Abstract
CD4+ CD25+ regulatory T cells produce the anti-inflammatory cytokines transforming growth factor (TGF)-β or interleukin (IL)-10. Regulatory T cells have been recognized to suppress autoimmunity and promote self-tolerance. These cells may also facilitate pathogen persistence by down-regulating the host defence response during infection with Mycobacterium tuberculosis. We evaluated TGF-β+ and IL-10+ lung CD4+ CD25+ T cells in a murine model of M. tuberculosis. BALB/c mice were infected with ∼50 colony-forming units of M. tuberculosis H37Rv intratracheally. At serial times post-infection, lung cells were analysed for surface marker expression (CD3, CD4, CD25) and intracellular IL-10, TGF-β, and interferon (IFN)-γ production (following stimulation in vitro with anti-CD3 and anti-CD28 antibodies). CD4+ lung lymphocytes were also selected positively after lung digestion, and stimulated in vitro for 48 h with anti-CD3 and anti-CD28 antibodies in the absence and presence of anti-TGF-β antibody, anti-IL-10 antibody or rmTGF-β soluble receptor II/human Fc chimera (TGFβsrII). Supernatants were assayed for elicited IFN-γ and IL-2. Fluorescence activated cell sorter analyses showed that TGF-β- and IL-10-producing CD4+ CD25+ T cells are present in the lungs of infected mice. Neutralization of TGF-β and IL-10 each resulted in increases in elicited IFN-γ, with the greatest effect seen when TGFβsrII was used. Elicited IL-2 was not affected significantly by TGF-β neutralization. These results confirm the presence of CD4+ CD25+ TGF-β+ T cells in murine pulmonary tuberculosis, and support the possibility that TGF-β may contribute to down-regulation of the host response.
Keywords: immunomodulation, interleukin (IL)-10, regulatory T cell, transforming growth factor (TGF)-β, tuberculosis
Introduction
Infections caused by Mycobacterium tuberculosis are characterized frequently by the inability of the host to completely eradicate the pathogen, as evidenced by the fact that one-third of the global population is infected with M. tuberculosis [1]. The human response to pulmonary mycobacterial infection depends primarily on cell-mediated immunity which frequently fails to clear the pathogen, despite a robust inflammatory response with lung necrosis and destruction in advanced cases. One contribution to host failure to clear this organism is concurrent immunosuppression, resulting in insufficient mycobactericidal activity. Immune responses to pathogens are normally counter-balanced by anti-inflammatory mechanisms, possibly serving to limit damage to host tissues from excessive inflammation, and to facilitate resolution of the process, thereby restoring normal organ function. In the setting of mycobacterial infections, which strongly polarize the host immune response to a CD4+ Th1 lymphocyte phenotype, one possible mechanism for counter-balancing the T helper 1 (Th1) response would be through regulatory T cells (Treg), a CD4+ CD25+ lymphocyte subset that produces the cytokines transforming growth factor (TGF)-β and/or interleukin (IL)-10. Overly vigorous down-regulation of the inflammatory response invoked by a pathogen could result in an insufficient host response, with failure to eliminate the organism.
Regulatory T cells consist of several subsets of CD4+ CD25+ lymphocytes. CD4+ CD25+ T cells that are induced in the periphery, rather than the thymus, secrete IL-10 (Tr1 Tregs) and/or TGF-β (Th3 Tregs), and function in tumour immunity and organ transplant rejection [2,3]. There is emerging evidence that induced Tregs may also have a role in infectious diseases, either in facilitating pathogen persistence [4–6] or modulating the host immune response to the infection [7,8]. IL-10 appears to have a minor role in the outcome of mycobacterial infections, especially M. tuberculosis [9–11]. To date, the role of CD4+ CD25+ Th3 T cells in infection with M. tuberculosis has not been characterized fully, but the cytokine TGF-β has been shown to be up-regulated in blood as well as lung granulomas in patients with tuberculosis, and to be permissive for mycobacterial growth, as well as depression of T cell responses [12–14]. Murine pulmonary tuberculosis, which shares certain features with human tuberculosis, is also characterized by granulomatous inflammation and organism persistence. We questioned whether CD4+ CD25+ Th3 Tregs may play a role in the persistence of M. tuberculosis in the lung, and so we examined the frequency and function of CD4+ CD25+ TGF-β- and IL-10-producing lung lymphocytes in a murine model of pulmonary tuberculosis.
Materials and methods
Animals
Specific pathogen-free BALB/c mice (Charles River Laboratories, Inc., Wilmington, MA, USA) were used for these experiments. Animals were housed in the Louisiana State University Health Sciences Center (LSUHSC) Pulmonary/Critical Care Medicine biocontainment level-3 laboratory and experiments were performed in the biocontainment level-3 laboratory in accordance with appropriate safety precautions recommended by the Centers for Disease Control (CDC) [15]. All animal procedures were approved by the LSUHSC Institutional Animal Care and Use Committee. All data represent groups of at least four mice unless otherwise stated.
M. tuberculosis H37Rv infection
M. tuberculosis H37Rv was obtained from ATCC (Rockville, MD, USA; catalogue no. 27294), and was grown in Middlebrook 7H11 broth at 37°C for 14 days. This culture was concentrated by centrifugation, gently sonicated at 95 watts for 10 s in a cup-horn sonicator, and stored in 0·1 ml aliquots at −80°C. At the time of inoculation, an aliquot was thawed, gently sonicated and diluted in endotoxin-free phosphate-buffered saline (PBS) to a concentration of 102 organisms/ml. Mice were anaesthetized lightly with ketamine/xylazine (200 mg/kg/10 mg/kg) intraperitoneally, the ventral surface of the neck swabbed with isopropyl alcohol, and a midline skin incision performed in a sterile fashion. The soft tissues of the neck were gently retracted laterally to expose the trachea. Mycobacteria were sonicated (as above) for 10 s prior to injection to achieve uniform single organism dispersion. Direct intratracheal (i.t.) injection was performed, injecting 100 µl of bacterial suspension (≈ 50 organisms) through a 30-gauge needle, followed by 0·3 ml of air. The incision was closed with a stainless steel surgical clip, and the animal was allowed to recover. An aliquot of the inoculum was plated for quantification. At serial time-points after injection, animals were killed and lungs were homogenized, serially diluted and plated in quadruplicate on Middlebrook 7H10 agar plates. The plates were incubated at 37°C for 17 days. At the end of the incubation period, the number of colony-forming units (CFU) present on plates containing between 20 and 200 CFUs/quadrant was counted and multiplied by the appropriate dilution in order to determine the number of CFU in the initial tissue homogenate.
Isolation of lung lymphocytes
Lung lymphocytes were isolated according to the following method [16]. Lungs were removed from mice in all groups at serial times after killing (days 7, 14, 21, 28 and 35 after infection) and the conducting airways were removed. Lung tissue was minced and placed in RPMI-1640 containing collagenase (150 U/ml) and DNAse (50 U/ml) for enzymatic digestion. Ten ml of the enzymatic solution was used for every 600 mg lung tissue, and the mixture was incubated at 37°C for 90 min with constant stirring. After incubation, the mixture was passed through a 70-µm nylon mesh, placed briefly in NH4Cl lysis buffer and resuspended in media. Cells were counted, the viability was checked with trypan blue, and they were then diluted to the desired concentration.
Analysis of CD3+ CD4+ CD25+ lung lymphocytes by flow cytometry
Lung lymphocyte populations were analysed by flow cytometry. For the initial flow cytometric assay, lung lymphocytes were pooled from the individual mice, and in a repeat experiment of selected time-points after infection (days 21, 28 and 35), lung CD4+ lymphocytes from the individual mice were assayed. Lymphocytes (in single cell suspensions, prepared as above) were stained for CD3 and CD4 surface marker expression with anti-CD3 monoclonal antibody (clone 145–2C11, conjugated with APC-Cy7); anti-CD4 monoclonal antibody (clone RM4-5, conjugated with Alexa Fluor 405; Caltag, Burlingame, CA, USA) and with anti-CD25 monoclonal antibody [clone PC61, conjugated with allanophycocyanin (APC)]. Except where stated, all monoclonal antibodies from BD Pharmingen (San Diego, CA, USA). Isotype monoclonal control antibodies were used to test for non-specific antibody binding. Cells (1 × 106) were incubated with 1 µg of each of the antibodies for 20 min at 4°C.
Assessment of intracellular TGF-β, IL-10 and IFN-γ production by the lung CD4+ CD25+ T cell phenotype was also performed. For these experiments, the lung cells were stimulated in vitro for 4 h (for IFN-γ assay) or 18 h (for TGF-β assay) with anti-CD3 (clone 145–2C11) and anti-CD28 (clone 37·51) antibodies in the presence of brefeldin (added to the final 4 h of the 18-h stimulation). These stimulation parameters were chosen after pilot experiments were performed to optimize the signals of the individual cytokines. Subsequently, cells were washed and stained for CD3, CD4 and CD25 surface marker expression (as detailed above) and then fixed with paraformaldehyde and permeabilized with saponin. Cells were next stained for intracellular TGF-β [clone A75-3, biotin-conjugated, followed by streptavidin conjugated with phycoerythrin (PE)-Texas red]; IL-10 (clone JES5–16E3, conjugated with PE); and IFN-γ (clone XMG1·2, conjugated with PE-Cy7). To determine optimal staining parameters, and as a positive control for intracellular IFN-γ and IL-10 detection, MiCK-1 and MiCK-2 cytokine positive control cells were analysed (BD Biosciences, San Diego, CA, USA), and for intracellular TGF-β staining the TGF-β-producing human colon carcinoma cell line [ATCC CRL-2159 (LS411N)] was used [17]. The cells were analysed with a fluorescence activated cell sorter (FACS) FACSAria flow cytometer using FACSDiva software (BD Biosciences).
Analysis of in vitro CD4+ lung lymphocyte TGF-β production and effect of IL-10 and TGF-β inhibition on CD4+ lymphocyte cytokine production
After single cell suspensions were obtained from the lungs of M. tuberculosis-infected mice, CD4+ lymphocytes were isolated from total lung cells by magnetic bead positive selection (Invitrogen, Carlsbad, CA, USA) [18,19]. CD4+ lymphocytes so isolated were > 90% pure for the lung population when checked by flow cytometry (data not shown). For the initial in vitro assay, lung CD4+ lymphocytes were pooled from the individual mice, and in a repeat experiment of selected time-points after infection (days 21, 28 and 35), lung CD4+ lymphocytes from the individual mice were assayed. CD4+ lymphocytes (1 × 105 cells/well) were plated in duplicate in a 96-well plate. Some wells were stimulated with anti-CD3 (clone 145–2C11) and anti-CD28 (clone 37·51) antibodies, and unstimulated cells were used as controls. To pairs of duplicate wells, one of the following was added: recombinant murine TGF-β soluble receptor II/human Fc chimera at a concentration of 5 µg/ml (TGFβsrII; R&D Systems, Inc., Minneapolis, MN, USA); anti-IL-10 antibody (clone JES052A5; R&D Systems, Inc.) at a concentration of 1 µg/well; anti-TGF-β antibody at either 1 µg/ml or 10 µg/ml (clone 1D11, R&D Systems, Inc.). As additional controls, to some sets of wells, isotype antibodies for anti-IL-10 or anti-TGF-β were added at the same concentrations as the respective neutralizing antibodies. After incubation of the cells for 48 h at 37°C, the supernatants were collected and saved at −80°C for assay for IFN-γ and IL-2 or TGF-β by enzyme-linked immunosorbent assay (ELISA) (as per the manufacturer's recommendations). The lower limits of sensitivity for these ELISAs were 3 pg/ml for TGF-β, 2 pg/ml for IFN-γ and 3 pg/ml for IL-2. As there is TGF-β present in the culture media, media blanks were performed and the TGF-β measured in the blank was subtracted from the samples.
Statistical analysis
Differences between groups (where applicable) were analysed analysis of variance (anova) with Tukey–Kramer HSD follow-up testing. A P-value of < 0·05 was considered significant [20].
Results
M. tuberculosis H37Rv infection
M. tuberculosis H37Rv grew progressively in the lungs of mice after intratracheal inoculation, reaching a phase of non-progressive infection after 28 days, as seen in Table 1.
Table 1.
Growth of Mycobacterium tuberculosis and CD3+ CD4+ T cell subsets in the lungs of infected mice.
| Mtb lung CFU | Day 7 1 ± 0·1 × 103 | Day 14 1·8 ± 0·2 × 104 | Day 21 6·3 ± 1 × 105 | Day 28 3·1 ± 1 × 106 | Day 35 2·8 ± 0·8 × 106 |
|---|---|---|---|---|---|
| T cell subsets (%) | |||||
| CD3+ 4+ | 23 | 35 | 41 | 29 | 29 |
| CD3+ 4+ 25+ | n.d. | n.d. | 2 | 11 | 3 |
| CD3+ 4+ 25+ IFN+ | 0·2 | 3·7 | 2·0 | 8·6 | 2·9 |
| CD3+ 4+ 25+ TGFβ+ | 6·7 | 3·5 | 3·0 | 3·8 | 6·4 |
| CD3+ 4+ 25+ IL-10+ | 4·1 | 5·8 | 3·0 | 1·8 | 1·1 |
Data represent pooled cells from groups at each time-point. IFN: interferon; TGF: transforming growth factor; IL: interleukin; n.d.: not done; CFU: colony-forming units.
Analysis of CD3+ CD4+ CD25+ lung lymphocytes by flow cytometry
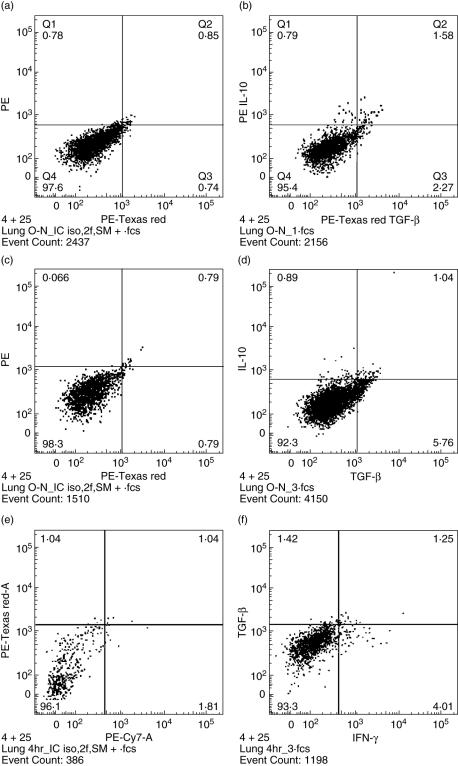
The percentage of CD3+ CD4+ lung lymphocytes rose until day 21 during M. tuberculosis infection, and then remained fairly constant over the times assayed (Table 1). The percentages of the CD3+ CD4+ CD25+ lung lymphocyte subsets over the course of the infection are also shown in Table 1. The CD3+ CD4+ CD25+ IFN-γ+ lymphocyte subset was also evaluated after the 4-h stimulation period, and percentages are displayed in Table 1. CD3+ CD4+ CD25+ IFN-γ+ cells increased and peaked at the day 28 time-point. The CD3+ CD4+ CD25+ TGF-β+ subset was analysed after an 18-h stimulation period. These cells were present at a slightly varying proportion throughout the time-points studied. Finally, CD3+ CD4+ CD25+ IL-10+ lymphocyte subset was also enumerated after the 18-h stimulation period, and was found to be at greatest prevalence (although low) at the early time-points after infection, and declined throughout the remaining time–course studied. Thus, IFN-γ-, TGF-β- and IL-10-producing CD4+ CD25+ lymphocytes are present in the lungs of mice after infection with M. tuberculosis. Representative flow cytometry dot plots (including isotype control plots) are shown in Fig. 1a–f.
Fig. 1.
Representative flow cytometry dot plots of intracellular transforming growth factor (TGF)-β, interleukin (IL)-10 and interferon (IFN)-γ cytokine staining in lung CD4+ CD25+ lymphocytes stimulated in vitro with anti-CD3 and anti-CD28 antibodies. (a) Isotype controls; (b) representative day 21 sample (18-h stimulation) with 2·37% IL-10+ cells and 3·85% TGF-β+ cells, both above the respective isotype antibody controls. (c) Isotype controls; (d) representative day 28 sample (18-h stimulation) with 1·93% IL-10+ cells and 6·80% TGF-β+ cells, both above the respective isotype controls. (e) Isotype controls; (f) representative day 28 sample (4-h stimulation) with 5·26% IFN-γ+ cells, exceeding the respective isotype control.
Analysis of CD4+ lung lymphocyte TGF-β production and effect of TGF-β inhibition on CD4+ lymphocyte cytokine production
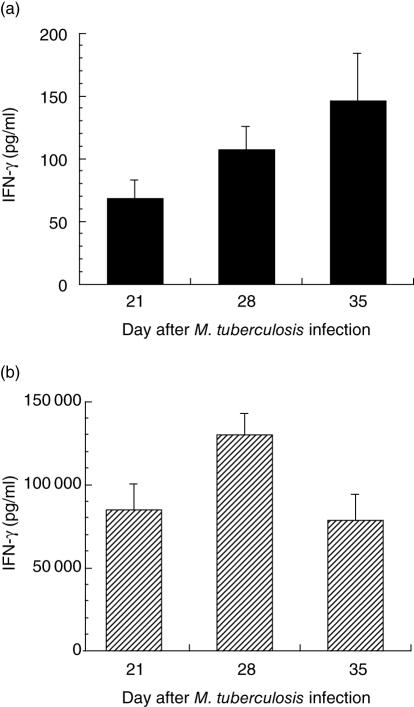
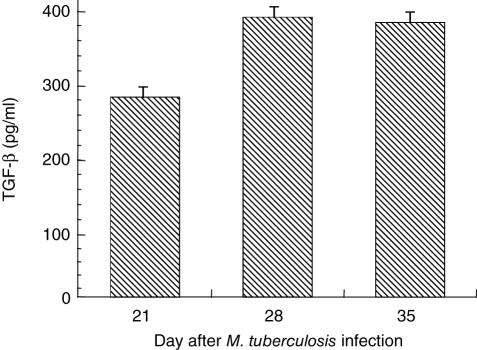
After positive selection by magnetic beads, lung lymphocytes enriched for CD4+ T cells were stimulated in vitro with anti-CD3 and anti-CD28 antibodies and IFN-γ, TGF-β and IL-2 elicitation studied. Detectable values for all three of these cytokines were confined generally to the latter three time-points, when the infection was maximal, so results from these times are presented. For elicited IFN-γ, unstimulated controls are shown in Fig. 2a, and with stimulation, levels were elevated at all the latter three time-points (Fig. 2b). TGF-β elicitation is shown in Fig. 3. Elicited TGF-β was present in the supernatants at day 21 and slightly increased further by days 28 and 35. The values shown are corrected for the media blank, and unstimulated controls were not different from the media blank sample. In our previous work, IL-10 was assayed in the stimulated CD4+ lymphocyte supernatants, and relatively low levels (≤ 300 pg/ml) were detectable at these same time-points [19]. The supernatants from unstimulated CD4+ lymphocytes had < 150 pg/ml IFN-γ (Fig. 2a) and < 40 pg/ml for IL-2 at all three time-points (data not shown).
Fig. 2.
(a) Interferon (IFN)-γ elicited from positively selected, unstimulated CD4+ lung lymphocytes at days 21, 28 and 35 after infection with Mycobacterium tuberculosis. There are only low levels of IFN-γ at all time-points. (b) IFN-γ elicited from positively selected CD4+ lung lymphocytes stimulated in vitro with anti-CD3 and anti-CD28 antibodies. There is substantially more IFN-γ elicited at each time-point after stimulation.
Fig. 3.
Transforming growth factor (TGF)-β elicited from positively selected CD4+ lung lymphocytes stimulated in vitro with anti-CD3 and anti-CD28 antibodies. Values corrected for media blank. Unstimulated cells did not produce TGF-β at levels above the media blank control.
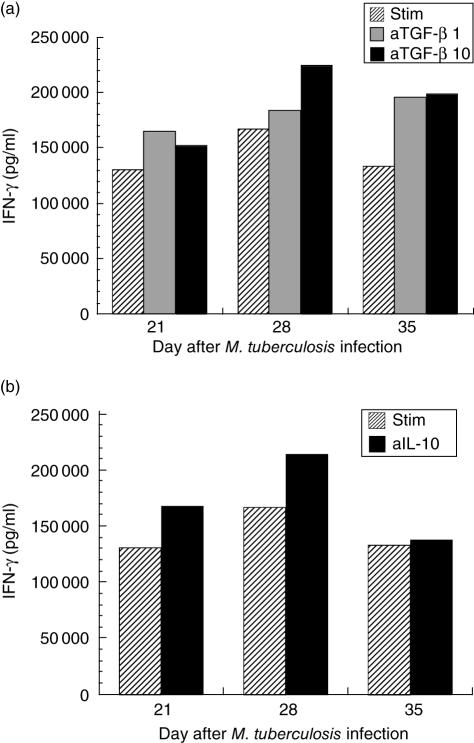
The effects of adding anti-TGF-β antibody (1 µg/ml and 10 µg/ml) to the pooled CD4+ lymphocyte cultures demonstrated that there was also a modest effect on up-regulating IFN-γ elicitation by those cells resulting in, at most, approximately 25% increase in elicited IFN-γ over the stimulated control values, with no differences between the two antibody concentrations (Fig. 4a). Addition of anti-IL-10 antibody to the stimulated CD4+ lymphocytes also resulted in a modest increase in IFN-γ elicitation from the cells (Fig. 4b), consistent with the published studies of M. tuberculosis infection in mice deficient in IL-10 [9–11]. CD4+ T cells incubated with isotype antibodies in lieu of anti-TGF-β or anti-IL-10 produced levels of IFN-γ very similar to the stimulated levels.
Fig. 4.
(a) Interferon (IFN)-γ elicited from positively selected pooled CD4+ lung lymphocytes stimulated in vitro with anti-CD3 and anti-CD28 antibodies, without (hatched bars) and with anti-transforming growth factor (TGF)-β antibody added to the wells at 1 µg/ml (grey bars) or 10 µg/ml (black bars) at days 21, 28 and 35 after infection with Mycobacterium tuberculosis. There is modest augmentation of the TGF-β elicited with either dose of anti-TGF-β antibody, but no dose–response evident. The maximum increase in IFN-γ is seen at the day 35 time (46% increase with anti-TGF-β 1 µg/ml and 49% increase with anti-TGF-β 10 µg/ml). (b) IFN-γ elicited from positively selected pooled CD4+ lung lymphocytes stimulated in vitro with anti-CD3 and anti-CD28 antibodies, without (hatched bars) and with anti-IL-10 antibody added to the wells at 1 µg/ml (black bars). Modest increases in IFN-γ are present at days 21 and 28 (both 29% above stimulated control), but no effect is evident at the day 35 time-point (3% above stimulated control).
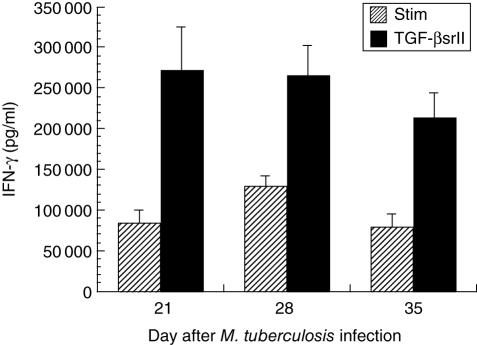
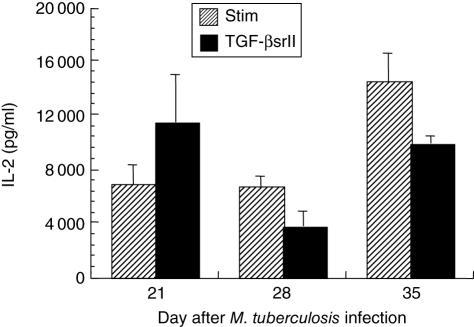
When TGFβsrII was added to the CD4+ lymphocyte cultures, significant, substantial increases in IFN-γ elicitation (≥ 100% of the stimulated controls for each time-point) were observed at days 21, 28 and 35 (Fig. 5). The more potent effect of TGFβsrII on facilitating the elicitation of IFN-γ reflects the fact that anti-TGF-β antibody is less efficient at inhibiting TGF-β than is the soluble receptor, which neutralizes TGF-β1 and TGF-β2 at the 1 nmol/l range [21]. Thus, when TGF-β is effectively neutralized in the setting of stimulation of lung CD4+ lymphocytes from mice infected with M. tuberculosis, the IFN-γ-producing capacity of those lymphocytes is markedly facilitated. IL-2 showed an inconsistent effect related to neutralization of TGF-β, with no significant change from the stimulated control samples (Fig. 6).
Fig. 5.
Interferon (IFN)-γ elicited from positively selected CD4+ lung lymphocytes stimulated in vitro with anti-CD3 and anti-CD28 antibodies, without (hatched bars) and with recombinant murine TGF-β soluble receptor II (TGFβsrII) added to the wells at 5 µg/ml (black bars). There are significant increases in IFN-γ production with TGFβsrII at each time-point: 223% of stimulated value at day 21 (P = 0·02 TGFβsrII versus stimulated (STIM)], 104% of stimulated value at day 28 (P = 0·02 TGFβsrII versus STIM) and 174% of stimulated value at day 35 (P = 0·007 TGFβsrII versus STIM).
Fig. 6.
Interleukin (IL)-2 elicited from positively selected CD4+ lung lymphocytes stimulated in vitro with anti-CD3 and anti-CD28 antibodies, without (hatched bars) and with recombinant murine TGF-β soluble receptor II (TGFβsrII) added to the wells at 5 µg/ml (black bars). There is an insignificant increase in elicited IL-2 with TGFβsrII at the day 21 time-point only (P = 0·25 stimulated (STIM) versus TGFβsrII), and no evident augmentation at days 28 and 35 post-infection.
Discussion
We show that TGF-β- and IL-10-producing CD4+ CD25+ lymphocytes can be recovered from the lungs of normal mice infected with virulent M. tuberculosis. Additionally, TGF-β and IL-10 can be elicited from a purified population of stimulated lung CD4+ lymphocytes. Furthermore, when inhibitors of IL-10 or TGF-β are added to the stimulated CD4+ lung lymphocyte cultures from the infected mice, IFN-γ production by those cells is subsequently enhanced. In keeping with the relatively minor role of IL-10 reported in mycobacterial infections [9–11], neutralization of IL-10 in the CD4+ lung lymphocytes resulted in a relatively modest increase in IFN-γ production. Neutralization of TGF-β in vitro resulted in marked augmentation of IFN-γ. Lack of a consistent effect of TGF-β neutralization on augmentation of IL-2 elicitation was found, due to perhaps the previous near maximal up-regulation of IL-2 gene expression in the previously activated CD4+ cells (in vivo), which may render them refractory to TGF-β inhibition of IL-2 promoter activity [22].
TGF-β, produced by cells of different lineages (including mononuclear cells) and also released by platelets, comprises a family of related proteins. The TGF-β1 isotype confers the greatest activity in mammals, and is critical for normal development, as mice lacking this protein have excessive systemic multi-organ inflammation and live only a short time if born alive [23]. TGF-β has significant regulatory effects on T lymphocytes, inhibiting proliferation and differentiation. Several mechanisms are responsible for these effects, including inhibition of IL-2 production and alteration of regulators of the cell cycle [22]. The differentiation of both Th1 and Th2 CD4+ lymphocytes is influenced negatively by TGF-β, through down-regulation of key transcription factors: Gata-3 in Th2 cells (leading to failure of differentiation of Th2 cells) and T-bet in Th1 cells (leading to alteration of the response of CD4+ T cells to IL-12, thereby depressing Th1 development) [24,25]. Thus, TGF-β counterbalances CD4+ effector T cell-driven inflammation.
Subsets of induced CD4+ CD25+ lymphocytes produce high levels of IL-10 (Tr1) or TGF-β (Th3). Both Tr1 and Th3 cells (inducible Tregs) seem to originate (possibly from naive CD4+ cells or CD4+ effector T cells) in the periphery (rather than the thymus) in response to specific stimuli, such as tumour cells or pathogens [2]. Tr1 and Th3 cells suppress lymphocytes via the secretion of IL-10 or TGF-β, respectively, rather than exclusively by cell–cell contact (as do natural, or central, Tregs). Tr1 and/or Th3 subset induction may depend on the nature of the antigenic stimulus, the innate cytokine milieu in which naive T cells encounter antigen in conjunction with the antigen presenting cell (i.e. dendritic cell [DC]), or pathogen-derived signals stimulating the DC innately via pattern recognition receptors, which may influence the state of maturation of the DC [26]. If the activating signals for the DC stimulate it to elaborate IL-10 (rather than IL-12), the naive CD4+ lymphocyte may be directed to a Treg phenotype, rather than that of a CD4+ Th1 or Th2 effector T cell. The location of antigenic encounter may also play a role in the induction of Tregs compared to effector T cells, with mucosal sites (i.e. gut or lung) thought to induce antigen-specific Tregs preferentially. This may facilitate tolerance to the plethora of antigenic encounters at these sites, where repeated immune reactions would be detrimental to the host [5].
Inducible Treg subsets may play roles in pathogen-driven inflammation, to limit immunologically mediated pathology and to facilitate restoration of normal organ function after an inflammatory reaction. These cells have been described in murine Pneumocystis carinii, Bordetella pertussis, onchocerciasis, feline immunodeficiency virus and human hepatitis C infection [4–7,27]. While no description of Tregs in murine M. tuberculosis infection has occurred prior to this time, TGF-β has been described to play a role in tuberculous infection. Guinea pigs infected with virulent M. tuberculosis and then treated with recombinant TGF-β1 demonstrate significantly enhanced bacterial burdens compared to the control guinea pigs [28]. Humans with pulmonary M. tuberculosis infection have enhanced TGF-β production by peripheral blood mononuclear cells (PBMCs) compared to uninfected individuals, and TGF-β has been detected by immunohistochemistry in cells comprising human lung granulomas [12]. PBMCs infected with M. tuberculosis are permissive for bacterial replication in a TGF-β dose-dependent fashion, and neutralization of this cytokine enhances bactericidal activity [29]. TGF-β can also be induced with purified protein derivative (PPD) or lipoarabinomannan from PBMCs from healthy, uninfected subjects [30,31]. Neutralization of the TGF-β produced by PBMCs of infected tuberculosis patients results in enhancement of both lymphocyte proliferation and IFN-γ production in vitro [14], suggesting that TGF-β may play animmunosuppressive role in the setting of infection. Recently described are CD4+ CD25+ lymphocytes, recoverable from the blood and lung lavage of patients with tuberculosis, that appear capable of suppressing IFN-γ production in the blood [13]. Damping the host immune response against M. tuberculosis, although ameliorating immunopathology, may confer an advantage to the pathogen that allows persistence.
We demonstrate that CD4+ CD25+ TGF-β-producing T cells are present in the lungs of mice infected with virulent M. tuberculosis. While others have shown that TGF-β is produced by monocytic cells in the setting of tuberculous infection and may play an immunomodulatory role, ours is the first report of functional CD4+ CD25+ regulatory T cells in murine pulmonary tuberculosis. We demonstratethat Tregs are present and that they produce TGF-β. When this TGF-β is efficiently neutralized in vitro, a substantial increase in IFN-γ can be elicited from CD4+ lymphocytes, suggesting that TGF-β produced by CD4+ CD25+ T cells actively down-modulates IFN-γ production from lung CD4+ effector T cells. IFN-γ is recognized to be the primary effector cytokine for the induction of host mycobactericidal activity [32,33]. It is possible, by suppressing the full expression of IFN-γ, that TGF-β is permissive for persistence of M. tuberculosis.
While we have identified CD4+ CD25+ TGF-β-producing T cells in the lungs of mice infected with M. tuberculosis, we have not yet screened exhaustively for other markers described as characteristic of Tregs. The glucocorticoid-induced tumour necrosis factor (TNF) receptor (GITR) and the co-stimulatory molecule cytotoxic T lymphocyte-associated antigen 4 [CTLA-4 (CD152)] are other surface markers expressed on Tregs, but not exclusively, as they may also be expressed on other subsets of activated T cells [34–36]. Forkhead box P3 (Foxp3) is a recently described transcription factor that is involved in the development of Tregs, but the expression of this transcription factor is not limited to CD4+ CD25+ cells, and can be found in CD4+ CD25– cells [37]. While it may be a marker of Tregs, the significance of expression in CD4+ CD25– T cells in the setting of pathogen-driven inflammation is uncertain at the current time. Further study is required to determine the expression of Foxp3 in relation to the TGF-β-producing CD4+ CD25+ T cells in our model. While we demonstrate that TGF-β can be elicited in vitro from lung cells highly enriched for CD4+ lung lymphocytes, the contribution of other TGF-β-producing mononuclear cells cannot be excluded entirely. However, the presence of CD4+ CD25+ TGF-β-producing lung lymphocytes, as shown in the flow cytometry analyses, and the elicitation of TGF-β from highly purified lung CD4+ lymphocytes in vitro are highly suggestive of a role for CD4+ CD25+ T cells in TGF-β production in our system. Finally, while we have demonstrated the production of TGF-β in lung CD4+ CD25+ T cells in our model (the neutralization of which results in significantly augmented IFN-γ production by CD4+ lung lymphocytes), we have not discerned whether the TGF-β effect is due to surface-bound TGF-β or secreted cytokine. Future studies would also be required to distinguish between these two possibilities, although assuming the CD4+ CD25+ T cells described are induced Tregs, the latter possibility would be the most feasible.
In summary, we have demonstrated that CD4+ CD25+ TGF-β-producing and CD4+ CD25+ IL-10-producing T cells are present in the lungs of normal mice infected with virulent M. tuberculosis. In vitro, when these cytokines are neutralized (especially so in the case of TGF-β), substantially more IFN-γ can be elicited from the lung CD4+ lymphocytes. These findings suggest the intriguing possibility that neutralizing the production of TGF-β in vivo in the setting of tuberculous infection may afford the host a significant advantage in eliminating a pathogen that frequently resists eradication.
Acknowledgments
This work was supported by the following grants: AA11760 (to C. M. M), HL076100 (to C. M. M and P. Z) and AA09803 (to S. N). The authors also wish to thank Connie Porretta of the LSUHSC Pulmonary/Critical Care Medicine Immunology Core Laboratory for expert assistance with all aspects of the flow cytometry assays in this work.
References
- 1.World Health Organization. Tuberculosis Fact Sheet. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/index.html (accessed 29 January 2007)
- 2.Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–7. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- 3.McHugh R, Shevach E. The role of suppressor T cells in regulation of immune responses. Molec Mechan Allerg Clin Immunol. 2002;110:693–702. doi: 10.1067/mai.2002.129339. [DOI] [PubMed] [Google Scholar]
- 4.Vahlenkamp T, Tompkins M, Tompkins W. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+CD25+ T regulatory cells. J Immunol. 2004;172:4752–61. doi: 10.4049/jimmunol.172.8.4752. [DOI] [PubMed] [Google Scholar]
- 5.McGuirk P, McCann C, Mills K. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordella pertussis. J Exp Med. 2002;195:221–31. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satoguina J, Mempel M, Larbi J, et al. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminthic infection (onchocerciasis) Microb Infect. 2002;4:1291–300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Carvalho T, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–91. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Montagnoli C, Bacci A, Bozza S, et al. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol. 2002;169:6298–308. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- 9.Roach D, Martin E, Bean A, Rennick D, Briscoe H, Britton W. Endogenous inhibition of antimycobacterial immunity by IL-10 varies between mycobacterial species. Scand J Immunol. 2001;54:163–70. doi: 10.1046/j.1365-3083.2001.00952.x. [DOI] [PubMed] [Google Scholar]
- 10.Murray P, Young R. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–95. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North R. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–8. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J. Enhanced production of TGF-β by blood monocytes from patients with active tuberuclosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–73. [PubMed] [Google Scholar]
- 13.Ribeiro-Rodrigues R, Co T, Rojas R, Toosi Z, Dietze R, Boom W, Maciel E, Hirsch C. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch C, Hussain R, Toosi Z, Dawood G, Shahid F, Ellner J. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193–8. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richmond J, McKinney R. Biosafety in Microbiological and Biomedical Laboratories. 2. Washington, DC: US. Government Printing Office; 1993. [Google Scholar]
- 16.Hoag K, Street N, Huffnagle G, Lipscomb M. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol. 1995;13:487–95. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 17.Garba M, Frelinger J. Intracellular cytokine staining for TGF-β. J Immunol Meth. 2001;258:193–8. doi: 10.1016/s0022-1759(01)00491-4. [DOI] [PubMed] [Google Scholar]
- 18.Anderson G, Jenkinson E, Moore N, Owen J. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993;362:70–3. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- 19.Mason C, Dobard E, Shellito J, Nelson S. CD4+ lymphocyte responses to pulmonary infection with Mycobacterium tuberculosis in naive and vaccinated BALB/c mice. Tuberculosis. 2001;81:327–34. doi: 10.1054/tube.2001.0306. [DOI] [PubMed] [Google Scholar]
- 20.Bourke G, Daly L, McGilvray J. Interpretation and uses of medical statistics. 3. Oxford: Blackwell Scientific Publications; 1985. [Google Scholar]
- 21.Suzuki E, Kapoor V, Cheung H, et al. Soluble type II transforming growth factor-β receptor inhibits established murine malignant mesothelioma tumor growth by augmenting host tumor activity. Clin Cancer Res. 2004;10:5907–18. doi: 10.1158/1078-0432.CCR-03-0611. [DOI] [PubMed] [Google Scholar]
- 22.Brabletz T, Pfeuffer I, Schorr E, Siebelt F, Wirth T, Serfling E. Transforming growth factor β and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol Cell Biol. 1993;13:1155–62. doi: 10.1128/mcb.13.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni A, Huh C, Becker D, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorelik L, Fields P, Flavell R. Cutting edge. TGF-β inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–7. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 25.Gorham J, Guler M, Fenoglio D, Gubler U, Murphy K. Low dose TGF-beta attenuates IL-12 responsiveness in murine Th1 cells. J Immunol. 1998;161:1664–7. [PubMed] [Google Scholar]
- 26.Mills K, McGuirk P. Antigen-specific regulatory T cells − their induction and role in infection. Semin Immunol. 2004;16:107–17. doi: 10.1016/j.smim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Cabrera R, Tu Z, Xu Y, et al. An immunoregulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 28.Dai G, McMurray D. Effects of modulating TGF-β1 on immune responses to mycobacterial infection in guinea pigs. Tuberc Lung Dis. 1999;79:207–14. doi: 10.1054/tuld.1998.0198. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch C, Yoneda T, Averill L, Ellner J, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-β1. J Infect Dis. 1994;170:1229–37. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 30.Toosi Z, Young T, Averill L, Hamilton B, Shiratsuchi H, Ellner J. Induction of transforming growth factor β1 by purified protein derivative of Mycobacterium tuberculosis. Infect Immun. 1995;63:224–8. doi: 10.1128/iai.63.1.224-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahl K, Shiratsuchi H, Hamilton B, Ellner J, Toosi Z. Selective induction of transforming growth factor β in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis. Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper A, Dalton D, Stewart T, Griffin J, Russell D, Orme I. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn J, Chan J, Triebold K, Dalton D, Stewart T, Bloom B. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Boden E, Henriksen K, Bour-Jordan H, Bi M, Bluestone J. Distinct roles of CTLA-4 and TGF-β in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–9. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontenot J, Rasmussen J, Williams L, Dooley J, Farr A, Rudensky A. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]