; (b) WNI children were six boys and four girls, age range 7–34 months. Two WNI children showed respiratory infections, two presented gastrointestinal infections and six had both respiratory and gastrointestinal infections; and (c) two boys and six girls were in the MNI group, age range 7–29 months. Three of these children showed gastrointestinal infections, two were diagnosed with respiratory infections and three with both gastrointestinal and respiratory infections. The severity of acquired malnutrition was assessed by clinical signs and symptoms of malnutrition and weight/height deficit according to the established values for Mexican children [11]. Three children had second-degree malnutrition (weight/height deficit > 25% and < 40% according to age). Three children had marasmus (weight/height deficit > 40%) and two were diagnosed with kwashiorkor (weight/height deficit > 25% due to the presence of oedema). All children were patients of the Maternity-Paediatric Xochimilco Hospital (Mexico City, Mexico), and the protocol was approved by the Medical Ethics Committee of the General Direction of Medical Services (DDF, Mexico).
Cell preparation and staining
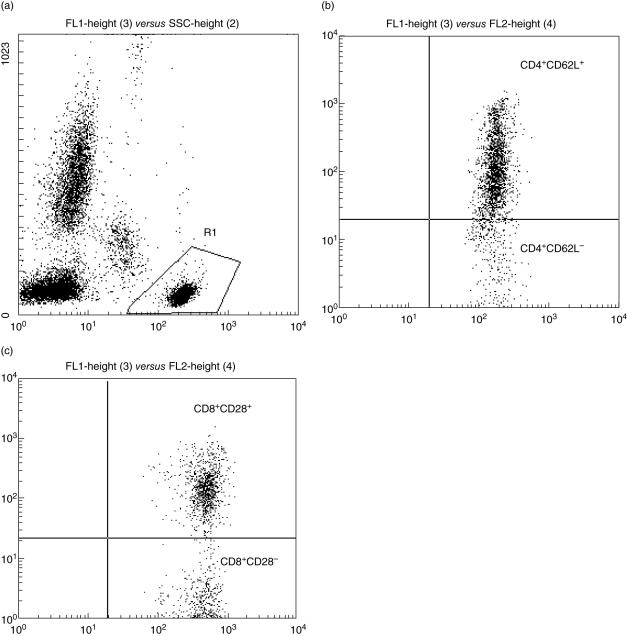
Heparinized peripheral blood was obtained from WN, WNI and MNI children. Sample preparation and staining for flow cytometry were conducted the same day that blood was withdrawn from patients. Lymphocyte subsets were evaluated simultaneously with monoclonal antibodies (MoAbs) conjugated directly to fluorescein isothiocyanate (FITC), phycoerythrin (PE) and peridinin chlorophyll (PerCP). First, cells were incubated with MoAbs for 20 min. Two ml of (1×) fluorescence activated cell sorter (FACS) lysing solution was then added and cells incubated for 10 min. Cells were washed and fixed in paraformaldehyde. All incubation steps were conducted at room temperature in the dark. Acquisition was performed using a FACScan flow cytometry instrument and analysed using CellQuest software (BD Biosciences, San Jose, CA, USA). Ten thousand events were acquired for each sample. Absolute subset cell numbers were determined by multiplying the total cell count by the percentage of cells exhibiting the indicate phenotype. Cell phenotype was identified using conjugated FITC-anti-CD4, PE-anti-CD62L (Leu8), PerCP-anti-CD3 and FITC-anti-CD8, PE-anti-CD28 and PerCP-anti-CD3 MoAbs (BD Biosciences). We acquired CD3+ cells first and then obtained CD4+ and CD8+ subsets. The gate was then restricted to analyse CD4 and CD8 effector positive cells (Fig. 1).
Fig. 1.
(a) The lymphocyte gate set in the FL-1-side-scatter distribution (R1) gate was selected to restrict the analysis of CD4 and CD8 positive cells. (b) CD4 positive effector T cells showing CD62L positive (upper right quadrant) and negative (lower right quadrant) phenotypes. (c) CD 28 positive T cells expressing CD28 antigen (upper right quadrant).
Statistical analysis
Data were analysed by multivariate variance analysis and expressed as mean ± standard error of the mean (s.e.m.). Proportions and absolute numbers of T cell subsets were compared using Student's t-tests. Statistical significance was set at P ≤ 0·05.
Results
Leucocyte counts
Mean total leucocyte counts were within normal ranges according to the children's ages. Total leucocyte counts were 8·29 ± 2·3 × 106 cells/ml in WN, 10·23 ± 3·0 × 106 cells/ml in WNI children and 10·90 ± 5·6 × 106 cells/ml in MNI children. Lymphocytes and neutrophils represented 54·9 ± 16% and 43·8 ± 11%, respectively, of the total leucocyte count in WN children. In WNI, lymphocytes and neutrophils amounted to 48·0 ± 23% and 48·6 ± 23%, respectively, whereas in MNI children those values were 55·1 ± 17% and 44·0 ± 16%. Mean haemoglobin concentration was 13·0 ± 1·2 g/dl in WN children, 12·0 ± 0·8 g/dl in WNI and 9·16 ± 1·0 g/dl in MNI children. Five cases of WNI and seven cases of MNI were found to have anaemia with haemoglobin values < 10 g/dl. Clinical characteristics at hospital admission for gastrointestinal infection were associated with diarrhoea, dehydration and fever. Respiratory infection showed coughing, fever and respiratory distress.
Lymphocyte subsets
The proportion of CD3+ T cells was significantly lower in WNI and MNI children than in WN children (P < 0·001, Table 1). Absolute values of CD3+ T cells were lower in the WNI group when compared to WN and MNI children (P < 0·05). We observed no significant differences in relative and absolute numbers of CD4+ T cells among the study group. Although the WNI children had a significantly higher mean percentages of CD8+ cells than WN (P < 0·05), both groups had mean absolute numbers of CD8+ than were not significantly different (Table 1).
Table 1.
Percentages and absolute values of peripheral lymphocytes in well-nourished (WN), well-nourished infected (WNI) and malnourished infected (MNI) children.
| WN (n = 11) | WNI (n = 10) | MNI (n = 8) | |
|---|---|---|---|
| CD3+ | 34·6 ± 2·3 | 20·5 ± 2·4a | 26·9 ± 2·7a |
| 1462 ± 217 | 786 ± 217a | 1407 ± 266 | |
| CD3+ CD4+ | 57·3 ± 2·6 | 54·1 ± 2·7 | 57·3 ± 3·0 |
| 2597 ± 308 | 2363 ± 292 | 2702 ± 377 | |
| CD4+ CD62L– | 6·5 ± 1·0b | 11·1 ± 1·0 | 7·4 ± 1·1b |
| 260 ± 73b | 489 ± 65 | 244 ± 92b | |
| CD3+ CD8+ | 29·1 ± 3·0 | 38·3 ± 3·0a | 34·3 ± 3·9 |
| 1509 ± 278 | 1697 ± 263 | 1661 ± 372 | |
| CD8+ CD28– | 23·9 ± 4·8b | 40·2 ± 5·0 | 23·1 ± 6·2b |
| 1145 ± 322 | 1792 ± 304 | 1054 ± 407 |
Absolute numbers (106) are indicated in bold type
versus WN group: CD3+, P < 0·001 for relative and P < 0·05 for absolute values; CD8+, P < 0·05 for relative values
versus WNI group: CD4+ CD62L–, P < 0·01 for relative and P < 0·04 for absolute values; CD8+ CD28–P < 0·05 for relative values.
The percentage of CD4+ CD62L– lymphocytes was higher in WNI than in WN and MNI children (P < 0·01, Table 1). Absolute numbers were significantly lower in MNI children compared to WNI children (P < 0·04). Significant differences in relative values of CD8+ CD28– cells were observed among the three study groups (P < 0·05).
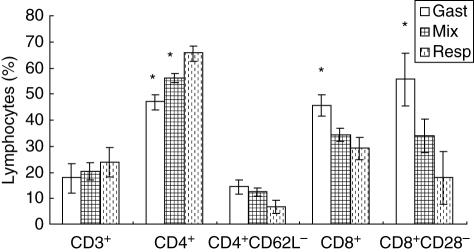
The mean values of CD4+, CD8+, CD4+ CD62L (Leu8–) and CD8+ CD28– T lymphocytes according to infection type are shown in Fig. 2. WNI children with respiratory infections (n = 2) presented higher values of CD4+ lymphocytes when compared to children with gastrointestinal infections (n = 2) or children with both infections (n = 6, P < 0·01). Absolute numbers of CD4+ lymphocytes in the three groups showed the same pattern change as that observed for relative values; however, differences were not statistically significant (data not shown). The mean percentage of CD8+ lymphocytes were significantly higher in children with gastrointestinal infections than by those with respiratory infections (P < 0·05). The absolute number of CD8+ cells was significantly higher in children with gastrointestinal infection than in children with respiratory infection or mixed infections (2785 ± 346, 1107 ± 346 and 1531 ± 200, respectively; P < 0·02).
Fig. 2.
Percentages of peripheral blood effectors lymphocytes in well-nourished infected (WNI) children according to infection type. Gastrointestinal infection (Gast); mixed infection (Mix); respiratory infection (Resp). Mean ± s.e. *Significant difference in WNI children with respiratory infection, CD4+ (P < 0·01), CD8+ (P < 0·05), CD8+ CD28– (P < 0·05).
We observed no significant differences in the percentage of CD4+ CD62L– cells between the three groups of children. In contrast to relative numbers, the mean absolute CD4+ CD62L– cells were significantly different between the study groups (848 ± 122, 448 ± 70 and 255 ± 122, respectively; P < 0·03). Children with gastrointestinal infection showed higher percentage values of CD8+ CD28– T cells than children with respiratory infection (Fig. 2, P < 0·05). The absolute numbers of CD8+ CD28– T cells in children with gastrointestinal infection also showed higher values compared to respiratory and mixed infection groups (gastrointestinal infection: 3407 ± 322; respiratory infection: 672 ± 322; both infections: 1430 ± 186; P < 0·001). MNI children showed no statistical differences in relative and absolute values of lymphocyte subsets when compared according to infection (gastrointestinal versus respiratory) or malnourishment type (second- and third-degree).
Discussion
To gain insight into the underlying immunodeficient mechanisms in malnourished infected children, we investigated the levels of effector T cells in Mexican children with gastrointestinal and respiratory infections. In agreement with our previous data and those of others, we found a significant decrease in CD3+ cells in WNI and MNI children compared to WN children [1,2]. In contrast, the CD8+ cell subset values obtained in the present study were higher in WNI children compared to WN groups, in disagreement with previous findings and our own data [2,3]. This discrepancy may be accounted for by differences in the infection type affecting the WNI group. Although we did not identify the bacteria involved in gastrointestinal infections, Campylobacter spp., Salmonella spp., Shigella spp. and Escherichia coli (enteropathogenic) are typical gastrointestinal pathogens in Mexican children [12]. The bacteria associated most frequently with respiratory infections are Streptococcus pneumoniae, Haemophilus influenzae and Moraxela (Branhamella) catarrhalis [13,14]. Further studies are warranted to ascertain whether activation of effector T cell subsets is associated with specific pathogens causing gastrointestinal and respiratory infections.
In order to determine possible changes in T cell subsets as a function of malnutrition and infection, we measured the percentage of CD4+ CD62L– and CD8+ CD28– cells in fresh blood samples of WN, WNI and MNI children. CD62L– and CD28– subsets possess phenotypical characteristics that have been associated with cytolytic function and these cells can secrete cytokines that play an important role in controlling infections [15,16]. Elevated percentages of CD4+ CD62L– and CD8+ CD28– circulating cells were detected in WNI children. We have reported previously an increased in vitro responsiveness to mitogen of lymphocytes isolated from WNI children [2]. A higher proportion of effector cells in WNI children than in WN and MNI children suggest that WNI children have circulating antigen-experienced cells, whereas MNI children may have an ineffective antigen-activated T cell system. WNI and MNI had infections disease associated with several clinical situations that needed hospitalization, but the percentage of effector cells in MNI children was similar to that observed in WN children (Table 1); this fact can be interpreted as an ineffective antigen experienced in malnourished children. We have reported lower levels of CD4+ CD45RO+ (memory circulating cells) in MNI children compared to WNI children [5]. Thus, a defective activation of T cell-mediated immune mechanisms against invading pathogens may be associated with an increased susceptibility to infections in MNI children.
To our knowledge, this is the first study reporting changes in the number and proportions of CD8+ CD28– and CD4+ CD62L– cells in malnourished children. Involvement of effector T cells in infectious diseases is well documented [17,18]. The CD8+ CD28– cell population consists of a subset of memory T cells whose phenotype and function are highly suggestive of effector T cells. CD8+ CD28– cells produce cytokines and express high levels of granzyme A and perforin [17,19]. They also contain antigen-specific memory CTL (cytotoxic T lymphocyte) and exert a potent cytolytic activity without requiring deliberate in vitro activation [20]. CD4+ CD62Llow memory/effector T cells are required for the generation and maintenance of an immune response and they had been associated with cytolytic activity in virus-positive patients [18].
Although we found significant differences in the number of lymphocyte subsets according to infection type in WNI children, the small sample sizes examined for the gastrointestinal and respiratory groups (n = 2) prevent us from concluding that our findings relate to a larger population of Mexican children with such infections. Further studies with larger sample sizes must be performed. WNI children with gastrointestinal infections also showed higher percentages of CD8+ and CD8+ CD28– T cells compared to children with respiratory infections. It is well known that CD8+ T cells play a crucial role in controlling infections by intracellular pathogens [21] and CD8+ CD28– T cells have been largely studied in viral and intracellular infections [22,23].
A higher number of CD8+ CD28– and CD4+ CD62L– cell subsets were found in children with gastrointestinal infections than in children with respiratory infections. These data suggest that gastrointestinal infections may require a more elaborate immune response that includes several cellular pathways. Alternatively, an increase in effector lymphocytes may constitute the normal response to the critical phase of infection. A time–course analysis of cell types and cytokine production and release in a larger sample of infected children is needed to answer these critical questions. Concerning children with respiratory infections, we observed higher levels of CD4+ cells compared to children with gastrointestinal infections. CD4+ CD62L– cells have been related to T helper 2 (Th2)-type immune responses and might play an essential role in basal airway hyperresponsiveness [24]. Moreover, CD4+ helper T cells might contribute to the optimal priming of CD8 T cells in chronically infected hosts and the phenotypic and functional maturation of CD8 T cell responses [25]. Changes in lymphocyte subsets associated with infection type were not found in malnourished children.
Conclusion
Expression of cell surface molecules regulates effector cell functions. In the present study, we found infection type- and malnutrition-related changes in CD4+ CD62L- and CD8+ CD28– T lymphocyte subsets in peripheral blood. Malnourished children showed impairment in the development of effector cell responses. Our data showed an enhanced production of peripheral blood CD4+ CD62L– and CD8+ CD28– T cells in WNI children, which might be an appropriate host response against pathogenic invasion of microbes associated with efficient induction mechanisms. Our data add evidence to demonstrate that the functionality of lymphocytes is affected by malnutrition, and contribute to understand more clearly the mechanisms of immunodeficiency observed in malnourished children.
References
- 1.Chandra RK. 1990 McCollum Award lecture. Nutrition and immunity: lessons from the past and new insights into the future. Am J Clin Nutr. 1991;53:1087–101. doi: 10.1093/ajcn/53.5.1087. [DOI] [PubMed] [Google Scholar]
- 2.Nájera O, Gonzalez C, Toledo G, et al. Early Activation of T, B and NK lymphocytes in infected malnourished and infected well-nourished children. J Nutr Immunol. 2001;5:85–7. [Google Scholar]
- 3.Najera O, Gonzalez C, Toledo G, Lopez L, Ortiz R. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infections. Clin Diagn Lab Immunol. 2004;11:577–80. doi: 10.1128/CDLI.11.3.577-580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez L, Gonzalez C, Flores L, Jimenez-Zamudio L, Graniel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol. 2005;12:502–7. doi: 10.1128/CDLI.12.4.502-507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Najera O, Gonzalez C, Toledo G, et al. CD45RA and CD45RO isoforms in infected malnourished and infected well-nourished children. Clin Exp Immunol. 2001;126:461–5. doi: 10.1046/j.1365-2249.2001.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefrancois L, Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Curr Opin Immunol. 2002;14:503–8. doi: 10.1016/s0952-7915(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 7.Roman E, Miller E, Harmsen A, et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–68. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodland DL, Dutton RW. Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol. 2003;15:336–42. doi: 10.1016/s0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 9.Altvater B, Pscherer S, Landmeier S, et al. CD28 co-stimulation via tumour-specific chimaeric receptors induces an incomplete activation response in Epstein–Barr virus-specific effector memory T cells. Clin Exp Immunol. 2006;144:447–57. doi: 10.1111/j.1365-2249.2006.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaddi E, Quiroz H, Balbaryski J, et al. [1-selectin expression on T lymphocytes and neutrophils in HIV infected children] Medicina (Buenos Aires) 2005;65:131–7. [PubMed] [Google Scholar]
- 11.Ramos-Galvan R. [The significance and use of somatometric reference values of weight and height in paediatric and epidemiologic practice] Bol Med Hosp Infant Mex. 1992;49:321–34. [PubMed] [Google Scholar]
- 12.Larrosa-Haro A, Ruiz-Perez M, Aguilar-Benavides S. [Utility of studying faces for the diagnosis and management of infants and preschool children with acute diarrhoea] Salud Publ Mex. 2002;44:328–34. [PubMed] [Google Scholar]
- 13.Nandi-Lozano E, Espinosa LE, Vinas-Flores L, Avila-Figueroa C. [Acute respiratory infections in children attending a child day care centre] Salud Publ Mex. 2002;44:201–6. [PubMed] [Google Scholar]
- 14.Solorzano-Santos F, Ortiz-Ocampo LA, Miranda-Novales MG, Echaniz-Aviles G, Soto-Nogueron A, Guiscafre-Gallardo H. [Prevalence of Streptococcus pneumoniae serotypes on nasopharyngeal colonization in children of Mexico City] Salud Publ Mex. 2005;47:276–81. doi: 10.1590/s0036-36342005000400004. [DOI] [PubMed] [Google Scholar]
- 15.Gondois-Rey F, Biancotto A, Bettendroffer MA, Blazkova J, Trejbalova K, Hirsch M. R5 variants of human immunodeficiency virus type 1 preferentially infect C. J Virol. 2006;80:854–65. doi: 10.1128/JVI.80.2.854-865.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schilbach K, Kerst G, Walter S, et al. Cytotoxic minor histocompatibility antigen HA-1-specific CD8+ effector memory T cells: artificial APCs pave the way for clinical application by potent primary in vitro induction. Blood. 2005;106:144–9. doi: 10.1182/blood-2004-07-2940. [DOI] [PubMed] [Google Scholar]
- 17.Acierno PM, Schmitz JE, Gorgone DA, et al. Preservation of functional virus-specific memory CD8+ T lymphocytes in vaccinated, simian human immunodeficiency virus-infected rhesus monkeys. J Immunol. 2006;176:5338–45. doi: 10.4049/jimmunol.176.9.5338. [DOI] [PubMed] [Google Scholar]
- 18.Aslan N, Yurdaydin C, Wiegand J, et al. Cytotoxic CD4 T cells in viral hepatitis. J Viral Hepatol. 2006;13:505–14. doi: 10.1111/j.1365-2893.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorentini S, Licenziati S, Alessandri G, et al. CD11b expression identifies CD8+CD28+ T lymphocytes with phenotype and function of both naive/memory and effector cells. J Immunol. 2001;166:900–7. doi: 10.4049/jimmunol.166.2.900. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006;36:842–54. doi: 10.1002/eji.200535730. [DOI] [PubMed] [Google Scholar]
- 22.Yonkers NL, Rodriguez B, Post AB, et al. HIV coinfection impairs CD28-mediated costimulation of hepatitis C virus-specific CD8 cells. J Infect Dis. 2006;194:391–400. doi: 10.1086/505582. [DOI] [PubMed] [Google Scholar]
- 23.Sud D, Bigbee C, Flynn JL, Kirschner DE. Contribution of CD8+ T cells to control of Mycobacterium tuberculosis infection. J Immunol. 2006;176:4296–314. doi: 10.4049/jimmunol.176.7.4296. [DOI] [PubMed] [Google Scholar]
- 24.Nakagome K, Dohi M, Okunishi K, et al. Antigen-sensitized CD4+CD62Llow memory/effector T helper 2 cells can induce airway hyperresponsiveness in an antigen free setting. Respir Res. 2005;6:46. doi: 10.1186/1465-9921-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–44. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]